Abstract
Background
Coronary artery disease (CAD) remains a leading cause of morbidity and mortality. Cytokines play a potential role in atherosclerosis pathogenesis and progression. We investigated the association between high sensitive C-reactive protein (hsCRP) and severity of CAD.
Methods
CAD patients were stratified according to hsCRP cut-off value into high levels hsCRP group (≥ 8.4 mg/L) and low levels hsCRP group (< 8.4 mg/L). Severity of CAD was assessed according to artery stenosis degree and the number of vessel involved. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS, version 23.0).
Results
The mean age was 60.3 ± 11.0 years. The level of hsCRP was increased and ranged from 0.2 to 1020.0 mg/L. Biochemical risk factors and severity of CAD didn't show significant differences between the two groups. In multivariate linear analysis, cardiac troponin I (cTnI) and serum amyloid A (SAA) were predictors of hsCRP. As shown in receiver operating characteristic (ROC) curve analysis performed in patients with ST-segment elevation myocardial infarction (STEMI) and compared to myonecrosis biomarkers, hsCRP (area under the curve (AUC): 0.905; 95%CI: 0.844–0.966; P < 0.001) could be a powerful predictor marker in evaluating the infarct size after myocardial infarction but not better than cTnI.
Conclusions
HsCRP levels were not associated with the severity of CAD but could be useful in the evaluation of myocardial necrosis in patients with STEMI.
Keywords: Coronary artery disease, High sensitive C-reactive protein, Severity
1. Introduction
Inflammation plays an essential role in the development, progression, and prognosis of coronary artery disease (CAD), and has been widely concerned as an independent risk factor for the development of CAD.[1] It is involved in many steps of atherosclerosis, and the activation of inflammatory responses may be a major contributor to plaque instability.[1],[2] The activation of proinflammatory cells and the upregulation of adhesion molecules increase the production of cytokines and procoagulant substances. Theses molecules promote the thickening or rupture of atherosclerotic plaques and lead to the occurrence and development of acute coronary syndrome (ACS).[1],[3] C-Reactive Protein (CRP) is a liver protein whose synthesis depends on several transacting cytokines of interleukin-6 (IL-6), IL-1 and tumor necrosis factor α (TNF-α).[4] Measurement of CRP is useful for the detection and evaluation of infection, tissue injury, inflammatory disorders, and associated diseases.[5] High concentrations of CRP were shown in patients with metabolic disorders such as insulin resistance, obesity, lipid metabolism and unstable angina (UA).[4] It has been suggested that high levels of high-sensitivity CRP (hsCRP) are associated with adverse events in various cardiovascular diseases, including heart failure, ischemic stroke, atrial fibrillation, type 2 diabetes mellitus (T2DM), hypertension and myocardial infarction (MI).[6] HsCRP levels are recognized as a strong independent risk marker for the identification of individuals at risk for future cardiovascular disease, which may be useful as an independent marker of prognosis for recurrent events in patients with stable coronary disease or ACS.[5] In this study, we investigate the relationship between hsCRP levels and severity of CAD in patients undergoing angiography.
2. Methods
2.1. Study patients
In this investigation, 310 patients with CAD undergoing coronary angiography in our University Hospital (Monastir, Tunisia) were enrolled. Patients were divided into two groups according to hsCRP cut-off levels. The first group with low hsCRP levels (< 8.4 mg/L) and the second group with high hsCRP levels (≥ 8.4 mg/L). The exclusion criteria of patients were the presence of severe respiratory disease, kidney disease, liver disease, concomitant inflammatory diseases such as infections and autoimmune disorders, or malignancy. Informed consent was obtained from all participants prior to the study. The study was approved by the National Committee for Medical and Research Ethics of Farhat Hached University Hospital (Sousse, Tunisia), which complied with the ethical principles of the WMA Declaration of Helsinki.
2.2. Angiographic diagnostic criteria
CAD was defined as the presence of 50% obstructive stenosis in at least one major coronary artery (left main coronary artery (LMCA), left anterior descending (LAD), left circumflex (LCx) or right coronary artery (RA) or major branches). Clinical characteristics of patients were recorded from hospital records. ST-elevation myocardial infarction (STEMI) was defined as the presence of chest pain (> 20 min) associated with persistent electrocardiographic elevation of the ST segment and increased concentrations of cardiac troponin I (cTnI) reflecting an acute total coronary occlusion. A rapid, complete, and sustained reperfusion by primary angioplasty or fibrinolytic therapy is mandatory. The diagnosis of non-ST segment elevation myocardial infarction (NSTEMI) was defined as the presence of persistent or transient ST-segment depression or T-wave inversion, flat T waves, pseudo-normalization of T waves, or no ECG changes at presentation. Patients have also ischemic symptoms associated with high concentrations of cTnI but without any marked change in the electrocardiogram. UA was defined as the presence of chest pain which was accelerated on exertion or rest angina. No elevation of cTnI concentrations was detected in the diagnosis. The exclusion criteria in this study were inflammatory diseases (infections, autoimmune disorders…), pregnancy, renal dysfunction, respiratory and liver disease.
2.3. The assessment of CAD severity
All patients were angiographically examined and underwent percutaneous coronary intervention (PCI). The severity of CAD was evaluated according to the number and the degree of coronary artery significant stenosis. Stenosis was defined as moderate (50%–70% stenosis), and severe (70%–99% stenosis). Patients were divided into single- and multi-vessel disease subgroups according to the stenosis vessels number.[7]
2.4. Laboratory tests
Serum samples were collected from study participants, and stored in aliquots at –80 °C pending use. Triglycerides (TG), total cholesterol (TC), high density lipoprotein-cholesterol (HDL-C), hsCRP, glucose, urea, creatinine, creatine phosphokinase (CPK) and creatine kinase (CK)-MB isoforms were measured using an analyser (Cobas Integra 400, Roche Diagnostic, Germany) for patients upon admission. Low density lipoprotein-cholesterol (LDL-C) was estimated by the Friedewald equation. Serum IL-6 levels was measured by electrochemiluminescence immunoassay (ECLIA) using a Cobas E601 analyzer (Roche Diagnostics, Germany). Serum amyloid A (SAA) was measured using an analyser (BN ProSpec, Siemens, Germany). Homocysteine was measured by Fluorescence polarization immunoassay (FPIA) using an analyser (Axym, Abbott, Germany).
2.5. Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS, version 23.0). Continuous variables were described as mean ± SD for normally distributed data or medians (minimum-maximum) for non-normally distributed data, as appropriate. Categorical data were summarized as frequencies or percentages. HsCRP was log transformed before analyses because its non-normally distribution. Differences in quantitative parameters between groups were performed using independent-samples t test or Mann–Whitney U test, as appropriate. The univariate and multivariate linear analysis were used to identify independent factors for hsCRP levels. The predictive values of different biomarkers for the presence of CAD and evaluating infarct size, were determined by constructing receiver operating characteristic (ROC) curves and the area under the curve (AUC) was calculated. P < 0.05 were considered as significant.
3. Results
3.1. Characteristics of the study subjects
The baseline characteristics of the 310 patients with CAD are listed in Table 1. The mean age of patients was 60.3 ± 11.0 years, and consisted of 74.8% men. The prevalence of cardiovascular risk factors were: diabetes mellitus (48.4%), hypertension (47.1%), smoking (41.0%) and menopause (90.8%).
Table 1. Baseline characteristics of the study population.
| Characteristics | All patients (n = 310) |
| Age, yrs | 60.3 ± 11.0 |
| Male | 232 (74.8%) |
| BMI, kg/m2 | 27.5 ± 4.3 |
| Smoker | 41.0% |
| Alcoholism | 2.6% |
| Menopause | 90.8% |
| Obesity | 22.6% |
| Hypertension | 47.1% |
| Diabetes | 48.4% |
| Dyslipidemia | 23.5% |
| Personal history of CAD | 33.2% |
| Personal history of ACS | 27.4% |
| Peripheral artery diseases | 1.6% |
| History of bypass surgery | 3.2% |
Data are expressed as mean ± SD or n (%). ACS: acute coronary syndrome; BMI: body mass index; CAD: coronary artery diseases.
3.2. Relationship between hsCRP levels and biochemical parameters
According to the hsCRP cut-off value, patients were divided into high levels group (hsCRP > 8.4 mg/L) and low levels group (hsCRP < 8.4 mg/L). In Table 2, only inflammatory parameters (white blood cells (WBC), IL-6 and SAA) were significantly increased in the high hsCRP levels group respectively (WBC: 10.3 × 103/µL, IL-6: 19.8 (4.3–3640.0) pg/mL and SAA: 18.3 (1.6–1020.0) mg/L). The other biochemical parameters didn't show any significant differences between the two groups.
Table 2. Biochemical characteristics of patients.
| Biochemical characteristics | All | Patients |
P | |
| Low < 8.4 mg/L | High ≥ 8.4 mg/L | |||
| Glucose, mmol/L | 7.9 (3.1–42.3) | 7.5 (3.6–42.3) | 7.9 (3.1–41.6) | 0.633 |
| HbA1c, % | 7.9 ± 2.4 | 7.9 ± 2.3 | 8.0 ± 2.6 | 0.754 |
| Urae, mmol/L | 5.7 (2.5–30.5) | 5.4 (2.5–14.0) | 6.3 (2.9–30.5) | 0.003* |
| Creatinin, µmol/L | 93.7 ± 28.9 | 90.9 ± 25.2 | 96.4 ± 30.0 | 0.134 |
| Uric Acid, µmol/L | 309.0 (82.0–817.0) | 305.0 (199.0–633.0) | 318.0 (82.0–817.0) | 0.911 |
| TC, mmol/L | 4.4 ± 1.4 | 4.4 ± 1.4 | 4.3 ± 1.4 | 0.424 |
| TG, mmol/L | 1.4 (0.5–12.1) | 1.4 (0.5–6.1) | 1.4 (0.5–12.1) | 0.642 |
| HDL-C, mmol/L | 0.9 (0.3–2.9) | 0.9 (0.3–2.9) | 0.9 (0.3–2.0) | 0.305 |
| LDL-C, mmol/L | 2.6 (0.0–6.7) | 2.5 (0.0–6.7) | 2.6 (0.1–5.0) | 0.876 |
| Lp(a), mg/dL | 11.0 (0.2–225.8) | 10.7 (0.2–225.8) | 11.8 (1.5–101.0) | 0.172 |
| ApoA1, mg/dL | 99.1 (0.0–189.0) | 100.5 (0.0–189.0) | 97.3 (22.5–187.0) | 0.056 |
| ApoB, mg/dL | 75.2 ± 28.7 | 69.8 (1.0–166.0) | 69.1 (11.1–152.0) | 0.624 |
| ApoB/ApoA1 | 0.8 ± 0.5 | 0.7 (0.1–5.1) | 0.7 (0.2–3.1) | 0.287 |
| Hcy, µmol/L | 18.8 (3.7–50.0) | 19.2 (7.7–50.0) | 17.5 (3.7–50.0) | 0.566 |
| HGB, g/dL | 13.7 ± 8.8 | 14.3 ± 11.0 | 12.7 ± 1.9 | 0.204 |
| WBC, 103/µL | 9.4 ± 3.2 | 8.7 ± 2.7 | 10.3 ± 3.6 | < 0.001 |
| SAA, mg/L | 6.7 (0.71–1020.0) | 4.6 (0.7–338.0) | 18.3 (1.6–1020.0) | < 0.001 |
| IL-6, pg/mL | 9.0 (1.5–3640.0) | 6.0 (1.5–1957.0) | 19.8 (4.3–3640.0) | < 0.001 |
| HsCRP, mg/L | 5.9 (0.2–1020.0) | 3.1 (0.2–8.4) | 20.0 (8.5–1020.0) | < 0.001 |
Data are expressed as the mean ± SD or median (minmum value – maximum value). ApoA-1: apolipoprotein A-1; ApoB: apolipoprotein B; HbA1c: glycated hemoglobin A1c; Hcy: homocysteine; HDL-C: high density lipoprotein cholesterol; HGB: hemoglobin; Hs-CRP: high sensitivity C-reactive protein; IL-6: interleukin 6; LDL-C: low density lipoprotein cholesterol; Lp(a): lipoprotein(a); TC: total cholesterol; TG: triglyceride; SAA: serum amyloid protein; WBC: white blood cells.
3.3. Relationship between hsCRP and severity of CAD
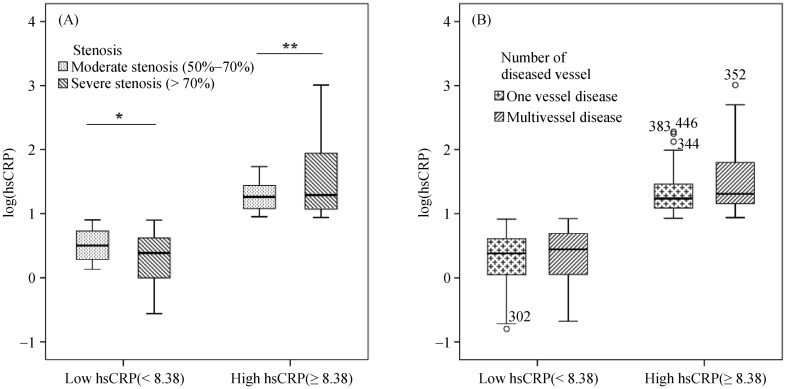
Clinical and angiographic characteristics of patients are presented in Table 3. 71.7% of patients had severe stenosis (P = 0.157). 56.7% of patients had multi vessel disease (P = 0.793). Both groups of high- and low-hsCRP levels were not associated with stenosis degree and involved vessel number. Heart rate levels in the high hsCRP levels patient group were significantly higher than levels shown in the other group (P = 0.045). To further evaluate the relation of hsCRP with the extent and severity of CAD, we measured its serum levels according to angiographic characteristics. HsCRP levels tended to be significantly associated with coronary total occlusion (P = 0.078) (Table 4). Comparing the logarithm (log) (hsCRP) values in the two patient groups between both stenosis degree and the number of vessel disease (Figure 1) showed no significant difference in each group, hsCRP levels were not associated with the severity of CAD.
Table 3. Clinical and angiographic characteristics of patients.
| Clinical and angiographic characteristics | Patients |
P | ||
| All | Low < 8.4 mg/L | High ≥ 8.4 mg/L | ||
| SBP, mmHg | 130 ± 22 | 132 ± 22 | 127 ± 21 | 0.065 |
| DBP, mmHg | 75 ± 13 | 75 ± 12 | 75 ± 15 | 0.947 |
| Heart rate, beats/min | 75.4 ± 16.2 | 73.4 ± 15.5 | 78.1 ± 17.3 | 0.045 |
| LVEF, % | 49.8 ± 14.7 | 53.5 ± 12.8 | 46.0 ± 15.7 | 0.056 |
| Vessel involved, % | ||||
| LMCA | 7.5 | 66.7 | 33.3 | 0.710 |
| LCx | 49.8 | 62.0 | 38.0 | 0.956 |
| LAD | 72.1 | 59.3 | 40.7 | 0.176 |
| RCA | 48.8 | 63.3 | 36.7 | 0.759 |
| Stenosis, % | ||||
| 50–70 | 28.3 | 69.2 | 30.8 | 0.157 |
| 70–99 | 71.7 | 53.0 | 47.0 | |
| Total occlusion, % | ||||
| No | 69.7 | 65.9 | 34.1 | 0.257 |
| Yes | 30.3 | 57.1 | 42.9 | |
| Vessel disease number, % | ||||
| One vessel | 43.3 | 63.2 | 36.8 | 0.793 |
| Multi vessel | 56.7 | 61.4 | 38.6 | |
Data are expressed as the mean ± SD or n (%). DBP: diastolic blood pressure; LAD: left anterior descending; LCx: left circumflex; LDL-C: low density lipoprotein cholesterol; LMCA: Left main coronary artery; LVEF: left ventricular ejection fraction; RCA: right coronary artery; SBP: systolic blood pressure.
Table 4. HsCRP levels distribution according to angiographic profile.
| Characteristics | HsCRP, mg/L |
P | |
| Median | Vmin–Vmax | ||
| Stenosis, % | |||
| 50%–70% | 5.1 | 0.2–54.3 | 0.338 |
| 70%–99% | 7.5 | 0.3–1020.0 | |
| Total occlusion | |||
| No | 4.4 | 0.2–1020.0 | 0.078 |
| Yes | 6.4 | 0.3–500.0 | |
| Number of stenotic coronaryarteries | |||
| Single vessel diseased | 4.5 | 0.16–190.0 | 0.385 |
| Multi-vessel diseased | 5.9 | 0.21–1020.0 | |
HsCRP: high C reactive protein. Values are expressed as mean ± SD.
Figure 1. HsCRP and severity of CAD in low and high patient group.
(A): HsCRP levels in high and low groups according to stenosis degree; and (B): HsCRP levels in high and low groups according to the number of vessel disease. *P > 0.05; **P > 0.05. CAD: coronary artery disease; HsCRP: high C reactive protein.
3.4. Independent predictors of hsCRP levels
Univariate linear regression analysis was performed to evaluate the contribution of potential confounders on altered hsCRP levels in CAD. These include age, gender, body mass index (BMI), diabetes, hypertension, dyslipidemia, smoking, history of CAD and the admission clinical and biochemical diagnosis. Results from Table 5 showed that heart rate and CPK were independent predictor factors of hsCRP levels. Multivariate analysis demonstrated that only cTnI and SAA were independent predictor factors of hsCRP levels (Table 5).
Table 5. Univariate and multivariate analysis for hsCRP.
| Factors | HsCRP |
|||
| Univariate |
Multivariate |
|||
| β (95% CI) | P | β (95% CI) | P | |
| Heart rate | 0.140 (0.008–1.485) | 0.048 | 0.071 (–0.227–1.289) | 0.168 |
| SAA | 0.035 (0.500–0.639) | < 0.001 | 0.792 (0.627–0.839) | < 0.001 |
| cTnI | 0.393 (0.307–0.607) | < 0.001 | 0.176 (0.055–1.485) | 0.012 |
| CPK | 0.323 (0.021–0.046) | < 0.001 | –0.105 (–0.030–0.004) | 0.124 |
Risk factors: Age, men, BMI, smoking, diabetes mellitus, dyslipidemia, hypertension, menopause, history of CAD, ejection fraction, heart rate, SBP, DBP, creatinine, HGB, glucose, Lp(a), TG, LDL-C, HDL-C, ApoA1, ApoB, homocysteine, IL-6, SAA, cTnI, CPK, CKMB. BMI: body mass index; CAD: coronary artery disease; CK-MB: creatine kinase MB; CPK: creatine phosphokinase; cTnI: cardiac troponin I; DBP: diastolic blood pressure; HDL-C: high density lipoprotein cholesterol; HGB: hemoglobin; HsCRP: high C reactive protein; LDL-C: low density lipoprotein cholesterol; Lp(a): lipoprotein (a); SAA: serum amyloid protein; SBP: systolic blood pressure; TG: triglyceride.
3.5. Levels of hsCRP in clinical groups
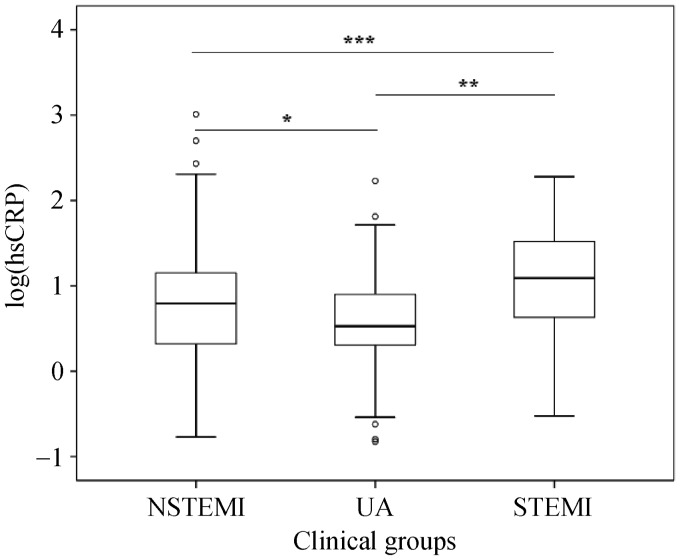
HsCRP levels measured in clinical groups UA (n = 107), NSTEMI (n = 111) and STEMI (n = 92) were respectively 8.2 ± 19.2, 28.6 ± 114.5 and 30.7 ± 45.4 mg/L; medians were 3.4, 6.2 and 12.3 mg/L, respectively. The hsCRP level was significantly different between the three patient groups, and the highest levels were shown in STEMI patients (Figure 2).
Figure 2. HsCRP levels in clinical subgroups.
UA (n = 107), NSTEMI (n = 111) and STEMI (n = 92). HsCRP: high C reactive protein; STEMI: ST-segment elevation myocardial infarction; NSTEMI: non ST-segment elevation myocardial infarction; UA: unstable angina. *P = 0.044, **P < 0.001, ***P < 0.001.
3.6. Prediction of infarct size in patients with MI by ROC curve analysis
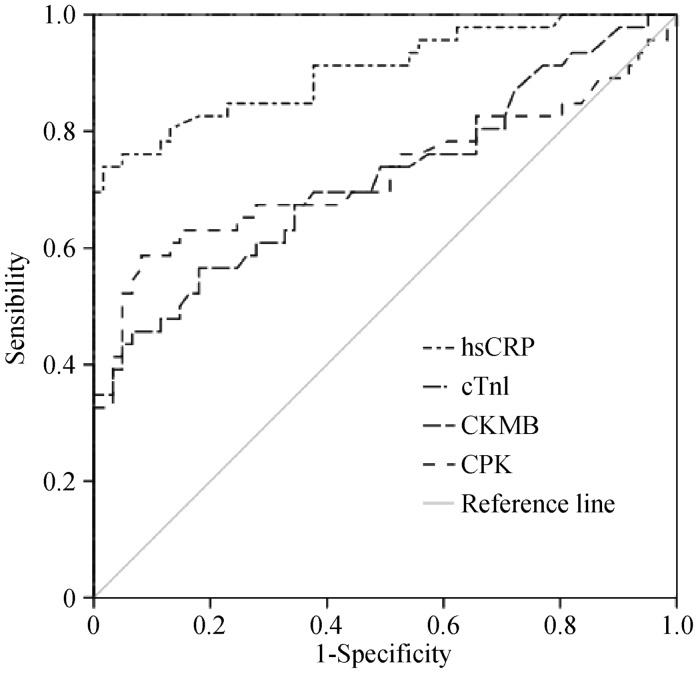
Despite the absence of correlation between hsCRP and cTnI and CPK and CK-MB in patients with MI (results are not shown), the analysis by ROC curve for hsCRP, cTnI and enzymatic markers (Figure 3) showed an AUC for hsCRP 0.905 (95% CI: 0.844–0.966; P < 0.001); CPK 0.727 (95% CI: 0.619–0.834; P < 0.001); CKMB 0.720 (95% CI: 0.618–0.821; P < 0.001); cTnI 1.00 (95% CI: 0.619–0.834; P < 0.001). These results suggest that hsCRP and cTnI were better than CKMB and CPK in predicting infarct size.
Figure 3. HsCRP in predicting infarct size in MI patients.
ROC curve analysis, area under the curve for hsCRP: 0.905 (95% CI: 0.844–0.966; P < 0.001); CPK: 0.727 (95% CI: 0.619–0.834; P < 0.001); CKMB: 0.720 (95% CI: 0.618–0.821; P < 0.001); cTnI: 1.00 (95% CI: 0.619–0.834; P < 0.001). CK-MB: creatine kinase MB; CPK: creatine phosphokinase; HsCRP: high C reactive protein; MI: myocardial infarction.
4. Discussion
In this study, hsCRP levels were increased in participants and tended to be associated with coronary occlusion. Stratifying patients into low and high hsCRP levels group according to the cut-off value, showed an association between hsCRP and other inflammatory markers as IL-6 and SAA. Specifically, multiple linear analysis showed that the increased level of hsCRP was associated with cTnI. We demonstrated that the severity of CAD, assessed by the stenosis degree and the number of vessel disease was not associated with hsCRP levels. The analysis by ROC curve for cTnI, hsCRP, andt enzymatic markers as CPK and CK-MB showed that cTnI and hsCRP were the best markers of MI which could predict the infarct size.
Inflammation is recognized as an essential factor contributing in all steps of atherosclerosis. Inflammatory markers are involved in ACS evolution by stimulating atheroma formation, destabilization of damaged atherosclerotic plaques, formation of occlusive thrombi and eventually thrombotic complications.[8],[9] It has been shown that CRP is useful in MI and UA prognosis and a strong independent marker in the prediction of cardiovascular disease and mortality risk.[10]–[12] Elevated hsCRP concentrations were associated with endothelial dysfunction, differentiation of macrophages, smooth muscle cell proliferation and plaque vulnerability.[13] CRP promotes angiotensin proatherogenic activity, alterates structure and function of arteries, stimulates heart and vascular repair, vascular thickness, rise of peripheral vascular resistance. CRP interferes with mechanisms of arterial blood pressure regulation leading to vascular endothelium dysfunction.[14],[15] The data reported in our study, didn't show significant association between hsCRP levels and severity and extent of CAD, even when patients were categorized in low and high hsCRP levels group. According to prior investigations, the relationship between hsCRP concentrations and coronary artery stenosis grade is controversial. Previous studies showed that hsCRP didn't correlate with stenosis degree.[16] Other studies showed that in CAD, hsCRP was associated with severity of coronary stenosis.[17],[18] These controversial findings could be attributed to the difference in risk profile between patients. In fact, patients with one vessel disease could have a multiple significant and- or long plaques and stenosis, high inflammatory markers levels and high prevalence and number of cardiovascular risk factors. The multifactorial feature of CAD and the differences between clinical outcomes symptoms could explain the difference in the relationship between hsCRP and extent and severity in CAD patients.[19] Many conventional cardiovascular risk factors, such as smoking, diabetes and hypertension, were associated with elevated hsCRP levels.[20] It has been suggested that inflammation, genetic factors, age, hypercholesterolemia and diabetes caused discordance between cardiovascular risk factor burden and the extent of CAD.[19] Also, it has been found that hypolipidemic drugs decrease hsCRP levels, which supports its involvement in inflammation mechanism in cardiovascular disease.[21] Smoking as example can cause cholesterol proteins alteration stimulate lipid peroxidation, and platelet aggregation, which cause endothelial dysfunction.[22] Our findings indicated that heart rate was significantly associated with increased levels of hsCRP. It has been proposed that increased heart rate may reflect a disorder in the autonomic nervous system.[23] Following a vagal nerve stimulation, tumor necrosis factor and other cytokines were lowly expressed from macrophages inducing inflammation inhibition. Increased heart rate generates elevated mechanical stress frequency on the vascular endothelium.[24]
Despite no significant association between hsCRP and angiographic risk profile was shown in our study, the increased levels of this cytokine support the concept that hsCRP is involved in different CAD outcomes pathophysiology. Furthermore, highest levels of hsCRP were significantly shown in STEMI patients compared to NSTEMI and UA patients. These results suggested a relationship between hsCRP levels and necrosis size after a MI. On the basis that necrosis biomarkers are used and available to estimate infarct size.[25]–[27] the analysis by ROC curve for cTnI, CK and CK-MB, and even more for hsCRP showed that hsCRP was a powerful marker but not better than cTnI in predicting infarct size. Cardiac troponins are the ideal biomarkers in ACS diagnosis and risk evaluation. Inflammatory biomarkers, such hsCRP may provide incremental prognostic report and assessing the extent and the severity of myocardial necrosis.[28]–[30] In fact, inflammatory responses during myocardial repair process may differ among patients profile, biomarkers short half-life, the time of patient admission and blood collection recruitment time. These factors could explain the no correlation between hsCRP levels and cardiovascular myonecrosis markers. Previous studies reported that higher hsCRP concentrations were associated with large myocardial necrosis acute phase of inflammatory response depends on ventricular function and repair, ischemic and reperfusion necrosis.[31]
In patients with STEMI, elevated levels of hsCRP were associated with myocardial damage size and major adverse cardiac events.[14] According to prior investigations and independently from other prognosis markers, early and highly expressed hsCRP in post infarction is associated with high risk of cardiac and early mechanical complication. This increase was not useful in the prognosis of reinfarction.[13],[31] Many studies suggested that hsCRP could be a predictor marker of postprocedural complications,[13] mortality risk stratification, all-cause death[19],[24] and total stroke.[32],[33]
The patients enrolled in this study come from a single center and the number of participants was limited which make this study unable to present the Tunisian population. Despite the common pathophysiology process, CAD outcomes differ in clinical symptoms which distort results. We had a single measurement of hsCRP in this study, a kinetic study of hsCRP could provide more information about its role in CAD even more in STEMI patients.
HsCRP levels were not associated with the severity of CAD assessed by the stenosis degree and the number of diseased coronary vessels. Highest levels of hsCRP in patients with STEMI strongly suggest its role in myocardial necrosis evaluation.
Acknowledgments
Our study was funded by research organizations in Tunisia (Ministry of Public Health and Ministry of Higher Education and Scientific Research). The authors are grateful to the patients and volunteers for their collaboration, the entire team for collecting blood samples from patients (Cardiology Department, Fattouma Bourguiba University Hospital, Monastir, Tunisia); and the staff members of the Biochemistry Laboratory (Farhat Hached University Hospital, Sousse, Tunisia) for their technical support.
References
- 1.Ma CY, Xu ZY, Wang SP, et al. Change of inflammatory factors in patients with acute coronary syndrome. Chin Med J. 2018;131:1444–1449. doi: 10.4103/0366-6999.233953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouzidi N, Betbout F, Maatouk F. Relationship of activin A levels with clinical presentation, extent, and severity of coronary artery disease. Anatol J Cardiol. 2017;18:402–409. doi: 10.14744/AnatolJCardiol.2017.7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razi MM, Abdali N, Asif SM, et al. Association of inflammatory cytokines/biomarkers with acute coronary syndrome and its correlation with severity and hospital outcome. J Clin Prev Cardiol. 2017;6:44–49. [Google Scholar]
- 4.Kaur R, Matharoo K, Sharma R, et al. C-reactive protein + 1059 GNC polymorphism in type 2 diabetes and coronary artery disease patients. Meta Gene. 2013;1:82–92. doi: 10.1016/j.mgene.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefanutti C, Mazza F, Steiner M, et al. Relationship between sustained reductions in plasma lipid and lipoprotein concentrations with apheresis and plasma levels and mRNA expression of PTX3 and plasma levels of hsCRP in Patients with HyperLp(a)lipoproteinemia. Mediators Inflamm. 2016;2016:4739512. doi: 10.1155/2016/4739512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L, Zou Y, Wang Y, et al. Prognostic significance of plasma high-sensitivity C-reactive protein in patients with hypertrophic cardiomyopathy. J Am Heart Assoc. 2017;6:e004529. doi: 10.1161/JAHA.116.004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogensen UM, Jensen T, Køber L, et al. Cardiovascular autonomic neuropathy and subclinical cardiovascular disease in normoalbuminuric type 1 diabetic patients. Diabetes. 2012;61:1822–1830. doi: 10.2337/db11-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sara JDS, Prasad M, Zhang M, et al. High-sensitivity C-reactive protein is an independent marker of abnormal coronary vasoreactivity in patients with non-obstructive coronary artery disease. Am Heart J. 2017;190:1–11. doi: 10.1016/j.ahj.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Vuković-Dejanović V, Bogavac-Stanojević N, Spasić S, et al. Association of serum Pentraxin-3 and high sensitivity C-reactive protein with the extent of coronary stenosis in patients undergoing coronary angiography. J Med Biochem. 2015;34:440–449. doi: 10.2478/jomb-2014-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toss H, Lindahl B, Siegbahn A, et al. Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease. FRISC Study Group. Fragmin during instability in coronary artery disease. Circulation. 1997;96:4204–4210. doi: 10.1161/01.cir.96.12.4204. [DOI] [PubMed] [Google Scholar]
- 11.James SK, Armstrong P, Barnathan E, et al. Troponin and C-reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndrome: a GUSTO-IV substudy. J Am Coll Cardiol. 2003;41:916–924. doi: 10.1016/s0735-1097(02)02969-8. [DOI] [PubMed] [Google Scholar]
- 12.Vaucher J, Marques-Vidal P, Waeber G, et al. Cytokines and hs-CRP levels in individuals treated with low-dose aspirin for cardiovascular prevention: a population-based study (CoLaus Study) Cytokine. 2014;66:95–100. doi: 10.1016/j.cyto.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez MC, Rexrode KM, Glynn RJ, et al. Association between high sensitivity C-reactive protein and total stroke by hypertensive status among men. J Am Heart Assoc. 2015;4:e002073. doi: 10.1161/JAHA.115.002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adukauskienė D, Čiginskienė A, Adukauskaitė A, et al. Clinical relevance of high sensitivity C-reactive protein in cardiology. Medecina. 2016;52:1–10. doi: 10.1016/j.medici.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Koenig W. High sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int J Cardiol. 2013;168:5126–5134. doi: 10.1016/j.ijcard.2013.07.113. [DOI] [PubMed] [Google Scholar]
- 16.Huziuk IM, Lelonek M. Severe multivessel coronary artery disease and high-sensitive troponin T. Kardiochir Torakochirurgia Pol. 2015;12:139–144. doi: 10.5114/kitp.2015.52855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoharan G, Ntalianis A, Muller O, et al. Severity of coronary arterial stenoses responsible for acute coronary syndromes. Am J Cardiol. 2009;103:1183–1188. doi: 10.1016/j.amjcard.2008.12.047. [DOI] [PubMed] [Google Scholar]
- 18.Seyedian SM, Ahmadi F, Dabagh R, et al. Relationship between high-sensitivity C-reactive protein serum levels and the severity of coronary artery stenosis in patients with coronary artery disease. ARYA Atheroscler. 2016;12:231–237. [PMC free article] [PubMed] [Google Scholar]
- 19.Marini A, Naka KK, Vakalis K, et al. Extent of coronary artery disease in patients undergoing angiography for stable or acute coronary syndromes. Hellenic J Cardiol. 2017;58:115–121. doi: 10.1016/j.hjc.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Zhong X, Cheng G, et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis. 2017;259:75–82. doi: 10.1016/j.atherosclerosis.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Nadrowski P, Chudek J, Skrzypek M, et al. Associations between cardiovascular disease risk factors and IL-6 and hsCRP levels in the elderly. Exp Gerontol. 2016;1:112–117. doi: 10.1016/j.exger.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Juonala M, Viikari JSA, et al. Exposure to parental smoking in childhood is associated with high C-reactive protein in adulthood: The cardiovascular risk in young finns study. J Atheroscler Thromb. 2017;24:31–41. doi: 10.5551/jat.40568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park WC, Seo I, Kim SH. Association between resting heart rate and inflammatory markers (white blood cell count and high-sensitivity C-reactive protein) in healthy korean people. Korean J Fam Med. 2017;38:8–13. doi: 10.4082/kjfm.2017.38.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whelton SP, Narla V, Blaha MJ, et al. Association between resting heart rate and inflammatory biomarkers (high sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the multi-ethnic study of atherosclerosis) Am J Cardiol. 2014;113:644–649. doi: 10.1016/j.amjcard.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ternant D, Ivanes F, Prunier F, et al. Revisiting myocardial necrosis biomarkers: assessment of the effect of conditioning therapies on infarct size by kinetic modeling. Sci Rep. 2017;7:10709. doi: 10.1038/s41598-017-11352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi T, Hirohata S, Uesugi T, et al. Relationship between activin A level and infarct size in patients with acute myocardial infarction undergoing successful primary coronary intervention. Clin Chim Acta. 2009;401:3–7. doi: 10.1016/j.cca.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Nordlander R, Nyquist O, Sylven C. Estimation of infarct size by creatine kinase. A comparison between maximal value, planimetry and computer calculation. Cardiology. 1981;68:201–205. doi: 10.1159/000173283. [DOI] [PubMed] [Google Scholar]
- 28.Nilsen DW, Mjelva ØR, Leon de la Fuente RA, et al. Borderline values of troponin-T and high sensitivity C-reactive protein did not predict 2-year mortality in TnT positive chest-pain patients, whereas brain natriuretic peptide did. Front Cardiovasc Med. 2015;2:16. doi: 10.3389/fcvm.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radović VV. Predictive value of inflammation and myocardial necrosis markers in acute coronary syndrome. Med Pregl. 2010;63:662–667. doi: 10.2298/mpns1010662r. [DOI] [PubMed] [Google Scholar]
- 30.Rashidinejad H, Hosseini SM, Moazenzadeh M, et al. Relationship between serum level of high-sensitive C-reactive protein and extension of myocardial involvement in patients with acute myocardial infarction. Rom J Intern Med. 2012;50:211–215. [PubMed] [Google Scholar]
- 31.He L, Tang X, Ling W, et al. Early C-reactive protein in the prediction of long-term outcomes after acute coronary syndromes: a meta-analysis of longitudinal studies. Heart. 2010;96:339–346. doi: 10.1136/hrt.2009.174912. [DOI] [PubMed] [Google Scholar]
- 32.Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC Med. 2010;8:34. doi: 10.1186/1741-7015-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiménez MC, Rexrode KM, Glynn RJ, et al. Association between High-sensitivity C-Reactive Protein and total stroke by hypertensive status among men. J Am Heart Assoc. 2015;4:e002073. doi: 10.1161/JAHA.115.002073. [DOI] [PMC free article] [PubMed] [Google Scholar]