Abstract
Introduction:
Esthesioneuroblastoma (ENB), also known as olfactory neuroblastoma, represents up to 3% of all sinonasal neoplasms. Hyams histological grading can be a promising tool in predicting metastases and establishing prognoses for this complex tumor.
Methods:
A systematic literature search was performed in PubMed, Ovid MEDLINE, and Cochrane databases. ENB patients with Hyams I-II or III-IV were categorized as low grade Hyams (LGH) or high grade Hyams (HGH), respectively. Binary and continuous random-effects models were applied to calculate Odds Ratios (OR) for the incidences of neck and distal metastases as well as for 5- and 10-year overall survival rates.
Results:
Of the 57 screened articles published from 1993–2018, 16 (525 patients) and 21 (563 patients) provided data for tumor metastases and overall survival rates, respectively. Neck metastasis was observed in 18.2% of HGH vs. 7.9% of LGH patients. Distant metastasis was noted in 20.7% of HGH vs. 8.9% of LGH patients. LGH patients had 5- and 10-year overall survival rates of 81.2% and 64.0%, respectively, compared to 60.9% and 40.6%, respectively, for HGH patients. In comparing HGH vs. LGHs, collective OR for neck and distant metastases were 2.08 (95% CI 1.09–3.99; p=0.03) and 2.37 (95% CI 1.07–5.26; p=0.03), respectively. Moreover, in comparing LGH vs. HGHs, collective OR for 5- and 10-year overall survival rates were 3.39 (95% CI 2.09–5.49; p<0.001) and 3.03 (95% CI 1.82–5.06; p<0.001), respectively.
Conclusion:
HGH ENBs, compared to LGH ENBs, are more likely to metastasize to neck or distal targets and to have lower overall survival rates.
Keywords: Esthesioneuroblastoma, Olfactory neuroblastoma, metastasis, survival, meta-analysis
Introduction
Esthesioneuroblastoma (ENB), also known as olfactory neuroblastoma, is a rare and malignant neuroectodermal tumor of the nasal cavity that was first described by Berger in 1924.1 It represents up to 3–5% of sinonasal neoplasms and has been associated with metastatic tendency, though overall prognosis is often favorable compared to other sinonasal malignancies.2–5 Previous uncertainties regarding the diagnosis and management of ENB can be attributed to its rarity in any single institution, varying biological activity and aggressiveness, susceptibility to misdiagnosis for other undifferentiated nasal cavity tumors, and the continuous evolution of treatment techniques.4,6,7 However, multi-institutional efforts and large population- or systems-based databases are changing this.8–11 Kadish and Dulguerov have proposed ENB classifications based on primary tumor extension and clinicoradiographic data, respectively.6,12 The only grading system based on the histologic maturation and differentiation was developed by Hyams in 1988 which categorized ENBs from grades 1 to 4 from well to least differentiated.13 However, the interpretation of this histopathological grading has not yet been fully established.
Hyams grading considers cellular architecture and pleomorphism, mitotic activity, and presence of necrosis, calcification, gland proliferation, and neurofibrillary matrix or rosettes.2 It is common to binarize Hyams grading into low-grade Hyams (LGH; Hyams I-II) or high-grade Hyams (HGH; Hyams III-IV) to analyze tumor characteristics and prognoses.14–17 While many studies have reported metastasis and survival of different Hyams-graded ENBs, an agreement on the relative risk or odds ratio in LGH vs. HGH has yet to be established. With an estimated 5-year survival of 65–73% for all ENBs,7,18 a 2001 meta-analysis demonstrated that mean survival for LGH and HGH can be broken down to 56% and 25%, respectively.7 Since histopathology is considered a potentially important prognostication,16,17 there is need for a comprehensive evaluation of survival likeliness in LGH compared to HGH ENBs. Furthermore, there is evidence suggesting that regional and distant metastases rates are different based on Hyams grading. This can play a significant role in surveillance and treatment regimen.11 We thus performed a comprehensive meta-analysis of all published articles to evaluate the odds ratio (OR) and strength of association of neck and distant metastasis as well as 5- and 10-year overall survival in LGH vs. HGH ENB patients.
Materials and methods
Institutional Review Board approval was deferred since only de-identified patient information accessible through the published literature was used. We performed a thorough literature search of the published articles in PubMed, Ovid MEDLINE, and Cochrane databases using “Hyams” and “esthesioneuroblastoma” or “olfactory neuroblastoma” keywords. Additionally, the included studies’ references were carefully assessed to ensure complete inclusion of all scientific publications containing histopathologic data. Each article was independently evaluated by two authors (K.G. and A.A.) to be considered for inclusion. Study inclusion, data extraction, and analyses are in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.19 Our search criteria resulted in 57 studies published from March 1993 to March 2019. Case reports, reviews, and studies with unclear Hyams classifications or outcomes were excluded (Figure 1). Koch et al.20 and Constantinidis et al.21 utilized the same patient cohort, thus only the latter study, which was in English, was utilized here. Furthermore, Van Gompel had two studies in 2012 and 2013 with possibly overlapping patients, thus only the former study was utilized for survival data and the latter study was utilized for metastasis data.22–23 Inclusion criteria mandated that studies report either of the outcome variables for both LGH and HGH tumors. Extracted data included patient demographics, Hyams classifications, eventual development and location of metastases, follow-up duration, and 5- and 10-year survival.
FIGURE 1.
Flowchart of study inclusion.
Overall survival was collected regardless of whether ENB was the cause of death. Some studies reported disease-free survival, but this was not analyzed due to the lower number of reports compared to overall survival. Neck metastasis was mostly indicated as metastasis “to the neck” or “cervical lymph node”, though it was sometimes deduced from reports of neck dissection. Distant metastasis locations consisted of dura, brain, breast, lung, and vertebrae; though, some were unspecified and simply reported as “distant” metastasis. We designated 5- and 10-year overall survival, as well as neck and distant metastasis, as primary outcome variables. These were compared between LGH and HGH ENBs using Review Manager v5.3 (Nordic Cochrane Centre, The Cochrane Collaboration, 2014) via binary random-effects models for OR calculation. A p value < 0.05 was considered significant. Forest plots and the corresponding ORs and 95% confidence intervals (CI) were obtained for each outcome variable. Funnel plots were created to assess for potential study bias.
Results
Of the 57 screened articles published from 1993–2018, 16 (525 patients) and 21 (563 patients) studies provided data for tumor metastases and overall survival rates, respectively. Patient demographics and outcome variables of all studies used in analyzing tumor metastasis and overall survival rates are listed in Table 1 and Table 2, respectively. The metastasis data contained up to 303 LGH and 222 HGH subjects, whereas survival data included up to 349 LGH and 214 HGH patients. Overall neck and distant metastasis rates for all ENB patients were 12.4% and 13.9%, respectively. Neck metastasis was observed in 18.2% of HGH and 7.9% of LGH patients. Distant metastasis was noted in 20.7% of HGH and 8.9% of LGH patients. The sites of distant metastases consisted of brain or dura (n=29; 39.7%), spine or bone (n=16; 21.9%), lung (n=3; 4.1%), breast (n=2; 2.7%), liver (n=2; 2.7%), and unspecified (n=21; 28.8%). LGH patients had 5- and 10-year overall survival rates of 80.2% and 66.8%, respectively, compared to 54.2% and 35.6%, respectively, for HGH patients.
Table 1:
Summary of patient demographics and outcome variables of studies used in the tumor metastasis meta-analysis.
| Study | Study Years | Sample Size (% F) | Mean Age (range, yr) | Mean Follow-up (range, mo) | Hyams Grade | Neck Metastasis (%) | Distant Metastasis (%) |
|---|---|---|---|---|---|---|---|
| Peng, 201826 | 2007–2015 | 10 (40) | 46.5 (26–66) | N/A (6–76) | Low | 1/7 (14.3) | 0/7 (0) |
| High | 1/3 (33.3) | 0/3 (0) | |||||
| Mays, 201844 | 1992–2012 | 35 (49) | 43 (13–71) | N/A (8–240) | Low | 1/24 (4.2) | 0/24 (0) |
| High | 0/10 (0) | 1/10 (10) | |||||
| Harvey, 201710 | N/A | 109 (46) | 49.2 | 128.6 (6–421) | Low | 5/58 (8.6) | 9/58 (15.5) |
| High | 5/51 (9.8) | 8/51 (15.7) | |||||
| Su, 201745 | 1993–2014 | 15 (47) | 46.1 (11–78) | 93.6 (1–266) | Low | 2/6 (33.3) | 5/6 (83.3) |
| High | 5/9 (55.6) | 6/9 (66.7) | |||||
| Zhang, 201642 | 2000–2010 | 13 (38) | 42.5 (15–69) | 67.4 (23–116) | Low | 0/6 (0) | 0/6 (0) |
| High | 3/7 (42.9) | 2/7 (28.6) | |||||
| Nalavenkata, 201611 |
1986–2013 | 113 (46) | 49.7 | 41.5* | Low | 2/43 (4.7) | 0/43 (0) |
| High | 4/38 (10.5) | 0/38 (0) | |||||
| Bell, 20152 | 1990–2013 | 124 (39) | 38.8 (7–90) | N/A (2–240) | Low | N/A | 7/75 (9.3) |
| High | N/A | 3/25 (12) | |||||
| Malouf, 201334 | 1979–2009 | 44 (48) | 42 (4–78) | 147.7 (1–356) | Low | 5/29 (17.2) | 0/29 (0) |
| High | 4/15 (26.7) | 8/15 (53.3) | |||||
| Van Gompel, 201323 |
1962–2012 | 8 (38) | 52.6 (36–84) | 27 (4–89) | Low | 1/2 (50) | 0/2 (0) |
| High | 1/6 (16.7) | 2/6 (33.3) | |||||
| Weinreb, 200946 | N/A | 20 (35) | 49.3 (20–79) | 58.6 (3–152) | Low | 0/15 (0) | 1/15 (6.7) |
| High | 0/5 (0) | 2/5 (40) | |||||
| Nakao, 200739 | 1979–2003 | 9 (44) | 49.8 (25–73) | 131.2 (6–325) | Low | 0/6 (0) | 0/6 (0) |
| High | 1/3 (33.3) | 1/3 (33.3) | |||||
| Constantinidis, 200421 |
1975–2000 | 26 (50) | 46.2 (10–84) | 90.6 (4–259) | Low | 0/11 (0) | 0/11 (0) |
| High | 2/11 (18.2) | 4/11 (36.4) | |||||
| Miyamoto, 200047 |
1970–1999 | 12 (42) | 51 (17–85) | N/A (2–60) | Low | 0/7 (0) | 2/7 (28.6) |
| High | 2/4 (50) | 0/4 (0) | |||||
| Eriksen, 200048 | 1977–1997 | 15 (40) | 48.7 (14–83) | 65 (8–139) | Low | 0/6 (0) | 2/6 (33.3) |
| High | 0/7 (0) | 4/7 (57.1) | |||||
| Tatagiba, 199549 | 1978–1992 | 8 (63) | 52.3 (29–70) | 45 (6–84) | Low | 1/7 (14.3) | 1/7 (14.3) |
| High | 0/1 (0) | 0/1 (0) | |||||
| Sakata, 199350 | 1978–1989 | 7 (29) | 43.3 (17–73) | 28.4 (2–120) | Low | 0/1 (0) | 0/1 (0) |
| High | 4/6 (66.7) | 5/6 (83.3) |
=median instead of mean.
Table 2:
Summary of patient demographics and outcome variables of studies used in the 5- and 10-year overall survival meta-analyses.
| Study | Study Years | Sample Size (% F) |
Mean Age (range, yr) |
Hyams Grade | 5-Year Survival (%) | 10-Year Survival (%) |
|---|---|---|---|---|---|---|
| Gram, 201851 | 2000–2016 | 14 (29) | 52.7 (17–81) | Low | 6/7 (85.7) | N/A |
| High | 1/2 (50) | N/A | ||||
| Peng, 201826 | 2007–2015 | 10 (40) | 46.5 (26–66) | Low | 4/7 (57.1) | N/A |
| High | 0/3 (0) | N/A | ||||
| Mays, 201844 | 1992–2012 | 35 (49) | 43 (13–71) | Low | 19/24 (79.2) | 19/24 (79.2) |
| High | 9/10 (90) | 5/10 (50) | ||||
| Turri-Zanoni, 201738 | 2001–2015 | 98 (52) | 51.2 (14–79) | Low | 48/51 (94.1) | 40/51 (78.4) |
| High | 11/16 (68.8) | 6/12 (50) | ||||
| Harvey, 201710 | N/A | 109 (46) | 49.2 | Low | 50/58 (86.2) | 32/58 (55.2) |
| High | 44/51 (86.3) | 27/51 (52.9) | ||||
| Su, 201745 | 1993–2014 | 15 (47) | 46.1 (11–78) | Low | 3/6 (50) | N/A |
| High | 7/7 (100) | N/A | ||||
| Zhang, 201642 | 2000–2010 | 13 (38) | 42.5 (15–69) | Low | 4/6 (66.7) | N/A |
| High | 2/7 (28.6) | N/A | ||||
| Bell, 20152 | 1990–2013 | 124 (39) | 38.8 (7–90) | Low | 60/75 (80) | 50/75 (66.7) |
| High | 19/25 (76) | 13/25 (52) | ||||
| Van Gompel, 201222 | 1962–2009 | 109 (44) | 49 (12–90) | Low | 35/47 (74.5) | 23/47 (48.9) |
| High | 22/40 (55) | 11/40 (27.5) | ||||
| Malouf, 201334 | 1979–2009 | 44 (48) | 42 (4–78) | Low | 13/14 (92.9) | N/A |
| High | 15/30 (50) | N/A | ||||
| Kaur, 201352 | 1995–2009 | 20 (14) | 51 (31–70) | Low | 7/8 (87.5) | N/A |
| High | 6/10 (60) | N/A | ||||
| Fukushima, 201253 | 1996–2009 | 12 (58) | 34.9 (19–82) | Low | 5/5 (100) | 5/5 (100) |
| High | 6/7 (85.7) | 6/7 (85.7) | ||||
| Weinreb, 200946 | N/A | 20 (35) | 49.3 (20–79) | Low | 8/9 (88.9) | N/A |
| High | 0/1 (0) | N/A | ||||
| Nakao, 200739 | 1979–2003 | 9 (44) | 49.8 (25–73) | Low | 5/6 (83.3) | 5/6 (83.3) |
| High | 0/3 (0) | 0/3 (0) | ||||
| Constantinidis, 200421 |
1975–2000 | 26 (50) | 46.2 (10–84) | Low | 5/7 (71.4) | 4/5 (80) |
| High | 2/9 (22.2) | 1/8 (12.5) | ||||
| Ingeholm, 200254 | 1978–2000 | N/A | 51.2 (13–82) | Low | 14/22 (63.6) | N/A |
| High | 4/10 (40) | N/A | ||||
| Miyamoto, 200047 | 1970–1999 | 12 (42) | 51 (17–85) | Low | 3/5 (60) | 0/4 (0) |
| High | 2/4 (50) | 0/3 (0) | ||||
| Eriksen, 200048 | 1977–1997 | 15 (40) | 48.7 (14–83) | Low | 4/6 (66.7) | 2/5 (40) |
| High | 1/6 (16.7) | 0/5 (0) | ||||
| McElroy, 199855 | 1970–1995 | 10 (40) | 48.2 (22–74) | Low | 4/4 (100) | N/A |
| High | 2/4 (50) | N/A | ||||
| Tatagiba, 199549 | 1978–1992 | 8 (63) | 52.3 (29–70) | Low | 4/5 (80) | N/A |
| High | 1/1 (100) | N/A | ||||
| Sakata, 199350 | 1978–1989 | 7 (29) | 43.3 (17–73) | Low | 2/2 (100) | 1/1 (100) |
| High | 1/5 (20) | 0/5 (0) | ||||
| Foote, 199356 | 1951–1990 | 49 (45) | 47.4 (3–79) | Low | 27/33 (81.8) | N/A |
| High | 5/14 (35.7) | N/A |
In comparing HGH vs. LGHs, collective OR for neck and distant metastases were 2.08 (95% CI 1.09–3.99; p=0.03) and 2.37 (95% CI 1.07–5.26; p=0.03), respectively (Figure 2). Moreover, in comparing LGH vs. HGHs, collective OR for 5- and 10-year overall survival rates were 3.39 (95% CI 2.09–5.49; p<0.001) and 3.03 (95% CI 1.82–5.06; p<0.001), respectively (Figure 3). Funnel plots assessing studies’ relative ORs showed nearly all studies falling within the 95% confidence boundaries (Figure 4).
FIGURE 2.
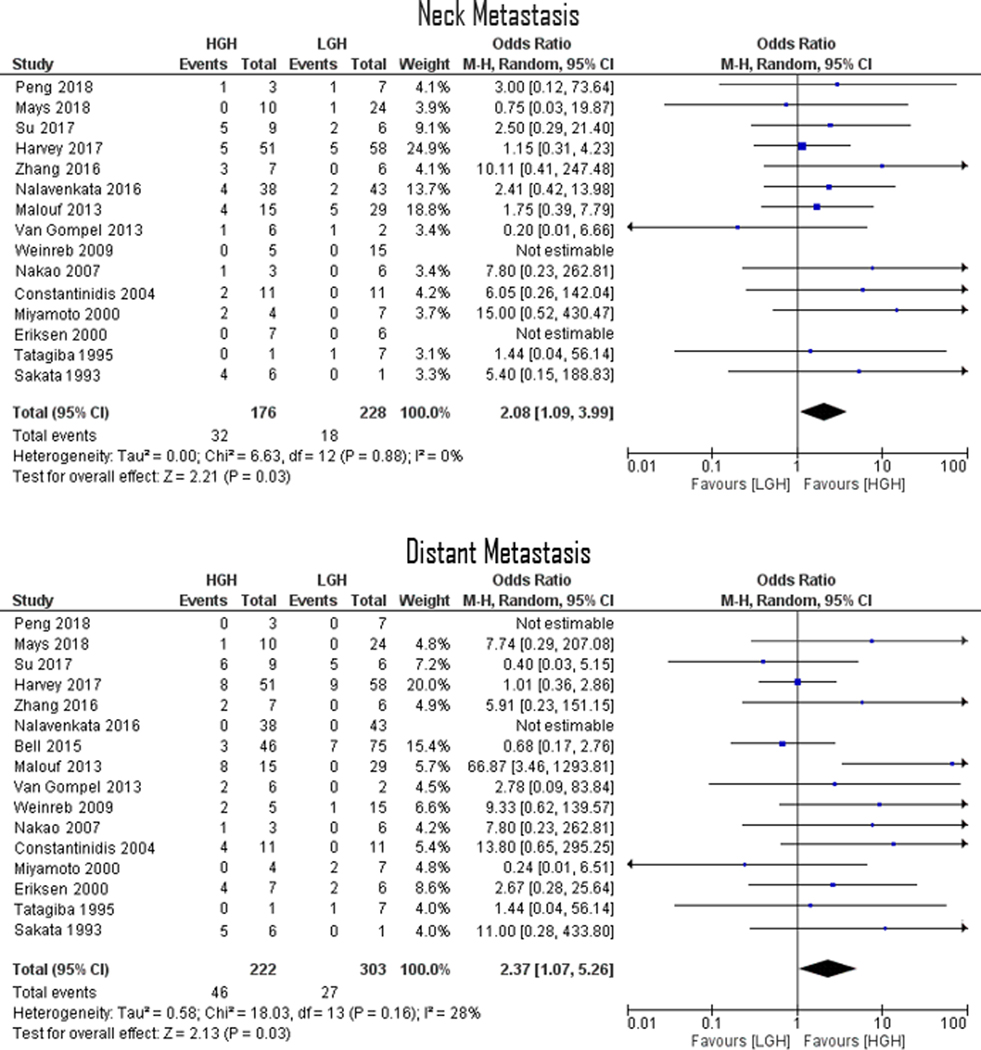
Forrest Plots demonstrating an overall 2.08 (p=0.03) and 2.37 (p=0.03) ORs for ENB neck metastasis and distant metastasis, respectively. Lines are representative of the 95% confidence interval and boxes represent the post-operative intervention rate. Each box’s size correlates to that study’s size effect.
FIGURE 3.
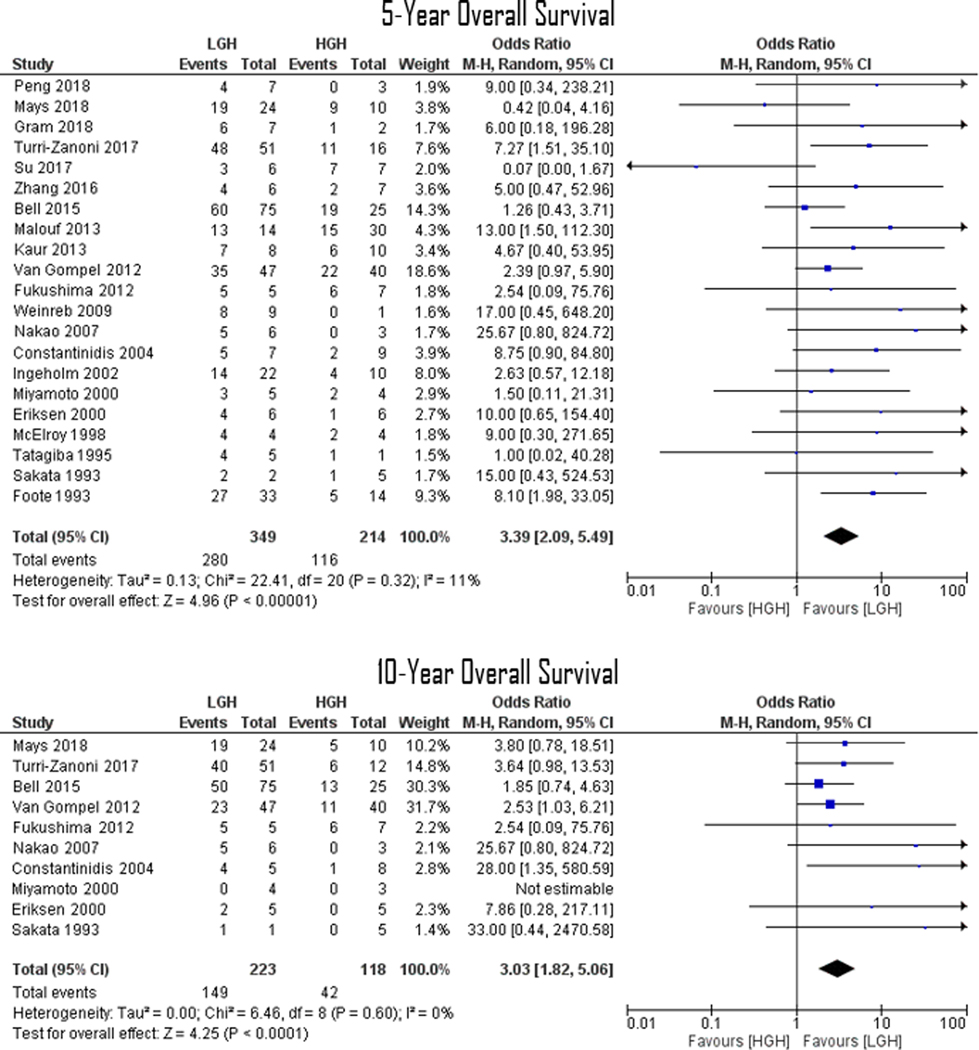
Forrest Plots demonstrating an overall 3.39 (p<0.001) and 3.03 (p<0.001) ORs for ENB 5- and 10-year overall survival, respectively. Lines are representative of the 95% confidence interval and boxes represent the post-operative intervention rate. Each box’s size correlates to that study’s size effect.
FIGURE 4.
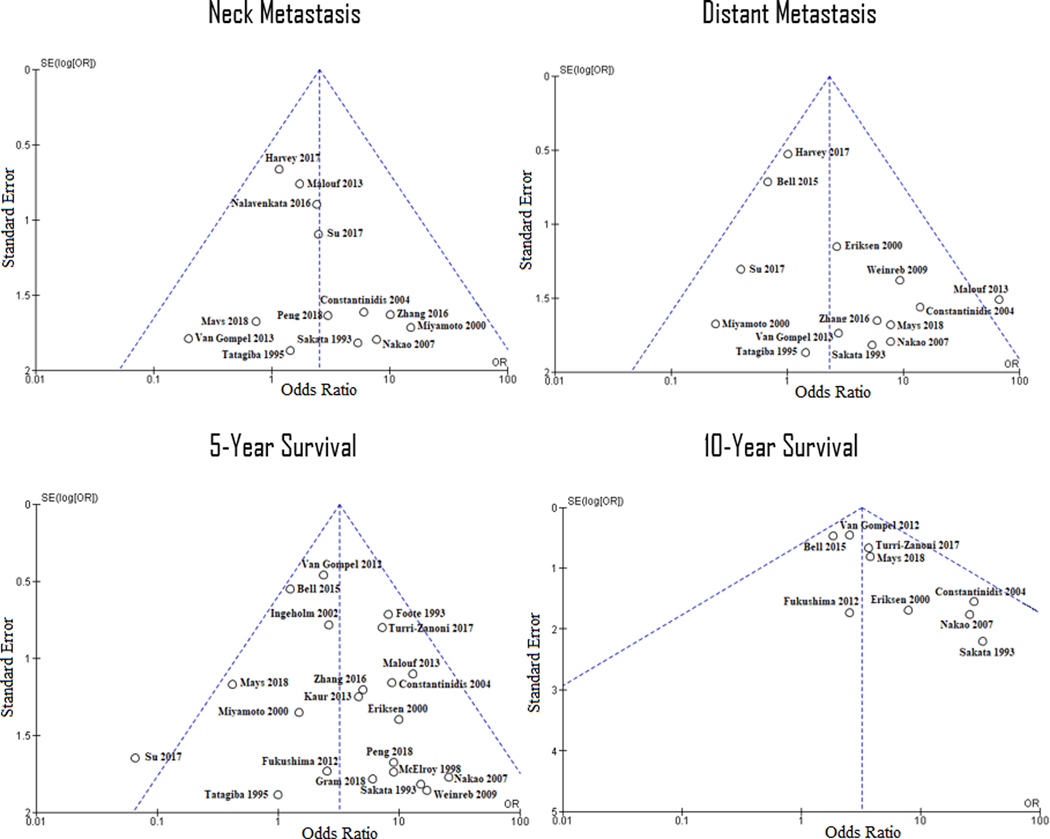
Funnel plots of the four outcome variables for the evaluation of study bias. Dotted lines represent 95% confidence interval with a fixed population treatment effect, and the presence of a study outside these boundaries may suggest systematic bias. The only studies falling outside the boundaries were Malouf et al. and Su et al. distant metastasis and 5-year survival data, respectively.
Discussion
In this meta-analysis, we demonstrated that compared to LGH ENB, HGH ENB is associated with significantly higher rates of both neck and distant metastasis as well as lower rates of 5- and 10-year overall survival. Specifically, our ORs demonstrated that HGH ENBs were 2.1 and 2.4 times more likely to metastasize to the neck and distally, respectively. Also, LGH patients were 3.4 and 3.0 times more likely to have survived during the 5- and 10-year follow up, respectively. The difference in all four outcome variables was statistically significant between low and high Hyams grades. Therefore, we believe that the histopathologic features of a newly-diagnosed or under-surveillance ENB may continue to serve as an important tool in discussions regarding management, prognosis, and possible complications.
In the case of any tumor, early diagnosis is an important contributor to deciding management and improving prognosis. However, late diagnosis of ENB, possibly due to nonspecific symptoms resembling chronic rhinosinusitis or other more common sinonasal entities, can lead to poorer prognosis.21,24 These indistinct symptoms can consist of unilateral nasal obstruction, loss of sense of smell, epistaxis, epiphora, and headache.25,26 This combined with the rare annual incidence of 0.4 per million people27 and variability in treatment response15,28 has made ENB a difficult entity to study in any single institution. There is also a moderate to high probability of extraprimary recurrence after therapy, making long-term follow up an important component of management.29,30 As a result, none of the main ENB classification systems has yet been deemed the gold standard for predicting metastasis or prognosis.
There exists a mix of studies that demonstrate either presence or absence of significant differences in survival based on Hyams grading.15,21,30,31 In a 2001 meta-analysis, Dulguerov suggested that Hyams grading was a significant prognosis factor with mean survivals of 56% and 25% for LGH and HGH, respectively.7 A 2014 population-based analysis of the largest ENB cohort revealed 84% LGH and 40% HGH for 5-year overall survival, and 67% LGH and 34% HGH for 10-year overall survival.17 These studies agree with our findings which demonstrated a 5-year survival rate of 80% and 54% and a 10-year survival rate of 67% and 36% for LGH and HGH, respectively. In a 2010 review by Kane, Hyams grading was also a significant prognostic factor in the context of different treatment modalities including surgery, radiotherapy, chemotherapy, or a combination thereof.16 Kane reported that in addition to Kadish grading and age, HGH was an independent predictor of poor survival with a 4.8 hazard ratio.16 Our ORs corroborated this information, showing LGH ENB patients to be 3.0–3.4 times more likely to survive after 5 and 10 years as compared to HGH ENB.
Both cervical and distant metastasis appear to be common in ENB regardless of grading or time from diagnosis.25 Neck metastasis and regional recurrence is reported in around 5%−20% of ENB patients.7,8,28,32,33 This is in accordance with our metastasis data showing 12–14% neck and distant metastasis. LGH ENB has been shown to be associated with local recurrence whereas HGH ENB can frequently involve higher T4 staging and brain metastasis.34 Two large-cohort analyses by VanGompel and Ball have suggested that neck metastasis is a predictor of survival.2,22 Accordingly, we displayed that HGH ENBs were more than twice likely to metastasize to both neck or distant targets, the same positive association as in the case of survival. Jiang and colleagues have argued for the efficacy of elective neck irradiation for ENB patients with clinically node-negative necks.35 Others have advocated for elective radiation of the N0 neck in higher grade or stage ENB cases.35–37 Hyams grading may be an important preoperative factor for decision-making in this scenario, as HGH ENBs were 2.1 times more likely to lead to eventual neck metastasis. Many physicians and institutions may also follow different preferences and guidelines for monitoring distant metastasis. Again, we believe that Hyams grading can play a valuable role in any systematic guideline. For instance, regular imaging may be considered for HGH ENBs as they may be 2.4 times more likely to metastasize distally. Aggressive treatments, such as induction chemotherapy and adjuvant radiotherapy, may also be more considered for patients with HGH ENBs. This has been alluded to by previous authors.2,22
To date, there are no universal guidelines for management of ENB, and many variables are considered in the multidisciplinary management of this rare malignancy. We thus recommend the LGH and HGH categorization of ENB as a constructive step in the comprehensive surveillance and management process. Multiple authors have suggested that Hyams grading could be utilized as an independent prognosticator.16,17,34,38 Despite its apparent impact on survival, there is now mounting evidence that this information may be useful for elective treatment of the neck. Nakao et al., Bell et al., and Ow et al. exhibited that nodal metastasis and early recurrence was correlated with poorer survival.2,39,40 Thus, our two main outcome variables, namely metastasis and survival, may be appropriately related.
The present study is not without limitations. There may be heterogeneity in correctly diagnosing ENB or accurately detecting and reporting metastasis. Some have suggested an increase in ENB diagnoses in recent decades likely due to improved differentiation of neuroendocrine malignancies.41 Others recognize the possibility of confusing ENBs with other sinonasal tumors such as undifferentiated or neuroendocrine carcinomas.2 There is also a possible lack of homogeneity in establishing Hyams grading per tumor. Some pathologists attest to the arbitrary nature of definitive grading for some ENB cases especially when a single tumor may have characteristics fitting multiple Hyams grades, such as the co-occurrence of necrosis and calcification.14,24 Biopsy may also lead to sampling error as different parts of the tumor may contain different Hyams grades.42,43 There might be a systematic difference in ENB treatment between institutions and time periods (i.e., decade of diagnosis), which may affect survival. It is worth noting that though all cases of dural metastasis were included as they were reported in the referenced studies, it is possible, if not otherwise specified, that some were not an indication of tumor progression but rather a result of local failure or lack of complete tumor resection. Additionally, it is very challenging to determine whether cervical metastases resulted from ENB primary recurrence, regional recurrence, or progression of disease. However, the data from the current study suggests that Hyams grading appears to demonstrate reliability as a marker of aggressive disease, which is associated with any of the above scenarios. There is also the possibility of study and reporting bias, as positive and favorable data may be more likely to be submitted for publication. However, we think that the existing internal validity within each study in comparing LGH and LGH ENBs will, to some degree, control for such confounding factors. Even when considering these limitations, this meta-analysis benefits from a large cumulative population of many different institutions, patient demographics, and management guidelines.
Conclusion
The literature suggests that HGH ENBs, compared to LGH ENBs, were more likely to metastasize to the neck or distantly and to have lower overall survival rates. HGHs were more than twice as likely to metastasize whereas LGHs were three times as likely to have overall 5- or 10-year survival. Thus, Hyams grading can be an important and valuable tool in the surveillance and management of ENB.
Footnotes
Conflict of Interest: None
Financial Disclosure: None
Level of Evidence: NA
References
- 1.Berger L. L’esthesioneuroepitheliome olfactif. Bull Assoc Fr Etud Cancer. 1924;13:410–21. [Google Scholar]
- 2.Bell D, Saade R, Roberts D, Ow TJ, Kupferman M, DeMonte F, Hanna EY. Prognostic utility of Hyams histological grading and Kadish-Morita staging systems for esthesioneuroblastoma outcomes. Head Neck Pathol. 2015. March 1;9(1):51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Vol 9 IARC; 2005. [Google Scholar]
- 4.Bradley PJ, Jones NS, Robertson I. Diagnosis and management of esthesioneuroblastoma. CURR OPIN OTOLARYNGO. 2003. April; 11(2):112–8. [DOI] [PubMed] [Google Scholar]
- 5.Broich I, Pagliari A, Ottaviani F. Esthesioneuroblastoma: a general review of the cases published since the discovery of the tumour in 1924. Anticancer Res. 1997;17:2683–706. [PubMed] [Google Scholar]
- 6.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970–1990. The Laryngoscope. 1992. August;102(8):843–9. [DOI] [PubMed] [Google Scholar]
- 7.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001. Nov 1;2(11):683–90. [DOI] [PubMed] [Google Scholar]
- 8.Kuan EC, Nasser HB, Carey RM, Workman AD, Alonso JE, Wang MB, John MA, Palmer JN, Adappa ND, Tajudeen BA. A Population‐Based Analysis of Nodal Metastases in Esthesioneuroblastomas of the Sinonasal Tract. Laryngoscope. 2018. September 8. [DOI] [PubMed] [Google Scholar]
- 9.Carey RM, Godovchik J, Workman AD, Kuan EC, Parasher AK, Chen J, Palmer JN, Adappa ND, Newman JG, Brant JA. Patient, disease, and treatment factors associated with overall survival in esthesioneuroblastoma. Int Forum Allergy Rhinol. 2017. Dec. Vol. 7, No. 12, pp. 1186–1194. [DOI] [PubMed] [Google Scholar]
- 10.Harvey RJ, Nalavenkata S, Sacks R, Adappa ND, Palmer JN, Purkey MT, Schlosser RJ, Snyderman C, Wang EW, Woodworth BA, Smee R. Survival outcomes for stage‐matched endoscopic and open resection of olfactory neuroblastoma. Head & neck. 2017. December;39(12):2425–32. [DOI] [PubMed] [Google Scholar]
- 11.Nalavenkata SB, Sacks R, Adappa ND, Palmer JN, Purkey MT, Feldman MD, Schlosser RJ, Snyderman CH, Wang EW, Woodworth BA, Smee R. Olfactory neuroblastoma: fate of the neck—a long-term multicenter retrospective study. Otolaryngol Head Neck Surg. 2016. February;154(2):383–9. [DOI] [PubMed] [Google Scholar]
- 12.Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma: a clinical analysis of 17 cases. Cancer. 1976; 37: 1571–76. [DOI] [PubMed] [Google Scholar]
- 13.Hyams VJ. Olfactory neuroblastoma (Case 6) In: Batsakis JG, Hyams VJ, Morales AR, editors. Special tumors of the head and neck. Chicago: ASCP Press; 1982. p. 24–9 [Google Scholar]
- 14.Gallagher KK, Spector ME, Pepper JP, McKean EL, Marentette LJ, McHugh JB. Esthesioneuroblastoma: updating histologic grading as it relates to prognosis. Ann Otol Rhinol Laryngol. 2014. May;123(5):353–8. [DOI] [PubMed] [Google Scholar]
- 15.Morita A, Ebersold M, Olsen KD, et al. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993;32(5):706–715. [DOI] [PubMed] [Google Scholar]
- 16.Kane AJ, Sughrue ME, Rutkowski MJ, et al. Posttreatment prognosis of patients with esthesioneuroblastoma. J Neurosurg. 2010;113(2):340–351. [DOI] [PubMed] [Google Scholar]
- 17.Tajudeen BA, Arshi A, Suh JD, St John M, Wang MB. Importance of tumor grade in esthesioneuroblastoma survival: a population-based analysis. JAMA Otolaryngol Head Neck Surg. 2014. December 1;140(12):1124–9. [DOI] [PubMed] [Google Scholar]
- 18.Platek ME, Merzianu M, Mashtare TL, Popat SR, Rigual NR, Warren GW, Singh AK. Improved survival following surgery and radiation therapy for olfactory neuroblastoma: analysis of the SEER database. Radiat Oncol. 2011. December;6(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151(4): 264–269. [DOI] [PubMed] [Google Scholar]
- 20.Koch M, Constantinidis J, Dimmler A, Strauss C, Iro H. Long-term experiences in the therapy of esthesioneuroblastoma. Laryngo-rhino-otologie. 2006. October;85(10):723–30. [DOI] [PubMed] [Google Scholar]
- 21.Constantinidis J, Steinhart H, Koch M, Buchfelder M, Schaenzer A, Weidenbecher M, Iro H. Olfactory neuroblastoma: the University of Erlangen-Nuremberg experience 1975–2000. Otolaryngol Head Neck Surg. 2004. May;130(5):567–74. [DOI] [PubMed] [Google Scholar]
- 22.Van Gompel JJ, Giannini C, Olsen KD, Moore E, Piccirilli M, Foote RL, Buckner JC, Link MJ. Long-term outcome of esthesioneuroblastoma: Hyams grade predicts patient survival. J Neurol Surg Part B Skull Base. 2012. February;73(S 01): A007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Gompel JJ, Carlson ML, Pollock BE, Moore EJ, Foote RL, Link MJ. Stereotactic radiosurgical salvage treatment for locally recurrent esthesioneuroblastoma. Neurosurgery. 2012. November 30;72(3):332–40. [DOI] [PubMed] [Google Scholar]
- 24.Levine PA, Gallagher R, Cantrell RW. Esthesioneuroblastoma: reflections of a 21-year experience. Laryngoscope. 1999;109:1539–43. [DOI] [PubMed] [Google Scholar]
- 25.Thompson LDR. Olfactory neuroblastoma. Head Neck Pathol. 2009;3(3):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng X, Liu Y, Peng X, Wang Z, Zhang Z, Qiu Y, Jin M, Wang R, Kong D. Clinical features and the molecular biomarkers of olfactory neuroblastoma. Pathol Res Pract. 2018. August 1;214(8):1123–9. [DOI] [PubMed] [Google Scholar]
- 27.Theilgaard SA, Buchwald C, Ingeholm P, Kornum Larsen S, Eriksen JG, Sand Hansen H. Esthesioneuroblastoma: a Danish demographic study of 40 patients registered between 1978 and 2000. Acta Otolaryngol. 2003;123(03):433–439 [DOI] [PubMed] [Google Scholar]
- 28.Devaiah AK, Andreoli MT. Treatment of esthesioneuroblastoma: a 16‐year meta‐analysis of 361 patients. Laryngoscope. 2009. July;119(7):1412–6. [DOI] [PubMed] [Google Scholar]
- 29.Zanation AM, Ferlito A, Rinaldo A, et al. When, how and why to treat the neck in patients with esthesioneuroblastoma: a review. Eur Arch Otorhinolaryngol. 2010;267(11):1667–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wertz A, Hollon T, Marentette LJ, Sullivan SE, McHugh JB, McKean EL. Surgical Treatment of Olfactory Neuroblastoma: Major Complication Rates, Progression Free and Overall Survival. J Neurol Surg Part B Skull Base. 2018. April;79(02):151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dias FL, Sá GM, Lima RA, Kligerman J, Leôncio MP, Freitas EQ, Soares JR, Arcuri RA. Patterns of failure and outcome in esthesioneuroblastoma. Arch Otolaryngol. 2003. November 1;129(11):1186–92. [DOI] [PubMed] [Google Scholar]
- 32.Rinaldo A, Ferlito A, Shaha AR, Wei WI, Lund VJ. Esthesioneuroblastoma and cervical lymph node metastases: clinical and therapeutic implications. Acta Otolaryngol. 2002; 122:215–221. [DOI] [PubMed] [Google Scholar]
- 33.Gore MR, Zanation AM. Salvage treatment of late neck metastasis in esthesioneuroblastoma: a meta-analysis. Arch Otolaryngol. 2009; 135:1030–1034 [DOI] [PubMed] [Google Scholar]
- 34.Malouf GG, Casiraghi O, Deutsch E, Guigay J, Temam S, Bourhis J. Lowand high-grade esthesioneuroblastomas display a distinct natural history and outcome. Eur J Cancer. 2013;49:1324–1334. [DOI] [PubMed] [Google Scholar]
- 35.Jiang W, Mohamed AS, Fuller CD, Kim BY, Tang C, Gunn GB, Hanna EY, Frank SJ, Su SY, Diaz E, Kupferman ME. The role of elective nodal irradiation for esthesioneuroblastoma patients with clinically negative neck. Pract radiat oncol. 2016. July 1;6(4):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monroe AT, Hinerman RW, Amdur RJ, Morris CG, Mendenhall WM. Radiation therapy for esthesioneuroblastoma: rationale for elective neck irradiation. Head Neck J Sci Spec. 2003. July;25(7):529–34. [DOI] [PubMed] [Google Scholar]
- 37.Demiroz C, Gutfeld O, Aboziada M, Brown D, Marentette LJ, Eisbruch A. Esthesioneuroblastoma: is there a need for elective neck treatment? Int J Radiat Oncol. 2011. November 15;81(4):e255–61. [DOI] [PubMed] [Google Scholar]
- 38.Turri-Zanoni M, Maragliano R, Battaglia P, Giovannardi M, Antognoni P, Lombardi D, Morassi ML, Pasquini E, Tarchini P, Asioli S, Foschini MP. The clinicopathological spectrum of olfactory neuroblastoma and sinonasal neuroendocrine neoplasms: refinements in diagnostic criteria and impact of multimodal treatments on survival. Oral Oncol. 2017. November 1;74:21–9. [DOI] [PubMed] [Google Scholar]
- 39.Nakao K, Watanabe K, Fujishiro Y, Ebihara Y, Asakage T, Goto A, Kawahara N. Olfactory neuroblastoma: long-term clinical outcome at a single institute between 1979 and 2003. Acta Oto-Laryngologica. 2007. January 1;127(sup559):113–7. [DOI] [PubMed] [Google Scholar]
- 40.Ow TJ, Hanna EY, Roberts DB, et al. Optimization of long-term outcomes for patients with esthesioneuroblastoma. Head Neck. 2014;36:524–530. [DOI] [PubMed] [Google Scholar]
- 41.Woods RS, Subramaniam T, Leader M, McConn-Walsh R, O’Neill JP, Lacy PD. Changing Trends in the Management of Esthesioneuroblastoma: Irish and International Perspectives. J Neurol Surg Part B Skull Base. 2018. June;79(03):262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Niu K, Zhu K, Xia C, Yan J, Zhao W, Wei J, Duan M, Zheng G. Long-term prognostic analysis after endoscopic endonasal surgery for olfactory neuroblastoma: a retrospective study of 13 cases. PloS one. 2016. November 2;11(11):e0166046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saade RE, Hanna EY, Bell D. Prognosis and biology in esthesioneuroblastoma: the emerging role of Hyams grading system. Curr Oncol Rep. 2015. January; 17(1):423. [DOI] [PubMed] [Google Scholar]
- 44.Mays AC, Bell D, Ferrarotto R, Phan J, Roberts D, Fuller CD, Frank SJ, Raza SM, Kupferman ME, DeMonte F, Hanna EY. Early Stage olfactory neuroblastoma and the impact of resecting dura and olfactory bulb. Laryngoscope. 2018. June;128(6):1274–80. [DOI] [PubMed] [Google Scholar]
- 45.Su SY, Bell D, Ferrarotto R, Phan J, Roberts D, Kupferman ME, Frank SJ, Fuller CD, Gunn GB, Kies MS, Glisson BS. Outcomes for olfactory neuroblastoma treated with induction chemotherapy. Head Neck. 2017. August;39(8):1671–9. [DOI] [PubMed] [Google Scholar]
- 46.Weinreb I, Goldstein D, Irish J, Perez-Ordonez B. Expression patterns of Trk-A, Trk-B, GRP78, and p75NRT in olfactory neuroblastoma. Hum Pathol. 2009. September 1;40(9):1330–5. [DOI] [PubMed] [Google Scholar]
- 47.Christopher Miyamoto R, Gleich LL, Biddinger PW, Gluckman JL. Esthesioneuroblastoma and sinonasal undifferentiated carcinoma: impact of histological grading and clinical staging on survival and prognosis. Laryngoscope. 2000. August;110(8):1262–5. [DOI] [PubMed] [Google Scholar]
- 48.Eriksen Lars Bastholt, Krogdahl Annelise S., Hansen Olfred, Karsten E. Joergensen JG. Esthesioneuroblastoma: what is the optimal treatment? Acta oncologica. 2000. January 1;39(2):231–5. [DOI] [PubMed] [Google Scholar]
- 49.Tatagiba M, Samii M, Dankoweit-Timpe E, Aguiar PH, Osterwald L, Babu R, Ostertag H. Esthesioneuroblastomas with intracranial extension: proliferative potential and management. Arq Neuro-psiquat. 1995. September;53(3B):577–86. [DOI] [PubMed] [Google Scholar]
- 50.Sakata N, Okamura J, Eguchi H, Ikuno Y, Tasaka H. Meningeal neuroblastoma after completing therapy. Pediatr Hemat Oncol. 1993. January 1;10(2):201–4. [DOI] [PubMed] [Google Scholar]
- 51.Gram SB, Grønhøj C, Mann H, Jakobsen KK, Kiss K, Bilde A, von Buchwald C. Patient characteristics of olfactory neuroblastoma: experience from a tertiary cancer centre 2000–2016 covering Eastern Denmark. Acta Path Micro Im. 2018. August;126(8):663–6. [DOI] [PubMed] [Google Scholar]
- 52.Kaur G, Kane AJ, Sughrue ME, Madden M, Oh MC, Sun MZ, Safaee M, El-Sayed I, Aghi M, McDermott MW, Berger MS. The prognostic implications of Hyam’s subtype for patients with Kadish stage C esthesioneuroblastoma. J Clin Neurosci. 2013. February 1;20(2):281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukushima S, Sugita Y, Niino D, Mihashi H, Ohshima K. Clincopathological analysis of olfactory neuroblastoma. Brain Tumor Pathol. 2012. October 1;29(4):207–15. [DOI] [PubMed] [Google Scholar]
- 54.Ingeholm P, Theilgaard SA, Buchwald C, Hansen HS, Francis D. Esthesioneuroblastoma: a Danish clinicopathological study of 40 consecutive cases. Acta Path Micro Im. 2002. September;110(9):639–45. [DOI] [PubMed] [Google Scholar]
- 55.McElroy EA, Buckner JC, Lewis JE. Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience. Neurosurgery. 1998. May 1;42(5):1023–7. [DOI] [PubMed] [Google Scholar]
- 56.Foote RL, Morita A, Ebersold MJ, Olsen KD, Lewis JE, Quast LM, Ferguson JA, O’Fallon WM. Esthesioneuroblastoma: the role of adjuvant radiation therapy. Int J Radiat Oncol. 1993. November 15;27(4):835–42. [DOI] [PubMed] [Google Scholar]


