Abstract
Background
Use of a microsensor has been suggested to monitor patching adherence. Application has been limited because the microsensor’s small size makes it easy to lose and a swallowing risk. We designed the Eye Patch Assistant (EPA) to hold the small microsensor in place and reduce the risk of loss or swallowing. This study reports the accuracy, precision, ease of use, and comfort for patching with EPA (patch+EPA) to monitor adherence.
Methods
Adults (N = 13) wore an adhesive patch alone or a patch+EPA for 2 hours each, recorded wear time, and completed an ease of use/comfort questionnaire; 30 children wore a patch or patch+EPA and completed the questionnaire. Sensor sampling interval was every 5 minutes or every 1 minute. Sensor accuracy and precision were evaluated by Bland-Altman analysis and 95% limits of agreement, and questionnaire scores compared by Wilcoxon tests.
Results
With 5-minute sampling, we found excellent accuracy for adults (mean actual vs recorded time difference, 1.4 minutes) and children (mean difference, −0.9 min). We found high precision for both adults and children (95% limits of agreement half widths of 6.4 minutes and 1.9 minutes, respectively). In adults, the ease of use score for the patch+EPA was lower than the patch (P < 0.01), but the comfort score for the patch+EPA was higher (P < 0.01). For children, scores did not differ significantly. The patch+EPA functioned well between 45° and 82°F.
Conclusions
The patch+EPA was well accepted and monitored adherence accurately and precisely.
Although patching the fellow eye is effective in treating many children with both moderate and severe amblyopia,1–3 adherence to treatment may be challenging for both the child and parent, and many children have residual amblyopia despite patching.4–6 Objective monitoring of patching adherence would be helpful for clinicians evaluating response to treatment and to researchers investigating dose–response relationships.
Researchers from the United Kingdom and the Netherlands have pioneered the monitoring of objective patching adherence7–12 using an “occlusion dose monitor” (ODM). The ODM developed in the United Kingdom used neonatal skin electrodes under the patch to monitor skin resistance10 and the ODM developed in the Netherlands was a temperature-sensitive disc worn on top of the patch.7–9 However, these ODMs are not available worldwide.
The TheraMon microsensor (Hargelsberg, Austria), originally developed for monitoring dental appliance adherence, was suggested for monitoring adherence to patching in 2013.13 The microsensor has several advantages over prior ODMs; it is the size of a shirt button, waterproof, measures skin temperature reliably, has battery life of up to 2 years, and can store data up to 100 days. 13,14 This microsensor appears ideal for objective monitoring of patching adherence, but its application has been limited because of several important practical challenges: (1) the size of the microsensor makes it hard to hold, easy to lose, and potentially easy to swallow by children or pets, (2) it is difficult to place in a consistent position on the adhesive patch and, (3) data on the accuracy, precision, and feasibility of the microsensor for monitoring patching adherence are lacking.
To overcome some of these challenges and to improve monitoring of adherence with patching using this microsensor, we designed the Eye Patch Assistant (EPA) to decrease the likelihood of the microsensors being lost or swallowed and to facilitate consistent placement of the microsensor on an adhesive patch. In this initial study of the EPA coupled with the TheraMon microsensor, we report the accuracy and precision of the patch+EPA and results of a feasibility/comfort questionnaire. Additionally, we report the accuracy and precision of the microsensor over a wide range of ambient temperatures.
Subjects and Methods
This research protocol and the informed consent forms were approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center, Dallas, and the Mayo Clinic, Rochester. Conduct of the study was compliant with the US Health Insurance Portability and Accountability Act of 1996. Verbal informed consent was obtained from adult participants and from a parent or guardian of children 3–12 years of age; verbal assent was also obtained from children 7–12 years of age.
The EPA was designed to facilitate consistent positioning of a TheraMon microsensor on the eyepatch for comfortable wear and prevent loss of the sensor. The EPA is a horseshoe-shaped device, injection-molded of biocompatible silicone material (Elastosil LR3003/80 A,B No:382WF; Wacker Chemical Corporation, Adrian, MI), that sticks to the inside of a regular size adhesive eye patch (Figure 1). It has a recessed opening positioned such that a TheraMon microsensor fits flush for positioning on the temple of the eye being patched. The open side of the EPA was positioned toward the nose to allow for comfortable spectacle wear over the eye patch. Once the patch was applied, the EPA insured that the microsensor was in contact with the skin. Skin temperature was recorded at preset time intervals (5-minute intervals at the Retina Foundation of the Southwest site and 1-minute intervals at the Mayo Clinic site) and stored in the sensor memory. The TheraMon reading station was used to transfer time-stamped temperature data to the TheraMon website, and a downloadable file with date, time, and temperature was extracted for offline analysis. Patch wear time was calculated as the sum of the time skin temperature was recorded at or above a threshold temperature of 82 °F.
FIG 1.
A, The Electronic Patching Assistant (EPA) fitted with an embedded microsensor. B, The EPA correctly positioned on the adhesive patch C, Subject wearing patch+EPA.
Accuracy, Precision, and Feasibility of Monitoring Patch Wear Time:
Adults vs Children Both visually normal and amblyopic adults were enrolled. Adults were asked to wear an eyepatch (patch) and, at a separate time (order randomized), an eyepatch plus the EPA with a microsensor embedded (patch+EPA). The microsensor was set to record skin temperature at 5- minute intervals. Each subject completed both testing conditions for 2 hours each and recorded their actual wear time based on a cell phone clock to the nearest second. At the end of each 2- hour session, the subject completed a 12-item questionnaire designed to assess ease of use (6 questions) and comfort (6 questions). See eSupplment 1 (available at jaapos.org). Equal numbers of questions were phrased in a positive and negative manner within the ease of use and comfort sets of questions. Answers were collected in a 5-point Likert-type scale: strongly disagree, disagree, neither agree nor disagree, agree, strongly agree and converted to scores of 0–4, with 4 indicating the greatest ease of use and greatest comfort. Scores were summed separately for the 6 ease of use questions and the 6 comfort questions, with possible sums of of 0–24 points for each set of questions.
The initial 20 child participants were randomized to either patch or patch+EPA while completing monocular vision tests (5–20 min). Wear time was recorded by research staff to the nearest second. The microsensor was set to record skin temperature at 5-minute intervals. At the end of wear time, the children completed the same questionnaire as the adults, with the exception that the answers were collected in a simpler, 3-point Likert-type scale: disagree (score of 0), neither agree nor disagree (score of 2), and agree (score of 4).
An additional 10 children (8 with amblyopia and 2 controls) were enrolled to evaluate different durations of wear time and a different recording interval of 1 minute. Children wore the patch+EPA for 5, 10, 15, 20, or 30 minutes. Actual wear time was recorded by research staff using a cell phone clock to the nearest second.
Effect of Ambient Temperatures
To explore whether monitoring skin temperature was useful in monitoring patching adherence over a wide range of ambient temperatures, two adults wore the patch+EPA for 30 minutes at prespecified outdoor temperatures ranging from 35° to 85°F. To explore the possibility that wearing a second microsensor would help distinguish between wearing and not wearing the patch in conditions at the extremes of our ambient temperature range, two adults wore the patch+EPA at the extremes of the tested temperature range (37°F or 87°F) along with a second microsensor (ambient sensor) mounted on their spectacle frames away from the skin to record the ambient air temperature (Figure 2).
FIG 2.
Patch+EPA coupled with a second sensor mounted on the temple of the spectacle frame to record ambient air temperature.
Statistical Analyses
Wear times were evaluated using the TheraMon software, and the raw data was exported to Excel. Using Bland-Altman analysis to compare microsensor-recorded wear times to actual wear times recorded by the clock, accuracy (bias) was estimated as the mean difference between the microsensor-recorded wear time and the actual wear time.15,16 Agreement between the microsensor-recorded time and actual wear time was estimated by 95% limits of agreement (LoA; range within which 95% of differences between microsensor measurement and clock measurement of patch wear time occurred).
For questionnaire data, ease of use, comfort, and total scores were compared for the patch versus patch+EPA using Wilcoxon signed-rank tests for adults (wearing both patches with and without the EPA) and Wilcoxon rank-sum tests for children (wearing either the patch alone or the patch with EPA).
Results
Accuracy, Precision, and Feasibility of Monitoring Patch Wear Time:
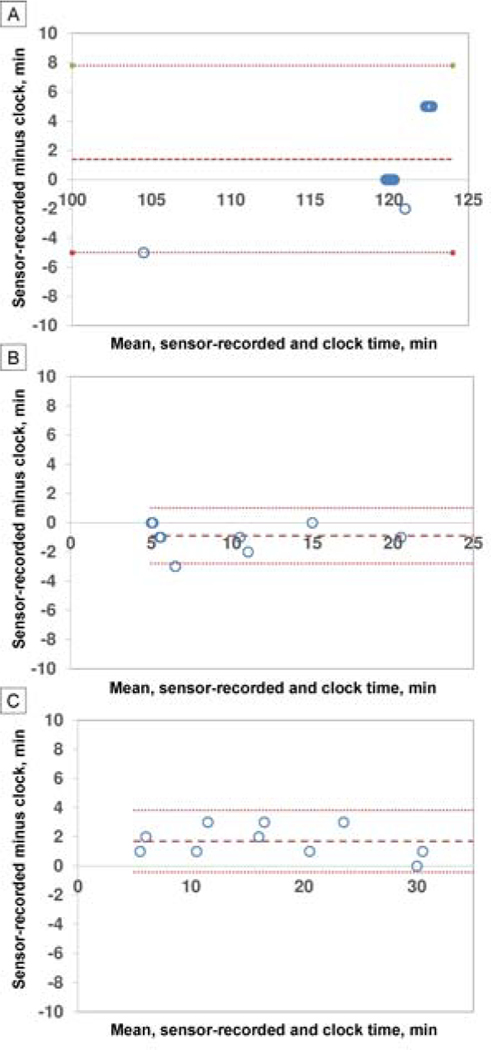
Adults vs Children Thirteen adults 16–69 years of age were enrolled; 11 were visually normal controls and 2 had amblyopia. A total of 30 children were enrolled: 18 with amblyopia and 12 controls. In adults, with 5-minute sampling over about 120 minutes, accuracy was excellent, with no significant difference between microsensor-reported and by-the-clock wear time (P = 0.15; Table 1). Precision was also excellent, with 95% LoA half-width of 6.4 minutes (Table 1; Figure 3A). The ease of use score in adults for the patch+EPA was lower (worse ease of use) than the patch (P < 0.01; Table 1), but the comfort score for the patch+EPA was higher (better comfort) (P < 0.01; Table 2).
Table 1.
Accuracy and precision of the patch and Eye Patch Assistant (EPA) for monitoring patch wear
| No. | Sampling interval, min | Actual wear time, min | Patch+EPA recorded time, min | Mean difference, min | 95% CI on difference, min | P value | 95% LoA | |
|---|---|---|---|---|---|---|---|---|
| Adults | 13 | 5 | 119.2 ± 3.7 | 120.5 ± 6.1 | 1.4 | −0.6 to 3.4 | 0.15 | −5.0 to 7.8 |
| Children | 20 | 5 | 8.5 ± 5.3 | 9.4 ± 5.4 | −0.9 | −1.6 to −0.2 | <0.05 | −2.8 to 1.0 |
| Children | 10 | 1 | 16.2 ± 9.2 | 17.9 ± 8.9 | 1.7 | 0.9 to 2.5 | <0.05 | −0.4 to 3.8 |
CI, confidence interval; LoA, limits of agreement.
FIG 3.
Bland-Altman analysis plots. In each figure, the middle, bold dashed line indicates the bias; the fine dash lines above and below indicate upper and lower 95% limits of agreement. A, Adults (N = 13) who wore the patch+EPA for 2 hours with a sampling interval of 5 minutes. B, children (n = 10) who wore the patch+EPA for 5–20 minutes with a sampling interval of 5 minutes. C, Children (n = 10) who wore the patch+EPA for 5, 10, 15, 20, or 30 minutes, with a sampling interval of 1 minute. Note: many data points overlapped and are shifted slighly to be visualized.
Table 2.
Questionnaire scores for the patch and patch+EPA
| No. | Patch | Patch + EPA | Mean difference | 95% CI on difference | P value | |
|---|---|---|---|---|---|---|
| Adults: ease of use | 13 | 18.3 ± 5.0 | 15.5 ± 5.2 | −2.8 | −4.4 to −1.7 | <0.01 |
| Adults: comfort | 13 | 12.7 ± 4.6 | 15.9 ± 4.2 | 3.2 | 1.4 to 5.1 | <0.01 |
| Children: ease of use | 20 | 16.8 ± 4.5 | 16.0 ± 4.8 | −0.8 | −5.0 to 3.4 | 0.75 |
| Children: comfort | 20 | 14.4 ± 5.0 | 14.0 ± 5.9 | −0.4 | −5.6 to 4.8 | 0.82 |
CI, confidence interval; EPA, Eye Patch Assistant.
In children, with 5-minute sampling over 5–20 minutes, there was a significant difference between microsensor-recorded and by-the-clock wear time, but the difference was small (mean difference, 0.9 minutes [P < 0.05]; Table 2), and therefore the accuracy was considered excellent. Bland-Altman analysis also showed excellent precision, with a 95% LOA half-width of 1.9 minutes (Table 1; and Figure 3B). Neither the ease of use score nor the comfort score for the patch+EPA differed significantly from the patch (Table 2).
In children, with a 1-minute sampling interval over 5–30 minutes, there was a significant difference between microsensor-recorded and by-the-clock wear time, but the difference was small (mean difference, 1.7 min [P < 0.05]; Table 1), and therefore the accuracy was considered excellent. Bland-Altman analysis also showed excellent precision, with a 95% LOA half-width of 2.1 minutes (Table 1, Figure 3C).
Effect of Ambient Temperatures
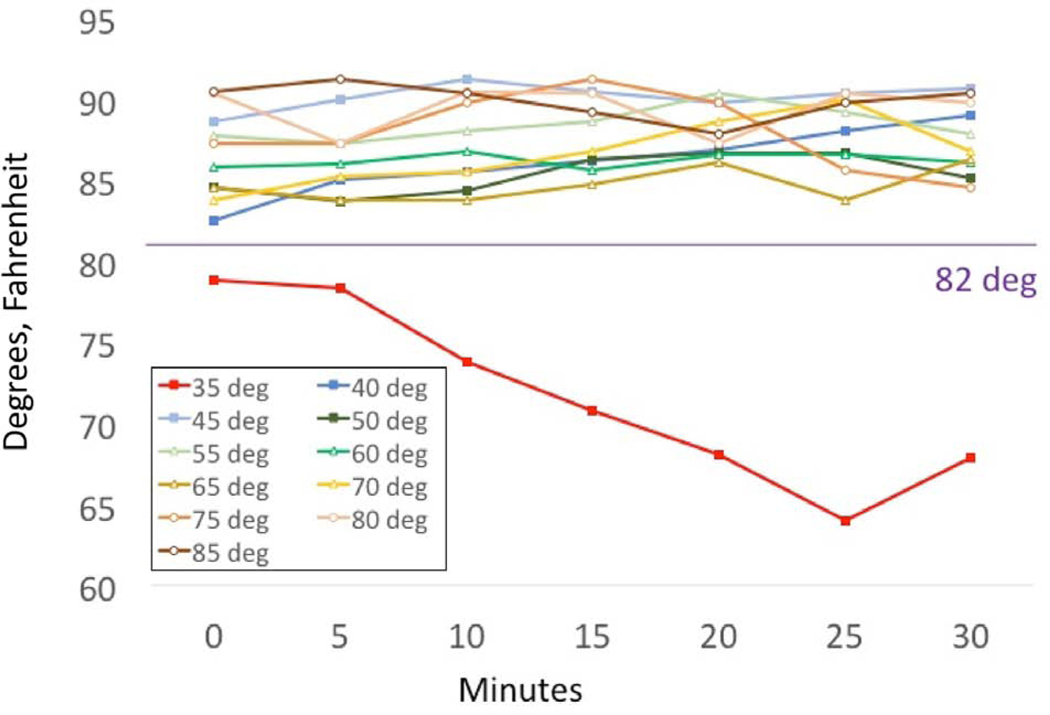
Data from 2 adults who wore the patch+EPA for 30 min at prespecified outdoor temperatures of 35°−85°F yielded similar findings (Figure 4). At temperatures of 40°−85°F, the patch+EPA consistently recorded skin temperatures above the threshold of 82°F when the patch was worn. At 35°F, the skin temperature recorded by the patch+EPA never reached the 82°F threshold; instead, it recorded temperatures in the range of 65°−78°F, which could be misinterpreted as not wearing the patch.
FIG 4.
Temperature record versus ambient temperature for an adult who wore the patch+EPA for 30 minutes at each ambient temperature.
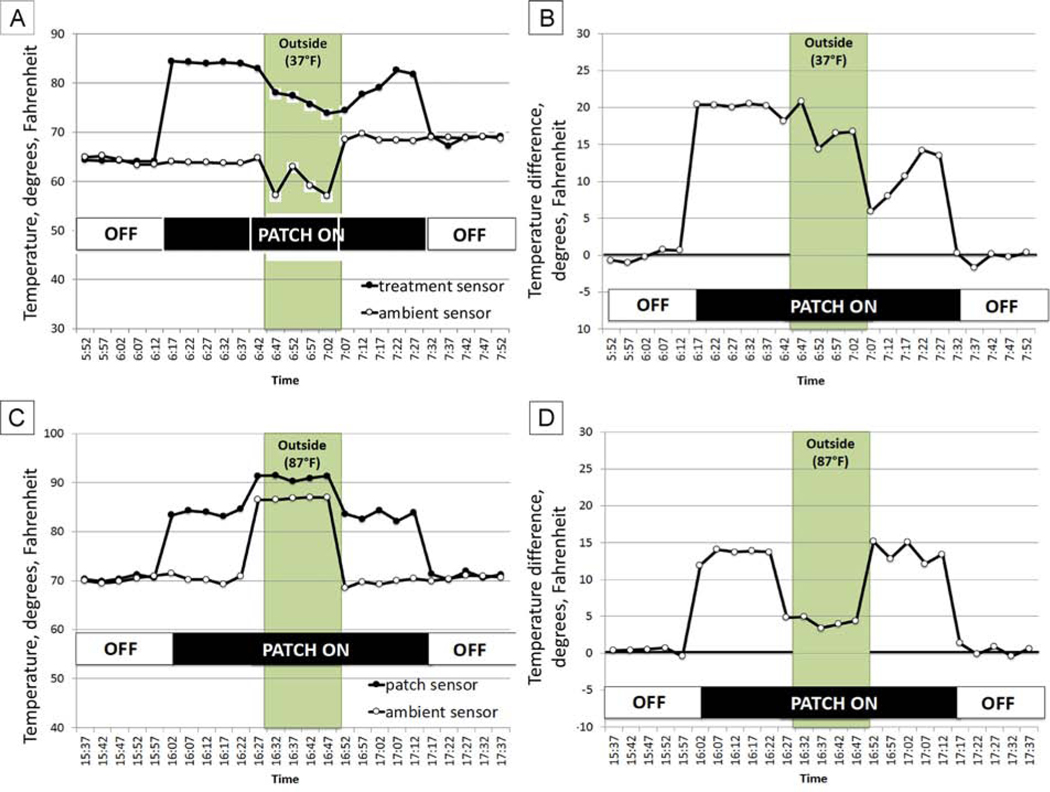
We explored whether use of two sensors, one to measure skin temperature under the patch and the other to measure ambient temperature, might be useful to extend the range of ambient temperatures in which the patch+EPA would still provide wear time data (Figure 2). As shown in Figure 5A, when the participant was outdoors in cold weather (37°F), the patch+EPA was unable to record temperatures ≥82°F, yet with the ambient sensor, we were able to detect that the patch was in place due to a marked temperature difference between the patch+EPA sensor and the ambient temperature sensor (Figure 5B).
FIG 5.
A, Temperatures recorded by the patch+EPA (filled symbols) and by an ambient temperature sensor mounted on spectacles (open symbols) when worn by an adult indoors (temperature, 65°−69°F; 6:12–6:42 and 7:05–7:7:32) and outdoors (temperature, 37°F; 6:42–7:05). B, Difference in temperature for the patch+EPA vs the ambient temperature sensor; patch wear time can be easily discerned. C, Temperatures recorded by the patch+EPA (filled symbols) and by an ambient temperature sensor (open symbols) when worn by an adult indoors (temperature, 70°−72°F; 16:00–16:30 and 16:55–17:15) and outdoors (temperature, 87 °F; 16:30–16:55). D. Difference in temperature for the patch+EPA versus the ambient temperature sensor; patch wear time can be easily discerned. Also note that, when not wearing the patch+EPA, both the patch+EPA and the ambient temperature sensor recorded similar temperatures.
In hot weather (>82°F), the patch+EPA sensor also continued to record temperatures ≥82°F even when it was not worn. As shown in Figure 5C, using two sensors also may address this limitation. When the participant was outdoors in hot weather (87°F), the patch+EPA consistently recorded temperatures ≥82°F. With the marked temperature difference between the patch+EPA sensor and the ambient temperature sensor, we were able to detect that the patch was in place (Figure 5D).
Discussion
The EPA was developed to facilitate use of the TheraMon microsensor in monitoring adherence of patching in children. Specifically, it was designed to faciltiate precise positioning of the sensor on the eyepatch, to prevent loss due to the small size of the sensor, and lower the risk of a child or pet swallowing the sensor. The sensor provided accurate and precise readings of patch wear time. Questionnaire results from adult subjects indicated that the patch+EPA was not as easy to use as the patch, but more comfortable; there were no significant differences between patch+EPA and patch for ease of use or comfort for children.
The TheraMon microsensor has programmable sampling rates. There is a trade-off between improved precision with increased sampling rate and recording time due to data storage limitations and battery life. For example, if the sampling interval is set to 5 minutes, the microsensor has a 33-day data storage capacity; if the sampling rate is set at 1-minute intervals, storage capacity is reduced to 6.7 days. This tradeoff must be considered when designing studies of patching adherence.
The TheraMon microsensor has a relatively long battery life. In previous studies using an ODM, patients had to have very frequent follow-up office visits, or home visits by researchers, to exchange the ODM every 10–20 days because of its very short battery life.8 Another study using the ODM only recorded adherence with patching for 1 week and used those data to represent adherence with patching during the entire treatment period.12 In later studies, Pradeep and colleagues17 used 4 3-week recordings. It is worth noting, however, that better adherence with patching was also associated with more frequent clinic visits18; if patients return for follow-up frequently, adherence with patching measured using an ODM might not reflect real-life adherence with the prescribed regime. Maconachie and colleagues19 used an updated version of an ODM—a glasses dose monitor (GDM)—to monitor glasses wear using a 10-minute sampling period over 6 weeks, but this required switching the GDM 4–5 times. Thus, use of the Theramon sensor in the current study may save human resources in clinic, reduce the office visit burdens for families, and more accurately represent wear times with a particular prescription of treatment.
To monitor patch adherence, the patch+EPA relies on measurement of skin temperature under the patch. Due to the nature of temperature measurement, there is potential for loss of accurate data when ambient temperature is extreme. With other ODMs, measurements of adherence with patching were obtained using temperature differentials between two surfaces; these previous studies assessed the influence of the ambient temperature range of 18°−38°C (64°- 100°F) and reported that the accuracy the temperature differential recorded by the ODM was affected by ambient temperatures >33°C (91°F).7–9 The Theramon microsensor used in our study has a similar problem in high ambient temperatures (>82 °F), but this limitation may be overcome by using a second sensor on spectacle frames to monitor ambient temperature. Previous studies with the ODM did not test the sensor’s function in cold temperatures.5,13 Our study may be the first to specifically address performance of temperature-sensitive ODMs in cold ambient temperatures. We found that the microsensor is affected by ambient temperatures of <45°F. Our proposed two-sensor approach may provide a means to address this issue. The ODM from The Netherlands5,13 also has two sensors, one for skin temperature and the other for environmental temperature, but both are mounted on the outside of the eyepatch, perhaps making it more difficult to detect the temperature differential in extreme ambient temperatures. The patch+EPA design includes one sensor under the patch in direct contact with the skin; a second sensor could be mounted on the temple of the worn spectacles, away from the skin.
The EPA was specifically designed to decrease the likelihood that the small microsensor could be lost or swallowed and to facilitate consistent placement of the microsensor on an adhesive patch.The TheraMon microsensor sits so firmly inside a recessed cavity of the EPA, making it difficult to remove. However, if used in a young child, a silicone sealant/adhesive may be used to form a permanent bond. The sensor and its battery are polyurethane-encapsulated over a very tight liquid-proof stainless steel housing. As a result, there is little risk in the unlikely event of blunt force trauma or if EPA and/or microsensor is swallowed.
A potential limitation of the Theramon sensor was noted in an adult pilot study by Schramm and colleagues14; the microsensor cannot reliably distinguish whether or not the patch is worn correctly. This is a limitation of essentially all previously reported ODMs. Although patch+EPA has some advantages over previous ODMs, the cooperation of patients and parents remains important, because it is impossible to detect exactly where the patch is positioned, whether the child is able to peek, whether the patch is placed on the wrong eye, or whether someone else is wearing the patch. Despite this inherent limitation, we are able to monitor adherence with patching in children using a patch+ EPA containing an embedded TheraMon microsensor, and the EPA is well accepted by adults and children. The sensor provides an accurate and precise record of patch wear under indoor and temperate outdoor environmental conditions.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Eye Institute (EY026664 [JW], EY022313 [EB], EY024333 [JMH, EB], and EY011751 [JMH]) and from The Pennsylvania Lions Sight Conservation and Eye Research Foundation Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scheiman MM, Hertle RW, Beck RW, et al. ; Pediatric Eye Disease Investigator Group. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol 2005;123:437–47. [DOI] [PubMed] [Google Scholar]
- 2.Wallace DK, Edwards AR, Cotter SA, et al. ; Pediatric Eye Disease Investigator Group. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology 2006;113:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Repka MX, Beck RW, Holmes JM, et al. ; Pediatric Eye Disease Investigator Group. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol 2003;121:603–11. [DOI] [PubMed] [Google Scholar]
- 4.Pediatric Eye Disease Investigator Group Writing Committee. Randomized trial to evaluate combined patching and atropine for residual amblyopia. Arch Ophthalmol 2011. 129:960–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart CE, Moseley MJ, Stephens DA, Fielder AR. Treatment dose-response in amblyopia therapy: the Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Invest Ophthalmol Vis Sci 2004;45:3048–54. [DOI] [PubMed] [Google Scholar]
- 6.Repka MX, Kraker RT2, Holmes JM, et al. ; Pediatric Eye Disease Investigator Group. Atropine vs patching for treatment of moderate amblyopia: follow-up at 15 years of age of a randomized clinical trial. JAMA Ophthalmol 2014;132:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopovska Y, Loudon SE, Cirina L, et al. Electronic recording of occlusion treatment for amblyopia: potential of the new technology. Graefes Arch Clin Exp Ophthalmol 2005;243:539–44. [DOI] [PubMed] [Google Scholar]
- 8.Fronius M, Bachert I, and Luchtenberg M. Electronic monitoring of occlusion treatment for amblyopia in patients aged 7 to 16 years. Graefes Arch Clin Exp Ophthalmol, 2009. 247(10): p. 1401–8. [DOI] [PubMed] [Google Scholar]
- 9.Fronius M, Bachert I, Lüchtenberg M. Occlusion treatment for amblyopia: assessing the performance of the electronic occlusion dose monitor. Strabismus 2006;14:65–70. [DOI] [PubMed] [Google Scholar]
- 10.Fielder AR, Irwin M, Auld R, Cocker KD, Jones HS, Moseley MJ. Compliance in amblyopia therapy: objective monitoring of occlusion. Br J Ophthalmol 1995;79:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart CE, Moseley MJ, Georgiou P, Fielder AR. Occlusion dose monitoring in amblyopia therapy: status, insights, and future directions. J AAPOS 2017;21:402–6. [DOI] [PubMed] [Google Scholar]
- 12.Tjiam AM, Holtslag G, Van Minderhout HM, et al. Randomised comparison of three tools for improving compliance with occlusion therapy: an educational cartoon story, a reward calendar, and an information leaflet for parents. Graefes Arch Clin Exp Ophthalmol 2013;251:321–9. [DOI] [PubMed] [Google Scholar]
- 13.Januschowski K, Bechtold TE, Schott TC, et al. Measuring wearing times of glasses and ocular patches using a thermosensor device from orthodontics. Acta Ophthalmol 2013;91:e635–40. [DOI] [PubMed] [Google Scholar]
- 14.Schramm C, Abaza A, Blumenstock G, et al. Limitations of the TheraMon®-microsensor in monitoring occlusion therapy. Acta Ophthalmol 2016;94:e753–6. [DOI] [PubMed] [Google Scholar]
- 15.Altman DG, Bland JM. Assessing agreement between methods of measurement. Clin Chem 2017;63:1653–4. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG, Bland JM. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- 17.Pradeep A, Proudlock FA, Awan M, Bush G, Collier J, Gottlob I. An educational intervention to improve adherence to high-dosage patching regimen for amblyopia: a randomised controlled trial. Br J Ophthalmol 2014;98:865–70. [DOI] [PubMed] [Google Scholar]
- 18.Wallace MP, Stewart CE, Moseley MJ, et al. ; Monitored Occlusion Treatment Amblyopia Study (MOTAS) Cooperatives; Randomized Occlusion Treatment Amblyopia Study (ROTAS) Cooperatives. Compliance with occlusion therapy for childhood amblyopia. Invest Ophthalmol Vis Sci 2013;54:6158–66. [DOI] [PubMed] [Google Scholar]
- 19.Maconachie GD, Farooq S, Bush G, Kempton J, Proudlock FA, Gottlob I. Association between adherence to glasses wearing during amblyopia treatment and improvement in visual acuity. JAMA Ophthalmol 2016;134:1347–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.