Abstract
Objective:
Study aims were to: 1) characterize, among pregnant Mexican women, gestational weight gain (GWG) trajectories, 2) assess associations of maternal dietary quality score (MDQS) with GWG during early-mid pregnancy, middle pregnancy, late pregnancy and prolonged pregnancy and 3) evaluate the association between MDQS and adequacy of GWG, throughout pregnancy.. We hypothesized that higher MDQS adherence is protective against insufficient or excessive GWG across pregnancy and that the association between MDQS adherence and GWG would vary by pre-pregnancy body mass index (BMI) category.
Research Methods & Procedures:
We analyzed data from 660 pregnant women participating in the Pregnancy Research on Inflammation, Nutrition and City Environments: Systematic Analyses (PRINCESA) cohort in Mexico City, 2009–2014. Repeated measures of dietary intake and mother’s weight were obtained during pregnancy. Individual GWG trajectories were modeled in a multilevel regression framework. Associations between MDQS (low, medium and high adherence) and GWG were investigated using mixed-effect regression models with linear splines.
Results:
Women with pre-pregnancy BMI of 30.0 kg/m2 or greater had a slower rate of GWG (RGWG) compared with other categories. A higher adherence to MDQS was protective against an insufficient (odds ratio (OR): 0.63; 95% confidence interval (CI) 0.42, 0.95; p=0.03)] and an excessive RGWG (OR: 0.62; 95% CI 0.41, 0.94; p=0.03) throughout pregnancy, adjusting for pre-pregnancy BMI, energy intake, maternal age, educational level, parity, fetal sex, marital status and physical activity. Associations between diet and RGWG differed by gestational period.
Conclusion:
A better quality diet, as measured by MDQS, was associated with appropriate GWG during pregnancy in the PRINCESA cohort.
Keywords: gestational weight gain, PRINCESA cohort, maternal diet, maternal diet quality score, dietary patterns, Mexico
Introduction
Epidemiological studies link women’s nutritional status and diet during pregnancy to later risk of non-communicable diseases among offspring [1]; these factors also influence the growth, metabolism and development of the fetus[2,3]. Gestational weight gain (GWG) is a complex phenotype that is influenced by maternal responses to pregnancy, such as gestational fat deposition and blood volume expansion, as well as fetal growth, placental size and amniotic fluid volume [4,5].
Independently of pre-pregnancy body mass index (prepregnancy-BMI), GWG has been shown to influence both maternal and infant outcomes[6]. Insufficient gestational weight gain (IGWG) increases the risk of preterm birth and low birth weight (LBW). In contrast, excessive gestational weight gain (EGWG) is associated with an increased risk of pregnancy-induced hypertension, preeclampsia, emergency caesarean delivery and macrosomia[5,7]. Additionally, women who gain excessive weight are at higher risk of postpartum weight retention, which may influence their susceptibility to developing overweight and obesity in the longer term and increase perinatal risks in subsequent pregnancies[6,7].
Multiple variables have been reported as predictors of EGWG, including nulliparity, pregestational overweight, low income, black or Hispanic ethnicity, unmarried status, limited education, and young maternal age[5,8]. Nevertheless, little is known about the association between modifiable risk lifestyle factors, such as diet, and GWG. Designing studies on how maternal diet can influence GWG is challenging due to the physiological adaptations that occur in pregnancy, the multiple interactions between nutrients and food groups, and the importance of the pre-pregnancy nutritional status of the mother, which is often difficult to characterize in cohorts that enroll women who are already pregnant. Current literature provides evidence that energy and protein intake is associated with GWG[9–12,12] but the roles of individual nutrients have not been fully elucidated [9–11,13]. With respect to food groups, those that have been reported as associated with EGWG are sweets, processed food[11,13,14], sweetened beverages[15,16], snacks, fish and bread[16]. Evaluation of diet quality to assess overall adequacy of dietary intake [17] has advantages over evaluation of nutrients or food groups separately, in part because intakes of different nutrients are often highly correlated [18]. However, despite these advantages, epidemiological studies that link a priori dietary pattern adherence to GWG are scarce, inconsistent and most of them utilized a cross-sectional design[14,18,19]. Hillesund et al [19] found a positive association between the New Nordic Diet score (fruits and vegetables, whole grains, potatoes and fish) and EGWG (odds ratio (OR) 0.93; 95% confidence interval (CI) 0.87–0.99; p=0.02). Shin et al [14] reported that the Healthy Eating Index-2005 (HEI-2005) was not determinant of adequate GWG, although inadequate intake of total vegetables (OR 3.8, CI 1.1–13.2, p = 0.03) and oils (OR 2.8, CI 1.2–6.4, p = 0.02) were associated with EGWG. Similarly, Rifas-Shiman et al [20] found that Alternate Healthy Eating Index, slightly modified for pregnancy (AHEI-P) assessed in the first trimester of pregnancy was not associated with EGWG (OR 0.99, 95% CI 0.94 to 1.04). Only one study [18] has evaluated GWG as a longitudinal outcome (but maternal diet was assessed only once), and concluded that an a priori-defined pattern based on national dietary recommendations of the Netherlands was not associated with GWG.
No study has simultaneously evaluated the maternal diet and GWG longitudinally during pregnancy. Evaluating repeated weight measurements during pregnancy in a cohort with detailed dietary intake data provides an opportunity to evaluate this association and avoid the limitations of cross-sectional studies or single measures of dietary intake.
HEI-2005 and AHEI-P have been used to evaluate the association between diet quality and a variety of pregnancy outcomes[20,21]; however, these a priori indices have been generated from studies done mostly in populations with little representation of Mexicans. In contrast, the new Maternal Dietary Quality Score (MDQS) was calculated using food components based principally on Mexican Dietary Guidelines (MDG) [28], so this score serves as indicator of a healthy, traditional and sustainable national diet.
The aims of the present study were to conduct a longitudinal, repeated measures analysis to: 1) characterize GWG trajectories among Mexican women, 2) assess the effect of MDQS on GWG during early-mid pregnancy (0–20 weeks), middle pregnancy (≥20 and <30 weeks), late pregnancy (30 to 40 weeks) and prolonged pregnancy (≥40 weeks of gestation) and 3) evaluate the association between MDQS and adequacy of GWG, throughout pregnancy.
Materials and methods
Study design
We analyzed data from a prospective cohort of pregnant women conducted in Mexico City, now known as the Pregnancy Research on Inflammation, Nutrition, & City Environment: Systematic Analyses (PRINCESA) cohort [22]. The main purpose of the cohort was to investigate the mechanisms by which exposure to air pollutants during pregnancy could lead to perinatal complications such as preterm birth and intrauterine growth restriction (IUGR).
From February 2009 to November 2014, 935 pregnant women who resided in diverse regions of metropolitan Mexico City were recruited at the Instituto Nacional de Perinatología (INPer), public health clinics throughout the city, and the Hospital Materno Infantil Inguarán (HMII), a perinatal hospital within the Mexico City government’s public hospital network. Human subjects approval for the study was obtained from the University of Michigan Institutional Review Board and the Ethics in Human Subjects and Research Committees of the participating Mexican institutions.
Inclusion criteria were 1) reliable recall of last menstruation; 2) agreement to prenatal visits every 4 weeks throughout their current pregnancy; 3) written consent for their inclusion in the study. Exclusion criteria were 1) previous presence of any medical or obstetric complication in the current pregnancy; 2) presence of multiple fetuses. Eligibility was determined at screening and confirmed at the first visit. Women who developed pregnancy complications such as gestational diabetes and preeclampsia were excluded and referred to a specialty hospital for follow-up. For the present study, two additional inclusion criteria were that the participants had: 1) at least one complete dietary recall in both the second and third trimesters of pregnancy; and 2) at least two measurements of gestational weight during pregnancy.
After screening for eligibility and acquiring informed consent at either the first visit or at health clinics during recruitment, women were seen monthly over the course of their pregnancies. Information on clinical, anthropometric, and biochemical parameters and maternal diet was collected at each visit by a dedicated team including certified medical staff and a dietitian.
Maternal Diet Quality Score (MDQS)
Data on maternal diet were collected through a multiple-step 24-hour dietary recall (24H-DR) in each prenatal visit (median time between visits: 5 weeks) by a dietitian with standardized training. The average number of dietary recalls during pregnancy was 5.8 (standard deviation (SD) 0.87, range 3–9 measurements). To evaluate diet quality, we built a Maternal Diet Quality Score (MDQS) based on the Mexican Dietary Guidelines (MDG)[23] and international recommendations for specific foods and nutrients. We included the following recommendations regarding nutrients and food groups: 1) polyunsaturated fats (PUFAS, >6% of energy intake) [24], 2) added sugars (<10% of total energy) [25], 3) fruits and vegetables (>400 grams/day)[25], 4) red meat (<500 grams/week) [26][27], 5) low fat dairy products (2 servings/day) [23], 6) legumes (2 servings/day) [26], and 7) foods high in saturated fat and/or added sugar (HSFAS) (<10% of energy intake) [28]. A value of 1 was assigned if the recommendation was met and 0 if the recommendation was not met for each of the seven individual recommendations. The scores for each recommendation were then summed with a maximum score of 7 if all recommendations were met and a minimum of 0 if no recommendations were met. We defined the 3 following categories of adherence: low (0–2 points), medium (3–4 points) and high (>5 points). Detailed methodology about the collection of maternal dietary data and its transformation to derive a priori and a posteriori dietary patterns and scores, and a finding that MDQS was associated with LBW in this cohort, are provided elsewhere (submitted, unpublished results).
Anthropometry
Maternal weight and height were measured at the first and consecutive visits (median time between visits: 5 weeks) by trained staff using standardized methods (Lohman technique)[29]. The average number of visits and body weight measurements during pregnancy was 5.8 (standard deviation (SD) 0.87, range 3–9 measurements); the first and the last weight measurements were carried out during the 11th (SD 1.7 weeks, range 2.4–13.6 weeks) and the 37th gestational week on average (SD 2.9 weeks, range 25.2–45.5 weeks) in the sample for this study. Pre-pregnancy weight was self-reported by participants. The pre-pregnancy BMI was calculated in kg/m2 and was categorized into five groups: underweight <18.5, normal >18.5–24.9, overweight 25–29.9, obesity 1: 30–34.9 and obesity 2: >35.
Rate of gestational weight gain (RGWG; kg/week) was calculated at each visit throughout the whole pregnancy as weight at the current visit minus weight from the previous visit, divided by follow-up duration (g/week). In the case of the first visit, RGWG was estimated using pre-pregnancy weight.[MM1] We categorized RGWG according to whether Institute of Medicine (IOM)[30] recommendations were met (insufficient, adequate and excessive) based on ranges of the mother’s pre-pregnancy BMI. The recommended weight gain in the first trimester of pregnancy (until 13 weeks) is 2.0 kg (0.17 kg/week), for all categories of maternal pre-pregnancy weight status[31]. Recommended weight gain in the second and third trimesters was based on the assumption that underweight, normal-weight, overweight, and obese women should gain weight within the normal range of 0.44–0.58 kg, 0.35–0.50 kg, 0.23–0.33 kg, and 0.17–0.27 kg per week, respectively[30]. We did not use the total weight gain as an outcome since the timing of the final measurement of weight gain varied between the mothers; that is, the monitoring of maternal weight gain ended at different time points depending on when in gestation the mother made her last clinic visit before giving birth.
Potential confounders
Maternal age, education and parity (number of pregnancies reported) were obtained using questionnaires that collected data on socio-demographic variables, obstetric history and detailed information about the pregnancy. Maternal education was grouped by whether the mother completed or did not complete 9 years of school (≥9 years or <9 years). Parity was divided into three groups (nulliparous, 1–2, and ≥3). Marital status was divided into two groups (married/partnered or divorced/single). Physical activity was assessed in each visit and categorized into whether the women met or did not meet the American College of Obstetricians and Gynecologists recommendations (≥150 min/week versus <150 min/week)[32].
Statistical Analysis
Maternal characteristics
Descriptive statistics were computed for socio-demographic variables and maternal characteristics. Differences between socio-demographic variables were compared using Fisher’s exact test and analysis of variance for categorical and continuous variables, respectively. Differences were considered significant at a significance level of p < 0.05.
Gestational weight gain trajectories
Appropriate modeling of longitudinal GWG patterns is essential to assess associations between this maternal variable and different exposures or outcomes, but it also represents several statistical challenges[33]. Linear spline regression models have recently been proposed as a method of representing change, which reduces the dimensionality of repeated measurements[34]. The shape of the trajectory of change is assumed to be piecewise linear, with knot points defining changes in the magnitude or direction of association of the response variable with time[34]. The selection of the number and location of knot points may be determined by the data or by prior knowledge to allow the linear splines to represent the shape of the change trajectory in a meaningful way[34].
In our sample, individual trajectories of RGWG (based on mother’s weight measurements at visit, without estimating fetal weight) were modeled in a multilevel linear spline regression framework (measurement occasion at level 1; individuals at level 2). We used the methodology proposed by Howe et al[33] which has been applied totally or partially in various epidemiological studies [34–39]. The shape of the trajectory was specified as a linear spline with 3 knots at 20, 30, and 40 weeks of gestation. The knots were placed to best reflect the observed data. Correlation between weight measurements within an individual was modeled by including random effects for the intercept and slope (for weeks of gestation) into the model. No additional correlation structure was assumed for the error terms. Subsequently, trajectories were smoothed using the “loess” option in ggplot2, a package in R, with a span value of 0.7.
Association between MDQS and weight gain trajectories
We performed linear splines mixed-effect (LSME) regression models to evaluate the association between MDQS during pregnancy and RGWG trajectories from first to last visit. Gestational age (weeks) was included in LME models as a random and fixed effect to adjust for the overall and individual variations in the RGWG over time and according to MDQS categories. All other covariates (gestational pre-pregnancy BMI, maternal age, educational level, parity, fetal sex, marital status and physical activity) were included as fixed effects. The principal advantages of using piecewise linear models are: 1) they make it easier to approximate smooth curves compared with quadratic models and 2) they have a more straightforward interpretation when evaluating the relationship between covariates and trajectories[40].
By using splines in modeling, we allowed MDQS exposure to affect all four slopes, one for each of the four segments of the spline. Thus, the effects on the difference in RGWG at early-mid pregnancy (0–20 weeks), middle pregnancy (≥20 and <30), late pregnancy (30 to 40) and prolonged pregnancy (≥40 weeks of gestation; comparing high and medium adherence to MDQS against the category of reference (low adherence), were assessed. No additional correlation structure was assumed for the error terms. We adjusted for the variables listed above as other covariates; additionally, we adjusted the models by energy intake (Kcal/day) because we were interested in the effect of diet quality independent of total energy intake.
Additionally, we implemented mixed-effect logistic regression models to explore the influence of the MDQS on the excessive or inadequate RGWG categories during pregnancy. These models were stratified by pre-pregnancy BMI categories and further adjusted for the confounders listed above as other covariates.
All models included measurements of MDQS and RGWG obtained from each visit.
Sensitivity analyses
We repeated the analyses of mixed-effect logistic regression models including only women for whom the number of total prenatal visits was ≥5 and ≥6. We evaluated the associations separately in women with a pre-pregnancy BMI below or above the cut-off point of 25 Kg/m2. Moreover, we compared the models using a pre-pregnancy BMI calculated from the self-reported pregestational weight and the BMI calculated from the weight obtained in visit 1 in women whose first measurement was carried out before week 14 (n=230).
All statistical analyses were performed in STATA (Stata for Mac 12.1, Drive East College; E.U), R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) and the R package ggplot2, version 2.2.1.
Results
Baseline characteristics of the study population (n=660) are listed in Table 1. MDQS ranged from 0 (reflecting no adherence at all) to 7 (reflecting maximum adherence). Mean (±SD) of MDQS was 2.86 (±1.08) for first, 3.06 (±1.25) for second, and 3.30 (±1.27) for third trimester and 3.11 (±2.19) for the whole pregnancy (averaged over the three trimesters). Mean (±SD) age was 25.0 (±5.8) years. On average, pregnant women with greater adherence (>5 points) to MDQS were older (25.8±6.7 vs. 24.0 ±5.2 years; P < 0.05) and more educated (>9 years of schooling, 15.7 vs. 12.7%, (p<0.05) compared with women with lower adherence (<3 points). We did not observe significant differences in the other baseline characteristics across the categories of MDQS.
Table 1.
Sociodemographic and maternal characteristics
| Characteristic | Inadequate RGWG N=196 (29.70%) | Adequate RGWG N=194 (29.39%) | Excessive RGWG N=270 (40.91%) |
|---|---|---|---|
| Maternal age, years (±SD) | 25.72(±5.89) | 24.83(±5.76) | 24.89(±5.76) |
| Pre-pregnancy BMI, Kg/m2 (±SD)b | 25.59(±6.10) | 24.17(±4.56) | 26.91 (±5.23) |
| Pre-pregnancy BMI categories (kg/m2), n(%)b | |||
| <18.5 | 17 (8.67) | 8 (4.12) | 4 (1.48) |
| >=18.5 <25 | 98 (50.00) | 124 (63.92) | 79 (29.26) |
| >=25 <30 | 48 (24.49) | 41 (21.13) | 126 (46.67) |
| >=30 | 33 (16.84) | 21 (10.82) | 61 (22.59) |
| Term of gestation, n(%) | |||
| Preterm | 20(10.58) | 18(9.52) | 27(10.07) |
| Term | 169 (89.42) | 171 (90.48) | 241 (89.93) |
| Parity, n(%)c | |||
| Nulliparous | 73 (37.24) | 90 (46.88) | 154 (56.30) |
| 1–2 | 64 (32.65) | 58 (30.21) | 65 (24.44) |
| >=3 | 57 (29.58) | 44 (22.92) | 53 (19.26) |
| Marital statusd | |||
| Single or divorced | 48 (24.49) | 41 (21.13) | 81 (30.00) |
| Married/partnered | 148 (75.51) | 151 (77.84) | 189 (70.00) |
| Education level, n(%) | |||
| <=9 years | 117(59.69) | 109(56.19) | 144(53.33) |
| >9 years | 79(40.31) | 85(43.81) | 126(46.67) |
| Baby sex, n(%) | |||
| Female | 90 (45.91) | 80 (41.23) | 121 (44.81) |
| Male | 90 (45.91) | 104 (53.60) | 131 (48.51) |
Values are n (%) or means ± SDs.
Significantly different (P value <0.05) between categories of rate of gestational weight gain rate (RGWG; Kg/week) during second and third trimesters defined according to Institute of Medicine(IOM) recommendations[30]. P-value refers Fisher’s exact test and analysis of variance for proportions and continuous variables, respectively.
One missing value in Inadequate RGWG category.
Two missing values in Adequate RGWG category..
16, 10 and 18 missing values in Inadequate, Adequate and Excessive RGWG categories respectively.
With respect to categories of RGWG, we found differences for maternal height (p<0.05), pre-pregnancy BMI (p<0.001) and parity (p< 0.05). We did not find differences for, age, maternal education, or baby’s sex (Table 1).
Gestational weight gain
The mean (±SD) maternal pre-pregnancy BMI was 25.72 kg/m2 (±5.23). About 17.42% of the participants were obese, 32.58% of them were overweight, and 4.49% were underweight. As to GWG, only 29.70% of women were in compliance with the RGWG level recommended by IOM; 29.39% of women gained below the recommended level and 40.91% of participants gained above that recommendation.
Particularly, women with pre-pregnancy BMI <18.5 Kg/m2 and >25 Kg/m2 did not experience the recommended RGWG during second and third trimesters; 58.62% of underweight women had a lower RGWG and 58.60% of overweight women had higher RGWG than the IOM recommendation (Figure 1).
Figure 1. Rate of gestational weight gain adequacy according to the recommendation of the Institute of Medicine (IOM) (2009).
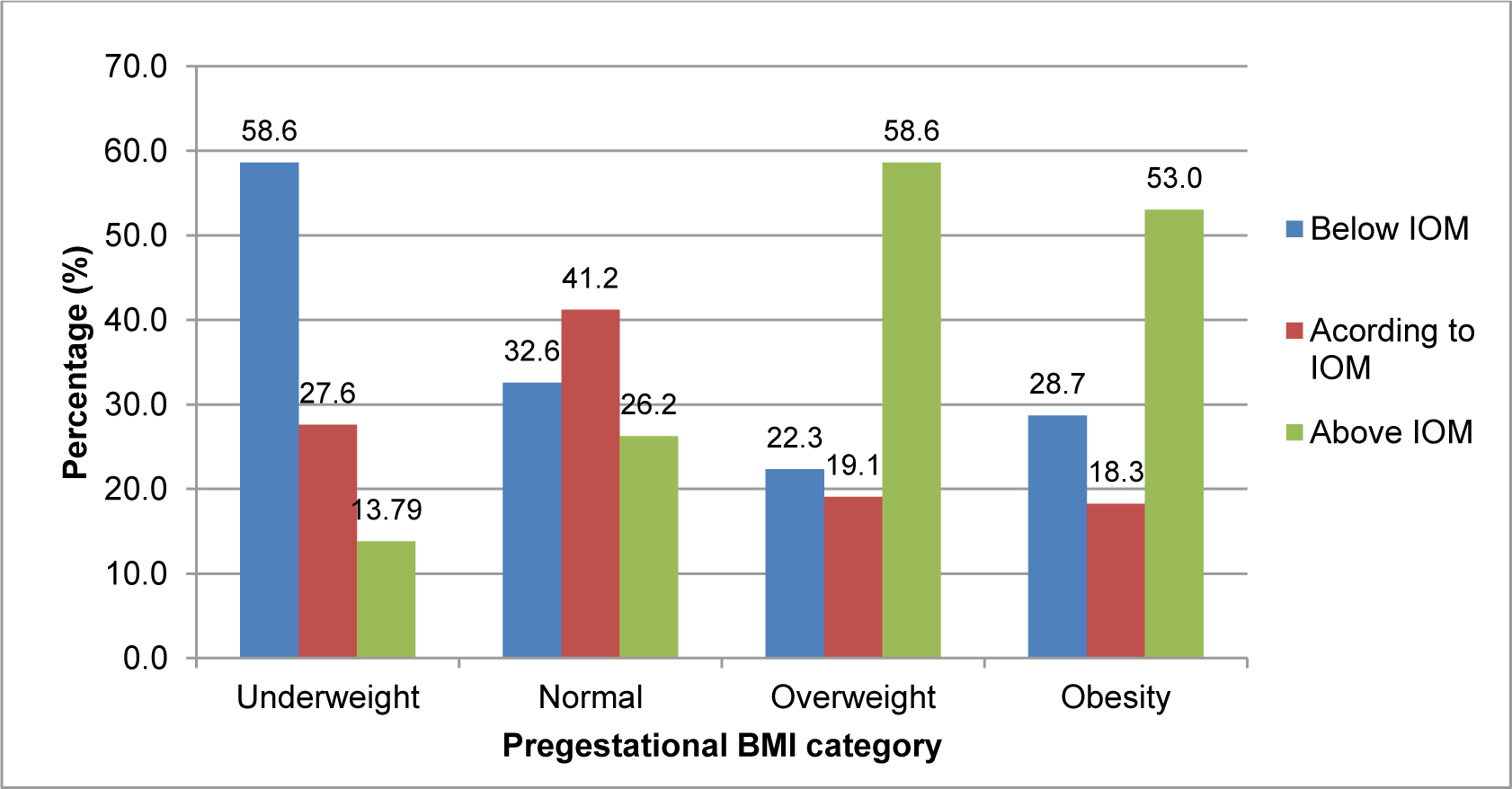
Values are in percentages (%). An adequate gestational weight gain rate (Kg/week) during second and third trimesters was defined according to Institute of Medicine(IOM) recommendations[30]. Abbreviations: BMI: body mass index.
Gestational weight gain (GWG) trajectories
The average RGWG differed across trimesters (p<0.001), with the lowest rate in the first [0.07; 95% CI −0.59, 1.10) kg/week] and the highest in the second [0.26; 95% CI −0.59, 1.10) kg/week] and third [0.39; 95% CI −0.40, 1.12) kg/week] trimesters. Figure 2 shows the average trajectory of the GWG across pregnancy predicted by the LSME regression modeling in the total sample (n = 660); the sigmoidal shape reflected that the majority of weight is gained in the second and early third trimesters of pregnancy.
Figure 2. Trajectory of rate of gestational weight in the study sample (n = 660).
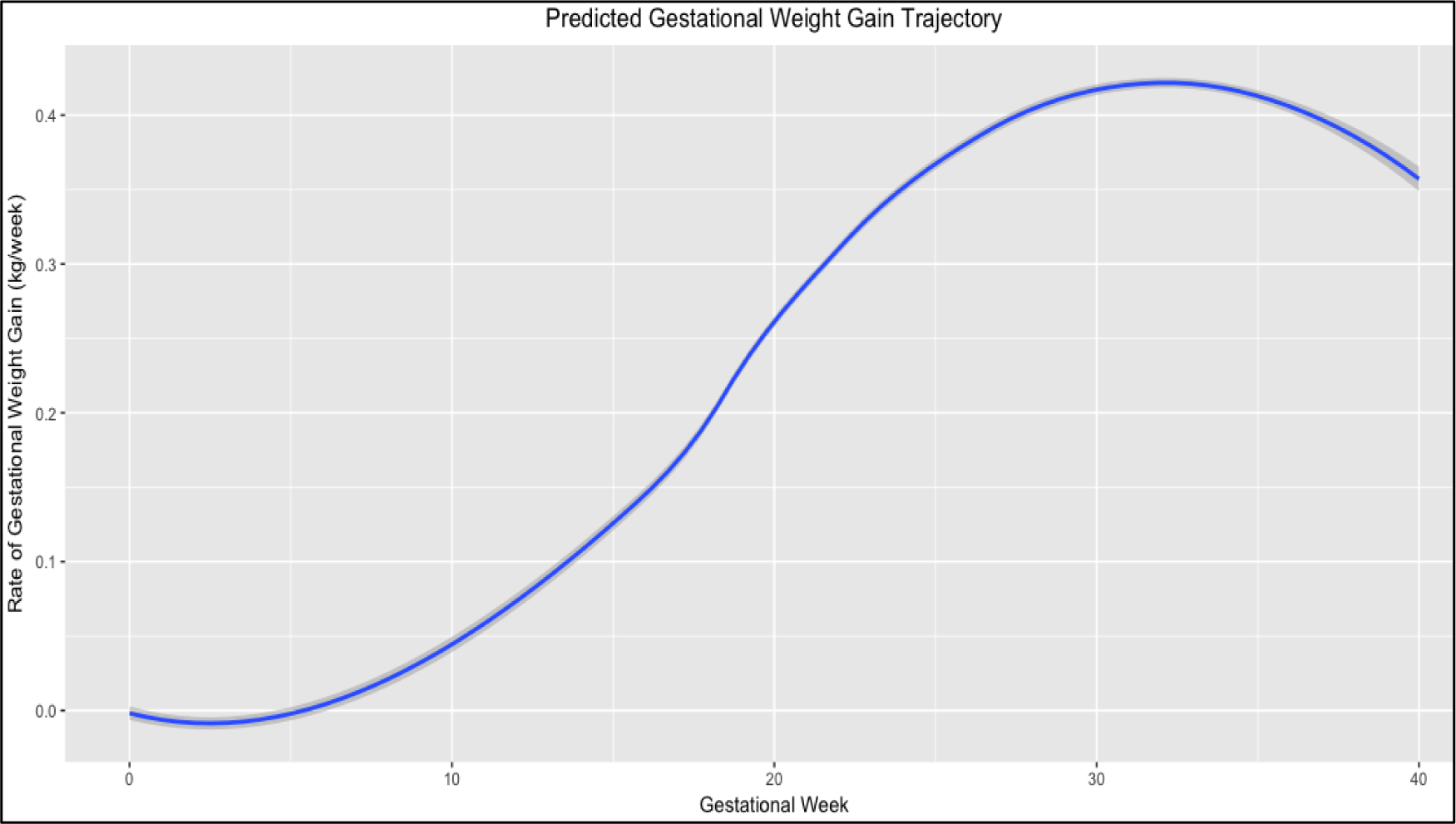
The figure shows the trajectory of the rate of gestational weight gain (RGWG) during pregnancy as estimated by the linear mixed regression model. Adjusted for gestational age, maternal age, educational level, parity, pregestational BMI, physical activity and fetal sex.
Figure 3 shows the GWG trajectories according to pre-pregnancy BMI category predicted by LSME. The GWG patterns of women with pre-pregnancy BMI of 30.0 kg/m2 or greater had a slower RGWG compared with women in the other pre-pregnancy BMI categories. There was no significant difference between trajectories of women with underweight or normal pre-pregnancy BMI.
Figure 3. Trajectories of rate of gestational weight by BMI category (n = 660).
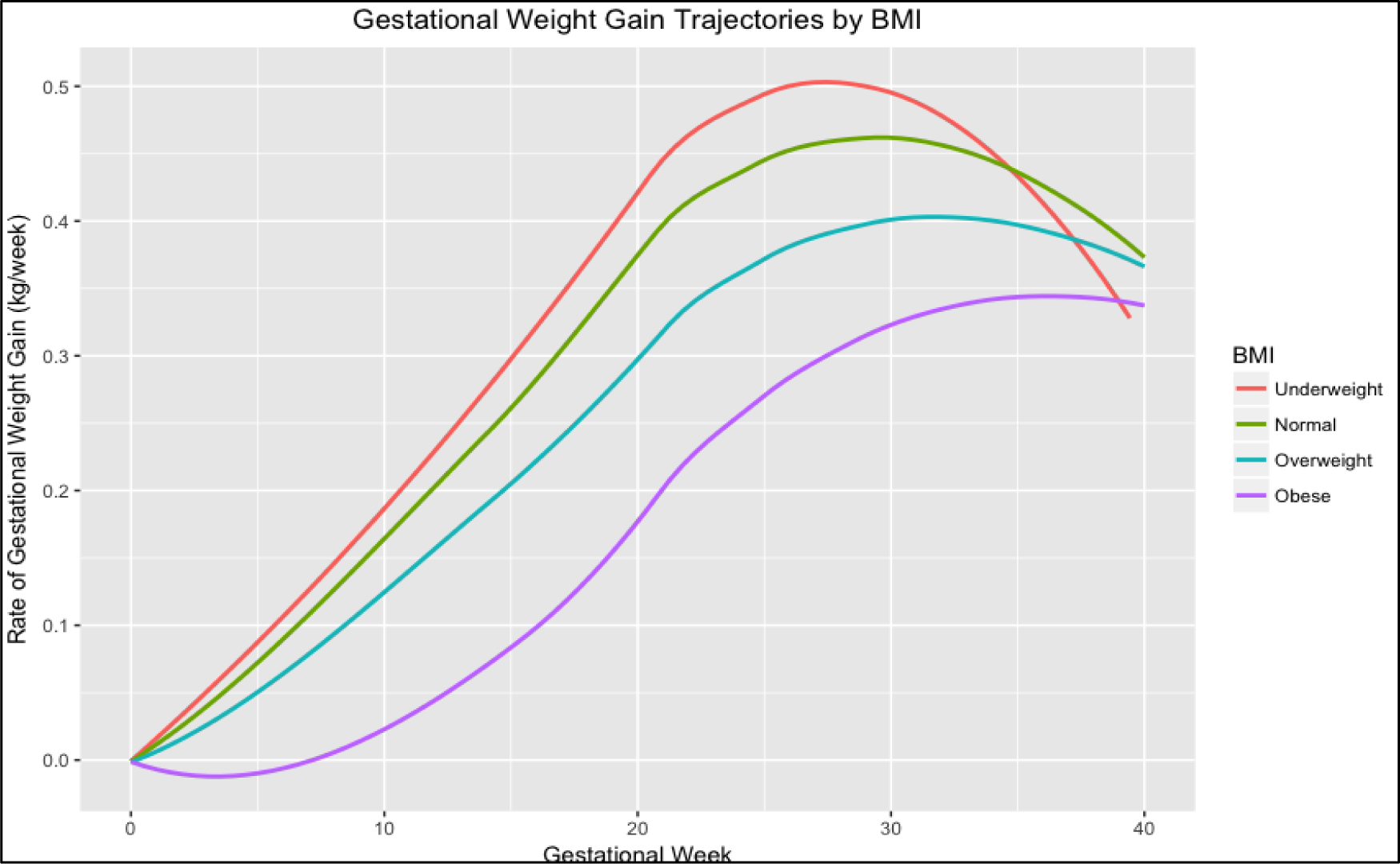
The figure shows the trajectories of the rate of gestational weight gain (RGWG) during pregnancy by category of BMI estimated by the linear mixed regression model and adjusted for gestational age, maternal age, educational level, parity, fetal sex, marital status and physical activity.
Association between MDQS and GWG trajectories
We found that MDQS was associated with the trajectories of RGWG across pregnancy after adjustment for potential confounders (Supplementary table 1).
Medium and high categories of MDQS (compared with low adherence) were positively associated with the RGWG in early-mid gestation, but only the medium adherence category was statistically significant (β: 0.0162; 95% CI 0.0005 0.0333; p= 0.058). Medium and high adherence categories were negatively associated with the RGWG in the middle stage of pregnancy [(β: −0.0266; 95% CI −0.0496, −0.0037; p= 0.023), (β: −0.0363; 95% CI −0.076, 0.037; p= 0. 076 (marginally significant))] In late pregnancy, both categories of adherence were positively related [(β: 0.0256; 95% CI 0.0077, 0.0436; p= 0.005) (β: 0.0472; 95% CI 0.0222, 0.0723; p<0.001)]. In prolonged pregnancy, medium and high adherence categories were negatively associated but only the category of high adherence was significantly associated with the RGWG (β: −0.182; 95% CI −0.360, −0.0045; p= 0.044). In other words, a better diet quality during pregnancy was associated with a faster RGWG in early-mid pregnancy (0–20 weeks), with a slower RGWG in the middle pregnancy (>20 and < 30 weeks gestation), with a speedier weight gain in late pregnancy (30–40 weeks), and with a slower RGWG in women with a prolonged pregnancy (>40 weeks).
With respect to the mixed-effect logistic regression models, we found that medium and high adherence to MDQS throughout pregnancy were each protective against an inadequate GWG [(OR: 0.74; 95% CI 0.56, 0.99; p=0.04) (OR: 0.63; 95% CI 0.42, 0.95; p=0.03)], respectively, and an excessive GWG [(OR: 0.77; 95% CI 0.57, 1.04; p=0.09) (OR: 0.62; 95% CI 0.41, 0.94; p=0.03)], respectively. The association between the medium category of adherence and an excessive GWG was only marginally significant. Table 2 shows the crude and adjusted models.
Table 2.
Association between MDQS and RGWG with spline knots at each period of pregnancy (n = 660).
| Crude modela | Adjusted modelb | ||||
|---|---|---|---|---|---|
| RGWG at each period | MDQS adherence | β (95% CI) | p | β (95% CI) | p |
| Early mid pregnancy (0–20 weeks) | Low | Ref | Ref | ||
| Medium | 0.011 (0.0021, 0.0209) | 0.017 | 0.0162 (−0.0005, 0.0333) | 0.058 | |
| High | 0.01281 (−0.0048,0.0305) | 0.156 | 0.0164 (−0.0148,0.0477) | 0.302 | |
| Middle pregnancy (20–30 weeks) | Low | Ref | Ref | ||
| Medium | −0.0206 (−0.0359, −0.0053) | 0.008 | −0.0266 (−0.0496, −0.0037) | 0.023 | |
| High | −0.0316 (−0.0576,−0.0056) | 0.017 | −0.0363 (−0.076, 0.0037) | 0.076 | |
| Late pregnancy (30–40 weeks) | Low | Ref | Ref | ||
| Medium | 0.0229 (0.0054, 0.0404) | 0.010 | 0.0256 (0.0077, 0.0436) | 0.005 | |
| High | 0.0462 (0.02193,0.0704) | <0.001 | 0.0472 (0.0222, 0.0723) | <0.001 | |
| Prolonged pregnancy (>40 weeks) | Low | Ref | Ref | ||
| Medium | −0.0740 (−0.2513, 0.1033) | 0.413 | −0.0747 (−0.257, 0.107) | 0.422 | |
| High | −0.1587 (−0.341, 0.2406) | 0.089 | −0.182 (−0.360, −0.00450) | 0.044 | |
Low adherence to MDQS is the reference (Ref.) category for diet and adequate RGWG is the reference category for adequacy of GWG in the logistic multilevel model.
Adjusted for gestational age.
Models adjusted for pre-pregnancy BMI, energy intake, gestational age, maternal age, educational level, parity, fetal sex, marital status and physical activity.
Abbreviations: RGWG: rate gestational weight gain; OR: odds ratio; CI: confidence interval; Pre- BMI: pregestational body mass index.
Sensitivity analyses
After repeating analyses in those women who attended more than 5 and 6 prenatal visits, we observed that the association between MDQS and the inadequate or excessive GWG remained significant. Additionally, the association between the medium category of adherence and excessive GWG became statistically significant (OR: 0.31; 95% CI 0.13, 0.74; p=0.008) (Supplementary table 2). We found that higher MDQS adherence was protective against inadequate or excessive GWG, independently if women entered pregnancy with a BMI less than or greater than 25 Kg/m2. (Table 4)
Table 4.
Association of MDQS with inadequate and excessive RWGW during pregnancy in women with a pre-pregnancy BMI <25 Kg/m2 and ≥ 25 Kg/m2.
| Pre- BMI | MDQS adherence | Adjusted modelb OR (%95 CI) | P | Adjusted modelb OR (%95 CI) | P | |
|---|---|---|---|---|---|---|
| <25 Kg/m2 | Low adherence | Ref. | Ref. | Ref. | ||
| Medium adherence | 0.426 (0.184, 0.985) | 0.046 | Ref. | 0.347 (0.126,0.952) | 0.040 | |
| High adherence | 0.295 (0.092, 0.946) | 0.040 | Ref. | 0.242 (0.059, 0.989) | 0.048 |
Low adherence to MDQS is the reference (Ref.) category for diet and adequate RGWG is the reference category for adequacy of GWG in the logistic multilevel model.
aaAdjusted for gestational age.
Models adjusted for pre-pregnancy BMI, energy intake, maternal age, educational level, parity, fetal sex, marital status and physical activity.
Abbreviations: RGWG: rate of gestational weight gain; OR: odds ratio; CI: confidence interval; Pre- BMI: pregestational body mass index.
The results of the mixed-effect logistic regression did not change greatly when comparing the models with the inclusion of pre-pregnancy BMI calculated from the self-reported pre-gestational weight versus the BMI calculated from the weight obtained in visit 1(Supplementary table 3).
Discussion
In this sample of pregnant women from the PRINCESA cohort, higher adherence to maternal dietary quality recommendations was protective against both inadequate and excessive GWG throughout pregnancy. These results are consistent with findings from the study carried out by Hillesund et al, which reported that normal-weight women with high as compared with low New Nordic Diet score adherence had lower adjusted odds of excessive gestational weight gain (OR=0·93; 95% CI 0·87, 0·99; p=0·024)[19]. On the other hand, the only other study [18] that has evaluated GWG as a longitudinal outcome concluded that a priori defined dietary patterns (Dutch Healthy Diet Index) were not consistently associated with any measure of GWG.
Results from cross-sectional studies that have evaluated the same association differ from our findings. For example, the “US healthy eating index of 2005” (HEI-2005)[ 21] and the AHEI-P [20] were not associated with GWG. However, the epidemiological design of these studies limits the comparison of results because the RGWG is not stable across pregnancy.
The very limited amount of literature on this topic suggests that further investigation is warranted, and that attention should be paid to standardizing, if possible, the measures used and the timing for taking them to facilitate comparisons. The inconsistent associations between a priori-defined dietary patterns and GWG reported in the few previous studies may be due to the use of different food groups and cut-off points to define score weights, as well as the timing for assessment of maternal diet and/or GWG.
Assessing the timing of weight gain in pregnancy is important because weight gain at key points during pregnancy has been associated with some adverse pregnancy and birth outcomes. For example, mid-gestational weight gain was associated with birth weight and neonatal adiposity[41]. GWG in early and late pregnancy was associated with gestational diabetes, gestational hypertension, macrosomia, and primary caesarean section[42]. Early gestation weight gain was additionally associated with pregnancy-induced hypertension and the RGWG in late pregnancy was also associated with preterm birth[42]. Our longitudinal analysis showed that higher adherence to MDQS was associated with slower weight gain in middle and prolonged late pregnancy and with a faster GWG in the early and late pregnancy. Further analyses to assess if these patterns are associated with clinical and birth outcomes are planned.
An important finding in the present study is that a large proportion of the women had a RWGW below or above the recommended ranges. Indeed, adherence to these recommendations has been reported as suboptimal in other populations in the last systematic review and meta-analysis of this topic [43]. Specifically, we found that 40.91% of participants gained weight at a rate above that recommended, similar to the percentages reported by previous studies carried out in Mexican (38%) [44] and Mexican-American women (45%)[45].
Strengths
The principal strengths of the present study include the prospective design that provided a valuable opportunity to assess maternal diet and GWG in a parallel approach during different stages of pregnancy. The MDQS was calculated using food components based on Mexican Dietary Guidelines (MDG) [23] and international recommendations, so this score could be a useful tool for evaluating overall diet quality of pregnant women who live in similar contexts.
In general, various studies have reported that women who follow healthy dietary patterns are more likely to be older, married and practice more regular physical activity, suggesting that healthy food choices are part of a larger healthy life-style pattern [19,20,46]. Hence, another advantage of our study is the availability of detailed information about potential confounders including maternal and socio-demographic variables.
Limitations
BMI classification should be based on pre-pregnancy weight; nevertheless, we did not find great differences in the estimations when comparing models that included the pre-pregnancy BMI calculated from the self-reported pre-gestational weight versus the BMI calculated from the weight obtained in visit 1 (for women whose first measurement was carried out before week 14). Also, we were not able to use the total GWG as an outcome because the final weight measurement was not provided at the same point in gestation for all the women.
Another limitation was the inability to obtain the different components of the weight gain, which includes both the mother’s weight gain and the growth of the fetus and other indicators of interstitial volume expansion (swelling or edema). However, the mother’s weight gain across pregnancy largely reflects fat gain, so the lack of detailed measures on the components is not likely to cause bias. Further, the relative weight contributed by the growth of the fetus, uterus, placenta and other maternal components will not be as pronounced up until gestational week 20 as compared to later in pregnancy[30].
The lack of weight measurement at each visit can be also considered a limitation, because fluid retention (swelling/edema) can affect the accuracy of weight measurements. However, swelling is expected in all pregnant women. Women with preeclampsia and other complicating conditions, which exacerbate this clinical symptom, were excluded from the PRINCESA cohort, so it is unlikely that extremely different degrees of edema at each visit based on these underlying conditions are present in this study.
With respect to MDQS, as in any dietary study, recall bias could have occurred, as well as other biases related to a- priori dietary pattern specification (food grouping, data treatment, plausible limits and validity). A limitation of using one 24-hr recall at each visit, without repeated measures in consecutive days is that we cannot capture the day-to-day variability in dietary intake, and therefore our measurement is subject to large within-person random error. However, the detailed information collected through dietary recalls provides more accurate estimates than other collection methods such as Food Frequency Questionnaires[47].
Conclusion
To our knowledge, this is the first study that has simultaneously evaluated the association between maternal dietary patterns and the GWG during pregnancy. Implementation of LSME models allowed capture of the overall and individual variations in the RGWG over time, according to MDQS categories. In summary, MDQS were protective against both inadequate and excessive GWG throughout pregnancy. Our results contribute to the scarce literature on maternal dietary patterns and weight gain throughout pregnancy. Beneficial changes in diet sustained during the entire pregnancy may prevent an inadequate or excessive GWG during pregnancy, and also may confer long-term benefits for mother and offspring. All pregnant women should be encouraged to have a healthier diet during the entire pregnancy to prevent negative maternal and fetal outcomes derived from rates of GWG lower or higher than recommended at specific points of the pregnancy.
Supplementary Material
Table 3.
Association of MDQS with inadequate and excessive RWGW during pregnancy.
| Inadequate RGWa | AdequateRGW | Excessive RGWb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MDQS adherence | Crude model OR (%95 CI) | P | Adjusted model OR (%95 CI) | P | Crude model OR (%95 CI) | P | Adjusted modelOR (%95 CI) | P | |
| Low adherence | Ref. | Ref. | Ref. | Ref. | Ref. | ||||
| Medium adherence | 0.834 (0.653, 1.065) | 0.146 | 0.742 (0.555, 0.991) | 0.044 | Ref. | 0.845 (0.649, 1.100) | 0.212 | 0.773 (0.572, 1.044) | 0.094 |
| High adherence | 0.765(0.534–1.095) | 0.143 | 0.630 (0.417, 0.953) | 0.031 | Ref. | 0.698 (0.479–1.018) | 0.062 | 0.623 (0.411, 0.942) | 0.025 |
Low adherence to MDQS is the reference (Ref.) category for diet and adequate RGWG is the reference category for adequacy of GWG in the logistic multilevel model.
Adjusted for gestational age.
Models adjusted for pre-pregnancy BMI, energy intake, gestational age, maternal age, educational level, parity, fetal sex, marital status and physical activity.
Abbreviations: RGWG: rate gestational weight gain; OR: odds ratio; CI: confidence interval; Pre- BMI: pregestational body mass index.
Highlights:
This study explored longitudinal effects of diet on gestational weight gain (GWG).
Trajectories of GWG varied by pre-pregnancy body mass index (BMI) category.
Associations between diet quality and GWG differed by period of gestation.
Overall, insufficient and excessive GWG was less likely with better diet quality.
Healthier diets in pregnancy may prevent negative maternal and fetal outcomes.
Acknowledgments
We are grateful for the support provided by the Capital Semilla of the Division of Social Studies and the Academic Vice-Presidency.of the Universidad Iberoamericana. We thank Ricardo Felix de Majo for data management support.
Funding: This work was supported by grants from the National Institute of Environmental Health Sciences R-01 ES 017022, R01 ES016932, and P30 ES017885.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- [1].Roberts VHJ, Frias AE, Grove KL. Impact of Maternal Obesity on Fetal Programming of Cardiovascular Disease. Physiology 2015;30:224–31. doi: 10.1152/physiol.00021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tsigga M, Filis V, Hatzopoulou K, Kotzamanidis C, Grammatikopoulou MG. Healthy Eating Index during pregnancy according to pre-gravid and gravid weight status. Public Health Nutr 2011;14:290–6. doi: 10.1017/S1368980010001989. [DOI] [PubMed] [Google Scholar]
- [3].Yan J Maternal pre-pregnancy BMI, gestational weight gain, and infant birth weight: A within-family analysis in the United States. Econ Hum Biol 2015;18:1–12. doi: 10.1016/j.ehb.2015.03.002. [DOI] [PubMed] [Google Scholar]
- [4].Warrington NM, Richmond R, Fenstra B, Myhre R, Gaillard R, Paternoster L, et al. Maternal and fetal genetic contribution to gestational weight gain. Int J Obes 2017;42:775–84. doi: 10.1038/ijo.2017.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Herring SJ, Oken E. Weight gain during pregnancy: Importance for maternal and child health. Ann Nestle 2010;68:17–28. doi: 10.1159/000298780. [DOI] [Google Scholar]
- [6].Wrottesley SV, Pisa PT, Norris SA. The influence of maternal dietary patterns on body mass index and gestational weight gain in urban black South African women. Nutrients 2017;9. doi: 10.3390/nu9070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Harrison CL. Gestational Weight Gain and its Association with Infant Birth Weight. Obesity 2017;25:1468–9. doi: 10.1002/oby.21912. [DOI] [PubMed] [Google Scholar]
- [8].Martin CL, Siega-riz AM, Sotres-alvarez D, Robinson WR, Daniels JL, Perrin EM, et al. Maternal Dietary Patterns during Pregnancy Are Associated with Child Growth in the First 3 Years of Life 1 – 3 2016. doi: 10.3945/jn.116.234336.The. [DOI] [PMC free article] [PubMed]
- [9].Blumfield ML, Hure AJ, MacDonald-Wicks L, Smith R, Collins CE. Systematic review and meta-analysis of energy and macronutrient intakes during pregnancy in developed countries. Nutr Rev 2012;70:322–36. doi: 10.1111/j.1753-4887.2012.00481.x. [DOI] [PubMed] [Google Scholar]
- [10].Tielemans MJ, Garcia AH, Santos AP, Bramer WM, Luksa N, Luvizotto MJ, et al. Macronutrient composition and gestational weight gain: A systematic review. Am J Clin Nutr 2016;103:83–99. doi: 10.3945/ajcn.115.110742. [DOI] [PubMed] [Google Scholar]
- [11].Olafsdottir AS, Skuladottir GV., Thorsdottir I, Hauksson A, Steingrimsdottir L. Maternal diet in early and late pregnancy in relation to weight gain. Int J Obes 2006;30:492–9. doi: 10.1038/sj.ijo.0803184. [DOI] [PubMed] [Google Scholar]
- [12].Diemert A, Lezius S, Pagenkemper M, Hansen G, Drozdowska A, Hecher K, et al. Maternal nutrition, inadequate gestational weight gain and birth weight: Results from a prospective birth cohort. BMC Pregnancy Childbirth 2016;16:1–9. doi: 10.1186/s12884-016-1012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].STUEBE AM, OKEN E, GILLMAN MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obs Gynecol 2009;6:247–53. doi: 10.1111/j.1743-6109.2008.01122.x.Endothelial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shin D, Bianchi L, Chung H, Weatherspoon L, Song WO. Is gestational weight gain associated with diet quality during pregnancy? Matern Child Health J 2014;18:1433–43. doi: 10.1007/s10995-013-1383-x. [DOI] [PubMed] [Google Scholar]
- [15].Guilloty NI, Soto R, Anzalota L, Rosario Z, Cordero JF, Palacios C. Diet, Pre-pregnancy BMI, and Gestational Weight Gain in Puerto Rican Women. Matern Child Health J 2015;19:2453–61. doi: 10.1007/s10995-015-1764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bärebring L, Brembeck P, Löf M, Brekke HK, Winkvist A, Augustin H. Food intake and gestational weight gain in Swedish women. Springerplus 2016;5. doi: 10.1186/s40064-016-2015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Borge TC, Aase H, Brantsæter AL, Biele G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children : a systematic review and meta-analysis 2017. doi: 10.1136/bmjopen-2017-016777. [DOI] [PMC free article] [PubMed]
- [18].Tielemans MJ, Erler NS, Leermakers ETM, van den Broek M, Jaddoe VWV, Steegers EAP, et al. A Priori and a Posteriori dietary patterns during pregnancy and gestational weight gain: The generation R study. Nutrients 2015;7:9383–99. doi: 10.3390/nu7115476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hillesund ER, Bere E, Haugen M, Øverby NC. Development of a New Nordic Diet score and its association with gestational weight gain and fetal growth - A study performed in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutr 2014;17:1909–18. doi: 10.1017/S1368980014000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary Quality during Pregnancy Varies by Maternal Characteristics in Project Viva: A US Cohort. J Am Diet Assoc 2009;109:1004–11. doi: 10.1016/j.jada.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Emond JA, Karagas MR, Baker ER, Gilbert-diamond D. Better Diet Quality during Pregnancy Is Associated with a Reduced Likelihood of an Infant Born Small for Gestational Age : An Analysis of the Prospective New Hampshire Birth Cohort Study 2018. doi: 10.1093/jn/nxx005. [DOI] [PMC free article] [PubMed]
- [22].ÓNeill MS, Osornio-Vargas A, Buxton MA, Sánchez BN, Rojas-Bracho L, Castillo-Castrejon M, et al. Air pollution, inflammation and preterm birth in Mexico City: Study design and methods. Sci Total Environ 2013;448:79–83. doi: 10.1016/j.scitotenv.2012.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].de Medicina AN. Guías alimentarias y de actividad física en contexto de sobrepeso y obesidad en la población mexicana. México DF: 2015. [Google Scholar]
- [24].Intakes R Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Washington, D.C.: National Academies Press; 2005. doi: 10.17226/10490. [DOI] [Google Scholar]
- [25].Organization WH. Diet, nutrition and the prevention of chronic diseases Report of a Joint WHO/FAO Expert Consultation. Geneva: World Health Organization; 2003. WHO Tech Rep Ser 2008. [Google Scholar]
- [26].Academia Nacional de Medicina. GUÍAS ALIMENTARIAS Y DE ACTIVIDAD FÍSICA. México: Intersistemas; 2015. [Google Scholar]
- [27].Glade M World Cancer Research Fund/American Institute for Cancer ResearchFood, Nutrition, Physical Activity and the Prevention of Cancer: A Global. Nutrition 2008. [Google Scholar]
- [28].Batis C, Aburto TC, Tania GS, Pedraza LS, Rivera JA. Adherence to Dietary Recommendations for Food Group Intakes Is Low in the Mexican Population 2016:1897–906. doi: 10.3945/jn.115.219626.1897S. [DOI] [PubMed]
- [29].Lohman TG, Roche AF, Martorell R, 1921-, Martorell R, 1947-. Anthropometric standardization reference manual. Human Kinetics Books; 1988. [Google Scholar]
- [30].Medicine I of. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press, National Academy of Sciences; 2009. [PubMed] [Google Scholar]
- [31].Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obs Gynecol 2009;21:521–6. doi: 10.1097/GCO.0b013e328332d24e.New. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Paper P. Nutrition and Lifestyle for a Healthy Pregnancy Outcome 2014:1–13.
- [33].Howe LD, Tilling K, Matijasevich A, Petherick ES, Santos AC, Fairley L, et al. Linear spline multilevel models for summarising childhood growth trajectories: A guide to their application using examples from five birth cohorts. Stat Methods Med Res 2016;25:1854–74. doi: 10.1177/0962280213503925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Macdonald-Wallis C, Lawlor DA, Palmer T, Tilling K. Multivariate multilevel spline models for parallel growth processes: Application to weight and mean arterial pressure in pregnancy. Stat Med 2012;31:3147–64. doi: 10.1002/sim.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fraser A, Tilling K, Macdonald C, Hughes R, Sattar N. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr 2011;93:1–4. doi: 10.3945/ajcn.110.008326.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Grajeda LM, Ivanescu A, Saito M, Crainiceanu C, Jaganath D, Gilman RH, et al. Modelling subject-specific childhood growth using linear mixed-effect models with cubic regression splines. Emerg Themes Epidemiol 2016;13:1–13. doi: 10.1186/s12982-015-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wu L Mixed Effects Models for Complex Data 2009;11:431. doi: 10.1201/9781420074086. [DOI] [Google Scholar]
- [38].Tilling K, MacDonald-Wallis C, Lawlor DA, Hughes RA, Howe LD. Modelling childhood growth using fractional polynomials and linear splines. Ann Nutr Metab 2014;65:129–38. doi: 10.1159/000362695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy 1 – 3 2013:1062–8. doi: 10.3945/ajcn.112.051706.INTRODUCTION. [DOI] [PMC free article] [PubMed]
- [40].Rabeth Hesketh SSA, editor. Multilevel and Longitudinal Modeling Using Stata Volume I: Continuous responses. 3rd ed. Texas: Stata Press; 2012. [Google Scholar]
- [41].Sommer C, Sletner L, Mørkrid K, Jenum AK, Birkeland KI. Effects of early pregnancy BMI, mid-gestational weight gain, glucose and lipid levels in pregnancy on offspring ‘ s birth weight and subcutaneous fat : a population-based cohort study 2015:1–9. doi: 10.1186/s12884-015-0512-5. [DOI] [PMC free article] [PubMed]
- [42].Cho E-H, Hur J, Lee K-J. Early Gestational Weight Gain Rate and Adverse Pregnancy Outcomes in Korean Women. PLoS One 2015;10:e0140376. doi: 10.1371/journal.pone.0140376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA - J Am Med Assoc 2017;317:2207–25. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zonana-Nacach A, Baldenebro-Preciado R, Ruiz-Dorado MA. Effects of maternal and neonatal gestational weight gain. [Spanish] Efecto de la ganancia de peso gestacional en la madre y el neonato. Salud Publica Mex 2010;52:220–5. doi: 10.1590/S0036-36342010000300006. [DOI] [PubMed] [Google Scholar]
- [45].Mielke RT. Determinants of excessive gestational weight gain in Mexican American women in Los Angeles 2010;Ph.D.:174 p-174 p 1p. [Google Scholar]
- [46].Shin D, Lee KW, Song WO. Dietary Patterns During Pregnancy are Associated with Gestational Weight Gain. Matern Child Health J 2016;20:2527–38. doi: 10.1007/s10995-016-2078-x. [DOI] [PubMed] [Google Scholar]
- [47].Natarajan L, Pu M, Fan J, Levine RA, Patterson RE, Thomson A, et al. Practice of Epidemiology Measurement Error of Dietary Self-Report in Intervention Trials 2010;172:819–27. doi: 10.1093/aje/kwq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


