Abstract
Background
Longevity, combined with a higher prevalence of obesity, particularly visceral obesity, has been associated with an increased risk of cardiovascular diseases. Insulin resistance (IR) is an important link between visceral obesity and cardiovascular diseases. An important association has been found between sagittal abdominal diameter, visceral obesity and IR. The objective of this study is to evaluate sagittal abdominal diameter as a marker of visceral obesity and correlate it with IR in older primary health care patients.
Methods
A cross-sectional study was performed with 389 patients over 60 years of age (70.6 ± 6.9), of whom 74% were female. Their clinical, anthropometric and metabolic profiles were assessed and their fasting serum insulin level was used to calculate the homeostasis model assessment insulin resistance (HOMA-IR). Sagittal abdominal diameter was measured in the supine position at the midpoint between the iliac crest and the last rib with abdominal calipers.
Results
Sagittal abdominal diameter was significantly correlated with anthropometric measures of general and visceral obesity and with HOMA-IR in both genders. There was no change in the association between sagittal abdominal diameter and HOMA-IR after adjusting for age, sex, diabetes and hypertension.
Conclusion
It is feasible to use sagittal abdominal diameter in older primary care patients as a tool to evaluate visceral obesity, which is an indicator of cardiovascular risk.
Keywords: Cardiovascular risk, Insulin resistance, Primary health care, Sagittal abdominal diameter
1. Introduction
According to the World Health Organization, the worldwide prevalence of overweight and obesity are approaching 39% and 13%, respectively, and continue to rise.[1],[2]
Obesity, especially visceral obesity, is associated with an increased risk of cardiovascular and metabolic diseases. Hypertension, diabetes mellitus, dyslipidemia, atherosclerotic disease and non-alcoholic fatty liver disease are frequent in individuals with visceral obesity.[3] Insulin resistance (IR) is an important link between visceral obesity and these diseases.[4]
Several studies have shown that anthropometric indices are an alternative, accessible, fast, non-invasive and inexpensive way to identify visceral obesity and IR.[5] Waist circumference (WC) has traditionally been used to measure visceral obesity.[6]–[8] However, aging causes a reduction of lean mass and an increase in body fat, especially in the abdominal region, which could impede accurate WC measurement.[9],[10] Sagittal abdominal diameter (SAD), also referred to as “abdominal height”, provides a better intra-abdominal or visceral obesity estimate than WC.[11] SAD is an anthropometric measure associated with IR, glucose intolerance, cardiovascular risk and general mortality.[12],[13]
Identifying older individuals at high cardiovascular risk through simple and non-invasive anthropometric measures is an important strategy for preventing cardiovascular disease. Thus, SAD has been considered a good method for assessing visceral obesity.[14]
The aim of this study was to assess visceral obesity with SAD and correlate it with IR in older primary health care patients.
2. Methods
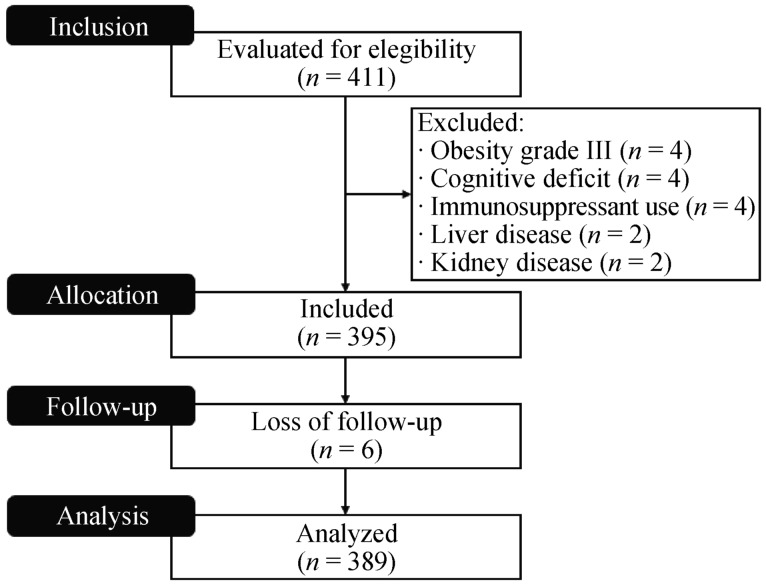
This cross-sectional, observational study included a convenience sample of 411 older patients (over 60 years old) assisted in a geriatric clinic, from March 2011 until March 2013. Patients with grade III obesity (four), hepatic (two) or renal insufficiency (four), cognitive deficit (two), or who were on corticosteroids or immunosuppressants (four) were excluded. Six patients lost the follow-up and were excluded from the analysis (Figure 1).
Figure 1. The Consort flow diagram of the studied population.
Patient's clinical information, anthropometric, and metabolic profiles were assessed and their fasting serum insulin levels were determined. With the patient seated and after at least five minutes of rest, blood pressure was measured in the left arm with an automatic OMRON HEM 742INT sphygmomanometer (OMRON, Bannockburn, IL, USA), and the mean of the last two measurements was used.[15] Weight (kg) and height (cm) were used to calculate the body mass index (BMI), presented in kg/m2, with a calibrated anthropometric scale (FILIZOLA, São Paulo, SP, Brazil) according to National Institute of Metrology guidelines. WC was measured at the midpoint between the iliac crest and the last rib at the end of expiration with the patient in the orthostatic position.[16] Neck circumference (NC), arm circumference (AC), thigh circumference (TC) and hip circumference (HC) were measured with an inelastic measuring tape (SANNY, São Bernardo do Campo, SP, Brazil). SAD or abdominal height, is the distance between the dorsum and the apex of the abdomen, which was measured in the supine position at the midpoint between the iliac crest and the last rib[17] with an abdominal caliper (Holtain Ltd., Crosswell, Wales, UK) that had a mobile stem and a fixed base. The SAD cut-offs for men and women were 20.5 cm and 19.3 cm, respectively.[11]
Fasting insulin levels were measured with the electrochemiluminescence method (ELECSYS, Roche, Japan). The homeostasis model assessment-insulin resistance (HOMA-IR) was calculated by multiplying the fasting glucose level (mg/dL) by the fasting insulin level (µU/mL) and dividing by 22.5; values > 2.71 were considered positive for IR.[18]
Risk scores and global cardiovascular risk stratification were calculated according to Framingham Heart Study criteria[19] and included the following variables: age, HDL cholesterol, total cholesterol, hypertension (treated or not), smoking and diabetes mellitus. Patients were stratified as low, intermediate, or high risk.
Statistical analyses were performed in SPSS 21.0 (SPSS, Chicago, IL, USA). The entire analysis was performed by gender due to the unbalance of the sample. Continuous variables were expressed as mean ± SD, while categorical variables were expressed as absolute numbers and percentages.
For comparisons between groups (categorical variables), we used chi-square tests with continuity correction and Fisher's exact test when necessary. Student's t-test was used to verify the existence of differences between means. Simple and multiple gamma regression (generalized linear model) with log-link, were used to identify factors associated with SAD. Simple gamma regression was applied to select the variables that could confuse the association between SAD and HOMA-IR (dependent variable) and multiple gamma regression aimed to test the independent effect of SAD on HOMA-IR. The generalized linear model gamma regression was chosen because HOMA-IR did not present a distribution nor was it approximately normal (P < 0.0001 value with the Kolmogorov Smirnov test).
To avoid multi-collinearity between anthropometric variables, we tested Spearman's correlation between them. The Rho of the correlation between SAD and anthropometric measurements ranged from 0.56 to 0.89. As our objective was not to test the association of SAD as HOMA-IR independently from other anthropometric measures, we chose to leave it as the only anthropometric measure in the multiple models. Bilateral tests with a significance level of 5% were used in all comparisons.
This study was approved by the institutional research ethics committee (0183.0.258.258.10) and all participants provided written informed consent.
3. Results
The sample was predominantly female (74%) and the mean age was 70.6 ± 6.9 years. The anthropometric data and cardiovascular risk factors are listed in Table 1. The mean waist circumference for women and men was 94.9 ± 12.2 and 100.0 ± 11.4 cm, respectively (P < 0.0001). SAD did not differ significantly between men and women.
Table 1. Metabolic profile and anthropometric and cardiovascular risk factors according to gender.
| Variable | Female, n = 290 | Male, n = 99 | *P-value |
| Age, yrs | 70.6 ± 6.9 | 69.8 ± 6.8 | 0.304 |
| SBP, mmHg | 137.1 ± 21.8 | 138.7 ± 18.9 | 0.522 |
| DBP, mmHg | 74.8 ± 10.0 | 77.9 ± 9.6 | 0.008 |
| Anthropometry | |||
| BMI, kg/m2 | 28.9 ± 5.6 | 28.4 ± 4.4 | 0.348 |
| NC, cm | 33.9 ± 2.9 | 39.4 ± 3.1 | < 0.0001 |
| AC, cm | 29.8 ± 4.2 | 30.0 ± 3.3 | 0.662 |
| WC, cm | 94.9 ± 12.2 | 100.0 ± 11.4 | < 0.0001 |
| SAD, cm | 21.9 ± 3.4 | 22.2 ± 3.2 | 0.465 |
| HC, cm | 101.7 ± 11.0 | 99.0 ± 8.6 | 0.029 |
| TC, cm | 50.1 ± 6.1 | 48.8 ± 5.2 | 0.054 |
| Laboratory | |||
| Fasting glucose, mg/dL | 105.1 ± 29.4 | 110.2 ± 38.1 | 0.174 |
| Cholesterol, mg/dL | 208.6 ± 44.6 | 188.6 ± 36.1 | < 0.0001 |
| Triglycerides, mg/dL | 137.1 ± 66.8 | 145.9 ± 80.0 | 0.271 |
| HDL-C, mg/dL | 57.9 ± 13.3 | 47.3 ± 11.6 | < 0.0001 |
| LDL-C, mg/dL | 122.9 ± 39.6 | 111.5 ± 32.6 | 0.01 |
| TSH, µU/mL | 2.5 ± 2.0 | 3.3 ± 7.6 | 0.132 |
| HOMA-IR | 3.7 ± 4.4 | 4.8 ± 10.9 | 0.147 |
| Risk factors, % | |||
| Smoking | 5 | 5 | 0.600 |
| Diabetes | 22 | 22 | 0.510 |
| Hypertension | 72 | 64 | 0.074 |
| MS-NCEP | 71 | 57 | 0.003 |
| Visceral obesity (SAD) | 75 | 67 | 0.058 |
| Cardiovascular risk, % | 0.011 | ||
| Low | 1.5 | 0 | |
| Medium | 24 | 11 | |
| High | 74.5 | 89 |
AC: arm circumference; BMI: body mass index; DBP: diastolic blood pressure; HC: hip circumference; HDL-C: high density lipoprotein cholesterol; HOMA-IR: homeostasis model assessment insulin resistance index; LDL-C: low density lipoprotein cholesterol; MS: metabolic syndrome; NC: neck circumference; NCEP: National Cholesterol Education Program; SAD: sagittal abdominal diameter; SBP: systolic blood pressure; TC: thigh circumference; WC: waist circumference. *Student's t-test and chi-square test.
SAD and other parameters were associated with HOMA-IR, in a statistically significant way, both in men and women, including all tested anthropometric measures (Table 2). In men, the crude association of HOMA-IR with SAD was 1.134 (95% CI: 1.093–1.176; P < 0.0001) and in women, 1.113 (95% CI: 1.086–1,141; P < 0.0001). In the multiple gamma regression (generalized linear model), the association was 1.114 (95% CI: 1.079–1.150; P < 0.0001) for men and 1.091 (95% CI: 1.068–1.114; P < 0.0001) for women (Table 3).
Table 2. Crude association between HOMA-IR and anthropometric and laboratory variables (A) and SAD with anthropometric and laboratory variables (B) by gender.
| (A) Outcome: HOMA-IR |
(B) Outcome: SAD |
|||
| Female Exp (B) (95% CI) | Male Exp (B) (95% CI) | Female Exp (B) (95% CI) | Male Exp (B) (95% CI) | |
| Age, yrs | 1.007 (0.994–1.021) | 0.974 (0.953–0.996) | 0.999 (0.997–1.002) | 0.998 (0.994–1.001) |
| BMI, kg/m2 | 1.063 (1.046–1.079) | 1.103 (1.074–1.134) | 1.025 (1.024–1.027) | 1.028 (1.025–1.031) |
| NC, cm | 1.156 (1.123–1.190) | 1.119 (1.082–1.158) | 1.038 (1.034–1.043) | 1.032 (1.026–1.038) |
| AC, cm | 1.074 (1.053–1.096) | 1.133 (1.092–1.175) | 1.028 (1.025–1.031) | 1.032 (1.027–1.038) |
| WC, cm | 1.034 (1.027–1.041) | 1.039 (1.029–1.050) | 1.011 (1.011–1.012) | 1.011 (1.010–1.012) |
| HC, cm | 1.022 (1.014–1.031) | 1.049 (1.034–1.064) | 1.012 (1.011–1.013) | 1.013 (1.011–1.015) |
| TC, cm | 1.022 (1.007–1.037) | 1.057 (1.030–1.084) | 1.018 (1.015–1.020) | 1.017 (1.013–1.021) |
| SBP, mmHg | 0.999 (0.995–1.003) | 0.996 (0.988–1.005) | 1.000 (0.999–1.001) | 1.000 (0.999–1.002) |
| DBP, mmHg | 0.995 (0.987–1.003) | 1.023 (1.008–1.037) | 1.002 (1.001–1.004) | 1.003 (1.000–1.006) |
| HOMA-IR | 1.012 (1.008–1.017) | 1.021 (1.015–1.027) | ||
| SAD | 1.113 (1.086–1.141) | 1.134 (1.093–1.176) | ||
| Fasting glucose, mg/dL | 1.016 (1.013–1.019) | 1.006 (1.003–1.010) | 1.001 (1.000–1.002) | 1.000 (0.999–1.001) |
| Cholesterol, mg/dL | 0.997 (0.995–0.998) | 1.000 (0.996–1.005) | 1.000 (0.999–1.000) | 1.000 (0.999–1.001) |
| Triglycerides, mg/dL | 1.003 (1.001–1.004) | 1.004 (1.002–1.006) | 1.000 (1.000–1.001) | 1.000 (1.000–1.001) |
| HDL-C, mg/dL | 0.984 (0.978–0.991) | 0.975 (0.963–0.987) | 0.997 (0.996–0.999) | 0.996 (0.994–0.998) |
| LDL-C, mg/dL | 0.996 (0.994–0.998) | 0.997 (0.993–1.002) | 1.000 (0.999–1.000) | 1.000 (0.999–1.001) |
AC: arm circumference; BMI: body mass index; DBP: diastolic blood pressure; HC: hip circumference; HDL-C: HDL cholesterol; HOMA-IR: homeostasis model assessment insulin resistance index; LDL-C: LDL cholesterol; NC: neck circumference; SAD: Sagittal abdominal diameter (simple gamma regression (generalized linear model) with Log-link); SBP: systolic blood pressure; TC: thigh circumference; WC: waist circumference.
Table 3. Adjusted association between HOMA-IR and SAD by sex.
| Interception | HOMA-IR |
|
| Men Exp (B) (95% CI) | Women Exp (B) (95% CI) | |
| 0.044 (0.016–0.122) | 0.095 (0.058–0.156) | |
| Fasting glucose, mg/dL | 1.007 (1.004–1.010) | 1.014 (1.011–1.017) |
| Triglycerides, mg/dL | 1.002 (1.001–1.004) | 1.001 (1.000–1.002) |
| SAD | 1.114 (1.079–1.150) | 1.091 (1.068–1.114) |
| DBP, mmHg | 1.010 (0.999–1.021) | |
DBP: diastolic blood pressure; HOMA-IR: homeostasis model assessment insulin resistance index; SAD: Sagittal abdominal diameter (multiple gamma regression (generalized linear model) with Log-link).
4. Discussion
In agreement with the literature, SAD was correlated with Homa-IR and anthropometric measurement of visceral obesity in the elderly older, regardless of sex, age, hypertension or diabetes.[14],[20]
Changes occur with aging, such as decreased body mass and stature, reduced fat free mass and changes in body fat compartments. This study's finding that decreased peripheral and increased visceral adipose tissue is aligned with SAD values applied to both genders.
Visceral adipose tissue is an important secretor of several adipokines involved in the genesis of IR and pro-inflammatory or prothrombotic states.[21],[22] Visceral obesity is also an important risk factor for cardiometabolic disorders and contributes to higher cardiovascular risk.[23]
SAD has been identified as a marker of visceral obesity internationally.[9],[11],[24]–[27] Van der Kooy, et al.[26] demonstrated that SAD correlated better with male visceral fat. However, later studies have found a better correlation between SAD and visceral fat in females.[28],[29] In the present study, SAD was correlated with IR regardless of gender.
Previous studies have demonstrated an association between SAD and IR.[3],[17],[25],[28] Ohrvall, et al.[30] presented a moderate correlation between SAD and fasting insulin levels in women (r = 0.46, P < 0.05). In the Bogalusa Heart study, SAD was a better predictor of blood glucose and insulin levels than other anthropometric measures.[21] Pouliot, et al.[27] concluded that SAD was significantly correlated with fasting hyperglycemia and other atherogenic metabolic disorders. According to Riserus, et al.,[22] SAD is a predictor of IR in obese men.
SAD results also extend to cardiovascular disease. In a control case study, Kahn, et al.,[17] observed an association between high SAD and coronary artery disease. Empana, et al.,[31] and Dahlen, et al.,[12] demonstrated a relationship between SAD and sudden death and between SAD and arterial stiffness, respectively. A prospective study of 981 men at the U.S. National Institutes of Health found that SAD was a strong predictor of overall mortality and cardiovascular disease in young adults.[10]
The strong association between SAD and insulin resistance in individuals with visceral fat, imply that may progress for cardiovascular events. The increasing prevalence of cardiovascular disease and elevated overweight and obese patients in worldwide should consider using SAD measurement tool for older people.[32] The predictive power of the SAD to evaluate visceral adiposity and association with risk factors of morbidity and mortality has been well established in the scientific literature. More than two thirds of deaths related to overweight or obesity were due to cardiovascular disease, thus become a significant public health challenge to recognize those older people with elevated cardiovascular risk to avoid an increase in hospital services demands.[33]
SAD is a relevant non-invasive cardiovascular risk marker that is easy to measure in the older and can be used in clinical practice to identify and stratify the individual risk of cardiovascular disease in primary care patients.
The main limitation of this study is the predominance of female and the probable reason would be that women seek more for medical care than men. The study did not compare imaging methods, such as computed tomography and nuclear magnetic resonance, which are considered the gold standards for identifying visceral obesity.
In conclusion, SAD is an anthropometric measure that correlates with visceral obesity as estimated by HOMA-IR and is feasible to identify cardiovascular risk in older primary care populations.
References
- 1.Agha M, Agha R. The rising prevalence of obesity: part A: impact on public health. Int J Surg Oncol. 2017;2:e17. doi: 10.1097/IJ9.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Obesity and overweight. 2006. [(accessed in March 20, 2019)]. www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 3.Britton KA, Massaro JM, Murabito JM, et al. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Col Cardiol. 2013;62:921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci. 2009;54:1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 5.Chang SA, Kim HS, Yoon KH, et al. Body mass index is the most important determining factor for the degree of insulin resistance in non-obese type 2 diabetic patients in Korea. Metabolism. 2004;53:142–146. doi: 10.1016/s0026-0495(03)00314-7. [DOI] [PubMed] [Google Scholar]
- 6.Storti KL, Brach JS, FitzGerald SJ, et al. Relationships among body composition measures in community-dwelling older women. Obesity. 2006;14:244–251. doi: 10.1038/oby.2006.31. [DOI] [PubMed] [Google Scholar]
- 7.Yan Q, Sun D, Li X, et al. Neck circumference is a valuable tool for identifying metabolic syndrome and obesity in Chinese elder subjects: a community-based study. Diabetes Metab Res Rev. 2014;30:69–76. doi: 10.1002/dmrr.2464. [DOI] [PubMed] [Google Scholar]
- 8.Seidell JC, Perusse L, Despres JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74:315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 9.Roriz AKC, Passos LCS, Oliveira CC, et al. Discriminatory power of indicators predictors of visceral adiposity evaluated by computed tomography in adults and older individuals. Nutr Hosp. 2014;29:1401–1407. doi: 10.3305/nh.2014.29.6.7185. [DOI] [PubMed] [Google Scholar]
- 10.Seidell JC, Andres R, Sorkin JD, Muller DC. The sagittal waist diameter and mortality in men: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord. 1994;18:61–67. [PubMed] [Google Scholar]
- 11.Sampaio LR, Simões EJ, Assis AMO, Ramos LR. Validity and reliability of the sagittal abdominal diameter as a predictor of visceral abdominal fat. Arq Bras Endocrinol Metab. 2007;51:980–986. doi: 10.1590/s0004-27302007000600013. [DOI] [PubMed] [Google Scholar]
- 12.Dahlen EM, Bjarnegard N, Lanne T, et al. Sagittal abdominal diameter is a more independent measure compared with waist circumference to predict arterial stiffness in subjects with type 2 diabetes - a prospective observational cohort study. Cardiovasc Diabetol. 2013;12:55–62. doi: 10.1186/1475-2840-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firouzi SA, Tucker LA, LeCheminant JD, Bailey BW. Sagittal abdominal diameter, waist circumference, and BMI as predictors of multiple measures of glucose metabolism: An NHANES Investigation of US adults. J Diabetes Res. 2018:3604108. doi: 10.1155/2018/3604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turcato E, Bosello O, Di Francesco V, et al. Waist circumference and abdominal sagittal diameter as surrogates of body fat distribution in the older: their relation with cardiovascular risk factors. Int J Obes Relat Metab Disord. 2000;24:1005–1010. doi: 10.1038/sj.ijo.0801352. [DOI] [PubMed] [Google Scholar]
- 15.Brazilian Society of Cardiology/Brazilian Society of Hypertension/Brazilian Society of Nephrology. VI Brazilian Guidelines of Hypertension. Arq Bras Cardiol. 2010;95:1–51. [Google Scholar]
- 16.World Health Organization. Report of a WHO Consultation. Geneva, Switzerland: 2000. Obesity: prevention and management of the global epidemic. [Google Scholar]
- 17.Kahn HS, Austin H, Williamson DF, Arensberg D. Simple anthropometric indices associated with ischemic heart disease. J Clin Epidemiol. 1996;49:1017–1024. doi: 10.1016/0895-4356(96)00113-8. [DOI] [PubMed] [Google Scholar]
- 18.Geloneze B, Vasques ACJ, Stabe CFC, et al. HOMA 1-IR and HOMA 2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study [BRAMS] Arq Bras Endocrinol Metab. 2009;53:281–287. doi: 10.1590/s0004-27302009000200020. [DOI] [PubMed] [Google Scholar]
- 19.D'Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 20.Kahn HS, Gu Q, Bullard KM, et al. Population distribution of the sagittal abdominal diameter [SAD] from a representative sample of US adults: comparison of SAD, waist circumference and body mass index for identifying dysglycemia. PLoS One. 2014;9:e108707. doi: 10.1371/journal.pone.0108707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustat J, Elkasabany A, Srinivasan S, Berenson GS. Relation of abdominal height to cardiovascular risk factors in young adults: the Bogalusa heart study. Am J Epidemiol. 2000;151:885–891. doi: 10.1093/oxfordjournals.aje.a010292. [DOI] [PubMed] [Google Scholar]
- 22.Risérus U, Arnlöv J, Brismar K, et al. Sagittal abdominal diameter is a strong anthropometric marker of insulin resistance and hyperproinsulinemia in obese men. Diabetes Care. 2004;27:2041–2046. doi: 10.2337/diacare.27.8.2041. [DOI] [PubMed] [Google Scholar]
- 23.Jaramillo LP, Pradilla LP. Role of the adipocyte for inflammation of the metabolic syndrome. Acta Med Colomb. 2005;30:137–140. [Google Scholar]
- 24.Yim JY, Kim D, Lim SH, et al. Sagittal abdominal diameter is a strong anthropometric measure of visceral adipose tissue in the Asian general population. Diabetes Care. 2010;33:2665–2670. doi: 10.2337/dc10-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gletsu-Miller N, Kahn HS, Gasevic D, et al. Sagittal abdominal diameter and visceral adiposity: correlates of beta-cell function and dysglycemia in severely obese women. Obes Surg. 2013;23:874–881. doi: 10.1007/s11695-013-0874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Kooy K, Leenen R, Seidell JC, et al. Abdominal diameters as indicators of visceral fat: comparison between magnetic resonance imaging and anthropometry. Br J Nutr. 1993;70:47–58. doi: 10.1079/bjn19930104. [DOI] [PubMed] [Google Scholar]
- 27.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 28.Zamboni M, Turcato E, Armellini F, et al. Sagittal abdominal diameter as a practical predictor of visceral fat. Int J Obes. 1998;22:655–660. doi: 10.1038/sj.ijo.0800643. [DOI] [PubMed] [Google Scholar]
- 29.Kullberg J, von Below C, Lönn L, et al. Practical approach for estimation of subcutaneous and visceral adipose tissue. Clin Physiol Funct Imaging. 2007;27:148–153. doi: 10.1111/j.1475-097X.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 30.Empana JP, Ducimetiere P, Charles MA, Jouven X. Sagittal abdominal diameter and risk of sudden death in asymptomatic middle-aged men. The Paris Prospective Study I. Circulation. 2004;110:2781–2785. doi: 10.1161/01.CIR.0000146395.64065.BA. [DOI] [PubMed] [Google Scholar]
- 31.Ohrvall M, Berglund L, Vessby B. Sagittal abdominal diameter compared with other anthropometricmeasurements in relation to cardiovascular risk. Int J Obes Relat Metab Disord. 2000;24:497–501. doi: 10.1038/sj.ijo.0801186. [DOI] [PubMed] [Google Scholar]
- 32.Iribarren C, Darbinian JA, Lo JC, et al. Value of the sagittal abdominal diameter in coronary heart disease risk assessment: cohort study in a large, multiethnic population. Am J Epidemiol. 2006;15:1150–1159. doi: 10.1093/aje/kwj341. [DOI] [PubMed] [Google Scholar]
- 33.Van den Heede K, Bouckaert N, Van de Voorde C. The impact of an ageing population on the required hospital capacity: results from forecast analysis on administrative data. Eur Geriatr Med. 2019;10:697–705. doi: 10.1007/s41999-019-00219-8. [DOI] [PubMed] [Google Scholar]