Abstract
Background
Endothelial dysfunction is the initial stage in atherosclerotic formation and progression and is associated with high serum uric acid (SUA) level. We hypothesized that reactive hyperemia index (RHI), which reflects endothelial function, is associated with SUA levels in elderly individuals with untreated mild hypertension.
Methods
We recruited 123 patients ≥ 60 years with untreated mild hypertension. The association between SUA level and RHI was analyzed using univariate correlation analysis and multiple regression analysis. The receiver operating characteristic (ROC) curve was performed to validate the cutoff value of SUA that can be used to predict endothelial dysfunction.
Results
The serum uric acid level significantly increased in the RHI < 1.67 group, and this result was still observed in the subgroup of men. RHI was inversely associated with SUA level (P = 0.006) and the association was still observed after adjusting for factors, such as age, sex, smoking status, and creatinine level (P = 0.014). In the subgroup analysis, a positive association was observed only in men. In the ROC curve analysis, the optimal cutoff values of SUA for predicting endothelial dysfunction was 293.5 µmol/L in elderly mild hypertension patients and 287.0 µmol/L in men.
Conclusion
A high SUA level was considered an independent predictor of endothelial dysfunction among elderly individuals, particularly men with untreated mild hypertension.
Keywords: Endothelial dysfunction, Hypertension, Reactive hyperemia index, Uric acid
1. Introduction
Hypertension is a common chronic disease in the elderly and the prevalence of hypertension of this condition increases gradually with aging.[1] In elderly individuals with hypertension, high blood pressure is an important cause of cardiovascular diseases and mortality.[2] The initial stage in atherosclerotic formation and progression is vascular endothelial dysfunction, which plays a key role in systemic organ damage and subsequently leads to the deterioration of prognosis.[3] Endothelial dysfunction is induced by both traditional and non-traditional risk factors.[4] Among these factors, serum uric acid (SUA) is considered significant.[5] Notably, hypertension is frequently accompanied by elevated SUA level.[6] UA is the final product of purine metabolism in humans, and a high SUA level is considered an independent risk factor of cardiovascular disease and metabolic syndrome, particularly in individuals with hypertension and diabetes.[7],[8]
Previous studies have revealed that elevated of SUA levels are associated with endothelial dysfunction.[9],[10] However, no study has evaluated the relationship between SUA and endothelial dysfunction assessed using reactive hyperemia index (RHI) among elderly patients with untreated mild hypertension. The current study aimed to investigate the cross-sectional relationship between SUA level and endothelial dysfunction using RHI in individuals aged ≥ 60 years with untreated mild hypertension. Moreover, a gender specific analysis was performed due to the remarkable differences in UA levels between men and women.
2. Methods
2.1. Subjects
We performed a retrospective analysis of 123 elderly patients from Cardiology Department of Xuanwu Hospital (China), who underwent EndoPAT testing. All patients were diagnosed with hypertension for the first time, or they stopped taking antihypertensive drugs for at least three months and were classified based on blood pressure (mild hypertension). To diminish some confounders that may affect endothelial function, we excluded patients receiving lipid-lowering drugs, those with poor glycemic control (fasting blood glucose ≥ 11.1 mmol/L); those with comorbidities (such as nephropathy and peripheral neuropathy), those with unstable angina, myocardial infarction within 6 months, heart failure, severe arrhythmia, stroke within 3 months, impaired liver function (alanine aminotransferase and/or aspartate aminotransferase exceeding the upper limit of normal value by one time or above) or impaired renal function (creatinine level exceeding the upper limit of normal value).
2.2. Blood pressure measurements
Clinic blood pressure was assessed three times using a mercury sphygmomanometer in while the patient was sitting after resting for at least 5 min. The mean of the three measured values was considered for analysis. Mild hypertension, the same as hypertension class I, was defined as systolic blood pressure (SBP) of 140–159 mmHg and/or diastolic blood pressure (DBP) of 90–99 mmHg.[11]
2.3. Clinical and biochemical data
Physical examination and laboratory tests were obtained from the medical records of all participants during their first visit at the outpatient cardiology department. During the physical examination, data about age, complications, use of medications, highest blood pressure, waist circumference, hip circumference, height and weight. Moreover, the following equations were used: body mass index (BMI) = weight (kg)/height (m)2; and waist-to-hip ratio (WHR) = waist circumference (cm)/hip circumference (cm). The levels of allanine aminotransferase, aspartate transaminase, fasting blood glucose, triglyceride, cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein and uric acid were measured at the clinic.
2.4. Endothelial dysfunction measurements
Endothelial function was assessed via peripheral arterial tonometry, which is a non-invasive method, using the EndoPAT 2000 device (Itamar Medical Ltd., Caesarea, Israel). Two flexible probes were placed on the index fingers of both hands along with inflatable cuffs and plethysmographic biosensors. The baseline data of the two sides were collected after resting for 30 min.[12] Then, cuff occlusion in the left forearm lasted for 5 min, which resulted in transitory ischemia. All data of both fingers were recorded in the process of occlusion and post-occlusion. In the course, the parameter of RHI was calculated via an automated analysis. Thus, an RHI ≥ 1.67 indicated a normal vascular endothelial function, whereas an RHI < 1.67 represented an abnormal vascular endothelial function.[13]
2.5. Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences software version 23. Continuous variables are expressed as mean ± SD and were compared using student's t-tests. Categorical variables are expressed as percentages and compared using χ2 test. SUA level and traditional risk factors were selected for univariate correlation analysis and entered into the multiple regression model. Finally, the ability of SUA level in predicting dysfunction among elderly patients was assessed the area under the receiving operative characteristic (ROC) curve. A P-value < 0.05 was considered statistically significant and all confidence intervals were within the 95% range.
3. Results
3.1. Clinical, and biochemical characteristics of the study population
Table 1 presents the clinical and biochemical characteristics of the study population. A total of 123 participants (62 men and 61 women) were included and the mean age was 69.27 ± 5.76 years (range 60–78 years). Among them, 54 (43.90%) had endothelial dysfunction, which was defined as RHI < 1.67. The SUA level for the RHI ≥ 1.67 group was significantly higher than that of the RHI < 1.67 group (298.04 ± 82.39 vs. 332.22 ± 79.60 µmol/L, respectively, P < 0.01). This difference was still observed in the subgroup of men, but not in women. Moreover, patients with an RHI ≥ 1.67 had a higher WHR (P < 0.01), and this result was also observed among women (P < 0.05). No significant differences were observed in terms of other clinical and biochemical data (P > 0.05).
Table 1. The clinical, and biochemical characteristics of the study population.
| Valuables | RHI ≥ 1.67 |
RHI < 1.67 |
Pa | Pb | Pc | ||||
| Total | Men | Women | Total | Men | Women | ||||
| N | 69 | 36 | 33 | 54 | 26 | 28 | 0.72 | - | - |
| Age, yrs | 69.32 ± 5.82 | 69.08 ± 5.84 | 69.58 ± 5.87 | 69.22 ± 5.76 | 69.15 ± 5.45 | 69.29 ± 6.13 | 0.93 | 0.96 | 0.85 |
| Smoker | 19 (23.19%) | 16 (44.44%) | 3 (9.09%) | 14 (25.93%) | 11 (42.31%) | 3 (10.71%) | 1.00 | 1.00 | 1.00 |
| BMI, kg/m2 | 25.17 ± 3.84 | 25.10 ± 4.37 | 25.27 ± 3.23 | 25.27 ± 3.23 | 25.69 ± 3.76 | 24.90 ± 2.65 | 0.88 | 0.58 | 0.63 |
| WHR | 0.89 ± 0.07 | 0.92 ± 0.06 | 0.87 ± 0.06 | 0.86 ± 0.06 | 0.89 ± 0.05 | 0.84 ± 0.06 | < 0.01 | 0.11 | < 0.05 |
| ALT, U/L | 23.24 ± 12.66 | 24.99 ± 14.69 | 21.33 ± 9.88 | 23.61 ± 12.31 | 27.15 ± 15.06 | 20.32 ± 13.96 | 0.87 | 0.57 | 0.67 |
| AST, U/L | 23..82 ± 5.30 | 24.27 ± 5.50 | 23.33 ± 5.10 | 24.70 ± 7.36 | 26.04 ± 9.19 | 23.46 ± 4.97 | 0.44 | 0.35 | 0.92 |
| Creatinine, mmol/L | 61.86 ± 14.45 | 68.78 ± 15.13 | 54.30 ± 8.97 | 63.85 ± 13.90 | 72.62 ± 11.48 | 55.71 ± 10.72 | 0.44 | 0.28 | 0.58 |
| FBP, mmol/L | 5.40 ± 0.73 | 5.38 ± 0.83 | 5.41 ± 0.75 | 5.53 ± 0.79 | 5.43 ± 0.83 | 5.62 ± 0.75 | 0.32 | 0.78 | 0.28 |
| TG, mmol/L | 2.06 ± 1.44 | 2.47 ± 1.78 | 1.61 ± 0.72 | 2.07 ± 1.43 | 2.44 ± 1.75 | 1.72 ± 0.96 | 0.97 | < 0.01 | 0.95 |
| TCH, mmol/L | 5.20 ± 0.77 | 5.13 ± 0.77 | 5.27 ± 0.78 | 5.24 ± 1.13 | 4.96 ± 1.17 | 5.50 ± 1.04 | 0.83 | 0.96 | 0.63 |
| LDL-C, mmol/L | 3.26 ± 0.72 | 3.14 ± 0.76 | 3.39 ± 0.66 | 3.27 ± 0.89 | 3.16 ± 0.83 | 3.36 ± 0.96 | 0.96 | 0.47 | 0.33 |
| HDL-C, mmol/L | 1.35 ± 0.32 | 1.30 ± 0.28 | 1.41 ± 0.35 | 1.44 ± 0.63 | 1.22 ± 0.29 | 1.66 ± 0.78 | 0.29 | 0.30 | 0.11 |
| UA, µmol/L | 298.04 ± 82.39 | 302.38 ± 94.30 | 293.30 ± 68.21 | 332.22 ± 79.60 | 375.31 ± 743.17 | 292.21 ± 63.52 | < 0.05 | < 0.01 | 0.95 |
| SBP, mmHg | 137.75 ± 11.99 | 136.00 ± 9.80 | 139.67 ± 13.91 | 138.74 ± 11.92 | 138.07 ± 10.46 | 139.36 ± 13.29 | 0.65 | 0.43 | 0.93 |
| DBP, mmHg | 85.93 ± 8.56 | 85.44 ± 7.17 | 86.45 ± 9.98 | 87.91 ± 13.11 | 90.65 ± 12.17 | 85.36 ± 13.29 | 0.32 | < 0.05 | 0.71 |
| MAP, mmHg | 103.20 ± 7.99 | 102.30 ± 6.50 | 104.19 ± 9.26 | 104.85 ± 11.07 | 106.46 ± 9.79 | 103.36 ± 12.13 | 0.34 | < 0.05 | 0.76 |
| PP, mmHg | 51.83 ± 12.21 | 50.44 ± 7.17 | 53.21 ± 13.96 | 50.83 ± 13.30 | 47.42 ± 15.92 | 54.00 ± 9.53 | 0.67 | 0.35 | 0.80 |
Data are presented as mean ± SD or n (%). aP-values derived from compare between the RHI ≥ 1.67 group and RHI < 1.67 group; bP-values derived from compare between the RHI ≥ 1.67 group and RHI < 1.67 group among the men; cP-values derived from compare between the RHI ≥ 1.67 group and RHI < 1.67 group among the women. ALT: alanine aminotransferase; AST: aspartate transaminase; BMI: body mass index; DBP: diastolic blood pressure; FBP: fasting blood glucose; HDL-C: high-density lipoprotein; HT: hypertension; LDL-C: low density lipoprotein cholesterol; MAP: mean arterial pressure; PP: pulse pressure; RHI: reactive hyperemia index; SBP: systolic blood pressure; TCH: cholesterol; TG: triglyceride; UA: uric acid; WHR: waist-to-hip ratio.
3.2. Univariate analysis and multiple regression analysis of RHI
Table 2 depicts that SUA level was an independent determinant of RHI. In the univariate analysis, RHI was negatively associated with SUA level. Furthermore, the multiple regression analysis revealed that SUA level (β = −0.288, P = 0.014) and WHR (β = 0.246, P = 0.024) were still considered the independent predictors of RHI after adjusting for other traditional cardiovascular risk factors. This model explained 13.5% of the variance of RHI.
Table 2. Univariate analysis and multiple regression analysis of RHI in patients with untreated mild hypertension in the elderly.
| Variable | Univariate analysis |
Multiple regression analysis (R2 = 0.135) |
||
| β | P | β | P | |
| Gender | 0.050 | 0.586 | 0.051 | 0.653 |
| Age | 0.099 | 0.275 | 0.077 | 0.443 |
| WHR | 0.079 | 0.383 | 0.246 | 0.024 |
| Smoke | 0.015 | 0.870 | 0.090 | 0.392 |
| Creatinine | –0.121 | 0.183 | –0.138 | 0.262 |
| FBP | –0.148 | 0.102 | –0.123 | 0.194 |
| TG | –0.093 | 0.305 | 0.058 | 0.591 |
| HDL-C | 0.057 | 0.531 | –0.001 | 0.991 |
| UA | –0.246 | 0.006 | –0.288 | 0.014 |
| SBP | –0.015 | 0.869 | –0.009 | 0.926 |
| DBP | –0.105 | 0.248 | –0.044 | 0.670 |
DBP: diastolic blood pressure; HDL-C: high-density lipoprotein; FBP: fasting blood glucose; WHR: waist-to-hip ratio; RHI: reactive hyperemia index; SBP: systolic blood pressure; TG: triglyceride; UA: uric acid.
In the subgroup analysis, RHI was still negatively associated with SUA level in men (Table 3), but not in woman (Table 4). The multiple regression analysis revealed that SUA level (β = −0.430, P = 0.008) remained an independent predictor of RHI in men.
Table 3. Univariate analysis and multiple regression analysis of RHI in men.
| Variable | Univariate analysis |
Multiple regression analysis (R2 = 0.307) |
||
| β | P | β | P | |
| Age | 0.113 | 0.383 | –0.013 | 0.924 |
| WHR | 0.063 | 0.625 | 0.149 | 0.315 |
| Smoke | 0.096 | 0.460 | 0.166 | 0.210 |
| Creatinine | –0.081 | 0.532 | –0.014 | 0.930 |
| FBP | –0.154 | 0.232 | –0.149 | 0.266 |
| TG | –0.065 | 0.616 | 0.266 | 0.108 |
| HDL-C | 0.274 | 0.031 | 0.257 | 0.097 |
| UA | –0.386 | 0.002 | –0.430 | 0.008 |
| SBP | –0.184 | 0.153 | –0.175 | 0.167 |
| DBP | –0.190 | 0.140 | –0.044 | 0.739 |
DBP: diastolic blood pressure; FBP: fasting blood glucose; HDL-C: high-density lipoprotein; RHI: reactive hyperemia index; SBP: systolic blood pressure; TG: triglyceride; UA: uric acid; WHR: waist-to-hip ratio.
Table 4. Univariate analysis and multiple regression analysis of RHI in women.
| Variable | Univariate analysis |
Multiple regression analysis (R2 = 0.148) |
||
| β | P | β | P | |
| Age | 0.090 | 0.491 | 0.119 | 0.441 |
| WHR | 0.141 | 0.278 | 0.225 | 0.185 |
| Smoke | –0.026 | 0.844 | –0.024 | 0.866 |
| Creatinine | –0.157 | 0.226 | –0.116 | 0.441 |
| FBP | –0.155 | 0.232 | –0.215 | 0.145 |
| TG | –0.141 | 0.279 | –0.151 | 0.307 |
| HDL-C | –0.032 | 0.809 | –0.064 | 0.686 |
| UA | –0.126 | 0.334 | –0.073 | 0.683 |
| SBP | 0.065 | 0.616 | 0.233 | 0.192 |
| DBP | –0.046 | 0.727 | –0.108 | 0.523 |
DBP: diastolic blood pressure; FBP: fasting blood glucose; HDL-C: high-density lipoprotein; RHI: reactive hyperemia index; SBP: systolic blood pressure; TG: triglyceride; UA: uric acid; WHR: waist-to-hip Ratio.
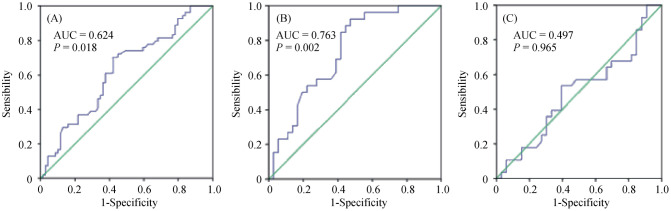
3.3. ROC curves of the serum uric acid for predicting endothelial dysfunction
The ROC curve is depicted in Figure 1. The ROC curve analysis revealed that the optimal cutoff value for SUA in predicting endothelial dysfunction among elderly individuals with mild hypertension was 293.5 µmol/L, with a sensitivity of 70.4% and a specificity of 58.0%, and the area under the curve was 0.624 (95% CI: 0.525–0.723, P = 0.018). In the subgroup analysis, the cutoff value was 287.0 µmol/L in men, with a sensitivity of 92.3% and a specificity of 58.8%, and the area under the curve was 0.763 (95% CI: 0.613–0.858, P = 0.002). However, the ROC curve analysis in women did not provide valuable information.
Figure 1. The receiver operating characteristic curves of the serum uric acid in the elderly (A), men (B) and women (C) for predicting endothelial dysfunction.
AUC: area under curve.
4. Discussion
Endothelial dysfunction is defined as an imbalance between vasodilation and vasoconstriction. This condition is mainly characterized by a decrease in the bioavailability of nitric oxide (NO), which plays a crucial role in maintaining vascular health by regulating vasomotor function.[14] Endothelial dysfunction is associated with traditional cardiovascular risk factors (e.g., diabetes and dyslipidemia)[15] and the use of statins, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and other drugs can affect endothelial function.[16] Based on the above mentioned factors, we eliminated the influence of other confounding factors based on the exclusion criteria.
This study used RHI assessed using EndoPAT 2000 to evaluate endothelial function. In previous clinical studies, the most commonly used technique is flow-mediated vasodilatation of the brachial artery.[17] However, its application depends on the experienced of operator.[18] The EndoPAT 2000 device using peripheral arterial tonometry (PAT) is easy-to-use and considered an operator-independent alternative for a noninvasive measurement of the endothelial function in the peripheral microcirculation.[19] Prior studied have shown that RHI is an independent predictor of cardiovascular event.[20]
This retrospective study first explored whether lower SUA levels were associated with better endothelial function among elderly patients with untreated mild hypertension. Result showed a negative and independent association between SUA level and endothelial dysfunction among elderly patients with untreated mild hypertension after adjusting for cardiovascular factors (P = 0.014). Furthermore, in subsequent subgroup analyses, a similar result was observed in men, but not in women. The multiple regression analysis reveals that SUA level was an independent predictor of RHI. However, the R2 of the model were only 0.135 and 0.307 in all participants and men, respectively. This result indicates that the index was significantly based on other unknown non-traditional clinical variables. In other populations with different clinical complications, the results about the association between SUA level and endothelial dysfunction were similar. Tanaka, et al.[3] have assessed both the cross-sectional and longitudinal association between SUA level and flow-mediated vasodilatation in participants with treated hypertension, and a linear negative association was observed at baseline and follow-up period. Several studies have shown a high SUA level is an independent predictor of endothelial dysfunction in participants with kidney disease.[21],[22] Moreover, Gaubert, et al.[10] demonstrated SUA level remained inversely associated with RHI, even after adjusting for traditional cardiovascular risk factors during the first episode of acute coronary syndrome. Meanwhile, we noted a difference in the relationship between SUA level and RHI in terms of sex, as observed in other studies in which positive results for the analysis of SUA were found only in men or women. The most likely explanation for this phenomenon is that SUA level is regulated by sex hormones and gene expression.[23],[24]
Prior studies have indicated that UA is involved in vascular tone and endothelium function via different mechanisms.[25] First, uric acid can reduce the level of L-arginine required for NO generation by enhancing the activity of arginase, and consequently reducing NO production and bioavailability.[26] Furthermore, uric acid also participates in C-reactive protein or calcium ion-induced instability of endothelial nitric oxide synthase (eNOS) mRNA in endothelial cells, which decreases eNOS expression and NO production.[26] Third, an increase of uric acid promotes oxidative stress and inflammation, which then enhances damage and even apoptosis of vascular endothelial cell.[27]–[29] In addition, several trials have reported that a lower uric acid level can improve endothelial function.[5] For example, allopurinol, a type of xanthine oxidase inhibitors, can improve endothelial dysfunction[30], as a direct consequence of the reduced UA levels, and superoxide anions mediated by xanthine oxidase.[31]
Finally, based on the ROC curve analysis, a SUA level ≥ 293.5 µmol/L and ≥ 287.0 µmol/L had a predictive value for endothelial dysfunction in all participants and in men, respectively. Such result indicates that the SUA level should be controlled more strictly to prevent the worsening of endothelial dysfunction among elderly patients with hypertension, particularly men. Some researchers proposed that the poor outcome associated with UA, which is free of crystal formation, can be observed even in the normal range.[32]
However, a paradoxically positive association was observed between RHI and WHR. Elevated WHR indicated central adiposity, one of important risk factor of cardiovascular diseases. To the best of our knowledge, the perplexing relationships between RHI and other cardiovascular risk factors, such as troponins level and carotid artery intima-media thickness, has been observed in previous trials.[17] Whether this result can be explained by the reverse epidemiology phenomenon or there is another mechanism underlying the relationship between RHI and WHR remains unknown. To obtain a more precise answer, this question must be subsequently assessed.
The current study had some limitations. First, this study had a cross-sectional design, and the causal relationship between SUA level and endothelial dysfunction could not be determined. Second, we only recruited elderly patients with hypertension who did not receive treatment, and several exclusion criteria were set to reduce the effect of other factors. Thus, only a small sample size was included, which might affected the statistical significance of the results. Third, the value of RHI was not measured repeatedly to reduce the random error, even though the RH-PAT method had been proven to have an excellent reproducibility.
In conclusion, a high SUA level can predict a lower RHI value, which is indicative of endothelial dysfunction. This condition is a known risk factor of adverse cardiovascular events in the elderly population with untreated mild hypertension, particularly men. For the abovementioned population, strict control of SUA level is likely to be beneficial for endothelial function. Therefore, when screening elderly patients with hypertension, particularly men, monitoring of the level of SUA, which is an inexpensive and easily obtainable biomarker, may provide information about endothelial dysfunction.
Acknowledgments
All authors had no conflicts of interest to disclose.
References
- 1.Wang Z, Chen Z, Zhang L, et al. Status of Hypertension in China: Results From the China Hypertension Survey, 2012–2015. Circulation. 2018;137:2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 2.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka A, Kawaguchi A, Tomiyama H, et al. Cross-sectional and longitudinal associations between serum uric acid and endothelial function in subjects with treated hypertension. Int J Cardiol. 2018;272:308–313. doi: 10.1016/j.ijcard.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Daiber A, Steven S, Weber A, et al. Targeting vascular (endothelial) dysfunction. Br J Pharmacol. 2017;174:1591–1619. doi: 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgi L, McMullan C, Wohlhueter A, et al. Effect of uric acid-lowering agents on endothelial function: a randomized, double-blind, placebo-controlled trial. Hypertension. 2017;69:243–248. doi: 10.1161/HYPERTENSIONAHA.116.08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghi C, Rosei EA, Bardin T, et al. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens. 2015;33:1729–1741. Discussion, 1741. doi: 10.1097/HJH.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 7.Choi H, Kim HC, Song BM, et al. Serum uric acid concentration and metabolic syndrome among elderly Koreans: The Korean Urban Rural Elderly (KURE) study. Arch Gerontol Geriatr. 2016;64:51–58. doi: 10.1016/j.archger.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Cicero AFG, Fogacci F, Giovannini M, et al. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci Rep. 2018;8:11529. doi: 10.1038/s41598-018-29955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirro M, Bianconi V, Schiaroli E, et al. Elevated serum uric acid levels are associated with endothelial dysfunction in HIV patients receiving highly-active antiretroviral therapy. Atherosclerosis. 2018;272:101–107. doi: 10.1016/j.atherosclerosis.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Gaubert M, Marlinge M, Alessandrini M, et al. Uric acid levels are associated with endothelial dysfunction and severity of coronary atherosclerosis during a first episode of acute coronary syndrome. Purinergic Signal. 2018;14:191–199. doi: 10.1007/s11302-018-9604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua Q, Fan L, Li J, et al. 2019 Chinese guideline for the management of hypertension in the elderly. J Geriatr Cardiol. 2019;16:67–99. doi: 10.11909/j.issn.1671-5411.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen AS, Butt JH, Holm-Yildiz S, et al. Validation of repeated endothelial function measurements using EndoPAT in stroke. Front Neurol. 2017;8:178. doi: 10.3389/fneur.2017.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jedlickova L, Merkovska L, Jackova L, et al. Effect of ivabradine on endothelial function in patients with stable angina pectoris: assessment with the Endo-PAT 2000 device. Adv Ther. 2015;32:962–970. doi: 10.1007/s12325-015-0253-x. [DOI] [PubMed] [Google Scholar]
- 14.Muller U, Matsuo Y, Lauber M, et al. Correlation between endothelial function measured by finger plethysmography in children and HDL-mediated eNOS activation -- a preliminary study. Metabolism. 2013;62:634–637. doi: 10.1016/j.metabol.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Peyton CC, Colaco MA, Kovell RC, et al. Erectile dysfunction is predictive of endothelial dysfunction in a well visit population. J Urol. 2016;195:1045–1050. doi: 10.1016/j.juro.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 16.Mudau M, Genis A, Lochner A, et al. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23:222–231. doi: 10.5830/CVJA-2011-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, Meng M, Chen J, et al. Reactive hyperemia index in patients on maintenance hemodialysis: cross-sectional data from a cohort study. Sci Rep. 2017;7:45757. doi: 10.1038/srep45757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azuma M, Chihara Y, Yoshimura C, et al. Association between endothelial function (assessed on reactive hyperemia peripheral arterial tonometry) and obstructive sleep apnea, visceral fat accumulation, and serum adiponectin. Circ J. 2015;79:1381–1389. doi: 10.1253/circj.CJ-14-1303. [DOI] [PubMed] [Google Scholar]
- 19.Deda L, Sochett EB, Mahmud FH. Physiological changes in blood pressure impact peripheral endothelial function during adolescence. Cardiol Young. 2015;25:777–779. doi: 10.1017/S1047951114001024. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzawa Y, Sugiyama S, Sumida H, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. J Am Heart Assoc. 2013;2:e000426. doi: 10.1161/JAHA.113.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanbay M, Yilmaz MI, Sonmez A, et al. Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am J Nephrol. 2011;33:298–304. doi: 10.1159/000324847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kocyigit I, Yilmaz M I, Orscelik O, et al. Serum uric acid levels and endothelial dysfunction in patients with autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2013;123:157–164. doi: 10.1159/000353730. [DOI] [PubMed] [Google Scholar]
- 23.Maloberti A, Maggioni S, Occhi L, et al. Sex-related relationships between uric acid and target organ damage in hypertension. J Clin Hypertens (Greenwich) 2018;20:193–200. doi: 10.1111/jch.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Techatraisak K, Kongkaew T. The association of hyperuricemia and metabolic syndrome in Thai postmenopausal women. Climacteric. 2017;20:552–557. doi: 10.1080/13697137.2017.1369513. [DOI] [PubMed] [Google Scholar]
- 25.Burnstock G. Purinergic signalling and endothelium. Curr Vasc Pharmacol. 2016;14:130–145. doi: 10.2174/1570161114666151202204948. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y J, Yoon Y, Lee K Y, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014;28:3197–3204. doi: 10.1096/fj.13-247148. [DOI] [PubMed] [Google Scholar]
- 27.Song C, Zhao X. Uric acid promotes oxidative stress and enhances vascular endothelial cell apoptosis in rats with middle cerebral artery occlusion. Biosci Rep. 2018;38:BSR20170939. doi: 10.1042/BSR20170939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valle M, Martos R, Canete M D, et al. Association of serum uric acid levels to inflammation biomarkers and endothelial dysfunction in obese prepubertal children. Pediatr Diabetes. 2015;16:441–447. doi: 10.1111/pedi.12199. [DOI] [PubMed] [Google Scholar]
- 29.Cicero AFG, Fogacci F, Giovannini M, et al. Interaction between low-density lipoprotein-cholesterolaemia, serum uric level and incident hypertension: data from the Brisighella Heart Study. J Hypertens. 2019;37:728–731. doi: 10.1097/HJH.0000000000001927. [DOI] [PubMed] [Google Scholar]
- 30.Cicero AFG, Pirro M, Watts GF, et al. Effects of allopurinol on endothelial function: a systematic review and meta-analysis of randomized placebo-controlled trials. Drugs. 2018;78:99–109. doi: 10.1007/s40265-017-0839-5. [DOI] [PubMed] [Google Scholar]
- 31.Dogan A, Yarlioglues M, Kaya MG, et al. Effect of long-term and high-dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press. 2011;20:182–187. doi: 10.3109/08037051.2010.538977. [DOI] [PubMed] [Google Scholar]
- 32.Leiba A, Vinker S, Dinour D, et al. Uric acid levels within the normal range predict increased risk of hypertension: a cohort study. J Am Soc Hypertens. 2015;9:600–609. doi: 10.1016/j.jash.2015.05.010. [DOI] [PubMed] [Google Scholar]