Abstract
Introduction
The Netherlands Maritime Special Operations Forces use closed circuit oxygen rebreathers (O2-CCR), which can cause pulmonary oxygen toxicity (POT). Recent studies demonstrated that volatile organic compounds (VOCs) can be used to detect POT in laboratory conditions. It is unclear if similar VOCs can be identified outside the laboratory. This study hypothesised that similar VOCs can be identified after O2-CCR diving in operational settings.
Methods
Scenario one: 4 h O2-CCR dive to 3 metres’ seawater (msw) with rested divers. Scenario two: 3 h O2-CCR dive to 3 msw following a 5 day physically straining operational scenario. Exhaled breath samples were collected 30 min before and 30 min and 2 h after diving under field conditions and analysed using gas chromatography-mass spectrometry (GC-MS) to reconstruct VOCs, whose levels were tested longitudinally using a Kruskal-Wallis test.
Results
Eleven divers were included: four in scenario one and seven in scenario two. The 2 h post-dive sample could not be obtained in scenario two; therefore, 26 samples were collected. GC-MS analysis identified three relevant VOCs: cyclohexane, 2,4-dimethylhexane and 3-methylnonane. The intensities of 2,4-dimethylhexane and 3-methylnonane were significantly (P = 0.048 and P = 0.016, respectively) increased post-dive relative to baseline (range: 212–461%) in both scenarios. Cyclohexane was increased not significantly (P = 0.178) post-dive (range: 87–433%).
Conclusion
VOCs similar to those associated with POT in laboratory conditions were identified after operational O2-CCR dives using GC-MS. Post-dive intensities were higher than in previous studies, and it remains to be determined if this is attributable to different dive profiles, diving equipment or other environmental factors.
Keywords: Volatile organic compounds, VOCs, Gas chromatography-mass spectrometry, GC-MS, Methyl alkanes, Oxygen rebreather diving, O2-CCR
Introduction
Exposure to a high pressure of oxygen (PO2) can induce cerebral and pulmonary oxygen toxicity (POT).[ 1 , 2] While recreational divers and patients receiving hyperbaric oxygen therapy are at risk, divers using closed circuit oxygen rebreathers (O2-CCR) may be at increased risk of POT due to the duration and PO2 of exposure.[ 2 , 3] For covert special operations forces (SOF) diving, the beneficial properties of O2-CCR, such as endurance and stealth, outweigh the potential health hazards associated with these diving systems.[ 4 - 7] To limit the risk of POT, oxygen exposure is restricted to a specific dose calculated according to mathematical models (such as units of pulmonary toxicity dose; UPTD).[ 8 - 10] However, the foundation of these models has been questioned.[ 3 , 11]
The most important flaw of the UPTD concept is the use of vital capacity as a marker of POT, which has substantial inter- and intrapersonal variation.[ 12 , 13] In addition, the correlation between UPTD and a decrease in vital capacity has been determined after ‘dry dives’ (i.e., inside a recompression chamber), which exclude factors related to diving that affect pulmonary function, such as immersion and hypothermia.[ 14 , 15]
The authors of the studies that first described the UPTD recognised this and assumed that newer techniques would overcome these limitations in due time.[ 9 , 10]
Exhaled breath analysis has recently been utilised to detect POT in ‘wet’ (i.e., diving) and ‘dry’ conditions. This technique seems able to distinguish between wet and dry hyperbaric hyperoxia and between oxygen and air diving.[ 16 - 18] Two separate groups of volatile organic compounds (VOCs) in exhaled breath have been detected: markers associated with inflammation, such as cyclohexane; and methyl alkanes, which are markers of lipoperoxidation.[ 17 , 18] These methyl alkanes originate from the membrane of alveoli in response to lipoperoxidation.[ 19]
While these markers fit within the pathophysiological framework of POT, the aforementioned studies were conducted in a controlled environment. This strengthens the methodological validity; however, it cannot be assumed that these findings translate to the real working environment of SOF divers. Many internal factors (e.g., continuous physical exertion, sleep deprivation and a very limited amount of food) and external factors (e.g., air contamination, weather conditions and no access to a clean environment during sample collection) can affect exhaled breath profiles and may limit the practical applicability of exhaled breath markers to determine POT in the field.[ 20 , 21]
This study aimed to determine exhaled breath profiles after simulated operational dives. We hypothesised that markers associated with inflammation and lipoperoxidation as reported in our previous studies (i.e., cyclohexane and methyl alkanes) can be detected after oxygen dives of practical relevance to military operations.
Methods
SETTING
This observational study was approved by the Ethics Committee of the University of Amsterdam (reference: 2017.183) and the Surgeon General of the Ministry of Defence. In accordance with the Declaration of Helsinki, all participants gave written informed consent on a voluntary basis, which could be retracted at any time without any consequences. According to privacy regulations, no study data were included in the medical files of the participants.
The study consisted of two scenarios, both of which simulated an operational dive of the Royal Netherlands Navy Maritime Special Operations Forces (NLMARSOF). Eligible for inclusion were healthy NLMARSOF divers, who were fit according to the European Diving Technical Committee standards, with the exception that pulmonary function tests were assessed using the reference values of the Global Lung Function Initiative.[ 22 , 23] Exclusion criteria were recent respiratory tract infection or use of medication. Participants were not exposed to hyperbaric conditions for at least 72 h prior to the start of the dive.
In the first scenario, no strenuous physical exercise (including sports) was performed on the day before the measurements were taken. In the second scenario, the divers completed a 5-day training exercise, which involved vigorous physical exertion. To avoid affecting the exhaled breath profiles, divers fasted for 1 h before the first measurement and within 1 h of sample collection, with the exception that drinking water was allowed.[ 24]
The scenarios were carried out in Q4 2018, with different individuals in both scenarios to avoid any carry-over effects.
Scenario one:
Divers were rested and well-fed, and completed a 4 h tactical dive to a maximum depth of 3 metres’ seawater (msw) (131.7 kPa) breathing 100% oxygen using Lambertsen Amphibious Respiratory (LAR) 5010 equipment (Dräger, Germany). This dive profile represented a 355 UPTD oxygen exposure. Water temperature was 11°C, with visibility estimated at 2–3 metres.
Scenario two:
Divers completed a 5-day training exercise, which involved vigorous physical effort including, but not limited to, kayaking 140 km, walking and running with approximately 50 kg of equipment and having very little sleep (estimated at 2–3 h per 24 h). After this exercise, a 3 h operational dive breathing 100% oxygen using LAR-5010 equipment up to a maximum depth of 3 msw was performed. This dive profile represented a 266 UPTD exposure. Due to the operational scenario, there were some restrictions regarding sample collection, which are described below. The diving location was the same as that used in scenario one, with a registered water temperature of 10°C and limited visibility due to night-time conditions.
MEASUREMENTS
The procedures for collection and analysis of exhaled breath samples have been published previously.[ 17 , 18] Briefly, exhaled breath samples were collected in accordance with European Respiratory Society (ERS) recommendations.[ 25] The diver breathed for 5 min through a disposable two-way non-rebreathing valve (Carefusion, Utrecht, the Netherlands) combined with an inspiratory VOC filter (Honeywell, USA in scenario one and Dräger, Germany in scenario two) to prevent contamination by exogenous particles. After 5 min, a single expiratory breath was collected in an empty uncoated aluminium balloon (Globos Nordic, Denmark). After collection, 500 mL of exhaled breath was pumped though a stainless-steel tube filled with Tenax™ GR 60/80 sorbent material (Camsco, Houston, USA) using a calibrated automatic air sampler pump (Gastec, Kanagawa, Japan) at a rate of 250 mL·min-1, resulting in entrapment of VOCs. Pre-dive measurements were performed 30 min before diving. Post-dive measurements were performed at 30 min and 2 h after diving.
Exhaled breath samples were analysed using gas chromatography-mass spectrometry (GC-MS) based on standardised procedures.[ 25] Briefly, the tubes were heated to 250°C for 15 min with a flow rate of 30 mL·min-1 using a thermal desorption unit (Markes, Sacramento, USA). VOCs were captured in a cold trap at 10°C, which was then heated rapidly to 300°C for 1 min. Thereafter, molecules were transferred via splitless injection into a 30 m gas chromatography column with a diameter of 0.25 mm (Restek, Bellefonte PA, USA) at a rate of 1.2 mL·min-1. Molecules were ionised using electron ionisation at 70 eV. Fragments were detected using a quadrupole mass spectrometer (GCMS-GP2010, Shimadzu, Japan) with a scan range of 37–300 Da. Ion fragments were used for statistical analysis. The predicted fragment ions were manually checked in the raw chromatograms, and the corresponding metabolites were tentatively identified based on National Institute of Standards and Technology (NIST) library matching, using the OpenChrom software package.[ 26] Metabolites were considered identified if the first five hits in the library were the same compound and all matching factors were higher than 90%.
STATISTICAL ANALYSIS
Our previous studies investigating VOCs after hyperbaric hyperoxia (PO2 192 kPa; 1 h) reported a 35% increase in emission of methyl alkanes. A 50% increase in emission was estimated in the present study. Using nQuery 7.0 (Statistical Solutions Ltd, Cork, Ireland) and assuming a power of 80% and a significance level of 0.05, a minimum sample of five participants was needed to detect such an increase.
After GC-MS analysis, an ion fragment peak table was generated with de-noising, alignment and peak detection (signal-to-noise ratio 100:1).[ 27] Subsequently, data were tested univariately using the Wilcoxon rank-sum test (i.e., pre-dive vs. 30 min post-dive and pre-dive vs. 2 h post-dive) to identify potentially relevant ion fragments. Then, ion fragments with retention times (± 2 s) that correlated (0.98 or higher) were selected. Compounds could be identified from this selection of ion fragments/retention times. As intensities of the GC-MS signal are commonly non normally distributed, the medians of the VOCs were longitudinally tested using a Kruskal-Wallis test with correction for repeated measurements using the FDR-concept as described by Benjamin and Hochberg.[ 28]
All statistical analyses were performed using the R software package (version 3.6.1, R Foundation for Statistical Computing, Austria), including surrogate variable analysis (SVA version 3.32.1) and Methods for the Behavioural, Educational and Social Sciences (MBESS version 4.6.0). A P-value of < 0.05 was considered statistically significant.
Results
This study included 11 male divers of the NLMARSOF. Their baseline characteristics are shown in Table 1. Two divers in scenario two were smokers. All other divers were non-smokers. The dives were completed without incidents.
Table 1. Baseline characteristics of the study population. Data are mean (standard deviation) .
| Total (n = 11) | Scenario one (n = 4) | Scenario two (n = 7) | |
| Age (years) | 28.0 (5.2) | 31.0 (7.7) | 25.8 (2.2) |
| Height (cm) | 184 (9.7) | 180 (4.7) | 186.9 (11.3) |
| Weight (kg) | 82 (7.6) | 78.8 (4.8) | 83.4 (8.7) |
In total, 26 GC-MS samples were collected: 12 in scenario one and 14 in scenario two. The 2 h post-dive sample could not be collected in scenario two due to operational limitations, meaning that only pre-dive and 30 min post-dive samples were collected. Analysis of these samples identified 3,826 ion fragments, of which 269 were significant (P < 0.05) after univariate testing. Overall, in contrast with previous studies under controlled (laboratory) conditions, the GC-MS signals contained a moderate amount of noise.
Of the 269 ion fragments, 99 had a retention time (± 2 s) with a correlation of ≥ 0.98. Following grouping of these fragments using the Standard Reference Dataset (NIST), four unique VOCs were identified (Figure 1): cyclohexane; 2,4-dimethylhexane; 3-methylnonane; and toluene.
Figure 1.
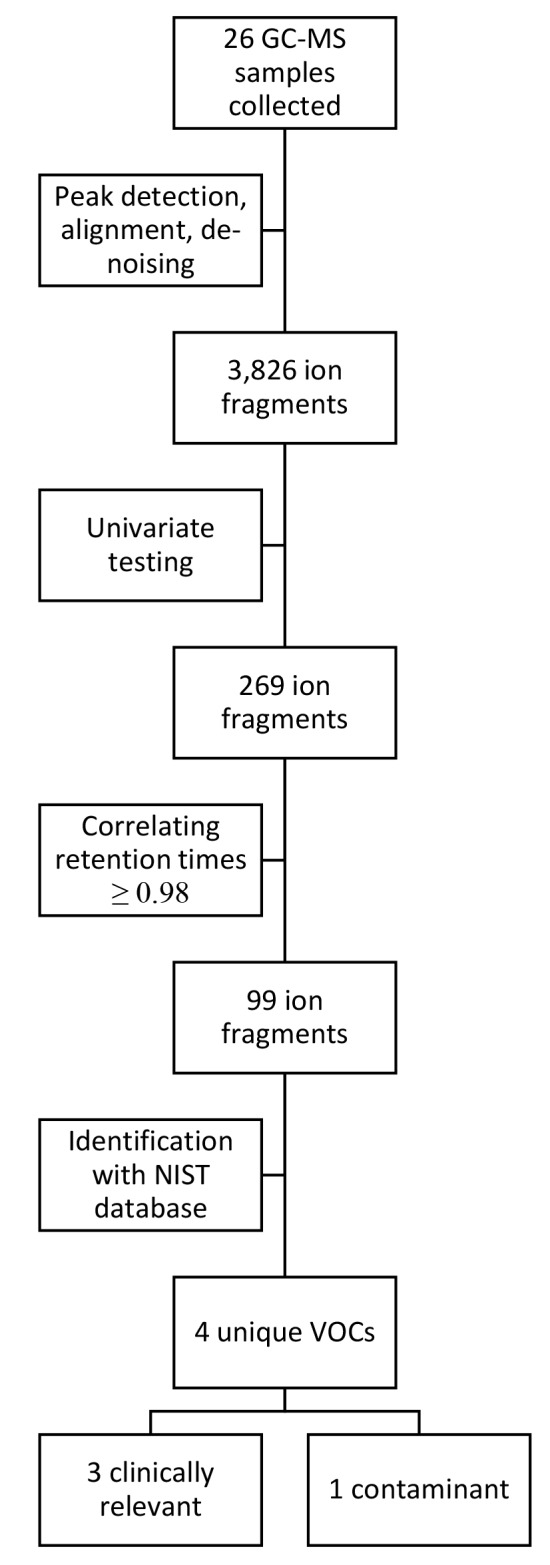
Overview of data and statistical analysis. GC-MS = gas chromatography-mass spectrometry; NIST = National Institute of Standards and Technology; VOC = volatile organic compound
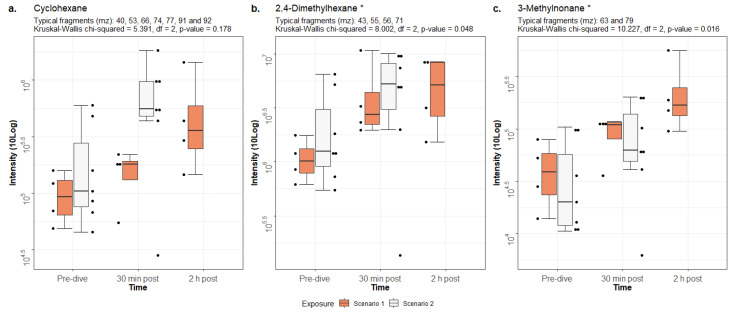
The intensities of cyclohexane, 2,4-dimethylhexane and 3-methylnonane were significantly higher post-dive than pre-dive in both scenarios (P < 0.05)(Figure 2). All values, relative to the baseline of scenario one, are displayed in Table 2. The increase (in percentage) is to the baseline of its respective scenario. Notably, the baselines of cyclohexane and 2,4-dimethylhexane were higher in scenario two than in scenario one, while the baseline of 3-methylnonane is higher in scenario one than in scenario two.
Figure 2.
Intensities of the identified VOCs with IQR. Raw data are plotted as dots next to their respective boxplot. Results of the Kruskal-Wallis test are shown above each figure. Significant differences between pre- and post-dive intensities are marked with an asterisk (*). Note that 2 h post-dive measurements were not collected in scenario two
Table 2. Intensities of the identified VOCs. Data are median (IQR) and relative to the baseline in scenario one for each compound. The increase in percentage is relative to its respective baseline. A. Cyclohexane (Kruskal-Wallis chi-squared = 5.391, df = 2, P = 0.178). B. 2,4-Dimethylhexane (Kruskal-Wallis chi-squared = 8.002, df = 2, P = 0.048). C. 3-Methylnonane (Kruskal-Wallis chi-squared = 10.227, df = 2, P = 0.016) .
| A. Cyclohexane | Pre-dive | 30 min post-dive | 2 h post-dive |
| Scenario one | 1.00 (0.67–1.37) | 1.87 (1.51–2.01) (+ 87%) | 3.82 (2.67–7.19) (+ 282%) |
| Scenario two | 1.09 (0.80–3.33) | 5.82 (5.04–10.20) (+ 433%) | – |
| B. 2,4-Dimethylhexane | Pre-dive | 30 min post-dive | 2 h post-dive |
| Scenario one | 1.00 (0.77–1.31) | 2.73 (2.18–5.03) (+ 273%) | 5.61 (2.69–8.16) (+ 461%) |
| Scenario two | 1.22 (0.91–3.43) | 5.12 (4.36–8.06) (+ 344%) | – |
| C. 3-Methylnonane | Pre-dive | 30 min post-dive | 2 h post-dive |
| Scenario one | 1.00 (0.60–1.47) | 2.70 (2.16–2.83) (+ 270%) | 4.15 (3.34–6.90) (+ 415%) |
| Scenario two | 0.49 (0.30–1.56) | 1.53 (1.23–3.60) (+ 212%) | – |
Toluene was only identified in scenario two (both pre- and post-dive) and should be considered a contaminant due to its exogenous origin.[ 29] To ensure that toluene did not originate from the LAR-5010 diving set or the VOC filter, we conducted an additional test in which a subject breathed pure oxygen for 4 h using the LAR-5010 rebreather at an ambient pressure of 101.3 kPa. Samples were collected pre- and post-exposure as described above. Small amounts of toluene were identified both pre- and post-exposure. In the sample with the highest signal intensity, the signal-to-noise ratio was 45:1 and thus this can be discarded as background noise. We conclude that this contamination is attributable to the nearby generator used to generate electricity, not to use of the LAR-5010 rebreather.
Discussion
This study identified similar VOCs in operational oxygen diving using an O2-CCR as in laboratory conditions, including markers of inflammation (cyclohexane) and lipoperoxidation of membranes (2,4-dimethylhexane and 3-methylnonane). The relatively low number of VOCs may be attributable to the overall ‘noisy’ GC-MS signals, which may have masked potential subtle changes. Toluene has not been previously identified in oxygen diving and should be considered a contaminant.
Cyclohexane, 2,4-dimethylhexane and 3-methylnonane were identified as clinically relevant in previous studies analysing exhaled breath after hyperbaric hyperoxic exposure.[ 16 - 18] Although a dose-response relationship has not been established, the exhaled concentrations of cyclohexane, 2,4-dimethylhexane and 3-methylnonane were much higher in the present study than in our previous studies, which used shorter and deeper dive profiles in a controlled environment.[ 16 , 17] In the present study, the intensities of the identified compounds increased by 87–482% after exposure to an inspired PO2 of 131.7 kPa for 3 and 4 h (Table 2). By contrast, the intensities of the same compounds only increased by 16–88% after exposure to a PO2 of 192.5 kPa for 1 h in a previous study.[ 17] This could indicate that time is more important than inspired PO2 in the development of POT or that other factors in operational diving affect or accelerate the development of POT.
None of the divers in our study experienced clinical symptoms of POT. It could be argued that this can be attributed to the relatively low hyperbaric hyperoxic exposure; scenario one (355 UPTD) provides 80% of the ‘daily limit’ of 450 UPTD, while scenario two (266 UPTD) gives 60%. However, clinical symptoms of POT are rarely reported in SOF diving, perhaps due to the covert nature of the operations.3 Recent data showed several divers having severe symptoms of POT after hyperbaric hyperoxic exposure (560 UPTD, or 125% of the daily limit) and repeated dry and wet hyperbaric hyperoxic exposures.[ 30 , 31] Knowledge of the transition from subclinical to clinical symptoms of POT is limited and it seems that clinical symptoms of POT can occur at exposures lower than the daily limit. We feel this further substantiates the need for an alternative parameter to express oxidative damage to the lung.
The baseline intensities of cyclohexane and 2,4-dimethylhexane were 9% and 22% higher in scenario two than in scenario one, respectively, while that of 3-methylnonane was 51% lower. It cannot be concluded with certainty whether this is because the intensities of cyclohexane and 2,4-dimethylhexane are increased in operation settings, possibly due to subclinical infections or other stressors, or whether this reflects individual variation. As there are no reference values for these VOCs, the clinical implication of these variations at baseline are unknown. The intensity of cyclohexane was almost 5-fold higher in scenario two than in scenario one at 30 min post-dive, even though the exposure was 1 h longer in scenario one than in scenario two. Conversely, the intensity of 3-methylnonane increased 212% in scenario two while in scenario one at 30 min post-dive the intensity increased 270%. Perhaps this can be attributed to the lower starting intensity. Although the intensities of the identified compounds were increased post-dive in both scenarios, we cannot explain the difference between scenarios one and two. It is possible that factors in operational diving, such as fatigue and little food, affect the development of POT. This should be investigated in future studies.
The identified VOC toluene is commonly present in working environments.[ 29 , 32 , 33] For clarification: exogenous VOCs may still be present in exhaled breath even after correct use of inspiratory VOC filters, as some particles may still reside in the alveolar space after 5 min of pre breathing through the filters.[ 29 , 34] This chemical compound is widely used as a solvent and in fuels, and prolonged exposure to it elicits a wide range of clinical symptoms.[ 33 , 35] In vitro experiments demonstrated that human epithelial lung cells (A549) are damaged upon exposure to toluene, but this is repaired within 24 h of exposure.[ 36] The effects of toluene on the pulmonary system in vivo are unclear.[ 35] We attribute the detection of toluene in the present study to exhaust fumes from the nearby diesel generator, not to use of LAR-5010 equipment and/or VOC filters. It is unclear to what extent toluene or exhaust fumes affected our results.
STRENGTHS AND LIMITATIONS
Caution is needed when comparing the results of this study with those of previous studies that used other dive profiles. However, the same compounds were identified post-dive, which strengthens the hypothesis that these compounds are associated with POT. In addition, the scenario used in the current study (3 or 4 h at an inspired PO2 of 131.7 kPa) more accurately mimics the circumstances in which SOF divers are deployed, perhaps making these results more relevant than those reported in our previous study (1 h at an inspired PO2 of 192.5 kPa).[ 17]
Fewer compounds were identified in the present study than in our previous studies. This may be attributable to the amount of noise in the GC-MS signals, which might have masked subtle changes in compounds. The total number of subjects was low, and the small number of samples was prone to bias. However, according to our sample size analysis, we included enough participants to provide sufficient statistical power at the group level. We must acknowledge that this could be confounded by inter- and intra-subject variability which we did not measure, and which is therefore unknown. Additionally, a Kruskal-Wallis test can be less reliable with small samples. We have performed additional tests with bias corrected and accelerated (BCa) bootstrap resampled data (n = 1,000) for cyclohexane, 2,4-dimethylhexane and 3-methylnonane, which generated P-values of 0.068, 0.018 and 0.006 respectively. While this increases statistical validity of the Kruskal-Wallis test, we feel that interpreting bootstrapped data should be done carefully and does not necessarily increase clinical validity, therefore, we did not report these values in the results section. We identified cyclohexane, 2,4-dimethylhexane and 3-methylnonane in our previous studies; therefore, despite the small sample size, noisy GC-MS signals, possible influence of toluene and two subjects that were smokers, the associations can be considered robust. Lastly, intensities of measured VOCs are dependent upon concentrations of the substances present in the exhaled breath. Even though the post dive samples were collected 30 min after emerging, we cannot rule out remaining changes in the functional dead space due to immersion or hyperoxia, thus affecting exhaled breath concentrations. Therefore, we cannot be sure that the intensities we have found are an accurate representation of the ‘true’ concentration. Usage of an internal standard could have overcome this, and we recommend using one in future studies.[ 29]
This study confirms our hypothesis that markers associated with inflammation and lipoperoxidation (i.e., cyclohexane and methyl alkanes) can be detected in exhaled breath after oxygen dives of practical relevance to military operations using GC-MS. These molecular markers could potentially form the basis of a field test for pulmonary oxygen toxicity. However, further work is required to associate VOC levels with clinically relevant pulmonary change following oxygen exposure, assess the sensitivity and specificity of the test, and to develop a measurement instrument suitable for deployment in the field.
Conclusion
The present study identified cyclohexane, 2,4-dimethylhexane and 3-methylnonane after operational 3 and 4 h dives with O2-CCR (LAR-5010). These compounds were previously noted after oxygen dives, which strengthens the hypothesis that they are related to POT. However, the signal intensities are higher than in a controlled environment; therefore, it remains to be established if these findings are solely due to oxygen diving or other factors in operational diving. In addition, further studies are required to determine which intensities can be regarded as safe and which risk reversible pulmonary damage when diving with increased oxygen mixtures.
Footnotes
Conflict of interest and funding: nil
Contributor Information
Thijs T Wingelaar, Diving Medical Centre, Royal Netherlands Navy, Den Helder, the Netherlands; Department of Anesthesiology, Amsterdam University Medical Centre, location AMC, Amsterdam, the Netherlands.
Paul Brinkman, Department of Pulmonology, Amsterdam University Medical Centre, location AMC, Amsterdam, the Netherlands.
Rigo Hoencamp, Department of Surgery, Alrijne Hospital, Leiderdorp, the Netherlands; Defence Healthcare Organisation, Ministry of Defence, Utrecht, the Netherlands; Leiden University Medical Centre, Leiden, the Netherlands.
Pieter-Jan AM van Ooij, Diving Medical Centre, Royal Netherlands Navy, Den Helder, the Netherlands; Department of Pulmonology, Amsterdam University Medical Centre, location AMC, Amsterdam, the Netherlands.
Anke-Hilse Maitland-van der Zee, Department of Pulmonology, Amsterdam University Medical Centre, location AMC, Amsterdam, the Netherlands.
Markus W Hollmnan, Department of Anesthesiology, Amsterdam University Medical Centre, location AMC, Amsterdam, the Netherlands.
Rob A van Hulst, Department of Anesthesiology, Amsterdam University Medical Centre, location AMC, Amsterdam, the Netherlands.
References
- Lorrain-Smith J. The pathological effects due to increase of oxygen tension in the air breathed . J Physiol. 1899;24:19–35. doi: 10.1113/jphysiol.1899.sp000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooij PJAM, Sterk PJ, van Hulst RA. Oxygen, the lung and the diver: friends and foes? Eur Respir Rev 2016; 25: 496- 506. 10.1183/16000617.0049-2016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingelaar TT, van Ooij PJAM, van Hulst RA. Oxygen toxicity and special operations forces diving: hidden and dangerous . Front Psychol. 2017;8:1263. doi: 10.3389/fpsyg.2017.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RD. Lambertsen and O2: beginnings of operational physiology . Undersea Hyperb Med. 2004;31:21–31. [PubMed] [Google Scholar]
- Donald K. Oxygen and the Diver . Welshpool (UK): The SPA Ltd; 1992. [Google Scholar]
- Acott K. Oxygen toxicity: a brief history of oxygen in diving . SPUMS Journal. 1999;29:150–5. [Google Scholar]
- Butler FK Jr. Closed-circuit oxygen diving in the U.S. Navy . Undersea Hyperb Med. 2004;31:3–20. [PubMed] [Google Scholar]
- Arieli R, Yalow A, Goldenshluger A. Modeling pulmonary and CNS O2 toxicity and estimation of parameters for humans. J Appl Physiol (1985). 2002;92:248–56. doi: 10.1152/japplphysiol.00434.2001. [DOI] [PubMed] [Google Scholar]
- Bardin H, Lambertsen CJ. A quantitative method for calculating pulmonary toxicity: use of the ‘unit pulmonary toxicity dose’ (UPTD). Institute for Environmental Medicine: University of Pennsylvania; 1970. Available from: http://archive.rubicon-foundation.org/xmlui/handle/123456789/10898. [cited 2019 June 20]. [Google Scholar]
- Clark JM, Lambertsen CJ. Pulmonary oxygen tolerance in man and derivation of pulmonary oxygen tolerance curves. Institute for Environmental Medicine: University of Pennsylvania; 1970. Available from: http://archive.rubicon-foundation.org/xmlui/handle/123456789/3863. [cited 2019 June 20]. [Google Scholar]
- van Ooij PJAM, Hollmann MW, van Hulst RA, Sterk PJ. Assessment of pulmonary oxygen toxicity: relevance to professional diving; a review . Respir Physiol Neurobiol. 2013;189:117–28. doi: 10.1016/j.resp.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Hruby J, Butler J. Variability of routine pulmonary function tests . Thorax. 1975;30:548–53. doi: 10.1136/thx.30.5.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harabin AL, Homer LD, Weathersby PK, Flynn ET. An analysis of decrements in vital capacity as an index of pulmonary oxygen toxicity . J Appl Physiol. 1987;63:1130–5. doi: 10.1152/jappl.1987.63.3.1130. [DOI] [PubMed] [Google Scholar]
- Pendergast DR, Lundgren CEG. The physiology and pathophysiology of the hyperbaric and diving environments . J Appl Physiol (1985). 2009;106:274–5. doi: 10.1152/japplphysiol.91477.2008. [DOI] [PubMed] [Google Scholar]
- Pendergast DR, Lundgren CEG. The underwater environment: cardiopulmonary, thermal, and energetic demands . J Appl Physiol (1985). 2009;106:276–83. doi: 10.1152/japplphysiol.90984.2008. [DOI] [PubMed] [Google Scholar]
- van Ooij PJAM, van Hulst RA, Kulik W, Brinkman P, Houtkooper A, Sterk PJ. Hyperbaric oxygen diving affects exhaled molecular profiles in men . Respir Physiol Neurobiol. 2014;198:20–4. doi: 10.1016/j.resp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Wingelaar TT, van Ooij PJAM, Brinkman P, van Hulst RA. Pulmonary oxygen toxicity in Navy divers: a crossover study using exhaled breath analysis after a one-hour air or oxygen dive at nine meters of sea water . Front Physiol. 2019;10:10. doi: 10.3389/fphys.2019.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingelaar TT, Brinkman P, van Ooij PJAM, Hoencamp R, Maitland-van der Zee AH, Hollmann MW, et al. Markers of pulmonary oxygen toxicity in hyperbaric oxygen therapy using exhaled breath analysis . Front Physiol. 2019;10:475. doi: 10.3389/fphys.2019.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M, Cataneo RN, Greenberg J, Grodman R, Gunawardena R, Naidu A. Effect of oxygen on breath markers of oxidative stress . Eur Respir J. 2003;21:48–51. doi: 10.1183/09031936.02.00053402. [DOI] [PubMed] [Google Scholar]
- Ajibola OF, Smith D, Spaněln P, Ferns GAA. Effects of dietary nutrients on volatile breath metabolites . J Nutr Sci. 2013:e34. doi: 10.1017/jns.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda OF, Guevara AJ, Contreras C, Lagos N, Berral FJ. Exhaled breath condensate analysis after long distance races . Int J Sports Med. 2012;33:955–61. doi: 10.1055/s-0032-1316314. [DOI] [PubMed] [Google Scholar]
- Wendling J, Nome T. Medical assessment of working divers . Fitness to dive Standards of European Diving Technology Committee. 1st ed. Biele-Biene; Hyperbaric Editions: 2004. Available from: http://www.edtc.org/EDTC-Fitnesstodivestandard-2003.pdf. [cited 2019 June 20]. [Google Scholar]
- Wingelaar TT, Clarijs P, van Ooij PA, Koch DA, van Hulst RA. Modern assessment of pulmonary function in divers cannot rely on old reference values . Diving Hyperb Med. 2018;48:17–22. doi: 10.28920/dhm48.1.17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Bergmann A, Steffens M, Trefz P, Ziller M, Miekisch W, et al. Impact of food intake on in vivo VOC concentrations in exhaled breath assessed in a caprine animal model . J Breath Res. 2015;9(4):047113. doi: 10.1088/1752-7155/9/4/047113. [DOI] [PubMed] [Google Scholar]
- Horvath I, Barnes PJ, Loukides S, Sterk PJ, Högman M, Olin AC, et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease . Eur Respir J. 2017;49:1600965. doi: 10.1183/13993003.00965-2016. [DOI] [PubMed] [Google Scholar]
- Wenig P, Odermatt J. OpenChrom: a cross-platform open source software for the mass spectrometric analysis of chromatographic data . BMC Bioinformatics. 2010;11:405. doi: 10.1186/1471-2105-11-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching and identification . Anal Chem. 2006;78:779–87. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing . J Roy Statist Soc Ser B. 1995; 1: 289- 300. Available from: https://www.jstor.org/stable/2346101 [cited 2019 June 20] [Google Scholar]
- Ahmed WM, Brinkman P, Weda H, Knobel HH, Xu Y, Nijsen TM, et al. Methodological considerations for large-scale breath analysis studies: lessons from the U-BIOPRED severe asthma project . J Breath Res. 2018;13(1):016001. doi: 10.1088/1752-7163/aae557. [DOI] [PubMed] [Google Scholar]
- Fothergill DM, Ross WL, Florian JP. Validation of an exhaled nitric oxic model of pulmonary hyperoxic stress . Presented at the Undersea and Hyperbaric Medicine Society Annual Scientific Meeting, Rio Grande, Puerto Rico; 2019. [Google Scholar]
- Willoughby C, Zhou H, Mahon R, Martin J, Fothergill DM, Hall A. Analysis of volatile organic compounds to predict hyperbaric pulmonary oxygen toxicity in US Navy divers. Leicestershire (UK): Breath Summit; 2019. [Google Scholar]
- Moreno T, Pacitto A, Fernandez A, Amato F, Marco E, Grimalt JO, et al. Vehicle interior air quality conditions when travelling by taxi . Environ Res. 2019;172:529–42. doi: 10.1016/j.envres.2019.02.042. [DOI] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration . Toluene - Occupational Exposure Limits . Available from: https://www.osha.gov/SLTC/toluene/exposure_limits.html. [cited 2019 June 20].
- Thekedar B, Oeh U, Szymczak W, Hoeschen C, Paretzke HG. Influences of mixed expiratory sampling parameters on exhaled volatile organic compound concentrations . J Breath Res. 2011;5(1):016001. doi: 10.1088/1752-7155/5/1/016001. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency . Toluene - CAS 108-88-3 . Available from: https://www.epa.gov/sites/production/files/2016-09/documents/toluene.pdf. [cited 2019 June 20].
- Pariselli F, Sacco MG, Ponti J, Rembges D. Effects of toluene and benzene air mixtures on human lung cells (A549) . Exp Toxicol Pathol. 2009;61:381–6. doi: 10.1016/j.etp.2008.10.004. [DOI] [PubMed] [Google Scholar]