Abstract
Glucocorticoid synthesis is a complex, multistep process that starts with cholesterol being delivered to the inner membrane of mitochondria by StAR and StAR-related proteins. Here its side chain is cleaved by CYP11A1 producing pregnenolone. Pregnenolone is converted to cortisol by the enzymes 3-βHSD, CYP17A1, CYP21A2 and CYP11B1. Glucocorticoids play a critical role in the regulation of the immune system and exert their action through the glucocorticoid receptor (GR). Although corticosteroids are primarily produced in the adrenal gland, they can also be produced in a number of extra-adrenal tissue including the immune system, skin, brain, and intestine. Glucocorticoid production is regulated by ACTH, CRH, and cytokines such as IL-1, IL-6 and TNFα. The bioavailability of cortisol is also dependent on its interconversion to cortisone which is inactive, by 11βHSD1/2. Local and systemic glucocorticoid biosynthesis can be stimulated by ultraviolet B, explaining its immunosuppressive activity. In this review, we want to emphasize that dysregulation of extra-adrenal glucocorticoid production can play a key role in a variety of autoimmune diseases including multiple sclerosis (MS), lupus erythematosus (LE), rheumatoid arthritis (RA), and skin inflammatory disorders such as psoriasis and atopic dermatitis (AD). Further research on local glucocorticoid production and its bioavailability may open doors into new therapies for autoimmune diseases.
Keywords: glucocorticoid biosynthesis, rheumatoid arthritis, multiple sclerosis, lupus erythematosus, psoriasis, atopic dermatitis, stress, hypothalamo-pituitary adrenal axis, immunoendocrinology
Introduction: Glucocorticoids, autoimmune, and inflammatory disorders in a nutshell.
The biosynthesis of steroid hormones starts from cholesterol, which in turn is derived from a number of sources including de novo synthesis, lipoprotein-derived cholesteryl esters, and cholesteryl esters stored in lipid droplets1–3. Hormonal regulation of steroid biosynthesis occurs within minutes (acute) to hours (chronic) and is primarily mediated by cAMP signaling3–7. Steroid hormones are largely synthesized in steroidogenic cells of the adrenal, ovary, testis, placenta, and brain; however, they are also produced in a number of extra-adrenal and -gonadal tissues. Glucocorticoids play critical roles in a wide variety of physiological processes, including regulation of various developmental and homeostatic pathways and display several immune functions3, 8. Their release and production are regulated primarily by ACTH (adrenocorticotropic hormone) and indirectly by CRH (corticotropin releasing hormone)1.
Autoimmune disease and skin inflammatory disorders represent a significant clinical problem affecting large segments of the population and the quality of life of affected patients and impose a significant cost to the economy, and the health care system in particular. While there are different factors underlying the etiology of multiple sclerosis (MS), lupus erythematosus (LE), rheumatoid arthritis (RA) and skin inflammatory disorders such as psoriasis and atopic dermatitis (AD), they are linked by one element, the diseases are a consequence of a dysfunctional/hyperactive immune system. Glucocorticoids are used worldwide to treat autoimmune disease and inflammatory disorders. Since the skin and systemic immune cells can produce glucocorticoids as well as their hormonal regulators, we are exploring the hypothesis that autoimmune and inflammatory diseases develop and progress due to a malfunction of local glucocorticosteroid signaling and that their regulators play a role in the development and progression of autoimmune and inflammatory diseases.
Glucocorticoid synthesis
a. Molecular and biochemical principles of glucocorticoid biosynthesis
Cholesterol transport into the inner mitochondria:
Glucocorticoid synthesis is a complex and multiregulated process that predominately takes place in the adrenal cortex. A schematic of this process is shown in Figure 1. It starts with the mobilization and delivery of cholesterol from the outer to the inner mitochondrial membrane, a process that is mediated by the steroidogenic acute regulatory protein (StAR; also called STARD1) and also involves StAR related lipid transfer domain containing 3 (STARD3), also known as metastatic lymph node protein, clone 64 or MLN64), and possibly the translocator protein (TSPO; known previously as peripheral benzodiazepine receptor, PBR).
Figure 1.
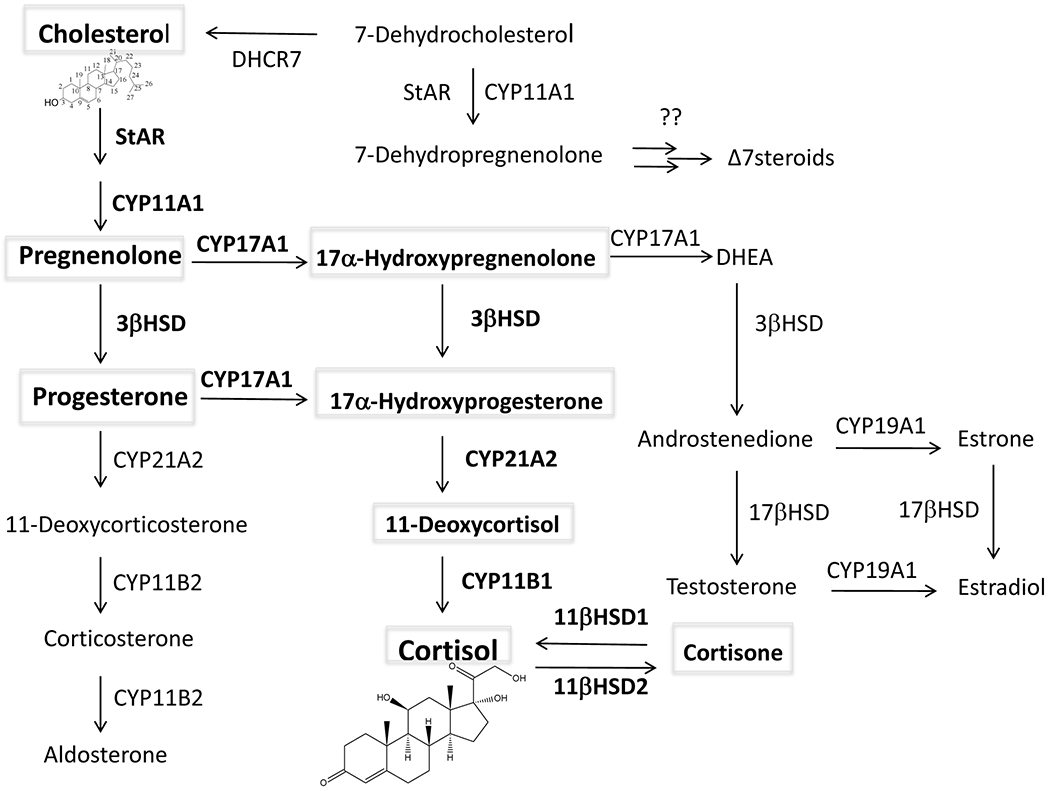
The biochemical pathway of steroidogenesis. Glucocorticoid synthesis is in bold. DHCR7: 7-delta reductase; 3βHSD: 3β-hydroxysteroid dehydrogenase
The mitochondrial StAR protein plays an indispensable role in the regulation of steroid hormone biosynthesis, i.e. the transfer of cholesterol from the outer mitochondrial membrane to the inner membrane site where CYP11A1 converts it to pregnenolone3, 4, 9, 10. Regulation of the expression, activation, and/or degradation of StAR is influenced by cAMP/protein kinase A (PKA), protein kinase C (PKC), as well as a host of other signaling pathways3, 4, 11–16. Therefore, control of StAR expression involves the interaction of a diversity of hormones and signaling pathways that coordinate the cooperation and interaction of various transcriptional regulators, as well as a number of post-transcriptional events that govern mRNA and protein expression2, 17, 18. Regardless of the regulatory events, there is a tight correlation between the synthesis of steroids and the synthesis of StAR mRNA/protein in a variety of classical and non-classical steroidogenic tissues3, 19. StAR has been implicated in virtually all cholesterol- and/or steroid led processes that involve endocrine, autocrine, and paracrine events3, 20–24.
STARD3 has a significant homology with the StAR protein and belongs to the START domain subfamily of 15 proteins (STARD1-STARD15), and it is localized in late endosomes and lysosomes25, 26. The START domain proteins, STARD1 and STARD3–6 bind a variety of sterols including cholesterol, 25-hydroxycholesterol and oxysterols, and are involved in intracellular cholesterol trafficking, lipid metabolism, and signal transduction27, 28. There is increasing evidence that STARD3 plays an important role in the intracellular transport of cholesterol from endosomes to the mitochondria for sustaining steroidogenesis. STARD3 is ubiquitously expressed in tissues suggesting a role in a variety of sterol mediated regulatory processes. In tissues such as the human placenta that do not express StAR, cholesterol delivery to CYP11A1 is mediated by STARD325, 29. It is assumed that STARD3 may deliver cholesterol to the mitochondria through transient interactions between the START domain and the outer mitochondrial membrane, as occurs for the StAR protein30. Taken together, STARD3, by transporting cholesterol from late endosomes and/or lysosomes to the mitochondria, influences steroidogenesis.
Translocator protein (TSPO) is ubiquitously expressed in tissues, most abundantly in mitochondria of steroid producing cells. Several studies reported that it plays a key role in controlling steroid biosynthesis31–35. TSPO binds cholesterol with high affinity and has been implicated in the transport of cholesterol to the inner mitochondrial membrane. Aberrant expression of TSPO has been linked to various complications and multiple diseases, including neurodegeneration, brain injury, ischemia reperfusion injury, and cancers36–40. The association of upregulation of TSPO expression with neuronal damage and inflammation makes it an important biomarker for neurodegenerative diseases. However, serum pregnenolone levels and pregnenolone synthesis by isolated mitochondria were found to be unaltered in global TSPO knockout mice which cast doubts over an essential role of TSPO in steroidogenesis41.
b. Glucocorticoid Biosynthesis:
The biochemistry of glucocorticoid biosynthesis is well established. This biochemical pathway is shown in Figure 1. In the inner mitochondrial membrane, CYP11A1 converts cholesterol to pregnenolone, a precursor of all steroids1, 42. CYP11A1 can also convert 7-dehydrocholesterol to 7-dehydropregnenolone and hydroxylates vitamin D, ergosterol and lumisterol to their corresponding hydroxyderivatives, with some side chain cleavage also occurring with lumisterol43–49. Pregnenolone can either serve as substrate for 3β-hydroxysteroid dehydrogenase (3-βHSD), which converts it to progesterone or be converted to 17α-hydroxypregnenolone by the enzyme CYP17A1)50, 51. The former reaction involves the oxidation of the 3β-hydroxyl group to a ketone group and the movement of the double bond from C5 to C4 through an isomerization reaction1. Progesterone is then converted to corticosterone by the actions of CYP21A2 and CYP11B1, while these same enzymes convert 17α-hydroxyprogesterone to cortisol1, 52. 7-Dehydropregnenolone can be metabolized by steroidogenic enzymes to the corresponding Δ7steroids (androgens and estrogens) as demonstrated experimentally47, 53, 54, and predicted from the steroid profile in Smith-Lemli-Opitz syndrome55–58. However, Δ7 glucocorticoids cannot be produced from 7-dehydropregnenolone53. Since cortisol is the predominant glucocorticoid in humans, the manuscript will focus on cortisol.
Peripheral glucocorticoid bioavailability is also dependent on the two enzymes, 11β-Hydroxysteroid dehydrogenase type 1 (11βHSD1) and 11β-Hydroxysteroid dehydrogenase type 2 (11βHSD2)59, 60. 11βHSD1 can act as both an activator of glucocorticoids by reducing cortisone to cortisol and as an inactivator by oxidizing cortisol to cortisone, depending on the NADPH and NADP levels1, 59. 11βHSD2 on the other hand acts only as an oxidase, converting the hydroxy group at C11 of cortisol to a ketone, generating cortisone1, 59, 60. One of the roles of 11βHSD2 is to prevent nonselective binding of cortisol to the mineralocorticoid receptor, thus enabling aldosterone to be the dominant mineralocorticoid59.
Hypothalamic-pituitary adrenal (HPA) axis: CRH and ACTH
a. Overview of hypothalamic pituitary adrenal (HPA) axis:
The HPA axis is the main regulator of the stress response as well as for systemic glucocorticoid production61, 62. CRH is the key regulator of the HPA and is produced in the paraventricular nucleus (PVN) of the hypothalamus63. Under stress it is released to the hypophysial portal vessels and after entering the anterior pituitary gland, it binds to the CRH receptor type 1 (CRH-R1) on the corticotrophs. Here it stimulates the expression, synthesis, and processing of proopiomelanocortin (POMC) including production and release of ACTH from corticotrophs 61–64. After entering the circulation, ACTH binds to the G-protein coupled 7-transmemebrane receptor, MC-2 (melanocortin type 2 receptor), in the zona fasciculata of the adrenal cortex. Then, via cAMP dependent mechanisms it stimulates the transport of cholesterol into the mitochondria and the synthesis and activity of steroidogenic enzymes resulting in increased production and secretion of cortisol and corticosterone65, 66 . Glucocorticoids inhibit POMC expression, ACTH secretion and production of CRH in a negative feedback loop67. Figure 2 shows a scheme for the regulation of the HPA axis.
Figure 2.
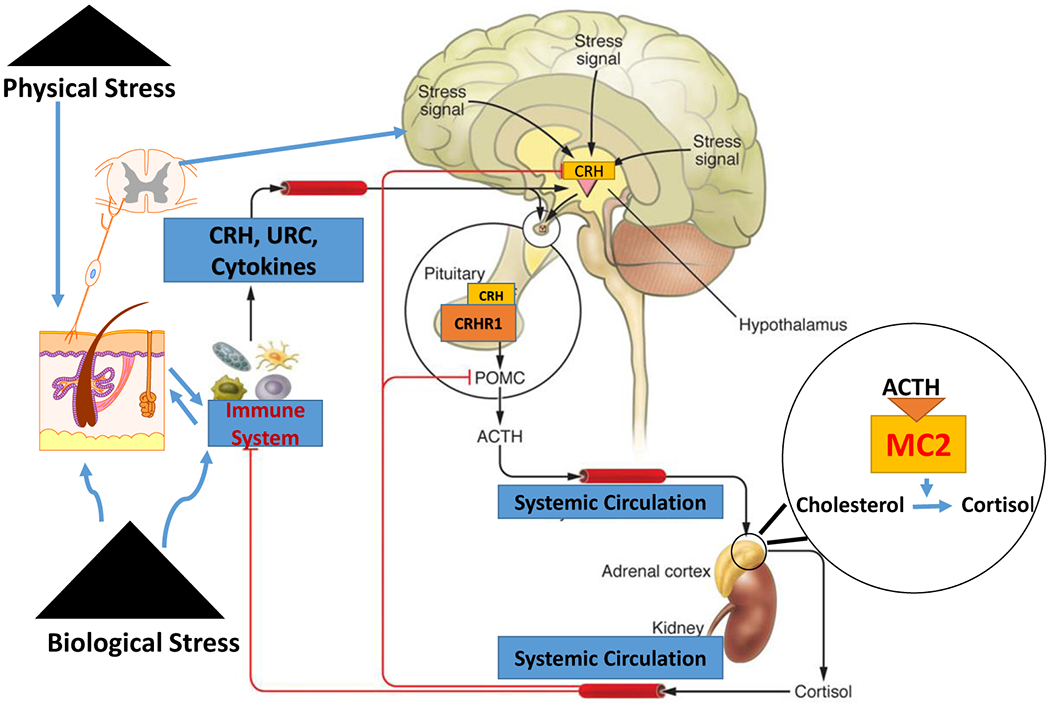
The functional organization of the hypothalamic-pituitary-adrenal axis with inputs from the immune system and the skin. Physical and biological stress promotes the release of stress signals in both the brain, the skin, and immune cells, resulting in the hypothalamic release of CRH, which in turn stimulates the release of ACTH and POMC expression and processing in the anterior pituitary. ACTH binds to the MC-2 receptor in the zona fasciculata of the adrenal cortex and stimulates the transport of cholesterol into the mitochondria and stimulates the production cortisol. Glucocorticoids not only regulate body homeostasis but also act in a negative feedback loop for CRH and POMC expression. Re-use of some elements of schematic figure from Dr Slominski Commentary205 is with permission from the Journal of Clinical Investigation.
b. CRH:
In the central HPA axis, various stressors cause the release of CRH from the hypothalamus which then indirectly regulates immune and inflammatory reactions through secretion of ACTH from the pituitary which subsequently stimulates glucocorticoid secretion by the adrenal glands68 . Inflammatory cytokines, including IL-1, TNFα and IL-6, stimulate the hypothalamus to secrete CRH69. CRH is also produced in various peripheral tissues including immune cells70, skin71, and other organs72, 73. The net effect of central CRH is immunosuppressive through activation of the HPA axis, while the direct effect of locally produced CRH is pro-inflammatory70, 71, 74–76. However, indirect immunosuppressive effects through stimulation of local production of POMC peptides and glucocorticoids are possible77–80.
CRH, in addition to acting on CRH-R1 also acts on CRH-R2, and both receptors are widely distributed in the body 81, 82 including skin 71, 83 and the immune system84. CRH receptors are coupled to different second messengers including cAMP, IP3 (inositol triphosphate), and Ca2+ 71, 81. There are different alternatively spliced isoforms of CRH-R1 and CRH-R2 with different functions 71, 79, 81, 85, 86. CRH related peptides including urocortin 1–3 are also produced centrally and peripherally and these show different affinities for CRH-R1 and CRH-R2 87, 88.
c. ACTH:
ACTH is synthesized as a part of the ~30 kD POMC precursor that undergoes cell-specific post translational processing by protein convertase 1 (PC1) to cleave the 39 amino acid (aa) ACTH peptide, as well as other neuropeptide precursors that are further processed by PC2 to melanocyte stimulating hormone-γ (γ-MSH), β-MSH and β-endorphin peptides64, 89. ACTH can also be cleaved by PC2 and further processed to produce the 13aa α-MSH peptide. ACTH interacts not only with MC-2 as an exclusive ligand for this receptor, but also with other MC receptors (MC1, MC3–5) to regulate different functions including melanogenesis (via MC-1)90–92. ACTH can also act directly as an immunosuppressor64, 93.
Glucocorticoid Receptor:
The glucocorticoid receptor (GR; NR3C1), a member of the nuclear receptor superfamily, mediates the action of glucocorticoids. It contains 4 domains: an N-terminal transactivation domain (NTD), central DNA-binding domain (DBD), a C-terminal ligand binding domain (LBD), and a hinge domain that connects the DBD with the LBD52, 94–96. There are two signaling pathways for GR: classical and non-classical94, 97, 98. GR is localized to the cytoplasm in association with a chaperone complex. In the classical GR signaling pathway, interaction with activating ligands induces a conformational change in GR and dissociation from the chaperone complex. GR subsequently translocates into the nucleus where it binds to GRE (glucocorticoid-responsive elements) and regulates the transcription of target genes94, 97. The non-classical GR signaling pathway is characterized by rapid signaling which is transcription independent, and is dependent on various types of kinases98.
Selected autoimmune and skin inflammatory disorders: an overview
a. Multiple sclerosis:
Multiple sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system99, 100. Multiple sclerosis affects about 1 in 400 adults with women being twice as likely to be affected by the disease than men101, 102. One likely mechanism giving rise to multiple sclerosis is that the overactive T helper cells (Th1 and Th17 cells) promote inflammation that results in demyelination103. The demyelination leads to the damage of the blood brain barrier (BBB), thus resulting in immune cells such as macrophages, T cells, and B cells infiltrating the brain and causing further inflammation and the eventual formation of scar tissue103, 104.
The main types of multiple sclerosis are relapsing/remitting MS (RMMS), secondary-progressive MS (SPMS), primary progressive MS (PPMS), and progressive- relapsing MS103, 105, 106. Progressive- relapsing MS used to be a subtype, but in 2013 was removed due to it being considered as repetitive105. RMMS is the most common type of MS representing about 85% of MS cases, followed by PPMS which represents 8–10% of MS cases103.
b. Lupus erythematous
Systemic lupus erythematosus (SLE) is an autoimmune disease that presents with multiple symptoms; however, the effects of the disease seem to result from the formation and deposition of autoantibodies 107–109. The causes of the disease remain to be further investigated; however, there are multiple etiological factors (genetic, immunological, hormonal, etc) involved in the disease107, 110. The disease seems to be related to the dysregulation of the Th2 cells as there is an increase in Th2 cytokines (IL-4, IL-5, and IL-13) in SLE patients108. SLE also happens to affect females more than men110, 111. Fortunately, because of better treatment the survival rate for SLE has improved significantly from a 5% survival rate over a 5 year period in the 1950s to a 99% survival rate over a 5 year period in the 2010s111.
c. Rheumatoid arthritis:
Rheumatoid arthritis (RA) is an autoimmune disease that is present in 1% of the population112. This disease adversely affects the quality of life and productivity of the patient, and there are high costs for therapy and its monitoring for toxicity113. RA is characterized by chronic synovitis, which shows a predilection for diarthrodial joints, particularly the metacarpophalangeal (MCP) and proximal interphalangeal joints (PIP joints)112. A preponderance of evidence indicates that an antigen-driven immune process against one or more proteins found in cartilage sustains synovial inflammation in RA114, 115. Each DMARD and biologic used to treat RA has the potential to cause unique serious adverse events and morbidities or mortalities. These include lung fibrosis, fulminant infections, inflammatory demyelination, liver cirrhosis, development of skin cancer or melanoma, retinal damage and triggering the onset of other autoimmune diseases such as vasculitis, MS and SLE112, 116–120.
d. Psoriasis
Psoriasis is a chronic inflammatory disorder of the skin affecting 1-9% of the population121–123. The etiology of this diseases is multifactorial with a crucial role assigned to the malfunctioning of the immune system with the dysregulation of T cells, particularly of the Th1 and Th17 lineages121, 123, 124. Although the exact mechanisms need further investigation, it is accepted that the disease progression is driven by cytokines, particularly IL-17 and IL-23123, 124. In addition, stress and deregulation of local and systemic neuroendocrine functions have been implicated in the etiology and natural history of psoriasis 71, 125–127. Currently, the main treatment for mild psoriasis encompasses the use of corticosteroids with or without additional topical vitamin D derivatives128. Ultraviolet light therapy is also used to treat psoriasis129. More recently, biologics that can target the IL-23/IL-17 pathways as well as JAK inhibitors that target IL-12 and IL-23 cytokines have been used in the therapy of this disease130, 131.
e. Atopic dermatitis
Atopic dermatitis is the most common skin inflammatory disease, affecting millions of people132. Like psoriasis the exact mechanism of this disease needs to be further investigated, although some of its causes include dysregulation of filaggrin and epidermal barrier function, and dysregulation of Th1 and Th2 effector cells132, 133. The acute phase of atopic dermatitis is mediated by Th2, while the chronic phase is mediated by Th1 cells133. Treatments for atopic dermatitis include the use of corticosteroids, cyclosporine, and more recently biologics that target IL-5 and IL-13, and potentially JAK inhibitors131.
Expression of CRH and POMC in the immune system and the skin
a. Immune system
Peripheral CRH can be synthesized by cells of the immune system, somatic cells and by peripheral afferent type sensory fibers and postganglionic sympathetic nerves134, 135. Many tissues (e.g. skin, synovium of patients with RA, colonic mucosa of patients with ulcerative colitis, ovaries, cardiovascular system, eyes, uterus, adipose tissue, thymus, bladder, liver, stomach and kidney) express CRH and/or CRH receptors (CRHR)64, 136–145. The most extensively studied site has been the skin of humans and mice which revealed the presence of not only CRH and CRH-R1/2, but also peptides derived from POMC64.
Whether an HPA-like axis is present and operative in synovium, gastrointestinal tract or other extracranial locations requires further studies. Although some authors reported expression of a truncated POMC mRNA in fibroblasts from human synovium, they failed to detect POMC protein in osteoarthritis synovial tissue and proper melanocortin signaling146. However, latter studies by these authors indicated a role for POMC signaling including MSH and ACTH in osteoarticular tissues with anti-inflammatory actions147, 148. Also, truncated POMC mRNA can be translated into full-length POMC protein that is processed to the corresponding downstream neuropeptides149–151. The POMC-derived peptides, ACTH and β-endorphin (β-End), are expressed in the synovium of RA patients and are produced by lipopolysaccharide stimulated B lymphocytes152. Analysis by double immunostaining of arthritic synovial tissue from Lewis rats with adjuvant arthritis showed that both ACTH and CRH colocalized in fibroblast-like cells and in mononuclear cells152. Various stimulants or stressors including phytohemagglutinin, concanavalin A, and IL-2, induce lymphocytes to express CRH mRNA and/or CRH protein153. Human monocyte- derived dendritic cells produce CRH mRNA and protein when stimulated by the intestinal commensal bacteria bacteroides vulgatus and fusobacterium varium154. Production of PGE2 by explants of RA synovial tissue is increased in the presence of CRH155.
Locally produced CRH modulates both pro-inflammatory and anti-inflammatory processes. This is supported by its ability to stimulate production of IL-6 from blood monocytes, increase leukocyte IL-1 and IL-2 secretion, suppress LPS- induced IL-1 and IL-6 by peripheral blood mononuclear cells, stimulate lymphocyte expansion, and IL-2 receptor expression, inhibit splenocyte proliferation induced by IL-2, facilitate NK cell mediated cell lysis; and stimulate the of production of ACTH and B-END by leukocytes156–161. CRH induces macrophages and mast cells to produce and release VEGF, IL-1β and IL-684 and human peripheral blood CD14+ monocytes to produce TNFα and dysfunction of vascular endothelium162. CRH upregulates IL-4 production by human Th2 T cells, downregulates IFN-ɣ production by human Th1 T cells and downregulates IL-10 production by FoxP3- negative human peripheral blood T regulatory cells163.
The different effects of CRH on inflammation and immune function may be influenced by different CRH receptors being expressed on different types of leukocytes or by somatic cells in different tissues84. The effects of CRH are mediated via two different receptors, CRH-R1 and CRH-R2, which are members of class B1 of G-protein coupled receptors that exhibit approximately 70% overall homology at the amino acid sequence level but only about 47% homology in the N-terminal extracellular domain164. CRH binds to CRH-R1 with greater affinity than it binds to CRH-R2165. Alternative splicing gives rise to at least 8 spliced variants of CRH-R1 and at least 3 spliced variants of CRH-R284, 85, 166. Both the pro-inflammatory and anti-inflammatory effects have been reported to be mediated by either CRH-R1 or CRH-R2 indicating that the ultimate effect of signaling via these receptors is determined by factors other than the specific type of CRH-R. For example, CRH via CRH-R1 induces mast cells and macrophages to produce IL-6, IL-1β, TNFα and VEGF and promotes vasculitis but has also been shown to block IL-1α-stimulated prostaglandin synthesis by fibroblasts84. Similarly, in the early stages of inflammation, CRH via CRH-R2, suppresses production of TNFα by macrophages activated by LPS, but has the opposite effect on LPS-induced macrophage TNFα production in late stage inflammation167.
b. Skin
Since the initial detection of CRH, CRH-R1, and CRH-R2 in human168–173 and murine168, 174–176 skin, a flurry of reports documented their regulated expression in the mammalian skin (reviewed in71, 79, 177–180). CRH and urocortins acting on cutaneous CRH-R1 and CRH-R2 can affect skin functions in a context-dependent fashion71, 75, 79, 181, 182. The direct CRH effects are predominantly anti-proliferative, pro-differentiation, barrier building and pro-inflammatory. However, indirect effects through activation of POMC or glucocorticoid signaling can be anti-inflammatory71, 79, 80 (see below). It should be noted that since the original discoveries on POMC expression and production of POMC peptides by skin cells149, 183–185, it has been widely established that skin cells can produce different POMC peptides in a context dependent fashion under different stimuli to regulate different skin functions, including downregulation of pro-inflammatory responses64, 186.129, 178
Extra-adrenal glucocorticoid biosynthesis
a. General overview with a list of steroidogenically active organs
It has been reported that glucocorticoids can be synthesized in many non-adrenal and non-gonadal tissues, such as the brain, intestine, lung, skin, spleen, placenta, adipose tissue and the immune system, as well as in a variety of cancer cells42, 52, 187–189. Table 1 shows the distribution of the proteins involved in the initial rate-controlling steps of steroidogenesis, CYP11A1 and StAR, as well as other downstream steroidogenic enzymes reported to be in these tissues including immune cells (Figure 3), at least at the level of mRNA expression. Some of the tissues listed such as bone, endometrium and mammary gland appear to primarily produce sex steroids and the production of corticosteroids from cholesterol remains to be established
Table 1.
Extra-adrenal and extra-gonadal expression of CYP11A1, StAR and other steroidogenic enzymes
| Tissue or cell typea | CYP11A1 expression | StAR expression | Other steroidogenic enzymes expressedb | Major type(s) of steroid produced | References |
|---|---|---|---|---|---|
| Adipocytes (human subcutaneous abdominal and omental and/or mouse 3T3-L1 preadipocytes) | mRNA, protein, activity | mRNA | CYP11B1, CYP11B2, CYP17A1, CYP19A1, CYP21A2, HSD3B1, HSD11B1, HSD17B3, HSD17B5, HSD17B7; SRD5A2 | sex steroids, corticosteroids | 254–256 |
| Bone, osteoblasts | mRNA, protein | not investigated | CYP17A1, CYP19A1, HSD3B, HSD17B2, HSD17B4 | estrogens | 257–259 |
| Brain | mRNA, protein, activity | mRNA, protein | CYP11B1, CYP17A1, CYP21A2, CYP2D6 (21-hydroxylase), HSD3B, HSD11B2 | pregnenolone sulfate, DHEA-sulfate, corticosteroids | 1, 19, 189, 260–264 |
| Colorectal, intestine (non-cancerous and cancerous) | mRNA, protein, activity | mRNA | CYP11B1, CYP21A2, HSD3B3, HSD11B1, CYP17A1 (human tumour) | corticosterone (mouse), cortisol | 189, 265–271 |
| Endometrial, endometriosis and tumors | mRNA, protein, activity | mRNA, protein | CYP17A1, CYP19A1, HSD3B2 | progesterone, androgen, estrogen | 272–274 |
| Heart (and blood vessels) | mRNA | mRNA | CYP11B1? CYP11B2? CYP21A2, HSD3B, HSD11B2 | aldosterone?c corticosterone (mouse) | 189, 275–279 |
| Kidney (rat) | mRNA protein, activity | mRNA, protein | HSD3B | pregnenolone, progesterone | 280, 281 |
| Lung | mRNA, activity | mRNA | CYP11B1, CYP21A2, HSD3B1, HSD3B3, HSD11B1 | aldosterone, corticosterone (mouse) | 265, 282, 283 |
| Lymphocytes macrophages and monocytes | mRNA, protein, activity | mRNA, protein | CYP21A2 | pregnenolone, cortisol? | 209, 284–288 |
| Mammary gland (including tumours) | mRNA | mRNA, protein | CYP17A1, CYP19A1 | progesterone, estrogen | 8, 18, 289–291 |
| Nasal mucosa | mRNA, protein, activity | not investigated | CYP11B1, CYP21A2, HSD3B, HSD11B1, HSD11B2, | cortisol | 292, 293 |
| Pancreas | mRNA, protein | mRNA, protein | CYP11B1 | pregnenolone? cortisol? | 294, 295 |
| Prostate | mRNA, protein, activity | mRNA, protein | CYP17A1, CYP19A1 HSD3B1, HSD3B2, HSD17B3, HSD17B5 | progesterone, androgens | 296–300 |
| Skin | mRNA, protein, activity | mRNA, protein | CYP11B1, CYP17A1, CYP21A2, HSD3B1, HSD11B1, HSD11B2, HSD17B | corticosteroids, androgens | 19, 188, 232, 301 |
| T-cells-activated (mouse) | mRNA, protein, activity | not investigated | not investigated | pregnenolone, corticosterone? | 208, 210, 211, 270, 302, 303 |
| Thymus (mouse) thymocytes and thymus epithelial cells | mRNA, activity | mRNA | CYP11B1, CYP17A1, CYP21A2, HSD3B | corticosterone | 189, 207, 208, 304 |
arefers, at least in part, to human tissues unless otherwise indicated
bexpression observed at least at the level of mRNA
cquestion mark indicates product is predicted but not confirmed experimentally
Figure 3:
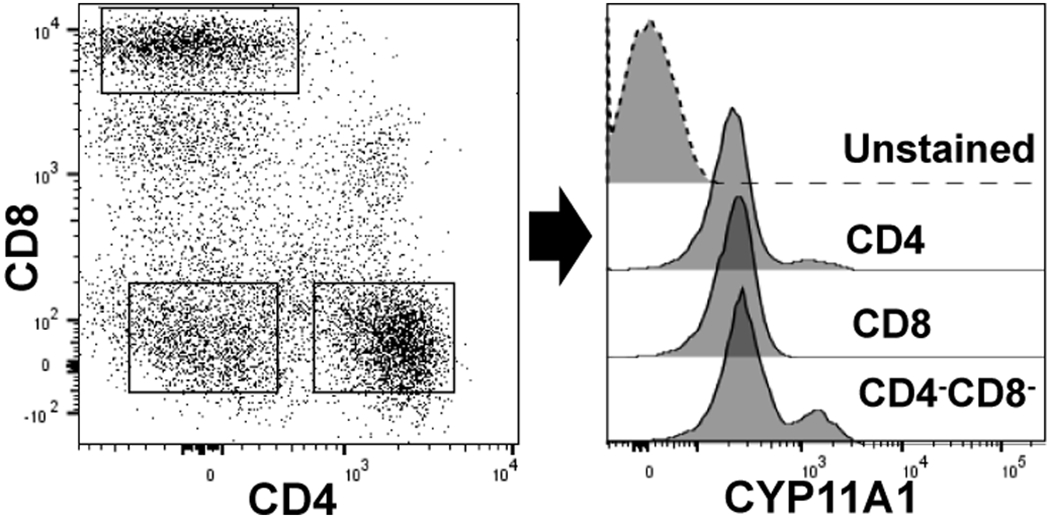
CYP11A1 expression in human peripheral blood mononuclear cells (PBMCs).The left dot plot shows CD4, CD8 T cells, and CD4−CD8− cells in PBMCs. The right histogram shows expression of CYP11A1 in gated CD4 cells, CD8 T cells, and CD4−CD8− cell populations verses the unstained PBMC. The blood was obtained from a healthy volunteer (IRB 160426001) and processed as described previously250. Intracellular staining for CYP11A1 (Cell signaling technology; Danvers, MA, USA) was performed in cells fixed with paraformaldehyde and permeabilized in methanol containing buffer 250, 251. Anti- Cyp11A1 was conjugated to APC-Cy7 (Abcam; Cambridge, UK) as per manufacturers protocol before use. Stained cells were analyzed using a BD-FACS Symphony flow cytometer (BD Biosciences, San Jose, CA). Data are representative of three independent experiments utilizing different donors.
b. Glucocorticoid biosynthesis in the skin
The skin has been shown to express all the CYP enzymes involved in steroid synthesis, including CYP11A1, CYP17A1, and CYP21A2 and StAR protein in both keratinocytes and sebaceous glands188, 190–193 (Table 1; Figure 4). Moreover, the skin has also been shown to express CRH and POMC170. The incubation of melanocytes with CRH caused the melanocytes to produce ACTH , and in turn ACTH stimulated the production of cortisol in melanocytes78. Similarly, fibroblasts can produce cortisol as shown by liquid chromatography/ mass spectrometry (LC/MS)194, and production of corticosterone can be stimulated by CRH and ACTH. Finally, the exposure of dermal fibroblasts to CRH stimulates POMC activity and corticosterone production77, with UVB activating cutaneous elements of the HPA195–198. Thus, there is evidence that a functional peripheral HPA-like axis is operative in the skin199, 200
Figure 4:
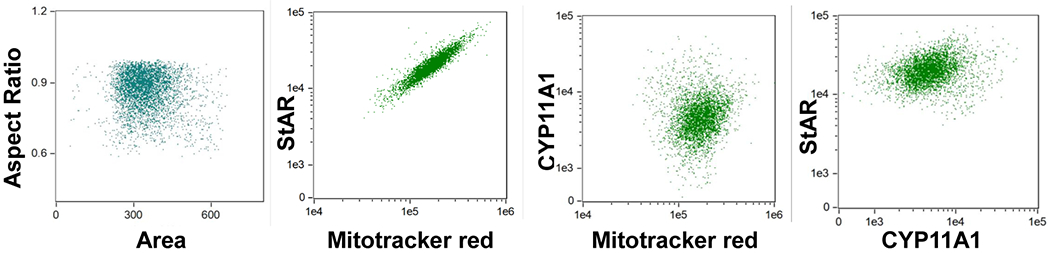
Expression of StAR and CYP11A1 in HaCaT cells (human epidermal keratinocytes). The intracellular expression of StAR and CYP11A1 in HaCaT cells was determined using Image Stream II (Amnis, Seattle, WA, USA) cytometer as described previously 252. Dot plots from left to right depict Area vs Aspect ratio (strategy to gate on single cells); StAR vs mitochondria; CYP11A1 vs mitochondria; and StAR vs CYP11A1. The 1:1 (diagonal) expression of StAR with mitochondria indicated their tightly linked expression and potential co-localization. Positive correlation between expression of CYP11A1 and mitochondria and between StAR and CYP11A1 indicate linked expression with each other and perhaps co-localization in the mitochondria. The HaCaT keratinocytes were detached and processed as previously described253. The cells were fixed and stained with antibodies to Cyp11A1 (Cell signaling technology; Danvers, MA, USA) StAR (Santa Cruz; Dallas, TX, USA), and Mitotracker Red (CMX Ros Invitrogen; Carlsbad, CA, USA) at 10 nM as described previously252. Data were analyzed using IDEAS software (Amnis, Seattle, WA, USA).
Glucocorticoid production in the skin is regulated by CRH, ACTH, IL-1β, UV light, and by 11βHSD1 and 11βHSD2 enzymes52, 188, 192. Stress to the skin either by inflammation or injury causes the stimulation of ACTH and POMC production in the skin64 . UVB exposure of the skin has been found to cause production of CRH, ACTH, β-END, and cortisol195. The corticosteroids produced in the skin appear to play a role in countering the inflammatory response of the skin192, 201. However, glucocorticoids produced locally can have a negative effect on barrier function and would healing and promote skin infection202–206.
c. Glucocorticoid biosynthesis by the immune system
The thymus, a place where T lymphocyte maturation occurs, has been found to produce glucocorticoids187 (see Table 1). In fact, de novo synthesis of steroids in the thymus was discovered in the mid 1990s by Vacchio et al.207. Vacchio also demonstrated the presence of the steroidogenic enzymes CYP11A1 and CYP11B1 by immunohistochemistry. In addition, thymic epithelial cells produced pregnenolone and deoxycorticosterone. Peripheral T cells have also been reported to produce steroids, particularly pregnenolone208. Importantly, there are recent reports showing the expression of CYP11A1 in human209 and murine208, 210, 211 T cells. We have also observed the expression of CYP11A1 in CD4 and CD8 human T lymphocyte and non-T cells (B cells and monocytes) as shown in Figure 3.
d. The role of the Glucocorticoid Receptor in the immune system
Inhibition of the expression of pro-inflammatory cytokines and synthesis by glucocorticoid is mediated by the binding of the glucocorticoid-GR complex to GREs in the promoter regions of these genes (e.g. IL-1α and IL-1β). This can block the binding and function of other transcription factors (e.g. nuclear factor-kappa-B (NF-κB), and activator protein-1(AP-1)) essential for transcriptional activation of proinflammatory mediators212–216. Inversely, NF-kB can also inhibit the function of the GR in a dose-dependent manner217 indicating that these pathways mutually affect each other. The protein glucocorticoid-induced leucine zipper (GILZ) has been found to play a role in some of the anti-inflammatory effects of glucocorticoids218–220. These effects include, but are not limited to inhibiting NF-κB, Ras/Raf, and AP-1 dependent pathways218, 220, 221.
Another mechanism for glucocorticoid-GCR mediated inhibition of inflammation is the recruitment of other transcription factors to promoter sequences of genes that code for proteins with anti-inflammatory properties (e.g. IL-10, NF-κB, IL-1 RII, GILZ, lipocortin-1, alpha-2-macroglobulin, and secretory leukocyte-protease inhibitor)212–214, 220, 221. Glucocorticoids can also mediate their anti-inflammatory effects at the post-translational level by decreasing the stability of mRNAs for IL-1,IL-2, IL-6, IL-8, TNFα and GMCSF, or increasing the stability of a number of other mRNAs. The latter include mRNAs for various enzymes (e.g. angiotensin - converting enzyme and neutral – endopeptidase) that degrade the vasodiliatory peptide (e.g. bradykinin), annexin-1( lipocortin-1, macrocortin and/or lipomodulin ) which has anti-inflammatory action by inhibiting phospholipase A2 leading to reduced generation of arachidonic acid from membrane phospholipids, and by decreasing the stability of cyclooxygenase-2 mRNA resulting in reduced production of PGE2222
The global effects of glucocorticoids on leukocytes and endothelial cells lead to a decrease in the adherence of leukocytes to the endothelium of blood vessels which reduces their extravasation into areas of inflammation, thus reducing the inflammatory response223–225. Proliferation of B cells and T cells is inhibited as well as the production of immunoglobulins (B cells) and Th1 and Th2 cytokines (T cells). There is less inhibition of Th2 production than Th1 and there is attenuation of natural killer (NK) cell activation177, 226, 227. Glucocorticoids act on eosinophils to increase their apoptosis directly, or via reducing the production of IL-5228. Mast cell degranulation, cytokine production and their adherence to the endothelium are inhibited by glucocorticoids177, 229. Glucocorticoids reduce the number of circulating monocytes and cause activation of antigen presentation functions of monocytes/macrophages/dendritic cells177, 230
Dysregulated local glucocorticoid synthesis in the etiology of autoimmune and inflammatory disorders
a. Skin inflammatory disorders
Psoriasis and atopic dermatitis:
The dysregulation of skin steroidogenesis may play a role in both psoriasis and atopic dermatitis188, 231, 232. Glucocorticoids act by blocking the production of IL-4 and IL-5231, 233. Hannen et al. reported that in the skin, the expression of several enzymes involved in steroid synthesis such as CYP11A1 and CYP17 are reduced in psoriasis, as well as the enzymes 11βHSD1, 11βHSD2, and the GR128. They further demonstrated that StAR and MLN64 expression is reduced in skin of both atopic dermatitis and psoriasis patients193. Tiala et al. reported that CCHCR1, a gene that plays a role in steroidogenesis and vitamin D metabolism, is downregulated in psoriasis234. Another study reported that deficient in situ synthesis of glucocorticoids in psoriatic skin was associated with increased inflammation235. The above data support the hypothesis that defective glucocorticoid signaling contributes to the pathogenesis of psoriasis206.
b. Autoimmune disorders
i. Multiple sclerosis:
Local steroidogenesis might also play a role in the prevention of multiple sclerosis. Boghozian et al. found lower CYP17A1 expression levels as well as lower dehydroepiandrosterone (DHEA) levels in oligodendrocytes in both MS patients and animals with EAE (experimental autoimmune encephalomyelitis)236. This group also found increased expression of IL-1β and IFN-γ in MS patients. The results seem to suggest that DHEA may play a role in immunoregulation. Arnason et al. reported that ACTH can be beneficial for MS patients, although Miller et al found ACTH to be a candidate for the therapy of multiple sclerosis as early as 1961237, 238. Arnason et al. later described how melanocortins are anti-inflammatory and act by blocking NF-kB , and that melanocortins exert their anti-inflammatory effects through melanocortin receptors MC1R, MC3R, and MC5R237.
The expression of genes encoding certain enzymes producing sex hormones as well as the receptors for these hormones may also be implicated in the pathogenesis of multiple sclerosis101. For example, Luchetti et al. reported that the MS lesions in males display higher expression of mRNA for aromatase, estrogen receptor B, and TNF, while women with MS have increased expression of mRNA for 3β-hydroxysteroid dehydrogenase and the progesterone receptor101.
ii. Lupus erythematous:
The dysregulation of steroidogenesis could be a contributing factor to the pathogenesis of SLE. Corticosteroids are used in first line treatment of patients with SLE108, 188, 239. Glucocorticoids affect T cells (especially CD4) more rapidly than B cells239. Glucocorticoids affect the T cells by enhancing circulatory emigration, inducing apoptosis, inhibiting T cell growth factors, and impairing the release of cells from lymphoid tissue239. ACTH has been used since the 1950s as a treatment option for SLE240, 241. Vogl et al. have found that a number of steroids are lower in SLE patients than control patients, specifically progesterone, 17-hydropregnenolone, and cortisol242. Li et al compared pituitary hormone level in SLE patients verses the healthy controls and found that prolactin levels were increased in SLE patients110.
iii. Rheumatoid arthritis:
Steroidogenesis as well as the factors that regulate it may play an important role in the pathogenesis of rheumatoid arthritis. This is not surprising since 100–2000 genes are regulated by glucocorticoids243. Yoursi et al found that there are 32 steroid-like metabolites whose concentration differ significantly between RA patients and healthy controls244. These metabolites included DHEA, adrostenediol, and cortisol244.
Straub et al reported that serum levels of cortisol, DHEA, and DHEA-S levels were elevated in early rheumatoid arthritis patients compared to healthy individuals and correlated with elevated levels of the proinflammatory cytokines, IL-6 and TNF245. This group speculated that this might be due to RA patients having a deficiency in either CYP21A2 or CYP11A1245. In another report, Straub et al noted that the relatively low levels of steroids in RA patients in relation to proinflammatory cytokines was not due to increased renal clearance, and in fact the renal clearance of steroids, including androgens, was decreased in RA patients246.
Schlaghecke et al found that the PBMCs (peripheral blood mononuclear cells) in RA patients have a lower density of glucocorticoid receptors that healthy controls247. However, Schlaghecke later reported that this decreased GC density does not cause glucocorticoid resistance in RA patients248.
In a review article about the role of 11βHSD1 and 2 in RA, Edwards concluded that overactivity of 11βHSD1 can cause dysregulation of the HPA controlling cortisol production249. He also speculated that the proinflammatory cytokine, TNFα ,triggers the overactivity of 11βHSD1 and that anti-TNFα therapy can be beneficial in RA249. Finding out the exact mechanism by which steroidogenesis is dysregulated in RA may open doors for discovering new treatment options in RA. Specifically, the regulation of the local interconversion of cortisol and cortisone and/or glucocorticoid biosynthesis and CYP11A1 activity may be targeted in immune cells or their target organs.
Concluding remarks and future perspectives
Glucocorticoids play many roles in the maintenance of homeostasis in the body including displaying important immunosuppressive activity. Glucocorticoid synthesis is regulated by ACTH, CRH, and cytokines such as IL-1, IL-6 and TNFα in a context-dependent fashion. While some of the regulators such as ACTH directly display immunosuppressive effects, others such as CRH and cytokines have predominantly pro-inflammatory activity. Therefore, in peripheral organs dissociation of the actions of the higher-level regulators, CRH and proinflammatory cytokines, from the executive arm involving the synthesis of glucocorticoids, can lead to uncontrolled stimulation of the immune system. Furthermore, our view is that dysregulation of local (immune cells and or target organs for immune activity) glucocorticoid synthesis plays a pivotal role in several autoimmune diseases, including MS, LE, and RA, as well as proinflammatory skin diseases such as psoriasis and AD (Figure 5).
Figure 5.
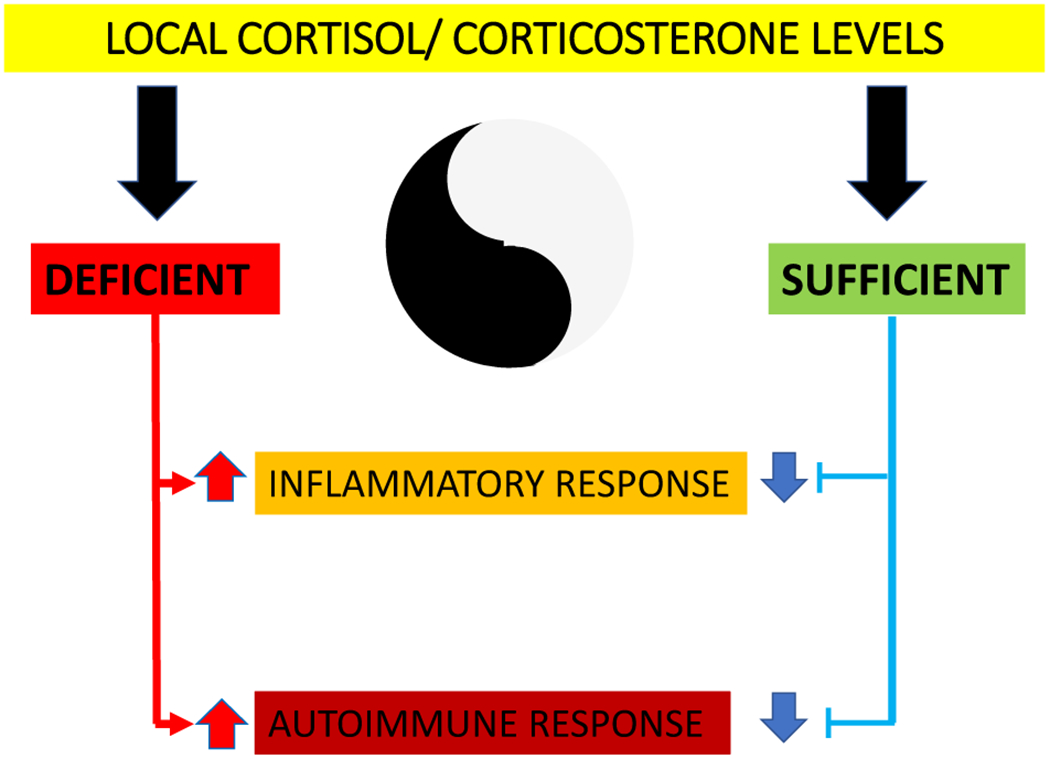
Local cortisol/corticosterone levels can control immune functions and inflammatory responses in a Yin/Yang manner.
Creative investigations on how to pharmacologically target local and endogenous glucocorticoid biosynthesis and glucocorticoid signaling should help to find future therapies/cures for inflammatory and autoimmune diseases. In particular, there needs to be targeted research aimed at increasing local cortisol/corticosterone levels through the activation of their local synthesis without systemic effects and/or by preventing their inactivation, and/or by stimulation of the activity of 11βHSD1/2. The precise delivery of factors regulating glucocorticoid biosynthesis to the target organs or immune cells should also be a focus of future research. Such agents that are able to directly or indirectly influence local cortisol levels may be chemically synthesized in an educated fashion or represent natural products identified by medicinal chemistry and computer modeling. In addition, the application of different types of physical factors such as ultraviolet B (UVB) radiation in a controlled fashion may represent an additional opportunity, since UVB is both immunosuppressive and also stimulates glucocorticoid biosynthesis. In conclusion, local cortisol levels can influence the development or regression of inflammatory (psoriasis, AD) and autoimmune diseases such as LE, MS and RA. Research aimed at modulating local levels of cortisol is necessary to provide new therapies to patients suffering from these devastating diseases.
Acknowledgement
The study was supported by NIH grants 1R01AR073004-01A1 and R01AR071189-01A1 and by a VA merit grant (no. 1I01BX004293-01A1) to ATS, internal (UAB) funds to ATS and CR and by the Intramural Research Program of the NIH, the NIEHS, NIH Z01-ES-101585 (to AMJ).
Footnotes
Competing interests
Authors declare no conflict of interest.
References
- 1.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011; 32(1): 81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manna PR, Cohen-Tannoudji J, Counis R, Garner CW, Huhtaniemi I, Kraemer FB et al. Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: its role in the regulation of the steroidogenic acute regulatory protein. J Biol Chem 2013; 288(12): 8505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manna PR, Stetson CL, Slominski AT, Pruitt K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016; 51(1): 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manna PR, Stocco DM. Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocr Metabol Disord 2005; 5(1): 93–108. [DOI] [PubMed] [Google Scholar]
- 5.Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 2005; 19(11): 2647–59. [DOI] [PubMed] [Google Scholar]
- 6.Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res 2011; 52(12): 2111–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo AF, Orlando U, Helfenberger KE, Poderoso C, Podesta EJ. The role of mitochondrial fusion and StAR phosphorylation in the regulation of StAR activity and steroidogenesis. Mol Cell Endocrinol 2015; 408: 73–9. [DOI] [PubMed] [Google Scholar]
- 8.Manna PR, Ahmed AU, Yang S, Narasimhan M, Cohen-Tannoudji J, Slominski AT et al. Genomic Profiling of the Steroidogenic Acute Regulatory Protein in Breast Cancer: In Silico Assessments and a Mechanistic Perspective. Cancers (Basel) 2019; 11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 1994; 269(45): 28314–22. [PubMed] [Google Scholar]
- 10.Miller WL. StAR search--what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol 2007; 21(3): 589–601. [DOI] [PubMed] [Google Scholar]
- 11.Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod 2009; 15(6): 321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller WL. Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol 2013; 379(1-2): 62–73. [DOI] [PubMed] [Google Scholar]
- 13.Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 2002; 23(2): 141–74. [DOI] [PubMed] [Google Scholar]
- 14.Manna PR, Joshi L, Reinhold VN, Aubert ML, Suganuma N, Pettersson K et al. Synthesis, purification and structural and functional characterization of recombinant form of a common genetic variant of human luteinizing hormone. Hum Mol Genet 2002; 11(3): 301–15. [DOI] [PubMed] [Google Scholar]
- 15.Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol 2002; 57(1-2): 3–18. [DOI] [PubMed] [Google Scholar]
- 16.Manna PR, Chandrala SP, Jo Y, Stocco DM. cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol 2006; 37(1): 81–95. [DOI] [PubMed] [Google Scholar]
- 17.Manna PR, Dyson MT, Jo Y, Stocco DM. Role of dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1 in protein kinase A- and protein kinase C-mediated regulation of the steroidogenic acute regulatory protein expression in mouse Leydig tumor cells: mechanism of action. Endocrinology 2009; 150(1): 187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manna PR, Ahmed AU, Vartak D, Molehin D, Pruitt K. Overexpression of the steroidogenic acute regulatory protein in breast cancer: Regulation by histone deacetylase inhibition. Biochem Biophys Res Commun 2019; 509(2): 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manna PR, Stetson CL, Daugherty C, Shimizu I, Syapin PJ, Garrel G et al. Up-regulation of steroid biosynthesis by retinoid signaling: Implications for aging. Mech Ageing Dev 2015; 150: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manna PR, Stocco DM. Crosstalk of CREB and Fos/Jun on a single cis-element: transcriptional repression of the steroidogenic acute regulatory protein gene. J Mol Endocrinol 2007; 39(4): 261–77. [DOI] [PubMed] [Google Scholar]
- 21.Manna PR, Dyson MT, Stocco DM. Role of basic leucine zipper proteins in transcriptional regulation of the steroidogenic acute regulatory protein gene. Mol Cell Endocrinol 2009; 302(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med (Maywood) 2009; 234(8): 880–907. [DOI] [PubMed] [Google Scholar]
- 23.Bose HS, Sugawara T, Strauss JF, 3rd, Miller WL. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. International Congenital Lipoid Adrenal Hyperplasia Consortium. N Engl J Med 1996; 335(25): 1870–8. [DOI] [PubMed] [Google Scholar]
- 24.Miller WR. Clinical, pathological, proliferative and molecular responses associated with neoadjuvant aromatase inhibitor treatment in breast cancer. J Steroid Biochem Mol Biol 2010; 118(4–5): 273–6. [DOI] [PubMed] [Google Scholar]
- 25.Watari H, Arakane F, Moog-Lutz C, Kallen CB, Tomasetto C, Gerton GL et al. MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis. Proc Natl Acad Sci U S A 1997; 94(16): 8462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Liu P, Dwyer NK, Christenson LK, Fujimoto T, Martinez F et al. MLN64 mediates mobilization of lysosomal cholesterol to steroidogenic mitochondria. J Biol Chem 2002; 277(36): 33300–10. [DOI] [PubMed] [Google Scholar]
- 27.Soccio RE, Breslow JL. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J Biol Chem 2003; 278(25): 22183–6. [DOI] [PubMed] [Google Scholar]
- 28.Strauss JF 3rd, Kishida T, Christenson LK, Fujimoto T, Hiroi H. START domain proteins and the intracellular trafficking of cholesterol in steroidogenic cells. Mol Cell Endocrinol 2003; 202(1-2): 59–65. [DOI] [PubMed] [Google Scholar]
- 29.Tuckey RC, Bose HS, Czerwionka I, Miller WL. Molten globule structure and steroidogenic activity of N-218 MLN64 in human placental mitochondria. Endocrinology 2004; 145(4): 1700–7. [DOI] [PubMed] [Google Scholar]
- 30.Rigotti A, Cohen DE, Zanlungo S. STARTing to understand MLN64 function in cholesterol transport. J Lipid Res 2010; 51(8): 2015–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhin AG, Papadopoulos V, Costa E, Krueger KE. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc Natl Acad Sci U S A 1989; 86(24): 9813–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krueger KE, Papadopoulos V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem 1990; 265(25): 15015–22. [PubMed] [Google Scholar]
- 33.Papadopoulos V, Mukhin AG, Costa E, Krueger KE. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J Biol Chem 1990; 265(7): 3772–9. [PubMed] [Google Scholar]
- 34.Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M et al. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids 1997; 62(1): 21–8. [DOI] [PubMed] [Google Scholar]
- 35.Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem 1997; 272(51): 32129–35. [DOI] [PubMed] [Google Scholar]
- 36.Batarseh A, Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol Cell Endocrinol 2010; 327(1-2): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin CJ, Kahlert J, Kassiou M, Rendina LM. The translocator protein (TSPO): a novel target for cancer chemotherapy. Int J Biochem Cell Biol 2013; 45(7): 1212–6. [DOI] [PubMed] [Google Scholar]
- 38.Maaser K, Grabowski P, Sutter AP, Hopfner M, Foss HD, Stein H et al. Overexpression of the peripheral benzodiazepine receptor is a relevant prognostic factor in stage III colorectal cancer. Clin Cancer Res 2002; 8(10): 3205–9. [PubMed] [Google Scholar]
- 39.Han Z, Junxu, Zhong N. Expression of matrix metalloproteinases MMP-9 within the airways in asthma. Respir Med 2003; 97(5): 563–7. [DOI] [PubMed] [Google Scholar]
- 40.Galiegue S, Casellas P, Kramar A, Tinel N, Simony-Lafontaine J. Immunohistochemical assessment of the peripheral benzodiazepine receptor in breast cancer and its relationship with survival. Clin Cancer Res 2004; 10(6): 2058–64. [DOI] [PubMed] [Google Scholar]
- 41.Banati RB, Middleton RJ, Chan R, Hatty CR, Kam WW, Quin C et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat Commun 2014; 5: 5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuckey RC. Progesterone synthesis by the human placenta. Placenta 2005; 26(4): 273–81. [DOI] [PubMed] [Google Scholar]
- 43.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem 2004; 271(21): 4178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J et al. Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17alpha,24-dihydroxyergosterol. Chem Biol 2005; 12(8): 931–9. [DOI] [PubMed] [Google Scholar]
- 45.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J 2005; 272(16): 4080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J 2006; 273(13): 2891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR et al. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol 2012; 44(11): 2003–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuckey RC, Slominski AT, Cheng CY, Chen J, Kim TK, Xiao M et al. Lumisterol is metabolized by CYP11A1: discovery of a new pathway. Int J Biochem Cell Biol 2014; 55: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EKY et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep 2015; 5: 14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. Modulation of the activity of human 17 alpha-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem J 1995; 308 ( Pt 3): 901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas JL, Myers RP, Strickler RC. Human placental 3 beta-hydroxy-5-ene-steroid dehydrogenase and steroid 5--−-4-ene-isomerase: purification from mitochondria and kinetic profiles, biophysical characterization of the purified mitochondrial and microsomal enzymes. J Steroid Biochem 1989; 33(2): 209–17. [DOI] [PubMed] [Google Scholar]
- 52.Phan TS, Merk VM, Brunner T. Extra-adrenal glucocorticoid synthesis at epithelial barriers. Genes Immun 2019. [DOI] [PubMed] [Google Scholar]
- 53.Sushko TA, Gilep AA, Yantsevich AV, Usanov SA. Role of microsomal steroid hydroxylases in Delta7-steroid biosynthesis. Biochemistry (Mosc) 2013; 78(3): 282–9. [DOI] [PubMed] [Google Scholar]
- 54.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PloS one 2009; 4(2): e4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo LW, Wilson WK, Pang J, Shackleton CH. Chemical synthesis of 7- and 8-dehydro derivatives of pregnane-3,17alpha,20-triols, potential steroid metabolites in Smith-Lemli-Opitz syndrome. Steroids 2003; 68(1): 31–42. [DOI] [PubMed] [Google Scholar]
- 56.Shackleton CH, Roitman E, Kratz LE, Kelley RI. Equine type estrogens produced by a pregnant woman carrying a Smith-Lemli-Opitz syndrome fetus. J Clin Endocrinol Metab 1999; 84(3): 1157–9. [DOI] [PubMed] [Google Scholar]
- 57.Shackleton CH, Roitman E, Kelley R. Neonatal urinary steroids in Smith-Lemli-Opitz syndrome associated with 7-dehydrocholesterol reductase deficiency. Steroids 1999; 64(7): 481–90. [DOI] [PubMed] [Google Scholar]
- 58.Shackleton CH, Roitman E, Kratz LE, Kelley RI. Midgestational maternal urine steroid markers of fetal Smith-Lemli-Opitz (SLO) syndrome (7-dehydrocholesterol 7-reductase deficiency). Steroids 1999; 64(7): 446–52. [DOI] [PubMed] [Google Scholar]
- 59.Holmes MC, Seckl JR. The role of 11beta-hydroxysteroid dehydrogenases in the brain. Mol Cell Endocrinol 2006; 248(1-2): 9–14. [DOI] [PubMed] [Google Scholar]
- 60.White PC, Mune T, Agarwal AK. 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 1997; 18(1): 135–56. [DOI] [PubMed] [Google Scholar]
- 61.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol 2009; 5(7): 374–81. [DOI] [PubMed] [Google Scholar]
- 62.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev 1999; 79(1): 1–71. [DOI] [PubMed] [Google Scholar]
- 63.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981; 213(4514): 1394–7. [DOI] [PubMed] [Google Scholar]
- 64.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev 2000; 80(3): 979–1020. [DOI] [PubMed] [Google Scholar]
- 65.Chan LF, Metherell LA, Clark AJ. Effects of melanocortins on adrenal gland physiology. Eur J Pharmacol 2011; 660(1): 171–80. [DOI] [PubMed] [Google Scholar]
- 66.Clark AJ, Weber A. Adrenocorticotropin insensitivity syndromes. Endocr Rev 1998; 19(6): 828–43. [DOI] [PubMed] [Google Scholar]
- 67.Keller-Wood M, Shinsako J, Dallman MF. Interaction between stimulus intensity and corticosteroid feedback in control of ACTH. Am J Physiol 1984; 247(4 Pt 1): E489–94. [DOI] [PubMed] [Google Scholar]
- 68.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 2002; 53(4): 865–71. [DOI] [PubMed] [Google Scholar]
- 69.Nappi RE, Rivest S. Stress-induced genetic expression of a selective corticotropin-releasing factor-receptor subtype within the rat ovaries: an effect dependent on the ovulatory cycle. Biol Reprod 1995; 53(6): 1417–28. [DOI] [PubMed] [Google Scholar]
- 70.Karalis K, Muglia LJ, Bae D, Hilderbrand H, Majzoub JA. CRH and the immune system. J Neuroimmunol 1997; 72(2): 131–6. [DOI] [PubMed] [Google Scholar]
- 71.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev 2013; 34(6): 827–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalantaridou S, Makrigiannakis A, Zoumakis E, Chrousos GP. Peripheral corticotropin-releasing hormone is produced in the immune and reproductive systems: actions, potential roles and clinical implications. Front Biosci 2007; 12: 572–80. [DOI] [PubMed] [Google Scholar]
- 73.Kawahito Y, Sano H, Kawata M, Yuri K, Mukai S, Yamamura Y et al. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology 1994; 106(4): 859–65. [DOI] [PubMed] [Google Scholar]
- 74.Anton PM, Gay J, Mykoniatis A, Pan A, O’Brien M, Brown D et al. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc Natl Acad Sci U S A 2004; 101(22): 8503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zbytek B, Slominski AT. CRH mediates inflammation induced by lipopolysaccharide in human adult epidermal keratinocytes. J Invest Dermatol 2007; 127(3): 730–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science 1991; 254(5030): 421–3. [DOI] [PubMed] [Google Scholar]
- 77.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol 2005; 162(1-2): 97–102. [DOI] [PubMed] [Google Scholar]
- 78.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab 2005; 288(4): E701–6. [DOI] [PubMed] [Google Scholar]
- 79.Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J et al. Corticotropin releasing hormone and the skin. Front Biosci 2006; 11: 2230–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zbytek B, Pfeffer LM, Slominski AT. CRH inhibits NF-kappa B signaling in human melanocytes. Peptides 2006; 27(12): 3276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grammatopoulos DK, Ourailidou S. CRH Receptor Signalling: Potential Roles in Pathophysiology. Curr Mol Pharmacol 2017; 10(4): 296–310. [DOI] [PubMed] [Google Scholar]
- 82.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev 2006; 27(3): 260–86. [DOI] [PubMed] [Google Scholar]
- 83.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE et al. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J 2001; 15(10): 1678–93. [DOI] [PubMed] [Google Scholar]
- 84.Zhu H, Wang J, Li J, Li S. Corticotropin-releasing factor family and its receptors: pro-inflammatory or anti-inflammatory targets in the periphery? Inflamm Res 2011; 60(8): 715–21. [DOI] [PubMed] [Google Scholar]
- 85.Zmijewski MA, Slominski AT. Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim Pol 2010; 57(1): 1–13. [PMC free article] [PubMed] [Google Scholar]
- 86.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J 2001; 15(14): 2754–6. [DOI] [PubMed] [Google Scholar]
- 87.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab 2002; 13(10): 436–44. [DOI] [PubMed] [Google Scholar]
- 88.Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. J Clin Endocrinol Metab 2000; 85(2): 815–23. [DOI] [PubMed] [Google Scholar]
- 89.Castro MG, Morrison E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol 1997; 11(1): 35–57. [DOI] [PubMed] [Google Scholar]
- 90.Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B et al. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res 1996; 51: 287–317; discussion 318. [PubMed] [Google Scholar]
- 91.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science 1992; 257(5074): 1248–51. [DOI] [PubMed] [Google Scholar]
- 92.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 2004; 84(4): 1155–228. [DOI] [PubMed] [Google Scholar]
- 93.Blalock JE. The immune system as the sixth sense. J Intern Med 2005; 257(2): 126–38. [DOI] [PubMed] [Google Scholar]
- 94.Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol 2013; 132(5): 1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramamoorthy S, Cidlowski JA. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum Dis Clin North Am 2016; 42(1): 15–31, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sulaiman RS, Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in the eye. Steroids 2018; 133: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamamoto KR. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet 1985; 19: 209–52. [DOI] [PubMed] [Google Scholar]
- 98.Samarasinghe RA, Witchell SF, DeFranco DB. Cooperativity and complementarity: synergies in non-classical and classical glucocorticoid signaling. Cell Cycle 2012; 11(15): 2819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tillery EE, Clements JN, Howard Z. What’s new in multiple sclerosis? Ment Health Clin 2017; 7(5): 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hemmer B, Nessler S, Zhou D, Kieseier B, Hartung HP. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat Clin Pract Neurol 2006; 2(4): 201–11. [DOI] [PubMed] [Google Scholar]
- 101.Luchetti S, van Eden CG, Schuurman K, van Strien ME, Swaab DF, Huitinga I. Gender differences in multiple sclerosis: induction of estrogen signaling in male and progesterone signaling in female lesions. J Neuropathol Exp Neurol 2014; 73(2): 123–35. [DOI] [PubMed] [Google Scholar]
- 102.Compston A, Coles A. Multiple sclerosis. Lancet 2002; 359(9313): 1221–31. [DOI] [PubMed] [Google Scholar]
- 103.Dargahi N, Katsara M, Tselios T, Androutsou ME, de Courten M, Matsoukas J et al. Multiple Sclerosis: Immunopathology and Treatment Update. Brain Sci 2017; 7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler 2003; 9(6): 540–9. [DOI] [PubMed] [Google Scholar]
- 105.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83(3): 278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 2015; 14(2): 183–93. [DOI] [PubMed] [Google Scholar]
- 107.Maidhof W, Hilas O. Lupus: an overview of the disease and management options. P T 2012; 37(4): 240–9. [PMC free article] [PubMed] [Google Scholar]
- 108.Cutolo M, Sulli A, Villaggio B, Seriolo B, Accardo S. Relations between steroid hormones and cytokines in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis 1998; 57(10): 573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Furie R, Mitrane M, Zhao E, Das M, Li D, Becker PM. Efficacy and tolerability of repository corticotropin injection in patients with persistently active SLE: results of a phase 4, randomised, controlled pilot study. Lupus Sci Med 2016; 3(1): e000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, May W, McMurray RW. Pituitary hormones and systemic lupus erythematosus. Arthritis Rheum 2005; 52(12): 3701–12. [DOI] [PubMed] [Google Scholar]
- 111.Kuhn A, Bonsmann G, Anders HJ, Herzer P, Tenbrock K, Schneider M. The Diagnosis and Treatment of Systemic Lupus Erythematosus. Dtsch Arztebl Int 2015; 112(25): 423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kahlenberg JM, Fox DA. Advances in the medical treatment of rheumatoid arthritis. Hand Clin 2011; 27(1): 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 2018; 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stuart JM, Postlethwaite AE, Townes AS, Kang AH. Cell-mediated immunity to collagen and collagen alpha chains in rheumatoid arthritis and other rheumatic diseases. Am J Med 1980; 69(1): 13–8. [DOI] [PubMed] [Google Scholar]
- 115.Watson WC, Cremer MA, Wooley PH, Townes AS. Assessment of the potential pathogenicity of type II collagen autoantibodies in patients with rheumatoid arthritis. Evidence of restricted IgG3 subclass expression and activation of complement C5 to C5a. Arthritis Rheum 1986; 29(11): 1316–21. [DOI] [PubMed] [Google Scholar]
- 116.Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration 2004; 71(4): 301–26. [DOI] [PubMed] [Google Scholar]
- 117.Fromont A, De Seze J, Fleury MC, Maillefert JF, Moreau T. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine 2009; 45(2): 55–7. [DOI] [PubMed] [Google Scholar]
- 118.Kerbleski JF, Gottlieb AB. Dermatological complications and safety of anti-TNF treatments. Gut 2009; 58(8): 1033–9. [DOI] [PubMed] [Google Scholar]
- 119.Ramos-Casals M, Brito-Zeron P, Munoz S, Soria N, Galiana D, Bertolaccini L et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007; 86(4): 242–51. [DOI] [PubMed] [Google Scholar]
- 120.Nalbant S, Ozyurt M, Yildirim M, Kuskucu M. Pulmonary tuberculosis and tuberculous arthritis of knee joint associated with rheumatoid arthritis treated with anti-tumor necrosis factor (TNF)-alpha medication: a case report. Rheumatol Int 2012; 32(9): 2863–6. [DOI] [PubMed] [Google Scholar]
- 121.Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun 2007; 8(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 122.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification, Management of P et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133(2): 377–85. [DOI] [PubMed] [Google Scholar]
- 123.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol 2009; 9(10): 679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang LH, Zinselmeyer BH, Chang CH, Saunders BT, Elvington A, Baba O et al. Interleukin-17 Drives Interstitial Entrapment of Tissue Lipoproteins in Experimental Psoriasis. Cell Metab 2019; 29(2): 475–487 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Snast I, Reiter O, Atzmony L, Leshem YA, Hodak E, Mimouni D et al. Psychological stress and psoriasis: a systematic review and meta-analysis. Br J Dermatol 2018; 178(5): 1044–1055. [DOI] [PubMed] [Google Scholar]
- 126.Pietrzak D, Pietrzak A, Grywalska E, Kicinski P, Rolinski J, Donica H et al. Serum concentrations of interleukin 18 and 25-hydroxyvitamin D3 correlate with depression severity in men with psoriasis. PLoS One 2018; 13(8): e0201589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Slominski A On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br J Dermatol 2009; 160(2): 229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hannen R, Udeh-Momoh C, Upton J, Wright M, Michael A, Gulati A et al. Dysfunctional Skin-Derived Glucocorticoid Synthesis Is a Pathogenic Mechanism of Psoriasis. J Invest Dermatol 2017; 137(8): 1630–1637. [DOI] [PubMed] [Google Scholar]
- 129.Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018; 159(5): 1992–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ronholt K, Iversen L. Old and New Biological Therapies for Psoriasis. Int J Mol Sci 2017; 18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Damsky W, King BA. JAK inhibitors in dermatology: The promise of a new drug class. J Am Acad Dermatol 2017; 76(4): 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol 2014; 134(4): 769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol 2011; 2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jancso N, Jancso-Gabor A, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother 1967; 31(1): 138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Basbaum AI, Levine JD. The contribution of the nervous system to inflammation and inflammatory disease. Can J Physiol Pharmacol 1991; 69(5): 647–51. [DOI] [PubMed] [Google Scholar]
- 136.Crofford LJ, Sano H, Karalis K, Friedman TC, Epps HR, Remmers EF et al. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J Immunol 1993; 151(3): 1587–96. [PubMed] [Google Scholar]
- 137.Kawahito Y, Sano H, Mukai S, Asai K, Kimura S, Yamamura Y et al. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut 1995; 37(4): 544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mastorakos G, Bouzas EA, Silver PB, Sartani G, Friedman TC, Chan CC et al. Immune corticotropin-releasing hormone is present in the eyes of and promotes experimental autoimmune uveoretinitis in rodents. Endocrinology 1995; 136(10): 4650–8. [DOI] [PubMed] [Google Scholar]
- 139.Seres J, Bornstein SR, Seres P, Willenberg HS, Schulte KM, Scherbaum WA et al. Corticotropin-releasing hormone system in human adipose tissue. J Clin Endocrinol Metab 2004; 89(2): 965–70. [DOI] [PubMed] [Google Scholar]
- 140.Devetzis V, Zarogoulidis P, Kakolyris S, Vargemezis V, Chatzaki E. The corticotropin releasing factor system in the kidney: perspectives for novel therapeutic intervention in nephrology. Med Res Rev 2013; 33(4): 847–72. [DOI] [PubMed] [Google Scholar]
- 141.Paschos KA, Chouridou E, Koureta M, Lambropoulou M, Kolios G, Chatzaki E. The corticotropin releasing factor system in the liver: expression, actions and possible implications in hepatic physiology and pathology. Hormones (Athens) 2013; 12(2): 236–45. [DOI] [PubMed] [Google Scholar]
- 142.Czimmer J, Tache Y. Peripheral Corticotropin Releasing Factor Signaling Inhibits Gastric Emptying: Mechanisms of Action and Role in Stress-related Gastric Alterations of Motor Function. Curr Pharm Des 2017; 23(27): 4042–4047. [DOI] [PubMed] [Google Scholar]
- 143.Hanna-Mitchell AT, Wolf-Johnston A, Roppolo JR, Buffington TC, Birder LA. Corticotropin-releasing factor family peptide signaling in feline bladder urothelial cells. J Endocrinol 2014; 222(1): 113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mastorakos G, Webster EL, Friedman TC, Chrousos GP. Immunoreactive corticotropin-releasing hormone and its binding sites in the rat ovary. J Clin Invest 1993; 92(2): 961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mastorakos G, Scopa CD, Kao LC, Vryonidou A, Friedman TC, Kattis D et al. Presence of immunoreactive corticotropin-releasing hormone in human endometrium. J Clin Endocrinol Metab 1996; 81(3): 1046–50. [DOI] [PubMed] [Google Scholar]
- 146.Bohm M, Apel M, Lowin T, Lorenz J, Jenei-Lanzl Z, Capellino S et al. alpha-MSH modulates cell adhesion and inflammatory responses of synovial fibroblasts from osteoarthritis patients. Biochem Pharmacol 2016; 116: 89–99. [DOI] [PubMed] [Google Scholar]
- 147.Lorenz J, Seebach E, Hackmayer G, Greth C, Bauer RJ, Kleinschmidt K et al. Melanocortin 1 receptor-signaling deficiency results in an articular cartilage phenotype and accelerates pathogenesis of surgically induced murine osteoarthritis. PLoS One 2014; 9(9): e105858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bohm M, Grassel S. Role of proopiomelanocortin-derived peptides and their receptors in the osteoarticular system: from basic to translational research. Endocr Rev 2012; 33(4): 623–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Slominski A, Paus R, Mazurkiewicz J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia 1992; 48(1): 50–4. [DOI] [PubMed] [Google Scholar]
- 150.Mazurkiewicz JE, Corliss D, Slominski A. Spatiotemporal expression, distribution, and processing of POMC and POMC-derived peptides in murine skin. J Histochem Cytochem 2000; 48(7): 905–14. [DOI] [PubMed] [Google Scholar]
- 151.Ermak G, Slominski A. Production of POMC, CRH-R1, MC1, and MC2 receptor mRNA and expression of tyrosinase gene in relation to hair cycle and dexamethasone treatment in the C57BL/6 mouse skin. J Invest Dermatol 1997; 108(2): 160–5. [DOI] [PubMed] [Google Scholar]
- 152.Miyazaki S, Yoshikawa T, Hashiramoto A, Yamada R, Tsubouchi Y, Kohno M et al. ACTH expression in synovium of patients with rheumatoid arthritis and Lewis rats with adjuvant arthritis. Mod Rheumatol 2002; 12(3): 206–12. [DOI] [PubMed] [Google Scholar]
- 153.Smith EM. Neuropeptides as signal molecules in common with leukocytes and the hypothalamic-pituitary-adrenal axis. Brain Behav Immun 2008; 22(1): 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koido S, Ohkusa T, Kan S, Takakura K, Saito K, Komita H et al. Production of corticotropin-releasing factor and urocortin from human monocyte-derived dendritic cells is stimulated by commensal bacteria in intestine. World J Gastroenterol 2014; 20(39): 14420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McEvoy AN, Bresnihan B, FitzGerald O, Murphy EP. Cyclooxygenase 2-derived prostaglandin E2 production by corticotropin-releasing hormone contributes to the activated cAMP response element binding protein content in rheumatoid arthritis synovial tissue. Arthritis Rheum 2004; 50(4): 1132–45. [DOI] [PubMed] [Google Scholar]
- 156.Leu SJ, Singh VK. Stimulation of interleukin-6 production by corticotropin-releasing factor. Cell Immunol 1992; 143(1): 220–7. [DOI] [PubMed] [Google Scholar]
- 157.Singh VK, Leu SJ. Enhancing effect of corticotropin-releasing neurohormone on the production of interleukin-1 and interleukin-2. Neurosci Lett 1990; 120(2): 151–4. [DOI] [PubMed] [Google Scholar]
- 158.Hagan P, Poole S, Bristow AF. Immunosuppressive activity of corticotrophin-releasing factor. Inhibition of interleukin-1 and interleukin-6 production by human mononuclear cells. Biochem J 1992; 281 ( Pt 1): 251–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh VK. Stimulatory effect of corticotropin-releasing neurohormone on human lymphocyte proliferation and interleukin-2 receptor expression. J Neuroimmunol 1989; 23(3): 257–62. [DOI] [PubMed] [Google Scholar]
- 160.Leu SJ, Singh VK. Modulation of natural killer cell-mediated lysis by corticotropin-releasing neurohormone. J Neuroimmunol 1991; 33(3): 253–60. [DOI] [PubMed] [Google Scholar]
- 161.Kavelaars A, Ballieux RE, Heijnen CJ. The role of IL-1 in the corticotropin-releasing factor and arginine- vasopressin-induced secretion of immunoreactive beta-endorphin by human peripheral blood mononuclear cells. J Immunol 1989; 142(7): 2338–42. [PubMed] [Google Scholar]
- 162.Song JP, Chen X, Yang G, Geng XR. Corticotropin releasing hormone activates CD14(+) cells to induce endothelial barrier dysfunction. Cell Biol Int 2013. [DOI] [PubMed] [Google Scholar]
- 163.Oh SH, Park CO, Wu WH, Kim JY, Jin S, Byamba D et al. Corticotropin-releasing hormone downregulates IL-10 production by adaptive forkhead box protein 3-negative regulatory T cells in patients with atopic dermatitis. J Allergy Clin Immunol 2012; 129(1): 151–9 e1–6. [DOI] [PubMed] [Google Scholar]
- 164.Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br J Pharmacol 2012; 166(1): 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reul JM, Holsboer F. On the role of corticotropin-releasing hormone receptors in anxiety and depression. Dialogues Clin Neurosci 2002; 4(1): 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM et al. A soluble mouse brain splice variant of type 2alpha corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci U S A 2005; 102(7): 2620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tsatsanis C, Androulidaki A, Dermitzaki E, Gravanis A, Margioris AN. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-alpha release from macrophages via induction of COX-2 and PGE2. J Cell Physiol 2007; 210(3): 774–83. [DOI] [PubMed] [Google Scholar]
- 168.Slominski A, Ermak G, Hwang J, Mazurkiewicz J, Corliss D, Eastman A. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochim Biophys Acta 1996; 1289(2): 247–51. [DOI] [PubMed] [Google Scholar]
- 169.Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J Clin Endocrinol Metab 1998; 83(3): 1020–4. [DOI] [PubMed] [Google Scholar]
- 170.Slominski A, Szczesniewski A, Wortsman J. Liquid chromatography-mass spectrometry detection of corticotropin-releasing hormone and proopiomelanocortin-derived peptides in human skin. J Clin Endocrinol Metab 2000; 85(10): 3582–8. [DOI] [PubMed] [Google Scholar]
- 171.Slominski AT, Roloff B, Zbytek B, Wei ET, Fechner K, Curry J et al. Corticotropin releasing hormone and related peptides can act as bioregulatory factors in human keratinocytes. In Vitro Cell Dev Biol Anim 2000; 36(3): 211–6. [DOI] [PubMed] [Google Scholar]
- 172.Quevedo ME, Slominski A, Pinto W, Wei E, Wortsman J. Pleiotropic effects of corticotropin releasing hormone on normal human skin keratinocytes. In Vitro Cell Dev Biol Anim 2001; 37(1): 50–4. [DOI] [PubMed] [Google Scholar]
- 173.Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. J Invest Dermatol 2004; 122(1): 235–7. [DOI] [PubMed] [Google Scholar]
- 174.Roloff B, Fechner K, Slominski A, Furkert J, Botchkarev VA, Bulfone-Paus S et al. Hair cycle-dependent expression of corticotropin-releasing factor (CRF) and CRF receptors in murine skin. FASEB J 1998; 12(3): 287–97. [DOI] [PubMed] [Google Scholar]
- 175.Slominski AT, Botchkarev V, Choudhry M, Fazal N, Fechner K, Furkert J et al. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system?”. Annals of the New York Academy of Sciences 1999; 885: 287–311. [DOI] [PubMed] [Google Scholar]
- 176.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J 2005; 19(10): 1332–4. [DOI] [PubMed] [Google Scholar]
- 177.O’Kane M, Murphy EP, Kirby B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp Dermatol 2006; 15(3): 143–53. [DOI] [PubMed] [Google Scholar]
- 178.Paus R, Langan EA, Vidali S, Ramot Y, Andersen B. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med 2014; 20(10): 559–70. [DOI] [PubMed] [Google Scholar]
- 179.Theoharides TC, Stewart JM, Taracanova A, Conti P, Zouboulis CC. Neuroendocrinology of the skin. Rev Endocr Metab Disord 2016; 17(3): 287–294. [DOI] [PubMed] [Google Scholar]
- 180.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology 2004; 145(2): 941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone stimulates NF-kappaB in human epidermal keratinocytes. J Endocrinol 2004; 181(3): R1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol 2006; 206(3): 780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Slominski A POMC gene expression in mouse and hamster melanoma cells. FEBS Lett 1991; 291(2): 165–8. [DOI] [PubMed] [Google Scholar]
- 184.Slominski A, Wortsman J, Mazurkiewicz JE, Matsuoka L, Dietrich J, Lawrence K et al. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J Lab Clin Med 1993; 122(6): 658–66. [PubMed] [Google Scholar]
- 185.Schauer E, Trautinger F, Kock A, Schwarz A, Bhardwaj R, Simon M et al. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest 1994; 93(5): 2258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Luger TA, Paus R, Slominski A, Lipton J. The proopiomelanocortin system in cutaneous neuroimmunomodulation. An introductory overview. Annals of the New York Academy of Sciences 1999; 885: xi–xiv. [DOI] [PubMed] [Google Scholar]
- 187.Talaber G, Jondal M, Okret S. Extra-adrenal glucocorticoid synthesis: immune regulation and aspects on local organ homeostasis. Mol Cell Endocrinol 2013; 380(1-2): 89–98. [DOI] [PubMed] [Google Scholar]
- 188.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol 2013; 137: 107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab 2011; 301(1): E11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab 1996; 81(7): 2746–9. [DOI] [PubMed] [Google Scholar]
- 191.Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z et al. Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1). J Invest Dermatol 2003; 120(6): 905–14. [DOI] [PubMed] [Google Scholar]
- 192.Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem 2011; 286(12): 10265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Commun 2011; 404(1): 62–7. [DOI] [PubMed] [Google Scholar]
- 194.Slominski A, Zbytek B, Szczesniewski A, Wortsman J. Cultured human dermal fibroblasts do produce cortisol. J Invest Dermatol 2006; 126(5): 1177–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab 2011; 301(3): E484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Skobowiat C, Nejati R, Lu L, Williams RW, Slominski AT. Genetic variation of the cutaneous HPA axis: an analysis of UVB-induced differential responses. Gene 2013; 530(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol 2013; 168(3): 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Skobowiat C, Postlethwaite AE, Slominski AT. Skin Exposure to Ultraviolet B Rapidly Activates Systemic Neuroendocrine and Immunosuppressive Responses. Photochem Photobiol 2017; 93(4): 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol 2012; 212: v, vii, 1–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wierzbicka JM, Zmijewski MA, Antoniewicz J, Sobjanek M, Slominski AT. Differentiation of Keratinocytes Modulates Skin HPA Analog. J Cell Physiol 2017; 232(1): 154–66. [DOI] [PubMed] [Google Scholar]
- 201.Bigas J, Sevilla LM, Carceller E, Boix J, Perez P. Epidermal glucocorticoid and mineralocorticoid receptors act cooperatively to regulate epidermal development and counteract skin inflammation. Cell Death Dis 2018; 9(6): 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Slominski AT, Zmijewski MA. Glucocorticoids Inhibit Wound Healing: Novel Mechanism of Action. J Invest Dermatol 2017; 137(5): 1012–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jozic I, Vukelic S, Stojadinovic O, Liang L, Ramirez HA, Pastar I et al. Stress Signals, Mediated by Membranous Glucocorticoid Receptor, Activate PLC/PKC/GSK-3beta/beta-catenin Pathway to Inhibit Wound Closure. J Invest Dermatol 2017; 137(5): 1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest 2007; 117(11): 3339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Slominski A A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest 2007; 117(11): 3166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Slominski AT, Brozyna AA, Tuckey RC. Cutaneous Glucocorticoidogenesis and Cortisol Signaling Are Defective in Psoriasis. J Invest Dermatol 2017; 137(8): 1609–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J Exp Med 1994; 179(6): 1835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Talaber G, Jondal M, Okret S. Local glucocorticoid production in the thymus. Steroids 2015; 103: 58–63. [DOI] [PubMed] [Google Scholar]
- 209.Costa B, Pini S, Gabelloni P, Da Pozzo E, Abelli M, Lari L et al. The spontaneous Ala147Thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology 2009; 150(12): 5438–45. [DOI] [PubMed] [Google Scholar]
- 210.Jia Y, Domenico J, Takeda K, Han J, Wang M, Armstrong M et al. Steroidogenic enzyme Cyp11a1 regulates Type 2 CD8+ T cell skewing in allergic lung disease. Proc Natl Acad Sci U S A 2013; 110(20): 8152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Oka H, Emori Y, Hayashi Y, Nomoto K. Breakdown of Th cell immune responses and steroidogenic CYP11A1 expression in CD4+ T cells in a murine model implanted with B16 melanoma. Cell Immunol 2000; 206(1): 7–15. [DOI] [PubMed] [Google Scholar]
- 212.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS Jr., Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 1995; 270(5234): 283–6. [DOI] [PubMed] [Google Scholar]
- 213.Almawi WY, Beyhum HN, Rahme AA, Rieder MJ. Regulation of cytokine and cytokine receptor expression by glucocorticoids. J Leukoc Biol 1996; 60(5): 563–72. [DOI] [PubMed] [Google Scholar]
- 214.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995; 270(5234): 286–90. [DOI] [PubMed] [Google Scholar]
- 215.Gottlicher M, Heck S, Herrlich P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J Mol Med (Berl) 1998; 76(7): 480–9. [DOI] [PubMed] [Google Scholar]
- 216.Rhen T, Cidlowski JA. Estrogens and glucocorticoids have opposing effects on the amount and latent activity of complement proteins in the rat uterus. Biol Reprod 2006; 74(2): 265–74. [DOI] [PubMed] [Google Scholar]
- 217.McKay LI, Cidlowski JA. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol Endocrinol 1998; 12(1): 45–56. [DOI] [PubMed] [Google Scholar]
- 218.Ronchetti S, Migliorati G, Riccardi C. GILZ as a Mediator of the Anti-Inflammatory Effects of Glucocorticoids. Front Endocrinol (Lausanne) 2015; 6: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.D’Adamio F, Zollo O, Moraca R, Ayroldi E, Bruscoli S, Bartoli A et al. A new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/CD3-activated cell death. Immunity 1997; 7(6): 803–12. [DOI] [PubMed] [Google Scholar]
- 220.Cannarile L, Delfino DV, Adorisio S, Riccardi C, Ayroldi E. Implicating the Role of GILZ in Glucocorticoid Modulation of T-Cell Activation. Front Immunol 2019; 10: 1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bereshchenko O, Migliorati G, Bruscoli S, Riccardi C. Glucocorticoid-Induced Leucine Zipper: A Novel Anti-inflammatory Molecule. Front Pharmacol 2019; 10: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med 2005; 353(16): 1711–23. [DOI] [PubMed] [Google Scholar]
- 223.Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med 1976; 84(3): 304–15. [DOI] [PubMed] [Google Scholar]
- 224.Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med 1993; 119(12): 1198–208. [DOI] [PubMed] [Google Scholar]
- 225.Fauci AS, Murakami T, Brandon DD, Loriaux DL, Lipsett MB. Mechanisms of corticosteroid action on lymphocyte subpopulations. VI. Lack of correlation between glucocorticosteroid receptors and the differential effects of glucocorticosteroids on T-cell subpopulations. Cell Immunol 1980; 49(1): 43–50. [DOI] [PubMed] [Google Scholar]
- 226.Slade JD, Hepburn B. Prednisone-induced alterations of circulating human lymphocyte subsets. J Lab Clin Med 1983; 101(3): 479–87. [PubMed] [Google Scholar]
- 227.Settipane GA, Pudupakkam RK, McGowan JH. Corticosteroid effect on immunoglobulins. J Allergy Clin Immunol 1978; 62(3): 162–6. [DOI] [PubMed] [Google Scholar]
- 228.Wallen N, Kita H, Weiler D, Gleich GJ. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol 1991; 147(10): 3490–5. [PubMed] [Google Scholar]
- 229.Andrade MV, Hiragun T, Beaven MA. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J Immunol 2004; 172(12): 7254–62. [DOI] [PubMed] [Google Scholar]
- 230.Shodell M, Shah K, Siegal FP. Circulating human plasmacytoid dendritic cells are highly sensitive to corticosteroid administration. Lupus 2003; 12(3): 222–30. [DOI] [PubMed] [Google Scholar]
- 231.Nikolakis G, Stratakis CA, Kanaki T, Slominski A, Zouboulis CC. Skin steroidogenesis in health and disease. Rev Endocr Metab Disord 2016; 17(3): 247–258. [DOI] [PubMed] [Google Scholar]
- 232.Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp Dermatol 2014; 23(6): 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sewell WA, Scurr LL, Orphanides H, Kinder S, Ludowyke RI. Induction of interleukin-4 and interleukin-5 expression in mast cells is inhibited by glucocorticoids. Clin Diagn Lab Immunol 1998; 5(1): 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tiala I, Suomela S, Huuhtanen J, Wakkinen J, Holtta-Vuori M, Kainu K et al. The CCHCR1 (HCR) gene is relevant for skin steroidogenesis and downregulated in cultured psoriatic keratinocytes. J Mol Med (Berl) 2007; 85(6): 589–601. [DOI] [PubMed] [Google Scholar]
- 235.Sarkar MK, Kaplan N, Tsoi LC, Xing X, Liang Y, Swindell WR et al. Endogenous Glucocorticoid Deficiency in Psoriasis Promotes Inflammation and Abnormal Differentiation. J Invest Dermatol 2017; 137(7): 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Boghozian R, McKenzie BA, Saito LB, Mehta N, Branton WG, Lu J et al. Suppressed oligodendrocyte steroidogenesis in multiple sclerosis: Implications for regulation of neuroinflammation. Glia 2017; 65(10): 1590–1606. [DOI] [PubMed] [Google Scholar]
- 237.Arnason BG, Berkovich R, Catania A, Lisak RP, Zaidi M. Mechanisms of action of adrenocorticotropic hormone and other melanocortins relevant to the clinical management of patients with multiple sclerosis. Mult Scler 2013; 19(2): 130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Miller H, Newell DJ, Ridley A. Multiple sclerosis. Treatment of acute exacerbations with corticotrophin (A.C.T.H.). Lancet 1961; 2(7212): 1120–2. [DOI] [PubMed] [Google Scholar]
- 239.Chatham WW, Kimberly RP. Treatment of lupus with corticosteroids. Lupus 2001; 10(3): 140–7. [DOI] [PubMed] [Google Scholar]
- 240.Fiechtner JJ, Montroy T. Treatment of moderately to severely active systemic lupus erythematosus with adrenocorticotropic hormone: a single-site, open-label trial. Lupus 2014; 23(9): 905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Harris-Jones JN. The role of ACTH and cortisone in the treatment of systemic lupus erythematosus. Postgrad Med J 1956; 32(365): 145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Vogl D, Falk W, Dorner M, Scholmerich J, Straub RH. Serum levels of pregnenolone and 17-hydroxypregnenolone in patients with rheumatoid arthritis and systemic lupus erythematosus: relation to other adrenal hormones. J Rheumatol 2003; 30(2): 269–75. [PubMed] [Google Scholar]
- 243.Straub RH, Cutolo M. Glucocorticoids and chronic inflammation. Rheumatology (Oxford) 2016; 55(suppl 2): ii6–ii14. [DOI] [PubMed] [Google Scholar]
- 244.Yousri NA, Bayoumy K, Elhaq WG, Mohney RP, Emadi SA, Hammoudeh M et al. Large Scale Metabolic Profiling identifies Novel Steroids linked to Rheumatoid Arthritis. Sci Rep 2017; 7(1): 9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Straub RH, Paimela L, Peltomaa R, Scholmerich J, Leirisalo-Repo M. Inadequately low serum levels of steroid hormones in relation to interleukin-6 and tumor necrosis factor in untreated patients with early rheumatoid arthritis and reactive arthritis. Arthritis Rheum 2002; 46(3): 654–62. [DOI] [PubMed] [Google Scholar]
- 246.Straub RH, Weidler C, Demmel B, Herrmann M, Kees F, Schmidt M et al. Renal clearance and daily excretion of cortisol and adrenal androgens in patients with rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis 2004; 63(8): 961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Schlaghecke R, Kornely E, Wollenhaupt J, Specker C. Glucocorticoid receptors in rheumatoid arthritis. Arthritis Rheum 1992; 35(7): 740–4. [DOI] [PubMed] [Google Scholar]
- 248.Schlaghecke R, Beuscher D, Kornely E, Specker C. Effects of glucocorticoids in rheumatoid arthritis. Diminished glucocorticoid receptors do not result in glucocorticoid resistance. Arthritis Rheum 1994; 37(8): 1127–31. [DOI] [PubMed] [Google Scholar]
- 249.Edwards C Sixty years after Hench--corticosteroids and chronic inflammatory disease. J Clin Endocrinol Metab 2012; 97(5): 1443–51. [DOI] [PubMed] [Google Scholar]
- 250.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med 2010; 16(4): 406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rowse AL, Naves R, Cashman KS, McGuire DJ, Mbana T, Raman C et al. Lithium controls central nervous system autoimmunity through modulation of IFN-gamma signaling. PLoS One 2012; 7(12): e52658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mier-Aguilar CA, Cashman KS, Raman C, Soldevila G. CD5-CK2 Signaling Modulates Erk Activation and Thymocyte Survival. PLoS One 2016; 11(12): e0168155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM Jr., Slominski AT. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol 2010; 223(1): 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li J, Daly E, Campioli E, Wabitsch M, Papadopoulos V. De novo synthesis of steroids and oxysterols in adipocytes. J Biol Chem 2014; 289(2): 747–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.MacKenzie SM, Huda SS, Sattar N, Fraser R, Connell JM, Davies E. Depot-specific steroidogenic gene transcription in human adipose tissue. Clin Endocrinol (Oxf) 2008; 69(6): 848–54. [DOI] [PubMed] [Google Scholar]
- 256.Byeon HR, Lee SH. Expression of Steroidogenesis-related Genes in Rat Adipose Tissues. Dev Reprod 2016; 20(3): 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Janssen JM, Bland R, Hewison M, Coughtrie MW, Sharp S, Arts J et al. Estradiol formation by human osteoblasts via multiple pathways: relation with osteoblast function. J Cell Biochem 1999; 75(3): 528–37. [DOI] [PubMed] [Google Scholar]
- 258.Rodriguez-Sanz M, Garcia-Giralt N, Prieto-Alhambra D, Servitja S, Balcells S, Pecorelli R et al. CYP11A1 expression in bone is associated with aromatase inhibitor-related bone loss. J Mol Endocrinol 2015; 55(1): 69–79. [DOI] [PubMed] [Google Scholar]
- 259.Liu L, Pathak JL, Zhu YQ, Bureik M. Comparison of cytochrome P450 expression in four different human osteoblast models. Biol Chem 2017; 398(12): 1327–1334. [DOI] [PubMed] [Google Scholar]
- 260.Corpechot C, Synguelakis M, Talha S, Axelson M, Sjovall J, Vihko R et al. Pregnenolone and its sulfate ester in the rat brain. Brain Res 1983; 270(1): 119–25. [DOI] [PubMed] [Google Scholar]
- 261.Watzka M, Bidlingmaier F, Schramm J, Klingmuller D, Stoffel-Wagner B. Sex- and age-specific differences in human brain CYP11A1 mRNA expression. J Neuroendocrinol 1999; 11(12): 901–5. [DOI] [PubMed] [Google Scholar]
- 262.Yu L, Romero DG, Gomez-Sanchez CE, Gomez-Sanchez EP. Steroidogenic enzyme gene expression in the human brain. Mol Cell Endocrinol 2002; 190(1-2): 9–17. [DOI] [PubMed] [Google Scholar]
- 263.King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K et al. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci 2002; 22(24): 10613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lavaque E, Sierra A, Azcoitia I, Garcia-Segura LM. Steroidogenic acute regulatory protein in the brain. Neuroscience 2006; 138(3): 741–7. [DOI] [PubMed] [Google Scholar]
- 265.Hostettler N, Bianchi P, Gennari-Moser C, Kassahn D, Schoonjans K, Corazza N et al. Local glucocorticoid production in the mouse lung is induced by immune cell stimulation. Allergy 2012; 67(2): 227–34. [DOI] [PubMed] [Google Scholar]
- 266.Fernandez-Marcos PJ, Auwerx J, Schoonjans K. Emerging actions of the nuclear receptor LRH-1 in the gut. Biochim Biophys Acta 2011; 1812(8): 947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Noti M, Sidler D, Brunner T. Extra-adrenal glucocorticoid synthesis in the intestinal epithelium: more than a drop in the ocean? Semin Immunopathol 2009; 31(2): 237–48. [DOI] [PubMed] [Google Scholar]
- 268.Cima I, Corazza N, Dick B, Fuhrer A, Herren S, Jakob S et al. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J Exp Med 2004; 200(12): 1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sidler D, Renzulli P, Schnoz C, Berger B, Schneider-Jakob S, Fluck C et al. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene 2011; 30(21): 2411–9. [DOI] [PubMed] [Google Scholar]
- 270.Wang M, Ramirez J, Han J, Jia Y, Domenico J, Seibold MA et al. The steroidogenic enzyme Cyp11a1 is essential for development of peanut-induced intestinal anaphylaxis. Journal of Allergy and Clinical Immunology 2013; 132(5): 1174–1183.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Huang SC, Lee CT, Chung BC. Tumor necrosis factor suppresses NR5A2 activity and intestinal glucocorticoid synthesis to sustain chronic colitis. Sci Signal 2014; 7(314): ra20. [DOI] [PubMed] [Google Scholar]
- 272.Tsai SJ, Wu MH, Lin CC, Sun HS, Chen HM. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab 2001; 86(12): 5765–73. [DOI] [PubMed] [Google Scholar]
- 273.Sugawara T, Nomura E, Fujimoto S. Expression of enzyme associated with steroid hormone synthesis and local production of steroid hormone in endometrial carcinoma cells. J Endocrinol 2004; 180(1): 135–44. [DOI] [PubMed] [Google Scholar]
- 274.Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab 2009; 94(2): 623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Young MJ, Clyne CD, Cole TJ, Funder JW. Cardiac steroidogenesis in the normal and failing heart. J Clin Endocrinol Metab 2001; 86(11): 5121–6. [DOI] [PubMed] [Google Scholar]
- 276.Kayes-Wandover KM, White PC. Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab 2000; 85(7): 2519–25. [DOI] [PubMed] [Google Scholar]
- 277.Casal AJ, Silvestre JS, Delcayre C, Capponi AM. Expression and modulation of steroidogenic acute regulatory protein messenger ribonucleic acid in rat cardiocytes and after myocardial infarction. Endocrinology 2003; 144(5): 1861–8. [DOI] [PubMed] [Google Scholar]
- 278.Ohtani T, Mano T, Hikoso S, Sakata Y, Nishio M, Takeda Y et al. Cardiac steroidogenesis and glucocorticoid in the development of cardiac hypertrophy during the progression to heart failure. J Hypertens 2009; 27(5): 1074–83. [DOI] [PubMed] [Google Scholar]
- 279.Anuka E, Yivgi-Ohana N, Eimerl S, Garfinkel B, Melamed-Book N, Chepurkol E et al. Infarct-induced steroidogenic acute regulatory protein: a survival role in cardiac fibroblasts. Mol Endocrinol 2013; 27(9): 1502–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Dalla Valle L, Toffolo V, Vianello S, Belvedere P, Colombo L. Expression of cytochrome P450scc mRNA and protein in the rat kidney from birth to adulthood. J Steroid Biochem Mol Biol 2004; 88(1): 79–89. [DOI] [PubMed] [Google Scholar]
- 281.Pagotto MA, Roldan ML, Pagotto RM, Lugano MC, Pisani GB, Rogic G et al. Localization and functional activity of cytochrome P450 side chain cleavage enzyme (CYP11A1) in the adult rat kidney. Mol Cell Endocrinol 2011; 332(1-2): 253–60. [DOI] [PubMed] [Google Scholar]
- 282.Provost PR, Tremblay Y. Genes involved in the adrenal pathway of glucocorticoid synthesis are transiently expressed in the developing lung. Endocrinology 2005; 146(5): 2239–45. [DOI] [PubMed] [Google Scholar]
- 283.Maron BA, Oldham WM, Chan SY, Vargas SO, Arons E, Zhang YY et al. Upregulation of steroidogenic acute regulatory protein by hypoxia stimulates aldosterone synthesis in pulmonary artery endothelial cells to promote pulmonary vascular fibrosis. Circulation 2014; 130(2): 168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ma Y, Ren S, Pandak WM, Li X, Ning Y, Lu C et al. The effects of inflammatory cytokines on steroidogenic acute regulatory protein expression in macrophages. Inflamm Res 2007; 56(12): 495–501. [DOI] [PubMed] [Google Scholar]
- 285.Taylor JM, Borthwick F, Bartholomew C, Graham A. Overexpression of steroidogenic acute regulatory protein increases macrophage cholesterol efflux to apolipoprotein AI. Cardiovasc Res 2010; 86(3): 526–34. [DOI] [PubMed] [Google Scholar]
- 286.Bai Q, Li X, Ning Y, Zhao F, Yin L. Mitochondrial cholesterol transporter, StAR, inhibits human THP-1 monocyte-derived macrophage apoptosis. Lipids 2010; 45(1): 29–36. [DOI] [PubMed] [Google Scholar]
- 287.Manna PR, Sennoune SR, Martinez-Zaguilan R, Slominski AT, Pruitt K. Regulation of retinoid mediated cholesterol efflux involves liver X receptor activation in mouse macrophages. Biochem Biophys Res Commun 2015; 464(1): 312–7. [DOI] [PubMed] [Google Scholar]
- 288.Zhou Z, Agarwal VR, Dixit N, White P, Speiser PW. Steroid 21-hydroxylase expression and activity in human lymphocytes. Mol Cell Endocrinol 1997; 127(1): 11–8. [DOI] [PubMed] [Google Scholar]
- 289.Iscan M, Klaavuniemi T, Coban T, Kapucuoglu N, Pelkonen O, Raunio H. The expression of cytochrome P450 enzymes in human breast tumours and normal breast tissue. Breast Cancer Res Treat 2001; 70(1): 47–54. [DOI] [PubMed] [Google Scholar]
- 290.Bulun SE, Lin Z, Zhao H, Lu M, Amin S, Reierstad S et al. Regulation of aromatase expression in breast cancer tissue. Annals of the New York Academy of Sciences 2009; 1155: 121–31. [DOI] [PubMed] [Google Scholar]
- 291.Tuzuner MB, Ozturk T, Eronat AP, Seyhan F, Kisakesen HI, Calay Z et al. Evaluation of Local CYP17A1 and CYP19A1 Expression Levels as Prognostic Factors in Postmenopausal Invasive Ductal Breast Cancer Cases. Biochemical genetics 2016; 54(6): 784–802. [DOI] [PubMed] [Google Scholar]
- 292.Jun YJ, Park SJ, Hwang JW, Kim TH, Jung KJ, Jung JY et al. Differential expression of 11beta-hydroxysteroid dehydrogenase type 1 and 2 in mild and moderate/severe persistent allergic nasal mucosa and regulation of their expression by Th2 cytokines: asthma and rhinitis. Clin Exp Allergy 2014; 44(2): 197–211. [DOI] [PubMed] [Google Scholar]
- 293.Park SJ, Kook JH, Kim HK, Kang SH, Lim SH, Kim HJ et al. Macrolides increase the expression of 11beta-hydroxysteroid dehydrogenase 1 in human sinonasal epithelium, contributing to glucocorticoid activation in sinonasal mucosa. Br J Pharmacol 2015; 172(21): 5083–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Baquie M, St-Onge L, Kerr-Conte J, Cobo-Vuilleumier N, Lorenzo PI, Jimenez Moreno CM et al. The liver receptor homolog-1 (LRH-1) is expressed in human islets and protects {beta}-cells against stress-induced apoptosis. Hum Mol Genet 2011; 20(14): 2823–33. [DOI] [PubMed] [Google Scholar]
- 295.Morales A, Vilchis F, Chavez B, Morimoto S, Chan C, Robles-Diaz G et al. Differential expression of steroidogenic factors 1 and 2, cytochrome p450scc, and steroidogenic acute regulatory protein in human pancreas. Pancreas 2008; 37(2): 165–9. [DOI] [PubMed] [Google Scholar]
- 296.Sakai M, Martinez-Arguelles DB, Aprikian AG, Magliocco AM, Papadopoulos V. De novo steroid biosynthesis in human prostate cell lines and biopsies. Prostate 2016; 76(6): 575–87. [DOI] [PubMed] [Google Scholar]
- 297.Bennett NC, Hooper JD, Lambie D, Lee CS, Yang T, Vesey DA et al. Evidence for steroidogenic potential in human prostate cell lines and tissues. Am J Pathol 2012; 181(3): 1078–87. [DOI] [PubMed] [Google Scholar]
- 298.Cai C, Balk SP. Intratumoral androgen biosynthesis in prostate cancer pathogenesis and response to therapy. Endocr Relat Cancer 2011; 18(5): R175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res 2011; 71(20): 6503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol 2008; 295(1-2): 115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Slominski AT, Zmijewski MA, Semak I, Zbytek B, Pisarchik A, Li W et al. Cytochromes p450 and skin cancer: role of local endocrine pathways. Anticancer Agents Med Chem 2014; 14(1): 77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Schedel M, Jia Y, Michel S, Takeda K, Domenico J, Joetham A et al. 1,25D3 prevents CD8+Tc2 skewing and asthma development through VDR binding changes to the Cyp11a1 promoter. Nature Communications 2016; 7(1): 10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Mahata B, Zhang X, Kolodziejczyk AA, Proserpio V, Haim-Vilmovsky L, Taylor AE et al. Single-cell RNA sequencing reveals T helper cells synthesizing steroids de novo to contribute to immune homeostasis. Cell Rep 2014; 7(4): 1130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Qiao S, Okret S, Jondal M. Thymocyte-synthesized glucocorticoids play a role in thymocyte homeostasis and are down-regulated by adrenocorticotropic hormone. Endocrinology 2009; 150(9): 4163–9. [DOI] [PubMed] [Google Scholar]


