Abstract
Analytic epidemiological studies cover a large spectrum of study methodologies, ranging from noninterventional observational studies (population-based, case–control, or cohort studies) to interventional studies (clinical trials). Herein, we review the different research methodologies or study designs and discuss their advantages and disadvantages in the context of chronic obstructive pulmonary disease (COPD) pharmacotherapy. Although randomized controlled trials (RCTs) are considered the “gold standard” for evaluating the efficacy and safety of an intervention, observational studies conducted in a real-world scenario are useful in providing evidence on the effectiveness of the intervention in clinical practice; understanding both efficacy and effectiveness is important from the clinician’s perspective. Pragmatic clinical trials that use real-world data while retaining randomization bridge the gap between explanatory RCTs and noninterventional observational studies. Overall, different study designs have their associated advantages and disadvantages; together, findings from all types of studies bring about progress in clinical research as elucidated through examples from COPD research in this paper.
Keywords: clinical trials, COPD, pharmacotherapy, study designs
Introduction
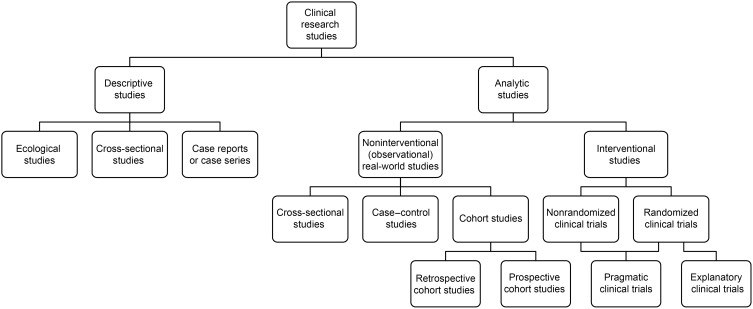
Clinical research studies can be broadly classified as descriptive (eg, ecological studies or case reports) or analytic (Figure 1).1 Analytic studies span a large spectrum, ranging from noninterventional and observational real-world studies to interventional studies.1–5 Observational studies include cross-sectional, case–control, and longitudinal cohort studies,6 and interventional studies include explanatory randomized controlled trials (RCTs) and pragmatic clinical trials (PrCTs), which bridge the gap between explanatory RCTs and real-world observational studies.2,7 In addition to the difference in study types, study designs vary in many respects (eg, methodologies, temporal relationship, number of subjects enrolled, eligibility criteria, characteristics of included subjects, interventions administered, duration, assessments, and outcomes). These variations lead to inherent advantages and disadvantages; however, ultimately, the various study types and resultant data complement each other and form the building blocks of the research process.
Figure 1.
Overview of study designs.
Here, we review the different study types and discuss their advantages and disadvantages (also summarized in the supplementary video), in general and in the context of chronic obstructive pulmonary disease (COPD) pharmacotherapy, for the benefit of clinicians with more limited research experience.
Process of Drug Development
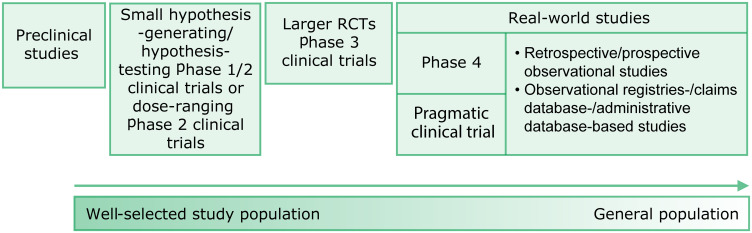
New drug development is a stepwise, rigorous, and prolonged process,8 typically involving preclinical studies, followed by phase 1 to 3 clinical trials, and phase 4 trials and other observational studies, which subsequently verify the results of phase 1 to 3 trials (Figure 2). The process may differ slightly for expanded indications (eg, additional age groups, other endpoints, and new diseases) for previously approved drugs.
Figure 2.
Research process for new drug development: possible sequence of research designs used.
Abbreviations: RCT, randomized controlled trial.
Study Designs
Explanatory Clinical Trials
Clinical trials are prospective studies in which patients receive an intervention. The designs of such studies increase in complexity with each phase of the study (Figure 2).8,9 In general, phase 1 trials include small, nonrandomized, and noncomparative studies, whereas phase 2 trials may include randomized and comparative/controlled interventional studies. Early phase 1 and 2 trials often test or modify initial hypotheses, which are further evaluated in phase 3 and 4 trials.
Phase 1 trials are primarily safety and pharmacokinetic/pharmacodynamic trials. These trials include a small number (20–80) of healthy volunteers who receive single or multiple doses of the investigational drug to determine dosing; document absorption, distribution, metabolism, and excretion (sometimes referred to as ADME studies); and identify short-term adverse effects.9
In phase 2 trials, safety and preliminary efficacy (the extent to which a drug can bring about its intended effect under ideal circumstances, such as in an RCT10) are assessed.9 Phase 2 trials are often blinded RCTs and include approximately 100 to 300 patients (ie, people with the disease under investigation) who receive either one or more doses of the investigational drug and/or standard-of-care treatment and/or placebo over a period of 1 to 4 weeks (to achieve steady state). Generally, the objective of the dose-ranging phase 2 trials is to determine the optimal dose(s) for evaluation in larger phase 3 trials in addition to assessing safety and preliminary efficacy.9
Phase 3 trials, comprising RCTs with specified eligibility criteria, are considered the “gold standard” for establishing the safety and efficacy of a drug.3,4 These RCTs are large scale—approximately 1000–3000 patients; can be single-, double-, or triple-blinded; and are often conducted over a prolonged period of time.9 Patients are randomized to receive one or more doses of the investigational drug, placebo, and/or a commercially available comparator agent for weeks, months, or even years. Treatment safety, efficacy, and adherence are monitored using objective, validated endpoints with the help of home diaries and periodic assessments during visits at regular intervals. The number of patients and duration of the trial may vary depending upon the disease under consideration, stage of drug development, duration of previous trials, and chronicity of the disease. For example, if the primary endpoint is a reduction in exacerbations of COPD, the frequency of which varies according to season, a year-long study is generally preferred to reduce the confounding effect of seasonality. These trials aim to meet regulatory agency approval requirements to evaluate the long-term safety and efficacy of clinically relevant doses of the investigational drug compared with placebo and/or a comparator agent, substantiated by sufficient statistical power generated with a large number of patients.
Phase 4 trials are usually prospective trials with active comparators (sometimes called head-to-head efficacy trials) or are open-label, noninterventional, observational studies conducted after regulatory approval; occasionally, these trials are agreed to by the study sponsor and are often required by regulatory approval authorities.11–13 The objective of these trials is to collect additional information about the safety (long-term risks and rare adverse events), efficacy of a drug on an expanded indication (eg, exacerbation reduction), effectiveness (the extent to which a drug achieves its intended effect in the usual clinical setting10), and optimal use of the investigational drug in the general patient population, as well as to evaluate the investigational drug in special patient populations that are usually excluded or difficult to include and follow-up in phase 3 RCTs.11
For rare diseases or orphan drugs, the number of phase 2 to 4 trials and the number of participants in each trial may be substantially reduced, and alternative clinical trial designs may be acceptable.14
Usually, statistical analyses in phase 2, 3, and 4 trials are conducted to assess whether or not the investigational drug has greater efficacy than placebo or an active control (a “superiority” design). Superiority evaluations require a prospective design with adequate statistical power, reliable objective endpoints, and adequate patient adherence. In superiority trials, usually an intent-to-treat (ITT) or an all-data-collected analysis is conducted15 by using appropriate analysis methods (eg, mixed models repeated measures [MMRM], analysis of variance [ANOVA], analysis of covariance [ANCOVA], Cox proportional hazard regression, or logistic regression16) to evaluate efficacy; however, other statistical methods may also be used. Traditionally, safety is assessed by the proportions of patients with adverse events, calculated as cumulative (at the end of the trial period) or cross-sectional (at each visit) percentages.16 Regulatory authorities often require two or more duplicate 12- to 24-week phase 3 efficacy trials and at least one phase 3 year-long safety trial (termed “pivotal trials”) to adequately characterize the safety and efficacy of a new drug for approval. Given the seasonality of COPD exacerbations, long-term phase 3 trials of up to 12 months duration are recommended for the evaluation of the effect of new investigational drugs on this outcome or for the expansion of approved indications for already commercially available drugs.17
Case Reports or Case Series
Reports of individual cases or a series of cases provide retrospective safety and efficacy details, as well as other clinical parameters (eg, quality of life [QoL]) derived from cases of interest in their natural clinical setting;18 they can be based on either on-label (supporting the approved indication) or off-label (demonstrating a potential new or expanded indication) use of a drug. These cases are easy to report and can generate new research questions, although the generalizability of their findings is limited because of selection bias and lack of controls.18
Real-World Observational Studies
Real-world observational studies include large-scale cross-sectional, cohort, and case–control studies that do not employ randomization6 and may be population based (Table 1). Case–control studies are retrospective, while cohort studies may be prospective or retrospective. In these studies, investigators solely observe treatment effects, generally using administrative health databases, claims databases, or registries;19 investigators have no control over the medical management of the patient or the data collected. These studies are characterized by the enrollment of real-world patients, lax controls on treatment adherence, use of concomitant medications or alternative therapies, and selection of endpoints for optimum feasibility; moreover, the endpoints may not be suitably objective or validated. Besides safety and clinical effectiveness, cost-effectiveness and other economical outcomes may also be assessed.19 When comparisons are made between the clinical effectiveness and/or safety of two or more different medications, propensity matching on selected clinical characteristics may be performed in an effort to minimize the impact of differences in these patient-related features. These studies are relatively cheaper and are especially useful when the disease of interest is rare, but can also be used when the disease of interest is common.
Table 1.
| RCTs | PrCTs | Real-World Observational Studies | |
|---|---|---|---|
| General information |
|
|
|
| Study population |
|
|
|
| Randomization |
|
|
|
| Comparability |
|
|
|
| Study setting/data sources |
|
|
|
| Assessment burden |
|
|
|
| Data collection |
|
|
|
| Adherence |
|
|
|
| Discontinuations/withdrawals |
|
|
|
| Statistical design and comparators |
|
|
|
| Follow-up data |
|
|
|
| Outcomes |
|
|
|
| Data quality |
|
|
|
| Generalizability |
|
|
|
| Validity |
|
|
|
| Precision |
|
|
|
| Cost |
|
|
|
| Value |
|
|
|
Abbreviations: COPD, chronic obstructive pulmonary disease; e-diary, electronic diary; e-health, electronic health; PrCT, pragmatic clinical trial; PRO, patient-reported outcome; RCT, randomized controlled trial.
Cohort studies6 are prospective or retrospective studies conducted to determine the incidence and natural history of a disease or condition. Exposure to putative risk factors precedes the outcomes, and multiple outcomes can be studied using one cohort. However, prospective cohort studies are expensive and have a substantial risk of attrition, whereas retrospective cohort studies may be impacted by recall bias.
Case–control studies6 are retrospective studies in which individuals with and without the disease or condition of interest are matched (eg, age, sex,20 duration of comorbid diseases, and severity markers for comorbid diseases). Severity matching is imperfect and can induce bias; therefore, both pre- and post-matched baseline severity characteristics should be reported. Case–control studies are useful for understanding exposure factors for rare diseases.6 In these studies, investigators review large healthcare database records and determine which individuals had the suspected exposure in the past. These studies are relatively cheaper (especially when compared with prospective cohort studies) and are feasible when the disease or condition of interest is rare. However, these studies are subject to biases such as sampling, observation, or recall bias.
Pragmatic Clinical Trials
Key Features vs RCTs and Real-World Studies
PrCTs have some features of both RCTs and real-world observational studies (Table 1):2,19,21 like RCTs, they use prospective study designs and randomization, and like real-world studies, they involve broadly inclusive populations, representative of patients receiving the treatment in everyday clinical practice, and are conducted by healthcare professionals in community-based settings, where regular patient management is ensured while still tracking some measures of treatment adherence. In PrCTs, relevant outcomes important to inform optimal healthcare treatment decisions are captured, and appropriate active comparators are generally included instead of placebo.22 In RCTs, the benefits of an intervention may be overestimated and the harms may be underestimated because they are performed with relatively small and highly selective patient populations at research sites with experienced investigators.2 Therefore, findings from RCTs should not be used in formulating usual practice guidelines without further evaluation.23 In contrast, PrCTs provide effectiveness and safety estimates in large, real-world, diverse patient populations using broad inclusion and relatively few exclusion criteria.21 Randomization in PrCTs confers some of the strengths of RCTs, such as credibility and limiting the allocation bias, to the PrCTs while providing external validity from the real-world component.24 PrCTs may also be designed to compare the effectiveness of alternative treatments or practice procedures not supported by the industry or the Food and Drug Administration (FDA)25 and allow recording of hospitalization and mortality throughout the study duration. Apart from objective clinical endpoints, inclusion of cost-effectiveness and adoption endpoints in PrCTs is critical for decision-making by health systems. Therefore, PrCTs can provide true risk/benefit assessments and value of a medicine in a routine care setting, allowing healthcare practitioners and payers to make informed decisions.23
The limitations of PrCTs should, however, be noted—depending on whether they more closely mimic RCTs or real-world observational studies, PrCTs may have weak controls on adherence to therapy,28 permit cross-over to alternative therapies,28 and/or lack or include fewer objective, procedure-based outcome measures compared to RCTs. PrCTs may rely on alternative endpoints22 based on in-office or telephone questionnaires, which may provide suggestive evidence in some cases. On the other hand, lower adherence to therapies in PrCTs compared with RCTs is more reflective of the real-world clinical scenarios in COPD29 and contributes to the enhanced external validity of the results.
As discussed, the key aspects of trial designs differ substantially between RCTs and PrCTs. Researchers can use the PRagmatic Explanatory Continuum Indicator Summary-2 (PRECIS-2) tool to make study design decisions befitting the intended use of the trial.26 The applicability of real-world evidence (RWE) in research is expanding. With the 21st Century Cures Act, drug manufacturers can submit RWE instead of RCT results to support the expansion of additional indications for previously approved drugs.27
Pragmatic Clinical Trials: Design Considerations
Study populations in PrCTs represent real-world populations likely to be prescribed treatment resembling routine clinical practice. With randomization and real-world use, the approximate effectiveness and safety of such interventions can be evaluated by establishing “superiority” vs usual care or standard treatment (ie, superiority trials),30 or by showing “noninferiority” vs well-established therapies (ie, comparative effectiveness trials).31
Active-controlled superiority trials may require larger sample sizes than placebo-controlled trials,32 sometimes requiring several hundred patients per treatment arm to have enough statistical power to detect a superiority benefit. When the “superiority” of an intervention vs a control arm is evaluated in a PrCT, a prespecified statistical analysis plan (similar to RCTs with analysis methods such as MMRM or ANCOVA) is followed, and significant improvements in primary and secondary endpoints are sought (typically with P<0.05).
In comparative effectiveness trials, a carefully selected “noninferiority limit” is prospectively specified to ensure that a similar high level of clinical benefit is achieved for both the new and established treatments.30 A noninferiority trial aims to establish that the intervention is not worse than its comparator by a prespecified degree, which is known as the “noninferiority limit or margin”.33 Statistical analyses need to be prespecified and of high rigor for noninferiority trials, with stringent noninferiority limits. High adherence to treatment and low use of confounding therapies are important. Otherwise, sloppy design or implementation features can lead to a false noninferiority finding, thus erroneously concluding an inferior treatment to be noninferior.15,34 Both per-protocol and ITT analyses are typically performed; however, unlike in superiority trials (where the ITT approach is considered conservative because it is likely to lead to a treatment effect closer to having no effect), the ITT approach is not conservative in noninferiority or equivalence trials because it can bias towards the null, which may lead to false claims of noninferiority or equivalence.35 Usually, the sample size required to demonstrate noninferiority in an active-controlled trial is substantially larger (sometimes impossibly larger) than that for a placebo-controlled superiority trial;15,30 small sample sizes may reduce the statistical power for proving noninferiority.30
When well-conducted PrCTs report key differences or strong evidence for noninferiority between treatments, the findings may be applicable to larger, real-world populations, and the generated RWE can help clinicians understand the effectiveness and safety of the drug in clinical practice settings.
Comparing and contrasting the characteristics of RCTs, PrCTs, and real-world observational studies (Table 1) help to understand how these designs complement each other and can cumulatively create comprehensive evidence. Ideally, clinical research should address questions that are relevant to the target audience. To that effect, researchers could use the effective dissemination and implementation frameworks proposed by leading funding agencies, such as the National Heart, Lung, and Blood Institute and Patient-Centered Outcomes Research Institute.36,37
Use of Different Research Methodologies in the Evaluation of COPD Pharmacotherapy
Determining an optimum treatment for COPD has been a conundrum for many decades. Researchers often focus on improving lung function, respiratory symptoms, and QoL; preventing and treating exacerbations; and minimizing morbidity and mortality.38–40 Prevention of exacerbations to reduce morbidity has been crucial in COPD studies conducted as early as the mid-twentieth century.38,39
Small Randomized/Nonrandomized Studies
Occasionally, small nonrandomized or randomized trials (pilot studies) with or without adequate statistical power are used to determine the feasibility of a therapeutic approach which, when successful, can lead to larger clinical trials. For example, a series of studies were conducted to assess the benefit of antibiotics in treating COPD exacerbations. Elmes et al conducted an RCT based on previous research which demonstrated that exacerbations of chronic bronchitis were usually associated with pathogenic bacterial proliferation.38 Benefits of prophylactic oxytetracycline at the beginning of a suspected COPD exacerbation were assessed (as days ill and days off work; no lung function reported) in patients with chronic bronchitis. Among 88 patients who reported 146 exacerbations, exacerbation-associated loss of work time in the intervention group was half of that in the control group. Three decades later, Anthonisen et al documented their findings in a similar but slightly larger RCT with a crossover component conducted over 3.5 years in 173 patients.41 Of the 362 exacerbations, 182 and 180 were treated with an antibiotic and a placebo, respectively. With a treatment success of 68.1% vs 55.0% (P<0.01), a significant benefit was observed with antibiotic vs placebo use. In a much larger, well-designed and appropriately powered, parallel-group, prospective RCT by Albert et al, approximately three further decades later, 1142 patients were randomized to receive azithromycin (n=570) or placebo (n=572) in addition to usual care for 1 year.42 Patients in the azithromycin group had a significantly longer median time to first exacerbation, fewer exacerbations, lower risk of acute exacerbations, and greater improvement in St. George’s Respiratory Questionnaire (SGRQ) scores than those in the placebo group (all P<0.05). Findings from a subgroup analysis showed that antibiotic efficacy was confined to former smokers. Results also indicated a significantly higher risk of hearing impairment in the azithromycin group (P<0.05).
Similar to small, nonrandomized or randomized trials, a series of individual RCTs (N-of-1 RCTs) may provide preliminary data that could be confirmed in larger clinical trials. In a series of 27 N-of-1 RCTs, long-term ambulatory oxygen therapy did not improve QoL (as measured by the Chronic Respiratory Questionnaire and the SGRQ) in patients with COPD; the general application of long-term ambulatory oxygen therapy was found to be not justifiable for patients with COPD and transient exertional hypoxemia who did not satisfy the criteria for mortality reduction.43 However, two patients were oxygen responders, leading the authors to conclude that select patients can be identified in N-of-1 RCTs who may benefit from long-term ambulatory oxygen therapy. Continuous oxygen therapy conferred a survival advantage specifically in patients with chronic obstructive lung disease with significant resting hypoxemia in the Nocturnal Oxygen Therapy Trial (NOTT) and Medical Research Council (MRC) studies.44,45 However, long-term supplemental oxygen did not improve clinical outcomes in the Long-Term Oxygen Treatment Trial (LOTT), a large, multicenter, parallel-group RCT of 738 patients with COPD and moderate (less severe) resting or exercise-induced desaturation who were followed for 1 to 6 years.46
Large Pivotal RCTs
Overall, well-conducted, large, pivotal RCTs have led to the approval of new drugs or drug combinations for COPD treatment (Table 2). The efficacy of single bronchodilators (long-acting β2-agonist [LABA] or long-acting muscarinic antagonist [LAMA]), dual bronchodilators (LABA + LAMA), and triple therapy (LABA + LAMA + inhaled corticosteroid [ICS]47) has been assessed in phase 3 and 4 clinical trials, which exemplify variations in RCTs with respect to design, population, and outcomes. The clinical development of tiotropium (an inhaled LAMA) and roflumilast (an oral phosphodiesterase-4 inhibitor), for example, exemplify the trajectory of different research methodologies—from preclinical studies through different phases of clinical trials.
Table 2.
Examples of Pivotal and Subsequent Randomized Controlled Trials for COPD Pharmacotherapies
| Study Name | Study Type | Study Results | Drug Comparators | Duration |
|---|---|---|---|---|
| Tiotropium (LAMA)48 | ||||
| Niewoehner et al48 | A parallel-group, randomized, double-blind, placebo-controlled trial (N=1829) | Tiotropium 18 µg vs placebo reduced COPD exacerbations and related healthcare utilization in patients with moderate-to-severe COPD | Tiotropium vs placebo | 6 months |
| UPLIFT49 | A phase 3, randomized, double-blind, parallel-group, multicenter, placebo-controlled trial (N=5993) | Tiotropium 18 µg improved lung function, quality of life, and exacerbations (vs placebo) over 4 years, but did not significantly reduce the rate of decline in FEV1 | Tiotropium vs placebo | 4 years |
| TIOSPIR61 | A phase 3, double-blind, parallel-group, multicenter, randomized controlled trial (N=17,135) | Tiotropium SMI 5 µg and 2.5 µg were noninferior to tiotropium DPI 18 µg for mortality risk (both P<0.05) and not superior for exacerbation risk (P=0.42 and P=0.56, respectively) in this trial of 17,135 COPD patients | Tiotropium SMI 5 µg and 2.5 µg vs tiotropium DPI 18 µg | 2.3 years mean follow-up |
| Tiotropium + olodaterol (LAMA + LABA)88,89 | ||||
| WISDOM76,77 | A phase 4, double-blind, parallel-group, multicenter, randomized controlled trial (N=2485) | Stepwise ICS withdrawal (fluticasone propionate) was noninferior to ICS continuation for risk of moderate or severe exacerbations in patients with severe or very severe COPD receiving tiotropium + salmeterol. ICS withdrawal resulted in a modest decline in trough FEV1; in patients with high baseline blood eosinophils, ICS withdrawal resulted in increased COPD exacerbations | ICS (fluticasone propionate) withdrawal vs ICS continuation in patients on triple therapy (fluticasone propionate + tiotropium + salmeterol) | 52 weeks |
| Indacaterol + glycopyrronium (LABA + LAMA)99 | ||||
| FLAME90 | A phase 3, double-blind, double-dummy, noninferiority, multicenter, randomized controlled trial (N=3362) | Indacaterol + glycopyrronium significantly lowered the annual rate of moderate or severe exacerbations and significantly increased the time to first moderate or severe exacerbation or time to first severe exacerbation vs salmeterol + fluticasone in COPD patients with a history of at least one exacerbation in the previous year | Indacaterol + glycopyrronium vs salmeterol + fluticasone | 52 weeks |
| SUNSET72 | A phase 4, double-blind, triple-dummy, parallel-group, multicenter, randomized controlled, switch trial (N=1053) | Direct switch from long-term triple therapy to indacaterol + glycopyrronium did not impact COPD exacerbation risk in low-risk populations; patients with eosinophil counts ≥300/µL at both screening and baseline were more likely to benefit from continuing triple therapy | Indacaterol + glycopyrronium vs triple therapy (tiotropium + salmeterol + fluticasone) | 26 weeks |
| Fluticasone furoate + umeclidinium + vilanterol (ICS + LAMA + LABA) | ||||
| IMPACT73 | A phase 3, double-blind, parallel-group, multicenter, randomized controlled trial (N=10,355) | Fluticasone furoate + umeclidinium + vilanterol in patients with COPD and FEV1 predicted <50% normal and ≥1 moderate or severe exacerbation or FEV1 predicted 50%–80% normal and ≥1 severe or ≥2 moderate COPD exacerbations resulted in significantly lower moderate or severe COPD exacerbation rates vs fluticasone furoate + vilanterol or umeclidinium + vilanterol in patients with symptomatic COPD |
Triple therapy (fluticasone furoate + umeclidinium + vilanterol) vs fluticasone furoate + vilanterol or umeclidinium + vilanterol | 52-week treatment period |
| Beclomethasone dipropionate + formoterol fumarate + glycopyrronium bromide (ICS + LABA + LAMA)78,79 | ||||
| TRILOGY74 | A phase 3, double-blind, parallel-group, multicenter, randomized controlled trial (N=1368) | In symptomatic COPD patients, triple therapy with beclomethasone dipropionate + formoterol fumarate + glycopyrronium bromide significantly improved predose and 2-hour postdose FEV1 vs beclomethasone dipropionate + formoterol fumarate dual therapy. Triple therapy also significantly reduced the adjusted annual moderate-to-severe exacerbation frequencies vs dual bronchodilator therapy | Triple therapy (beclomethasone dipropionate + formoterol fumarate + glycopyrronium bromide) vs beclomethasone dipropionate + formoterol fumarate | 52-week treatment period |
| Budesonide + glycopyrronium + formoterol fumarate (ICS + LAMA + LABA) | ||||
| KRONOS75 | A phase 3, double-blind, parallel-group, multicenter, randomized controlled trial (N=1902) | Budesonide + glycopyrronium + formoterol fumarate MDI triple therapy was efficacious and well tolerated and showed improvements, including reduced COPD exacerbation rates, vs corresponding dual bronchodilator therapies in symptomatic patients with moderate-to-very severe COPD, irrespective of exacerbation history | Budesonide + glycopyrronium + formoterol fumarate via MDI vs glycopyrrolate + formoterol fumarate or budesonide + formoterol fumarate via MDI, or open-label budesonide + formoterol fumarate DPI | 24 weeks |
| ETHOS47 | A phase 3, double-blind, parallel-group, multicenter, randomized controlled trial (N=8564 [actual enrollment]) | Completed; results awaited. The trial will assess the efficacy and safety of triple therapy with budesonide + glycopyrronium + formoterol fumarate aerosol provided as MDI vs corresponding dual bronchodilator and bronchodilator + ICS therapies for COPD exacerbations in patients with moderate-to-very severe COPD | Budesonide + glycopyrronium + formoterol fumarate (2 regimens with different doses) vs budesonide + formoterol fumarate or glycopyrronium + formoterol fumarate | 52 weeks |
Abbreviations: COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MDI, metered-dose inhaler; SMI, soft mist inhaler.
Niewoehner et al first demonstrated that tiotropium significantly reduced COPD exacerbations in patients with moderate-to-severe COPD in a clinical trial of 6-month duration.48 This finding was further corroborated in the UPLIFT trial, a much larger phase 3 trial with approximately 6000 patients with moderate-to-very severe COPD who were followed for 4 years.49 Historically, tiotropium was delivered as a dry powder inhaled through HandiHaler® (tiotropium dry powder inhaler [DPI]), but later, it became available as an inhalation solution delivered via Respimat®, a soft mist inhaler (SMI; tiotropium SMI). Further development led to the dual bronchodilator formulation of tiotropium + olodaterol (5 + 5 µg; a fixed-dose LAMA + LABA). The development of tiotropium began with ipratropium, the first approved anticholinergic that has been shown to be safe and efficacious in both COPD and asthma treatment.50 In initial in vitro studies, tiotropium—although structurally similar to ipratropium—had a substantially higher affinity (6- to 20-fold), greater M3 receptor selectivity, and a slower dissociation profile and, hence, longer duration of action than ipratropium.51,52 The high potency, slow onset, and long duration of effect of tiotropium were confirmed in the airways of guinea pigs and humans.53 The dose-related bronchodilator activity of tiotropium in COPD was demonstrated in an open-label, dose-escalation, crossover, pilot study of five single-inhalation doses of tiotropium (10–160 µg; N=6).54 The efficacy of tiotropium in COPD was further confirmed in a phase 2, 4-week, dose-ranging RCT (N=169); the once-daily, 18-µg dose was well tolerated and, thus, selected for long-term trials.55 In a larger RCT (N=288), 18 µg once-daily tiotropium DPI was significantly more efficacious than ipratropium (40 µg, four times daily), with a comparable safety profile over 13 weeks.56 Patients in this trial continued into a 1-year RCT and their results, combined with results from another identical 1-year RCT of patients with COPD, confirmed an acceptable safety profile and significantly greater efficacy of tiotropium 18 µg once daily vs ipratropium in improving dyspnea, reducing exacerbations, and improving health-related QoL and lung function.40
The efficacy and tolerability of different doses of once-daily tiotropium SMI (5 and 10 µg) were compared with that of 18 μg tiotropium DPI in preliminary studies,57,58 and results facilitated the transition from tiotropium DPI to tiotropium SMI. Based on the available breadth of evidence, tiotropium DPI was used as an active comparator to assess the efficacy and safety of once-daily tiotropium SMI in pivotal trials. Further key trials of tiotropium monotherapy or dual bronchodilator therapy with tiotropium + olodaterol (5 + 5 µg) are summarized in Table 2. Of interest, initial studies of 1-year duration on efficacy and safety of tiotropium SMI indicated a numerical imbalance in the all-cause mortality signal in the tiotropium groups compared with placebo.59,60 However, this finding directly conflicted with the results of UPLIFT, a large phase 3 trial with approximately 6000 patients, in which tiotropium DPI treatment was associated with significantly lower mortality compared with placebo during the 4-year study duration.49 Subsequently, TIOSPIR, a large phase 3 trial with over 17,000 patients, which compared mortality and other safety endpoints between tiotropium SMI and tiotropium DPI, corroborated the findings concerning the safety of tiotropium.61 With comparable cardiovascular safety profiles for tiotropium SMI and tiotropium DPI, TIOSPIR refuted the concern that tiotropium SMI may have additional cardiovascular risks. This highlights the importance of large, well-designed, and adequately powered phase 3/4 RCTs to establish reliable efficacy and safety for new pharmacotherapies.
Similar to the clinical development of tiotropium, preclinical studies62,63 of roflumilast were followed by phase 1 and 2 clinical trials in healthy volunteers and patients with COPD, respectively.64,65 In the phase 2, crossover study, roflumilast significantly reduced the absolute number of neutrophils and eosinophils in the sputum of patients with COPD, and improved postbronchodilator forced expiratory volume in 1 s (FEV1) vs placebo.65 Later, results of a large phase 3 RCT (N=1411) confirmed the efficacy and safety of roflumilast in patients with moderate-to-severe COPD: postbronchodilator FEV1 was improved and exacerbations were reduced.66 Results of another large RCT (N=1513), however, did not demonstrate a reduction in exacerbations despite a significant improvement in lung function.67 A pooled post hoc analysis of two previous replicate RCTs (including the one reported by Calverley et al67) revealed that patients with chronic bronchitis and severe airflow obstruction with or without concurrent ICS use were most likely to benefit from roflumilast.68 This understanding subsequently led to two multicenter RCTs in a specific subset of patients with COPD (ie, aged >40 years with severe airflow limitation, symptoms of chronic bronchitis, and history of exacerbations).69 Indeed, the results demonstrated significant efficacy with roflumilast vs placebo; prebronchodilator FEV1 was significantly improved, and moderate (glucocorticosteroid treated) or severe exacerbations were reduced. In the phase 3/4, postmarketing Roflumilast and Exacerbations in patients receiving Appropriate Combination Therapy (REACT) trial (N=1945), roflumilast significantly decreased the rate of moderate and severe exacerbations when added to baseline ICS + LABA or triple therapy (LABA + LAMA + ICS) over a period of 1 year in patients with severe COPD, according to prespecified secondary endpoints that considered the use of antibiotics in the definitions.70 The primary endpoint (without consideration of antibiotics in moderate-to-severe exacerbations) was not met using Poisson regression (prespecified primary analysis method), although it was met using a negative binomial regression analysis. In the phase 4 Roflumilast Effect on Exacerbations in Patients on Dual (LABA + ICS) Therapy (RE2SPOND) trial, roflumilast reduced the rate of moderate and/or severe exacerbations in patients at risk for exacerbations despite treatment with ICS + LABA with or without a LAMA, although the results were not significantly different between the roflumilast and placebo groups.71
Large phase 3 RCTs also helped establish robust scientific evidence for efficacy and safety of ICS-containing therapeutic combinations (eg, LABA + ICS in the FLAME trial, LAMA + LABA + ICS in the SUNSET,72 IMPACT,73 TRILOGY,74 and KRONOS75 trials) in unique patient populations with COPD.
In addition to evidence generated from clinical trials, practitioners, regulatory authorities, and formulary decision-makers appreciate RWE generated from observational and pragmatic trials. Examples of real-life COPD administrative database- or claims database-based studies and nonrandomized and randomized COPD PrCTs are summarized in Table 3.
Table 3.
Examples of Real-World Studies for COPD Pharmacotherapies
| Study Name | Study Type | Study Aim/Results |
|---|---|---|
| Observational studies | ||
| DACCORD80 | Real-life, prospective, noninterventional study in which patients were treated at the physician’s discretion (N=1258) |
|
| OPTIMO91 | Real-life, prospective study in which patients were treated at the physician’s discretion (N=914) |
|
| Samp et al 201792 | Retrospective observational study based on an insurance claims database that included COPD patients in the United States treated with LAMA + LABA or ICS + LABA (N=478,772) |
|
| Voorham et al 201893 | Matched historical cohort study conducted using records from the OPCRD and CPRD primary care databases (N=1647) |
|
| Price et al 201894 | Matched historical cohort study of real-life management of COPD patients with or without comorbid asthma Data from the OPCRD and CPRD primary care databases on patients prescribed the salbutamol comparator or a reference product were evaluated (N=1191) |
|
| Pragmatic nonrandomized controlled trial | ||
| Nyberg et al 201795 | Prospective, multicenter, 12-month trial with planned enrollment of 96 patients with COPD from six participating primary care units in Sweden (N=96) |
|
| Pragmatic randomized controlled trial | ||
| CRYSTAL96 | Prospective, multicenter, 12-week, open-label, PrCT in COPD patients with moderate airflow limitation (N=4389) |
|
| Salford Lung Study81,82 | Prospective, multicenter, 12-month, open-label, phase 3 PrCT in COPD patients receiving regular maintenance via inhaler therapy (N=2799) |
|
| AIRWISE85 | Prospective, multicenter, 12-month, open-label, phase 4 PrCT with a planned enrollment of 3200 patients across community-based sites (N=3200 estimated) |
|
| RELIANCE86 | Multicenter, 36-month, parallel-group, noninferiority, phase 3 study with a planned enrollment of 3200 patients |
|
Abbreviations: COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; OPCRD, Optimum Patient Care Research Database; PrCT, pragmatic clinical trial.
Over the years, findings from observational studies have increased our knowledge and rekindled the evaluation of clinical conundrums by using real-world data to understand the implications of changes in COPD management. For example, the use or withdrawal of ICS and its effects on COPD management have been evaluated in RCTs.72,73,75–79 In DACCORD, a real-life, prospective, noninterventional study, patients were treated at the discretion of the physician.80 The results were in agreement with similar clinical trials76,99 and demonstrated that the risk of exacerbation over 2 years was not increased following ICS withdrawal in patients with low exacerbation risk. However, COPD phenotype groups who do benefit from ICS, including patients with elevated eosinophils and with a history of one or more exacerbations when FEV1 is compromised (<50% of predicted),77 have been identified in large RCTs.73,75,77
The findings from large, community-based PrCTs such as the Salford Lung Study in COPD81,82 have provided important RWE for COPD pharmacotherapy. The aim of the Salford Lung Study, a 12-month, open-label, randomized (1:1) phase 3 PrCT, was to evaluate the safety and effectiveness of fluticasone furoate + vilanterol (FF + VI; an ICS + LABA combination) vs usual care in patients with COPD and a history of exacerbations (N=2799) in a real-world setting (75 general practices in Salford and South Manchester, United Kingdom).81,82 Use of FF + VI was associated with significantly lower rates of moderate or severe exacerbations without increasing the risk of serious adverse events.82 However, the rate of first moderate or severe exacerbation in the time-to-event analysis was not significantly different between groups (hazard ratio for FF + VI vs usual care: 0.93; 95% CI: 0.85–1.02). Limitations of this study included the fact that COPD diagnosis was not confirmed by spirometry,81,83 and that the study population for FF + VI did not match the target population approved by the regulatory agency.84 In addition, 22% of the subjects receiving FF + VI switched back to their previous regimen.83 Although the latter finding is reflective of real-world settings, it complicates the ITT analyses.83 Owing to such limitations and the possibility of the Hawthorne effect, ie, altered behavior of patients from awareness of being observed, the classification of the Salford Lung Study as a PrCT and the credibility of its results have been questioned in the literature.84 Planned and ongoing PrCTs such as the Assessment In a Real World Setting of the Effect of Inhaled Steroid-based Triple Therapy Versus the Combination of Tiotropium and Olodaterol on Reducing COPD Exacerbations (AIRWISE)85 and Roflumilast or Azithromycin to Prevent COPD Exacerbations (RELIANCE) trials86 will also add to the accumulating RWE for COPD pharmacological treatments.
Efficacy and safety results of RCTs have been traditionally considered important by clinicians and regulatory authorities alike. However, generalizability of these results is limited because of highly restrictive inclusion and exclusion criteria. On the contrary, effectiveness and long-term safety results provided by real-world studies are accommodative of real-world patient populations and routine clinical practice but have multiple sources of bias for comparisons of multiple treatment arms and questionable internal validity. Considering the strengths and limitations of RCTs and real-world studies, clinicians should make data-driven decisions taking into account results from both types of clinical studies. Moreover, RCT and real-world observational study designs should be complementary in nature, such that, taken together, they provide more robust clinical evidence compared with individual study types.87
Conclusion
In summary, different study designs have their associated advantages and disadvantages. However, when used in concert, findings from various types of studies bring about progress in clinical research. Although RCTs are considered the “gold standard” for evidence on the safety and efficacy of an intervention, observational studies conducted in real-world settings provide evidence on the effectiveness of that intervention in clinical practice. PrCTs help to bridge the gap between classical explanatory RCTs and real-world studies, with a study design that leverages the advantage of randomization in a real-world scenario, thus providing a clearer picture of the safety and effectiveness of a drug.
Acknowledgments
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no direct compensation related to the development of the manuscript. Writing, editorial support, and formatting assistance were provided by Vidula Bhole, MD, MHSc, Saurabh Gagangras, PhD, and Maribeth Bogush, MCI, PhD, of Cactus Life Sciences (part of Cactus Communications), which was contracted and compensated by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) for these services. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Funding Statement
Financial support for the preparation of this article was provided by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Disclosure
Dr. Tashkin has participated as an advisory board member and speaker for Boehringer Ingelheim, AstraZeneca, and Sunovion. He has also worked as a consultant for Mylan and Innoviva/Theravance. He also reports personal fees from AstraZeneca, outside the submitted work. Dr. Amin has worked as a consultant and/or speaker for Boehringer Ingelheim, Sunovion, BMS, Pfizer, Portola, and AstraZeneca. He also reports personal fees from Boehringer Ingelheim, Sunovion, BMS, Pfizer, Portola, and Astra Zeneca, outside the submitted work. Dr. Kerwin has participated in consulting, advisory boards, and speaker panels for, or has received travel reimbursement from, Amphastar, AstraZeneca, Boehringer Ingelheim, Cipla, GlaxoSmithKline, Mylan, Novartis, Oriel, Pearl, Sunovion, Teva, and Theravance. He has conducted multicenter clinical research trials for approximately 40 pharmaceutical companies. The authors report no other conflicts of interest in this work.
References
- 1.Centers for Disease Control and Prevention. Descriptive and analytic studies; 2012. Available from: https://www.cdc.gov/globalhealth/healthprotection/fetp/training_modules/19/desc-and-analytic-studies_ppt_final_09252013.pdf. Accessed February26, 2019.
- 2.Zuidgeest MGP, Goetz I, Groenwold RHH, Irving E, van Thiel GJMW, Grobbee DE. Series: pragmatic trials and real world evidence: paper 1. Introduction. J Clin Epidemiol. 2017;88:7–13. doi: 10.1016/j.jclinepi.2016.12.023 [DOI] [PubMed] [Google Scholar]
- 3.Clancy MJ. Overview of research designs. Emerg Med J. 2002;19(6):546–549. doi: 10.1136/emj.19.6.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimes DA, Schulz KF. An overview of clinical research: the lay of the land. Lancet (London, England). 2002;359(9300):57–61. doi: 10.1016/S0140-6736(02)07283-5 [DOI] [PubMed] [Google Scholar]
- 5.U.S._Department_of_Health_and_Human_Services_Centers_for_Disease_Control_and_Prevention_CDC. Principles of Epidemiology in Public Health Practice. An Introduction to Applied Epidemiology and Biostatistics. 3 ed. U.S. Department of Health and Human Services_Centers for Disease Control and Prevention (CDC); 2012. Available from: https://www.cdc.gov/ophss/csels/dsepd/ss1978/SS1978.pdf. Accessed December12, 2018.
- 6.Mann CJ. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20(1):54–60. doi: 10.1136/emj.20.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sackett DL. Explanatory and pragmatic clinical trials: a primer and application to a recent asthma trial. Pol Arch Med Wewn. 2011;121(7–8):259–263. [PubMed] [Google Scholar]
- 8.U.S._Department_of_Health_and_Human_Services_Food_and_Drug_Administration. The drug development process 2018. Available from: https://www.fda.gov/ForPatients/Approvals/Drugs/default.htm. Accessed May7, 2020.
- 9.United States Food and Drug Administration. Inside clinical trials: testing medical products in people; 2014. Available from: https://www.fda.gov/drugs/drug-information-consumers/inside-clinical-trials-testing-medical-products-people. Accessed March12, 2020.
- 10.Hill TP. Conducting phase IV clinical studies: a moral imperative? ecancermedicalscience. 2012;6:276. doi: 10.3332/ecancer.2012.276 [DOI] [Google Scholar]
- 11.Haque A, Daniel S, Maxwell T, Boerstoel M. Postmarketing surveillance studies-an industry perspective on changing global requirements and implications. Clin Ther. 2017;39(4):675–685. doi: 10.1016/j.clinthera.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 12.Suvarna V. Phase IV of drug development. Perspect Clin Res. 2010;1(2):57–60. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Zhang Y, Ye X, Guo X, Zhang T, He J. Overview of phase IV clinical trials for postmarket drug safety surveillance: a status report from the ClinicalTrials.gov registry. BMJ Open. 2016;6(11):e010643. doi: 10.1136/bmjopen-2015-010643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S._Department_of_Health_and_Human_Services_Food_and_Drug_Administration. Guidance for industry expedited programs for serious conditions – drugs and biologics 2014. Available from: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM358301.pdf. Accessed May7, 2020.
- 15.United States Food and Drug Administration. Non-inferiority clinical trials to establish effectiveness: guidance for industry 2016. Available from: https://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf. Accessed March 14, 2019.
- 16.Peduzzi P, Henderson W, Hartigan P, Lavori P. Analysis of randomized controlled trials. Epidemiol Rev. 2002;24(1):26–38. doi: 10.1093/epirev/24.1.26 [DOI] [PubMed] [Google Scholar]
- 17.Eriksson G, Calverley PM, Jenkins CR, et al. The effect of COPD severity and study duration on exacerbation outcome in randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2017;12:1457–1468. doi: 10.2147/COPD.S130713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayre JW, Toklu HZ, Ye F, Mazza J, Yale S. Case reports, case series - from clinical practice to evidence-based medicine in graduate medical education. Cureus. 2017;9(8):e1546. doi: 10.7759/cureus.1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrison LP Jr., Neumann PJ, Erickson P, Marshall D, Mullins CD. Using real-world data for coverage and payment decisions: the ISPOR real-world data task force report. Value Health. 2007;10(5):326–335. doi: 10.1111/j.1524-4733.2007.00186.x [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Statistics notes: matching. BMJ (Clinical Research Ed). 1994;309(6962):1128. doi: 10.1136/bmj.309.6962.1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624 [DOI] [PubMed] [Google Scholar]
- 22.Gamerman V, Cai T, Elsäßer A. Pragmatic randomized clinical trials: best practices and statistical guidance. Health Serv Outcomes Res Methodol. 2018;19:23–25. doi: 10.1007/s10742-018-0192-5 [DOI] [Google Scholar]
- 23.Woodcock A, Bakerly ND, New JP, et al. The Salford Lung Study protocol: a pragmatic, randomised phase III real-world effectiveness trial in asthma. BMC Pulm Med. 2015;15:160. doi: 10.1186/s12890-015-0150-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins R, Bowman L, Landray M, Peto R. The magic of randomization versus the myth of real-world evidence. N Engl J Med. 2020;382(7):674–678. doi: 10.1056/NEJMsb1901642 [DOI] [PubMed] [Google Scholar]
- 25.Anderson ML, Griffin J, Goldkind SF, et al. The Food and Drug Administration and pragmatic clinical trials of marketed medical products. Clin Trials. 2015;12(5):511–519. doi: 10.1177/1740774515597700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ (Clinical Research Ed). 2015;350:h2147. doi: 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 27.Goble JA. The potential effect of the 21st Century Cures Act on drug development. J Manag Care Spec Pharm. 2018;24(7):677–681. doi: 10.18553/jmcp.2018.24.7.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buesching DP, Luce BR, Berger ML. The role of private industry in pragmatic comparative effectiveness trials. J Comp Eff Res. 2012;1(2):147–156. doi: 10.2217/cer.12.9 [DOI] [PubMed] [Google Scholar]
- 29.Bogart M, Stanford RH, Laliberte F, Germain G, Wu JW, Duh MS. Medication adherence and persistence in chronic obstructive pulmonary disease patients receiving triple therapy in a USA commercially insured population. Int J Chron Obstruct Pulmon Dis. 2019;14:343–352. doi: 10.2147/COPD.S184653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang IK, Morikawa T. Design issues in noninferiority/equivalence trials. Drug Inf J. 1999;33(4):1205–1218. doi: 10.1177/009286159903300424 [DOI] [Google Scholar]
- 31.Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13(2):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moller HJ. Effectiveness studies: advantages and disadvantages. Dialogues Clin Neurosci. 2011;13(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Althunian TA, de Boer A, Groenwold RHH, Klungel OH. Defining the noninferiority margin and analysing noninferiority: an overview. Br J Clin Pharmacol. 2017;83(8):1636–1642. doi: 10.1111/bcp.13280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials. 2011;12:106. doi: 10.1186/1745-6215-12-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: the importance of rigorous methods. BMJ (Clinical Research Ed). 1996;313(7048):36–39. doi: 10.1136/bmj.313.7048.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patient-Centered Outcomes Research Institute. PCORI dissemination & implementation framework. Available from: https://www.pcori.org/sites/default/files/PCORI-Dissemination-Implementation-Framework.pdf. Accessed March9, 2020.
- 37.National Heart, Lung, and Blood Institute Strategic Visioning. Setting an agenda together for the NHLBI of 2025. Available from: https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.115.015712. Accessed March9, 2020. [DOI] [PMC free article] [PubMed]
- 38.Elmes PC, Fletcher CM, Dutton AA. Prophylactic use of oxytetracycline for exacerbations of chronic bronchitis. Br Med J. 1957;2(5056):1272–1275. doi: 10.1136/bmj.2.5056.1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.May JR, Oswald NC. Long-term chemotherapy in chronic bronchitis. Lancet (London, England). 1956;271(6947):814–818. doi: 10.1016/s0140-6736(56)92245-0 [DOI] [PubMed] [Google Scholar]
- 40.Vincken W, van Noord JA, Greefhorst AP, et al. Improved health outcomes in patients with COPD during 1 yr’s treatment with tiotropium. Eur Respir J. 2002;19(2):209–216. doi: 10.1183/09031936.02.00238702 [DOI] [PubMed] [Google Scholar]
- 41.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi: 10.7326/0003-4819-106-2-196 [DOI] [PubMed] [Google Scholar]
- 42.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi: 10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nonoyama ML, Brooks D, Guyatt GH, Goldstein RS. Effect of oxygen on health quality of life in patients with chronic obstructive pulmonary disease with transient exertional hypoxemia. Am J Respir Crit Care Med. 2007;176(4):343–349. doi: 10.1164/rccm.200702-308OC [DOI] [PubMed] [Google Scholar]
- 44.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93(3):391–398. doi: 10.7326/0003-4819-93-3-391 [DOI] [PubMed] [Google Scholar]
- 45.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet (London, England). 1981;1(8222):681–686. Available from: https://pubmed.ncbi.nlm.nih.gov/6110912/ [PubMed] [Google Scholar]
- 46.Albert RK, Au DH, et al.; Long-Term_Oxygen_Treatment_Trial_Research_Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617–1627. doi: 10.1056/NEJMoa1604344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearl Therapeutics Inc. Study to assess the efficacy and safety of PT010 relative to PT003 and PT009 in subjects with moderate to very severe COPD (Ethos); 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT02465567. Accessed December03, 2018.
- 48.Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143(5):317–326. doi: 10.7326/0003-4819-143-5-200509060-00007 [DOI] [PubMed] [Google Scholar]
- 49.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 50.Gross NJ. Ipratropium bromide. N Engl J Med. 1988;319(8):486–494. doi: 10.1056/NEJM198808253190806 [DOI] [PubMed] [Google Scholar]
- 51.Haddad EB, Mak JC, Barnes PJ. Characterization of [3H]Ba 679 BR, a slowly dissociating muscarinic antagonist, in human lung: radioligand binding and autoradiographic mapping. Mol Pharmacol. 1994;45(5):899–907. [PubMed] [Google Scholar]
- 52.Disse B, Reichl R, Speck G, Traunecker W, Ludwig Rominger KL, Hammer R. Ba 679 BR, a novel long-acting anticholinergic bronchodilator. Life Sci. 1993;52(5–6):537–544. doi: 10.1016/0024-3205(93)90312-Q [DOI] [PubMed] [Google Scholar]
- 53.Takahashi T, Belvisi MG, Patel H, et al. Effect of Ba 679 BR, a novel long-acting anticholinergic agent, on cholinergic neurotransmission in guinea pig and human airways. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1640–1645. doi: 10.1164/ajrccm.150.6.7952627 [DOI] [PubMed] [Google Scholar]
- 54.Maesen FP, Smeets JJ, Costongs MA, Wald FD, Cornelissen PJ. Ba 679 Br, a new long-acting antimuscarinic bronchodilator: a pilot dose-escalation study in COPD. Eur Respir J. 1993;6(7):1031–1036. [PubMed] [Google Scholar]
- 55.Littner MR, Ilowite JS, Tashkin DP, et al. Long-acting bronchodilation with once-daily dosing of tiotropium (Spiriva) in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1136–1142. doi: 10.1164/ajrccm.161.4.9903044 [DOI] [PubMed] [Google Scholar]
- 56.van Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ. A randomised controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax. 2000;55(4):289–294. doi: 10.1136/thorax.55.4.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caillaud D, Le Merre C, Martinat Y, Aguilaniu B, Pavia D. A dose-ranging study of tiotropium delivered via Respimat Soft Mist Inhaler or Handihaler in COPD patients. Int J Chron Obstruct Pulmon Dis. 2007;2(4):559–565. [PMC free article] [PubMed] [Google Scholar]
- 58.van Noord JA, Cornelissen PJ, Aumann JL, Platz J, Mueller A, Fogarty C. The efficacy of tiotropium administered via Respimat Soft Mist Inhaler or HandiHaler in COPD patients. Respir Med. 2009;103(1):22–29. doi: 10.1016/j.rmed.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 59.Bateman E, Singh D, Smith D, et al. Efficacy and safety of tiotropium Respimat SMI in COPD in two 1-year randomized studies. Int J Chron Obstruct Pulmon Dis. 2010;5:197–208. [PMC free article] [PubMed] [Google Scholar]
- 60.Bateman ED, Tashkin D, Siafakas N, et al. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010;104(10):1460–1472. doi: 10.1016/j.rmed.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 61.Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat Inhaler and the risk of death in COPD. N Engl J Med. 2013;369(16):1491–1501. doi: 10.1056/NEJMoa1303342 [DOI] [PubMed] [Google Scholar]
- 62.Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, Beume R. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther. 2001;297(1):280–290. [PubMed] [Google Scholar]
- 63.Wollin L, Bundschuh DS, Wohlsen A, Marx D, Beume R. Inhibition of airway hyperresponsiveness and pulmonary inflammation by roflumilast and other PDE4 inhibitors. Pulm Pharmacol Ther. 2006;19(5):343–352. doi: 10.1016/j.pupt.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 64.David M, Zech K, Seiberling M, Weimar C, Bethke T. Roflumilast, a novel, oral, selective PDE4 inhibitor, shows high absolute bioavailability. J Allergy Clin Immunol Immunol. 2004;113(2):S220–S221. doi: 10.1016/j.jaci.2004.01.246 [DOI] [Google Scholar]
- 65.Grootendorst DC, Gauw SA, Verhoosel RM, et al. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax. 2007;62(12):1081–1087. doi: 10.1136/thx.2006.075937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast–an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet (London, England). 2005;366(9485):563–571. doi: 10.1016/S0140-6736(05)67100-0 [DOI] [PubMed] [Google Scholar]
- 67.Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–161. doi: 10.1164/rccm.200610-1563OC [DOI] [PubMed] [Google Scholar]
- 68.Rennard SI, Calverley PM, Goehring UM, Bredenbroker D, Martinez FJ. Reduction of exacerbations by the PDE4 inhibitor roflumilast-the importance of defining different subsets of patients with COPD. Respir Res. 2011;12:18. doi: 10.1186/1465-9921-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet (London, England). 2009;374(9691):685–694. doi: 10.1016/S0140-6736(09)61255-1 [DOI] [PubMed] [Google Scholar]
- 70.Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet (London, England). 2015;385(9971):857–866. doi: 10.1016/S0140-6736(14)62410-7 [DOI] [PubMed] [Google Scholar]
- 71.Martinez FJ, Rabe KF, Sethi S, et al. Effect of roflumilast and inhaled corticosteroid/long-acting beta2-agonist on chronic obstructive pulmonary disease exacerbations (RE(2)SPOND). A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(5):559–567. doi: 10.1164/rccm.201607-1349OC [DOI] [PubMed] [Google Scholar]
- 72.Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329–339. doi: 10.1164/rccm.201803-0405OC [DOI] [PubMed] [Google Scholar]
- 73.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 74.Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet (London, England). 2016;388(10048):963–973. doi: 10.1016/S0140-6736(16)31354-X [DOI] [PubMed] [Google Scholar]
- 75.Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi: 10.1016/S2213-2600(18)30327-8 [DOI] [PubMed] [Google Scholar]
- 76.Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi: 10.1056/NEJMoa1407154 [DOI] [PubMed] [Google Scholar]
- 77.Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398. doi: 10.1016/S2213-2600(16)00100-4 [DOI] [PubMed] [Google Scholar]
- 78.Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet (London, England). 2017;389(10082):1919–1929. doi: 10.1016/S0140-6736(17)30188-5 [DOI] [PubMed] [Google Scholar]
- 79.Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet (London, England). 2018;391(10125):1076–1084. doi: 10.1016/S0140-6736(18)30206-X [DOI] [PubMed] [Google Scholar]
- 80.Vogelmeier C, Worth H, Buhl R, et al. “Real-life” inhaled corticosteroid withdrawal in COPD: a subgroup analysis of DACCORD. Int J Chron Obstruct Pulmon Dis. 2017;12:487–494. doi: 10.2147/COPD.S125616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bakerly ND, Woodcock A, New JP, et al. The Salford Lung Study protocol: a pragmatic, randomised phase III real-world effectiveness trial in chronic obstructive pulmonary disease. Respir Res. 2015;16:101. doi: 10.1186/s12931-015-0267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vestbo J, Leather D, Diar Bakerly N, et al. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med. 2016;375(13):1253–1260. doi: 10.1056/NEJMoa1608033 [DOI] [PubMed] [Google Scholar]
- 83.Girodet P-O, Raherison C, Molimard M. Effectiveness of fluticasone furoate–vilanterol in COPD. N Engl J Med. 2016;375(26):2605–2607. doi: 10.1056/NEJMc1613713 [DOI] [PubMed] [Google Scholar]
- 84.Dal-Re R. Could phase 3 medicine trials be tagged as pragmatic? A case study: the Salford COPD trial. J Eval Clin Pract. 2018;24(1):258–261. doi: 10.1111/jep.12796 [DOI] [PubMed] [Google Scholar]
- 85.Boehringer_Ingelheim. The AIRWISE study: assessment in a real world setting of the effect of inhaled steroid-based triple therapy versus the combination of tiotropium and olodaterol on reducing chronic obstructive pulmonary disease (COPD) exacerbations [AIRWISE]; August 2017. [updated December 2017] Available from: https://clinicaltrials.gov/ct2/show/NCT03265145. Accessed January 2018.
- 86.National Institutes of Health U.S. National Library of Medicine. Roflumilast or azithromycin to prevent COPD exacerbations (RELIANCE) (RELIANCE; NCT04069312). Available from: https://clinicaltrials.gov/ct2/show/NCT04069312. Accessed March9, 2020.
- 87.Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018;33(34):e213. doi: 10.3346/jkms.2018.33.e213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–1319. doi: 10.1016/j.rmed.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 89.Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6(5):337–344. doi: 10.1016/S2213-2600(18)30102-4 [DOI] [PubMed] [Google Scholar]
- 90.Wedzicha JA, Zhong N, Ichinose M, et al. Indacaterol/glycopyrronium versus salmeterol/fluticasone in Asian patients with COPD at a high risk of exacerbations: results from the FLAME study. Int J Chron Obstruct Pulmon Dis. 2017;12:339–349. doi: 10.2147/COPD.S125058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossi A, Guerriero M, Corrado A. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15:77. doi: 10.1186/1465-9921-15-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Samp JC, Joo MJ, Schumock GT, Calip GS, Pickard AS, Lee TA. Comparative effectiveness of long-acting beta2 -agonist combined with a long-acting muscarinic antagonist or inhaled corticosteroid in chronic obstructive pulmonary disease. Pharmacotherapy. 2017;37(4):447–455. doi: 10.1002/phar.1913 [DOI] [PubMed] [Google Scholar]
- 93.Voorham J, Kerkhof M, Scuri M, et al. Late breaking abstract - comparative real world effectiveness of triple therapy versus dual bronchodilation in frequently exacerbating patients with COPD. Eur Respir J. 2018;52(suppl 62). doi: 10.1183/13993003.01675-2018 [DOI] [Google Scholar]
- 94.Price DB, Gefen E, Gopalan G, et al. Real-life effectiveness and safety of salbutamol Steri-Neb vs. Ventolin Nebules(R) for exacerbations in patients with COPD: historical cohort study. PLoS One. 2018;13(1):e0191404. doi: 10.1371/journal.pone.0191404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nyberg A, Wadell K, Lindgren H, Tistad M. Internet-based support for self-management strategies for people with COPD-protocol for a controlled pragmatic pilot trial of effectiveness and a process evaluation in primary healthcare. BMJ Open. 2017;7(7):e016851. doi: 10.1136/bmjopen-2017-016851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vogelmeier CF, Gaga M, Aalamian-Mattheis M, et al. Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial. Respir Res. 2017;18(1):140. doi: 10.1186/s12931-017-0622-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE special task force on real-world evidence in health care decision making. Value Health. 2017;20(8):1003–1008. doi: 10.1016/j.jval.2017.08.3019 [DOI] [PubMed] [Google Scholar]
- 98.Singal AG, Higgins PD, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5:e45. doi: 10.1038/ctg.2013.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rossi A, van der Molen T, Del Olmo R, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548–1556. doi: 10.1183/09031936.00126814 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Centers for Disease Control and Prevention. Descriptive and analytic studies; 2012. Available from: https://www.cdc.gov/globalhealth/healthprotection/fetp/training_modules/19/desc-and-analytic-studies_ppt_final_09252013.pdf. Accessed February26, 2019.
- U.S._Department_of_Health_and_Human_Services_Centers_for_Disease_Control_and_Prevention_CDC. Principles of Epidemiology in Public Health Practice. An Introduction to Applied Epidemiology and Biostatistics. 3 ed. U.S. Department of Health and Human Services_Centers for Disease Control and Prevention (CDC); 2012. Available from: https://www.cdc.gov/ophss/csels/dsepd/ss1978/SS1978.pdf. Accessed December12, 2018.
- U.S._Department_of_Health_and_Human_Services_Food_and_Drug_Administration. The drug development process 2018. Available from: https://www.fda.gov/ForPatients/Approvals/Drugs/default.htm. Accessed May7, 2020.
- United States Food and Drug Administration. Inside clinical trials: testing medical products in people; 2014. Available from: https://www.fda.gov/drugs/drug-information-consumers/inside-clinical-trials-testing-medical-products-people. Accessed March12, 2020.
- U.S._Department_of_Health_and_Human_Services_Food_and_Drug_Administration. Guidance for industry expedited programs for serious conditions – drugs and biologics 2014. Available from: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM358301.pdf. Accessed May7, 2020.
- United States Food and Drug Administration. Non-inferiority clinical trials to establish effectiveness: guidance for industry 2016. Available from: https://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf. Accessed March 14, 2019.
- Patient-Centered Outcomes Research Institute. PCORI dissemination & implementation framework. Available from: https://www.pcori.org/sites/default/files/PCORI-Dissemination-Implementation-Framework.pdf. Accessed March9, 2020.
- National Heart, Lung, and Blood Institute Strategic Visioning. Setting an agenda together for the NHLBI of 2025. Available from: https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.115.015712. Accessed March9, 2020. [DOI] [PMC free article] [PubMed]
- Pearl Therapeutics Inc. Study to assess the efficacy and safety of PT010 relative to PT003 and PT009 in subjects with moderate to very severe COPD (Ethos); 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT02465567. Accessed December03, 2018.
- Boehringer_Ingelheim. The AIRWISE study: assessment in a real world setting of the effect of inhaled steroid-based triple therapy versus the combination of tiotropium and olodaterol on reducing chronic obstructive pulmonary disease (COPD) exacerbations [AIRWISE]; August 2017. [updated December 2017] Available from: https://clinicaltrials.gov/ct2/show/NCT03265145. Accessed January 2018.
- National Institutes of Health U.S. National Library of Medicine. Roflumilast or azithromycin to prevent COPD exacerbations (RELIANCE) (RELIANCE; NCT04069312). Available from: https://clinicaltrials.gov/ct2/show/NCT04069312. Accessed March9, 2020.