Abstract
Abnormally invasive placenta (AIP) is a potentially severe condition. To date, arterial embolization in women with postpartum hemorrhage due to AIP is the treatment option for which highest degrees of evidence are available. However, other techniques have been tested, including prophylactic catheter placement, balloon occlusion of the iliac arteries and abdominal aorta balloon occlusion. In this systematic review, we provide an overview of the currently reported interventional radiology procedures that are used for the treatment of postpartum hemorrhage due to AIP and suggest recommendations based on current evidences. Owing to a high rate of adverse events, prophylactic occlusion of internal iliac arteries should be used with caution and applied when the endpoint is hysterectomy. On the opposite, when a conservative management is considered to preserve future fertility, uterine artery embolization should be the preferred option as it is associated with a hysterectomy rate of 15.5% compared to 76.5% with prophylactic balloon occlusion of the internal iliac arteries and does not result in fetal irradiation. Limited data are available regarding the application of systematic prophylactic embolization and no comparative studies with arterial embolization are available.
Keywords: Postpartum hemorrhage, embolization, therapeutic, placenta, balloon occlusion, interventional radiology
Introduction
Abnormally invasive placenta (AIP) is defined by an abnormal invasion of the placental chorionic villi beyond the decidua basalis (1). AIP is classically classified as accreta, increta and percreta, depending on the depth of penetration of the chorionic villi into the myometrium, with placenta percreta being the least common but most severe form of AIP (1). The incidence of AIP parallels that of cesarean deliveries, Between the 1960s and 2002, the incidence of AIP increased from 1 in 30,000 pregnancies to 1 in 533; thus corresponding to a nearly 60-fold increase in 5 decades (2). AIP is a potentially severe condition that can lead to significant maternal morbidity and mortality (2). The diagnosis of AIP can be made or suspected on the basis of imaging findings before delivery using ultrasound and magnetic resonance imaging which enables early identification of women with high risk of hemorrhage and plan the delivery (1,3-5).
The management of women presenting with AIP depends on local preferences. AIP can be managed using cesarean hysterectomy (the placenta is removed along with the uterus at the penalty of no future fertility), a full conservative management (the placenta is left in situ, thus preserving future fertility) or an extirpative approach (the placenta is removed along with invaded myometrium) (6-8).
Uterine artery embolization consists in occluding the uterine arteries and other arteries that participate to the uteroplacental complex vascularization using a percutaneous approach with specific occluding agents (9). However, in the setting of uterine artery embolization, postpartum hemorrhage due to AIP is challenging because of specific variables. First, it is often performed in emergency with potentially hemodynamically unstable women (9). Second, angiographic findings are often unusual, resulting in more complex and longer procedure time (10). Finally, AIP is a main cause of failed arterial embolization, with the need for repeat embolization in a non-negligible proportion of women (7,11,12).
Currently, arterial embolization in women with postpartum hemorrhage due to AIP is the therapeutic option that conveys highest degrees of evidence (10-15). However, other approaches have been tested, including prophylactic catheter placement, balloon occlusion of the iliac arteries and abdominal aorta balloon occlusion (16-20).
The purpose of this systematic review was to provide an overview of the currently reported interventional radiology procedures that are used for the treatment of postpartum hemorrhage due to AIP and suggest recommendations based on current evidences.
Search strategy, selection and data analysis
A computer-assisted literature search was performed by one radiologist to identify articles related to role of interventional radiology in the management of AIP. A literature search strategy was conducted first (Table 1). The MEDLINE, EMBASE, and Cochrane databases, from January 2008 to September 2019 inclusively were checked for relevant articles with the following MeSH terms and free keywords: “Abnormally invasive placenta”, “Placental abnormalities”, “Placenta accreta”, “Placenta accreta spectrum”, “Placenta increta”, “Placenta percreta”, “Placenta previa”, “Postpartum hemorrhage”, “Aorta, Abdominal”, “Balloon Occlusion”, “Common iliac artery”, “Embolization, Therapeutic”, ”Endovascular procedures”, “Iliac Artery”, “Internal iliac artery”, “Therapeutic Occlusion”, “Radiology, Interventional”, “Uterine artery” and “Uterine Artery Embolization”. An expanded search was used using Boolean operators. Review articles, letters, editorials, comments, case reports, unpublished articles, and articles without inclusion of raw data were not selected. When articles were considered eligible on the basis of their titles, their abstracts were analyzed to determine suitability for inclusion. The search was limited to studies published in English and involving humans. The list of articles was supplemented by cross-checking of the reference lists of all potentially relevant articles and by a hand search of references of all available reviews. An update was made to search for additional studies published after September 2019 before article submission. Table 2 shows details of the computer-assisted literature search.
Table1. Search strategy table.
| Patients | Boolean operator | Intervention |
|---|---|---|
| Abnormally invasive placenta [key word] | AND | Aorta, Abdominal [MeSH] |
| Placental abnormalities [key word] | Balloon Occlusion [MeSH] | |
| Placenta Accreta [MeSH] | Common iliac artery [key word] | |
| Placenta accreta spectrum [key word] | Embolization, Therapeutic [MeSH] | |
| Placenta increta [key word] | Endovascular Procedures [MeSH] | |
| Placenta percreta [key word] | Iliac Artery [MeSH] | |
| Placenta Praevia [MeSH] | Internal iliac artery [key word] | |
| Postpartum Hemorrhage [MeSH] | Intervention [key word] | |
| Therapeutic Occlusion [MeSH] | ||
| Radiology, Interventional [MeSH] | ||
| Uterine Artery [MeSH] | ||
| Uterine Artery Embolization [MeSH] |
MeSH indicates Medical Subject Heading. Limits: human and publication date from January 2007and September 2019 inclusively.
Table 2. Characteristics and results of the computer-assisted literature search strategy.
| Search terms | Numbers of hits | ||
|---|---|---|---|
| PubMed* | Embase† | Cochraneǂ | |
| #1. “Abnormally invasive placenta” [key word] | 105 | 87 | 7 |
| #2. “Placental abnormalities” [key word] | 206 | 208 | 14 |
| #3. “Placenta Accreta” [MeSH] | 1,247 | 2,308 | 61 |
| #4. “Placenta accreta spectrum” [key word] | 88 | 168 | 1 |
| #5. “Placenta increta” [key word] | 170 | 528 | 18 |
| #6. “Placenta percreta” [key word] | 307 | 763 | 18 |
| #7. “Placenta Previa” [MeSH] | 854 | 3,133 | 8 |
| #8. “Postpartum Hemorrhage” [MeSH] | 3,732 | 8,346 | 61 |
| #9. “Aorta, Abdominal” [MeSH] | 1,949 | 6,075 | 20 |
| #10. “Balloon Occlusion” [MeSH] | 1,141 | 5,025 | 10 |
| #11. “Common iliac artery” [key word] | 440 | 2,033 | 5 |
| #12. “Embolization, Therapeutic” [MeSH] | 13,924 | 2,210 | 7 |
| #13. “Endovascular Procedures” [MeSH] | 41,177 | 6,681 | 27 |
| #14. “Iliac Artery” [MeSH] | 1,762 | 6,268 | 8 |
| #15. “Internal iliac artery” [key word] | 468 | 1,921 | 1 |
| #16. “Therapeutic Occlusion” [MeSH] | 14,014 | 2,679 | 6 |
| #17. “Radiology, Interventional” [MeSH] | 544 | 10,920 | 60 |
| #18. “Uterine Artery” [MeSH] | 1,252 | 3,445 | 18 |
| #19. “Uterine Artery Embolization” [MeSH] | 943 | 1,223 | 3 |
| #1 and #13 | 0 | 2 | 0 |
| #3 and #13 | 11 | 12 | 0 |
| #3 and #16 | 233 | 5 | 0 |
| #6 and #13 | 2 | 3 | 0 |
| #8 and #10 | 259 | 35 | 4 |
| #8 and # 11 | 1 | 27 | 0 |
| #8 and #19 | 170 | 221 | 1 |
MeSH indicates Medical Subject Heading. *, limits: human, female and publication date from January 2007 and September 2019 inclusively. †, limits: human, women, article, publication date from January 2007 and September 2019 inclusively, and article. ǂ, limits: publication date from January 2007and September 2019 inclusively.
Two radiologists with an experience of 30 and 5 years in endovascular procedures independently checked each identified article for fulfillment of inclusion criteria. The full text of relevant articles was analyzed and disagreement resolved by consensus. Inclusion criteria were as follows: (I) articles were written in English; (II) an interventional radiology technique (i.e., uterine artery embolization, balloon occlusion or intra-arterial catheter placement) was used; (III) the study population had at least 10 women with confirmed AIP; and (IV) when data were presented in more than one article, the most recent one was selected. Studies reporting women with placenta previa only and those in which AIP could not be individualized from a more general group of women with “placenta previa”, “abnormal placentation” or “pernicious placenta previa” were excluded.
The same radiologists independently extracted relevant data about study characteristics. They include: (I) name of first author; (II) year of publication; (III) number of women with AIP; (IV) respective numbers of placentas accreta, increta and percreta; (V) type of intervention; (VI) Rate of hysterectomy; (VII) estimated blood loss (EBL); (VIII) rate of complications due to the intervention; and (IX) radiation dose.
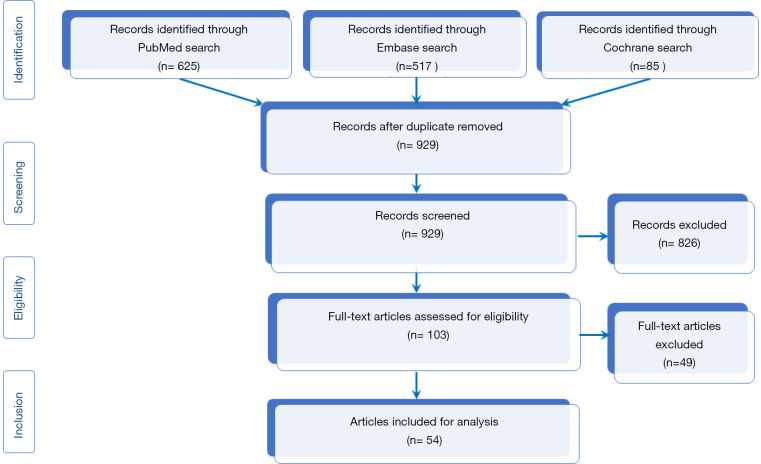
After exclusion of duplicate articles and those that did not fulfill the inclusion criteria a total of 54 articles were selected and ultimately analyzed (Table 3). Figure 1 shows the PRISMA flow diagram for the inclusion/exclusion of the studies.
Table 3. Main characteristics of studies included for analysis.
| First author, year, Ref | Number of AIP patients | AIP type A/I/P |
Type of intervention | Rate of hysterectomy (%) | Estimated blood loss (mL) | Complication rate (%) | Radiation dose | Major points |
|---|---|---|---|---|---|---|---|---|
| Shrivastava et al. 2007 (21) | 19 | 13/4/2 | PBO IIA (19/19) | 100 (19/19) | 2,700 (up to 8,000) | 16 (3/19) | NR | No differences in EBL and hysterectomy compared to no PBO |
| Tan et al. 2007 (22) | 11 | 3/1/7 | PBO IIA | 64 (7/11) | 2,011 (up to 5,000) | 0 (0/11) | N.R | Lower EBL with PBO (P=0.042) |
| Mok et al. 2008 (23) | 12 | 4/0/0 | PBO IIA (6/12) | 33 (4/12) | 6,415 (up to 14,000 | 0 (0/12) | NR | – |
| Yu et al. 2009 (24) |
11 | 4/3/4 | PUAE | 27 (3/11) | 3,190 (up to 13,000) | 0 (0/11) | N.R | – |
| Diop et al. 2010 (25) | 17 | Accreta* | UAE | 6 (1/17) | 1,929 | 0 (0/17) | NR | – |
| Sivan et al. 2010 (26) | 25 | 25/0/0 | PCIIA (25/25) + UAE (23/25) | 8 (2/25) | 2,000 (up to 9,000) | 0 (0/25) | NR | – |
| Carnevale et al. 2011 (27) | 21 | 18/0/3 | PBO IIA | 100 (21/21) | 1,671 (up to 4,000) | 10 (2/21) | NR | Thromboembolectomy in two women |
| Amsellem et al. 2011 (28) | 10 | Accreta* | PBO IIA | 40 (4/10) | 900±754 | 0 (0/10) | NR | The endpoint was a conservative approach |
| Jung et al. 2011 (29) | 17 | Accreta* | UAE | 18 (3/17) | 1,823 (up to 4,500) | 0 (0/17) | NR | – |
| Soyer et al. 2011 (9) | 12 | 4/2/6 | UAE | 18 (2/11) | > 1,000 | 8 (1/12) | Skin dose 175.89 cGy (range, 90.21–351.78) | Clinical success of UAE in 82% of women |
| Ballas et al. 2012 (30) | 59 | 24/0/35 | PBO IIA | NR | 2,165 | 3.4 (2/59) | NR | Case control study; EBL was greater in women with inflated balloons |
| Bouvier et al. 2012 (31) | 14 | 10/0/4 | UAE | 43 (6/14) | 2,242 (up to 3,500) | 0 (0/14) | NR | – |
| Li et al. 2012 (32) |
10 | 6/4/0 | UAE | 0 (0/10) | >500 | 10 (1/10) | NR | Secondary PPH conservative treatment |
| Panici et al. 2012 (33) | 15 | 13/2/0 | PBO AA | 13 (2/15) | 950 | 0 (0/15) | 0.1 mGy | Case control study; greater EBL without PBOAA (3,375 mL) |
| Claussen et al. 2013 (34) | 15 | 15/0/0 | PBO IIA | 53 (8/15) | 4,150 (up to 16,000) | 0 (0/15) | NR | EBL > 6,000 mL in 5 women |
| Hwang et al. 2013 (14) | 40 | Accreta* | UAE | 7.5 (3/40) | NR | 0 (0/40) | NR | Clinical success of UAE in 92.5% of women; one death due to DIC |
| Teixidor Viñas et al. 2014 (35) | 27 | 8/2/17 | PBO IIA [27]; PBO IIA + UAE [8] | 11 (3/27) | 1,920 (up to 12,000) | 4 (1/27) | FRD 4.4 mGy (range, 0.4–15.1) | Balloon rupture in one woman; resolutive IIA thrombosis in one woman |
| Cali et al. 2014 (36) | 30 | 12/0/18 | PBO IIA | 100 (30/30) | 846 (up to 1,700) | 0 (0/30) | NR | Lower EBL in women with placenta percreta by comparison with historical control group |
| Darwish et al. 2014 (37) | 32 | Accreta* | PBO IIA | 12.5 (4/32) | 1,9000 | NR | NR | Lower EBL with PBO by comparison with retrospective group |
| Chou et al. 2015 (38) | 12 | 1/6/5 | PBO IIA | 75 (9/12) | 1,902 (up to 8,000) | 17 (2/12) | NR | Popliteal thrombosis and EIA thrombosis (one woman each) |
| D’Souza et al. 2015 (39) | 10 | 0/4/6 | PBO IIA + UAE | 33 (3/10) | 1,200 (up to 4,000) | 0 (0/10) | NR | |
| Duan et al. 2015 (40) | 42 | 37/0/5 | PBO AA + UAE | 2.4 (1/42) | 586 | 0 (0/42) | FRD 4.2 mGy, | |
| Izbizki et al. 2015 (41) | 79 | 25/18/36 | PCUA + UAE | 94 (74/79) | NR | 0 (0/79) | NR | Resolving decreased temperature of right lower limb in one woman |
| Salim et al. 2015 (42) | 27 | Accreta* | PBO IAI [13]; Control [14] | 46 (6/13); 50 (7/14) | 4,950; 4,709 | 15 (2/13) | NR | Randomized trial; PBO IIA vs. control; no benefit with PBOIIA for EBL (P=0.72) |
| Omar et al. 2016 (43) | 14 | 5/4/5 | PBO IIA [11]; PBP CIA [3] | NR | 43,000±70,000 | 7 (1/14) | NR | No differences in EBL with control group |
| Wei et al. 2016 (18) | 45 | 22/20/3 | PBO AA | 9 (4/45) | 835 (up to 6,000) | 4 (2/45) | NR | Arterial thrombosis and ischemic injury of femoral nerve (one woman each) |
| Niola et al. 2016 (44) | 27 | A [21] P [6] | PUAE | 37 (10/27) | NR | 0 (0/27) | Skin dose 70.37 mGy (range, 18.53–234.27) | UAE was performed before delivery with the fetus in the uterus |
| Wu et al. 2016 (45) | 230 | 30/112/88 | PBO AA [203] + UAE [2] | 0 (0/230) | 921 | 0 (0/230) | FRD 5.1±3.0 mGy | – |
| Wang et al. 2016 (46) | 18 | 10/5/3 | UAE | 6 (1/18) | 1,372 | 0 (0/18) | NR | – |
| Angileri et al. 2017 (47) | 37 | 14/3/20 | PBO IIA | 0 (0/37) | 2,052 | 11 (4/37) | NR | Arterial thrombosis in 4 women |
| Cui et al. 2017 (48) | 38; 31 | 13/12/13; 11/14/6 | PBO AA [38] + UAE [12]; control group [31] | 5 (2/38); 10 (3/31) | 1,560±1,279; 2,145±1,160 | 8 (3/38) | FRD 3.3±1.1 mGy | No differences in EBL and hysterectomy rate. Arterial thrombosis (two women). UAE was needed in 12 women with PBO AA |
| Feng et al. 2017 (49) | 30 | 9/17/4 | PBO IIA (inflated in 27/30) | 43 (13/27) | 1,000 (up to 15,000) | 0 (0/30) | NR | No benefit of PBO IIA compared to historical control group |
| Pan et al. 2017 (50) | 26 19 |
18/7/1; 11/7/1 | PUA [26]; control group [19] | 23 (6/26); 37 (7/19) | 2,080; 2,800 | 4 (1/26) | FRD 30.6 mGy (range: 5.5-104) | No differences in EBL and hysterectomy rate; uterine necrosis with PUA in one woman |
| Wang et al. 2017 (51) | 57; 48 | Accreta*; Accreta* | PBO AA [57] + UAE [16]; PBO IIA [48] + UAE [14] | NR | 450; 619 | NR | NR | Prospective study comparing PBOIAI vs. PBOAA; EBL and FRD were lower with PBOAA |
| Wang et al. 2017 (52) | 10; 33 | Increta*; Increta* | PBO AA; control | 70 (7/10); 64 (21/33) | 1,000 2,000 |
NR | NR | Lower EBL with PBO AA |
| Xie et al. 2017 (53) | 30; 41 | 25/5 | PBO AA [30]; Control group [41] | 17 (5/30); 24 (10/41) | 961±784; 1,560±1,353 | 3.3 (1/30) | NR | Lower EBL with PBO AA. No differences in hysterectomy rates |
| Al-Hadethi et al. 2017 (54) | 25; 27 | PBO IIA; control group | 59 (16/25); 85 (23/27) | 2,455±1,444; 1,992±1,498 | 8 (2/25) | NR | Dissection of CFA and bilateral CIA dissection (one woman each) | |
| Luo et al. 2017 (55) | 29 | 25/0/4 | PBO AA [29] + UAE [2] | 17 (5/29) | 750 (up to 3,000) | 0 (0/29) | NR | Hysterectomy in 5 women with percreta |
| Dai et al. 2018 (56) | 20; 22 | Accreta*; Accreta* | PBO IIA [20] + UAE [2]; control group [20] + UAE [14] | 5 (1/22); 32 (7/22) | 2,900±2,352; 4,550±2,367 | 5 (1/20) | – | Thrombosis of EIA in one woman. Lower EBL with PBO |
| Mei et al. 2018 (57) | 20; 20 | 16/4/0; 16/4/0 | PBO IIA [20]; control group [20] | 0(0/20); 5 (1/20) | 800±359 (up to 1,800); 1,875±1,904 (up to 7,600) | 0 (0/20) | NR | Lower EBL with PBP IIA ± UAE; UAE was used when needed but no numbers were reported; no accreta in the study |
| Picel et al. 2018 (58) | 90; 61 | 20/20/50; 32/14/15 | PBO IIA; control group | 100 (90/90); 100 (61/61) | 2,360 (up to 2,500); 3,290 (up to 4,000) | 9 (8/80) | DAP 32.8 ìGym2 | Lower EBL with PBO IIA Dissection of oCIA; two pseudoaneurysms; acute limb ischemia |
| Ono et al. 2018 (59) | 29 | 17/7/5 | PBO CIA [29] | 100 (29/29) | 2,027±1,638 | 7 (2/29) | Skin dose 29.4±25.0 mGy | Balloon rupture in two woman |
| Blumenthal et al. 2018 (60) | 16; 19 | 4/3/9; 11/3/5 | PBO AA; control | 94 (15/16); 89 (17/19) | 2,007 (up to 20,000); 2,112 (up to 8,664) | 6 (1/16) | NR | No differences in EBL |
| Li et al. 2018 (61) | 199 | 39/60/13/29/52/6 | PBO [112] (IIA 37; CIA 42; AA 33); control group [87] | 11.6 (13/112); 32.8 (28/87) | 1,550; 3,500 | 1.8 (2/112) | 19.01 ± 13.33 mGy | Lower EBL with PBO IIA thrombosis in 2 women. Lower EBL with PBO in CIA and IAA |
| Huang et al. 2018 (62) | 11; 6 | 10/1/0; 3/1/2 | PUAE [11]; control group [6] | 9 (1/11); 33 (2/6) | 991.9±702; 3,448±1,767 | – | NR | – |
| Rosner-Tenerowicz et al. 2018 (63) | 13; 8 | Perceta*; Percreta* | PBO IIA [13]; Control group [8] | 100 (13/13); 100 (8/8) | 1,492±1,239; 2,963 ± 1,534 | 8 (1/13) | NR | Lower EBL with PBO IIA |
| Sun et al. 2018 (64) | 19 | Increta* | PBO AA | 31 (6/19) | 1,200 (up to 9,000) | 5 (1/19) | Skin dose 4.20±1.49 mGy | Thrombosis of IIA in one woman |
| Chodraui-Filho et al. 2019 (65) | 35 | 9/11/15 | PBO IIA + UAE | 100 (35/35) | 1,193 (up to 2,967) | 11 (4/35) | NR | Arterial thrombosis in two women. Gluteal muscle necrosis in one woman |
| Liu et al. 2019 (66) | 31 | 8/14/9 | PBO AA [31] + UAE [3] + OAE [3] | 3 (1/31) | 1,906±1,118 | 6 (2/31) | FRD 4.33±0.79 mGy | Thrombosis of right femoral artery in two women favorably treated with thrombolysis |
| Mei et al. 2019 (67) | 74; 100 | Accreta*; Accreta* | PBO AA [74] + UAE [23]; PBO IIA [100] + UAE [47] | 0 (0/74); 0 (0/100) | 600 (up to 2,500); 600 (up to 2,500) | 0 (0/74); 0 (0/100) | FRD 1.85 mGy (range, 2.9); FRD 25 mGy (range, 8–31) | No differences in EBL between the two procedures |
| Tokue et al. 2019 (68) | 42 | 33/6/3 | PBO IIA | 36 (15/42) | 3,706±3,852 | 0 (0/42) | FRD 25.5±8.2 mGy | Visualization of round ligament artery is associated with failed PBO IIA |
| Chen et al. 2019 (69) | 83; 31 | 30/0/53; 28/0/3 | PBO IIA [83]; control group [31] | 100 (83/83); 100 (31/31) | 3,000; 3,700 | NR | NR | PBO IIA has not impact of maternal outcome using a propensity score |
| Yuan et al. 2020 (70) | 28; 26 | 16/10/2; 18/7/1 | UAE [28]; control group [26] | 29 (8/28); 42 (11/26) | 1,325±871; 4,483±2,295 | 0 (0/28) | NR | Lower EBL in UAE group |
| Lee et al. 2020 (71) | 28 | 7/10/11 | PBOAA [12] or PBO IIA [16] | 100 (28/28) | 1,826 | 0 (0/28) | NR | Lower EBL with PBO |
*, o subgroup information was given. Table shows studies with more than 10 women with AIP. Studies were analyzed for the actual numbers of AIP and those that included women with AIP and placenta praevia were included when the actual number of AIP was ≥10. DR, fetal radiation dose; NR, not reported; DAP, dose area product; AA, abdominal aorta; AIP, abnormally invasive placenta; CIA, common iliac artery; DAP, dose area product; DIC, disseminated intravascular coagulopathy; FRD, fetal radiation dose; IIA, internal iliac artery; OAE, ovarian artery embolization; PBOAA, prophylactic balloon occlusion of abdominal aorta; PBOCIA, prophylactic balloon occlusion of common iliac artery; PBOIIA, prophylactic balloon occlusion of internal iliac arteries; PPH, postpartum hemorrhage; PUAE, prophylactic uterine artery embolization; PCUA, prophylactic catheterization of uterine arteries; PCIIA, prophylactic catheterization of IIA; UAE, uterine artery embolization.
Figure 1.
PRISMA flow diagram.
Arterial embolization
Arterial embolization has been used for years in the treatment of postpartum hemorrhage, with high success rates (14,72-78). By comparison with the more common uterine atony, AIP is a complex situation. The angiographic findings in women with AIP are greatly variable. Some women have undergone prior surgery such as arterial ligations resulting in recruitment of extra-uterine arteries (79,80). In addition, it is often hard to anticipate the actual degree of aggressiveness of AIP (accreta or percreta) that contributes to the technical difficulty. For these reasons, full pelvic angiogram is needed because it is crucial to understand the vascularization of the placenta that is left in place and because the vascularization is often complex. It is important to consider that AIP is often more technically demanding compared to uterine atony (81,82). This is because multiple vessels may contribute to the placental vascularization and some of them supply blood to organs that are sensitive to ischemia such as the bladder or the gastrointestinal tract. Due to its aggressive nature, the arterial network of the AIP is complex with multiple feeding vessels. Uterine arteries are always involved in the placenta vascularization, however other arteries such as ovarian, pudendal, obturator, sacral, and inferior epigastric arteries can also participate to the uteroplacental vasculature (9,10).
Arterial embolization in women with postpartum hemorrhage due to AIP has several goals. The primary objectives are to stop the distal bleeding and avoid surgical morbidity. The secondary objectives are to induce thrombosis of intervillous space, reduce the risk of further bleeding and improve the speed of placental resorption when conservative management is performed (9). The third objective is to preserve fertility and potential further pregnancies by avoiding uterine necrosis (83).
Choice of embolic agent
Several embolic agents can be used for the treatment of postpartum haemorrhage in women with AIP. Gelatin sponge, in the form of homemade torpedoes, is a temporary occlusive agent that was primarily or exclusively used by many authors (9,10,14,25,31,32,46,70). However, permanent or long lasting embolic agents such as monomeric n-butyl-2-cyanoacrylate (Histoacryl®, Glubran2®) (84-86), metallic coils (85), polyvinyl alcohol particles, and calibrated particles >700 µm were used in combination with gelatin sponge in some studies (9,14,25,31). Other embolic agents such as ethylene vinyl alcohol copolymer (Onyx®) (87) are available but their use in AIP has not been evaluated yet (88). In the study of Sentilhes et al., no associations were found between the rate of failure and the nature of the embolic material (12). However, each embolic agent may have specific advantages over another depending of the anticipated endpoint based on initial angiogram. Monomeric n-butyl-2-cyanoacrylate and calibrated particles are used for distal embolization while gelatin sponge torpedoes or metallic coils are used for proximal embolization (89).
Efficacy
Hwang et al. reported 40 women with AIP who underwent arterial embolization (14). Technical success was achieved in all women (100%), with a further initial clinical success rate of 82.5% (33/40). Three women with AIP underwent hysterectomy after arterial embolization failed to stop the bleeding within 24 hours (14). The other three women underwent successful repeat embolization, yielding overall 92.5% clinical success rate. Four women experienced pelvic pain, nausea and urticaria. There were no major complications (14). Soyer et al. reported 12 women with AIP (accreta, n=6; percreta, n=6) with primary (n=10) or secondary (n=2) postpartum hemorrhage (9). Arterial embolization was successful in 10/12 women after one (n=7) or two (n=3) embolization sessions and hysterectomy was needed in only 2 women. These two women had placenta percreta and bladder involvement (9). Sentilhes et al. reported 24% (4/17) of failed embolization in women with AIP and postpartum hemorrhage (12). Li et al. reported 10 women with AIP (6 accreta, 4 increta) managed conservatively who experienced secondary postpartum hemorrhage (32). Using gelatin sponge particules or pledgets, technical success rate of embolization was 100% (32). Bleeding was controlled in all women during follow-up [11±6.9 (SD) months; range 3–24 months], and no further bleeding occurred (32). One woman developed lower-extremity deep venous thrombosis on the side of artery access after uterine artery embolization with a favorable outcome after oral anticoagulation therapy, and no other major complications were reported (32).
Jung et al. reported 17 women with AIP for whom uterine artery embolization successfully controlled postpartum hemorrhage in 14 of them (82.4%) (29). Three women underwent hysterectomy after uterine artery embolization failed to stop the bleeding. No complications except fever lasting for 1–2 days were observed (29). Wang et al. have reported a hysterectomy rate of 6% and no complications (46).
A systematic review including 177 women with AIP reported an 89.8% success rates for arterial embolization, with 11.3% of women requiring further hysterectomy (90). The complication rate was 11%, including uterine necrosis, endometritis, and synechiae (91,92). One favorable effect of uterine artery embolization in AIP treated with a conservative approach is to reduce the resorption delay of the placenta (93). Soyer et al. reported a median resorption delay of 17 weeks after embolization compared to 32 weeks in the absence of embolization (P=0.036) (93). As reported by Hequet et al., retained placental tissues after arterial embolization can be removed using hysteroscopic resection (91).
Another option is to perform arterial embolization after cesarean delivery prior to hysterectomy in women with AIP (19). In a retrospective study, Wang et al. found lower EBL in seven women with AIP who received arterial embolization after cesarean delivery but before hysterectomy (1,500 mL; range, 500–2,000 mL) by comparison with those who underwent cesarean hysterectomy alone (2,000 mL; range, 1,000–4,500 mL) (P=0.04) but no differences in transfusion requirements (P=0.10) and length of intensive care unit stay (P=0.07) (19). However, this approach resulted in significant decrease in median EBL (P=0.004), transfusion requirements (P=0.009), and length of intensive care unit stay (P=0.04) only in the specific subgroup of women with placenta increta (18). A compelling benefit of this approach is the absence of fetal radiation as the entire procedure is performed after cesarean delivery (19).
In woman with AIP who are undergoing a gravid hysterectomy because of a nonviable fetus, the concern for blood loss and morbidity is high. In this setting, pre-hysterectomy uterine artery/pelvic embolization can aid in devascularization of the uteroplacental vasculature, rendering a dry surgical bed (94). This can help obviate injury to surrounding vessels and reduce periprocedural blood loss. However, the actual benefit of this approach should be assessed with further studies (94).
Prophylactic procedures
Prophylactic uterine artery catheterization and/or prophylactic embolization
Prophylactic bilateral uterine artery catheterization consists in selective catheterization prior to cesarean delivery in order to expedite treatment, should embolization be needed (41). This approach has been first described by Sumigama et al. (95). These researchers observed a marked decreased blood loss using a “stepwise treatment” in four women with AIP (95). Prophylactic uterine artery embolization has been originally proposed by Yu et al. with the purpose of minimizing the risk of massive blood loss during delivery in women with AIP (24). This technique consists in placing catheters in the uterine arteries to perform arterial embolization before delivery (24).
To date, limited data are available regarding the application of prophylactic catheter placement and embolization. One evidence is that the prophylactic approach yields conflicting results in terms of blood loss and hysterectomy rate. Izbizky et al. performed prophylactic bilateral uterine artery catheterization and further embolization when needed in the management of 95 women with suspected AIP; of them 79 women (79/95, 83%) had actually AIP, 92 (92/95; 97%) had catheterization and 83 (83/92; 87%) had further embolization (41). Complications, including bleeding requiring blood transfusion (49%) and bladder surgery (37%) were reported but there were no major complications attributable to the endovascular procedures (41). One minor complication in the form of transient paresthesia and decreased temperature of lower limb was reported, presumably related to embolization with uneventful follow-up. Clinical success rate was 86%, with no maternal deaths, but 14% of patients received large-volume blood transfusion (41). Giurazza et al. reported systematic uterine artery embolization in 69 women with placental abnormalities before cesarean hysterectomy (96). Although 36 women (52.2%) did not require blood transfusion, 30 women (43.5%) required hysterectomy (96). Pan et al. evaluated the potential of prophylactic intraoperative arterial embolization during cesarean delivery in women with AIP (50). They found no differences in EBL between the 26 women with AIP who received prophylactic intraoperative arterial embolization (2,080 mL) and the 19 women with AIP who did not (2,800 mL) (P=0.005). Prophylactic intraoperative arterial embolization resulted in a lower EBL only for the subgroup of woman who did not undergo hysterectomy (50). In addition, prophylactic intraoperative arterial embolization did not alter the need for hysterectomy and massive blood transfusion (50). Of note, in this study, the total mean fetal absorbed radiation dose was 30.6 mGy (range, 5.9–104.0 mGy) and adverse events due to prophylactic intraoperative arterial embolization were reported in 11/26 women (42%), including transient buttock pain (4 women) and uterine necrosis (one woman) (50). Yuan et al. reported that prophylactic uterine artery embolization during cesarean delivery in women with placenta accrete resulted in significantly lower EBL by comparison with a control group (70).
Chou et al. reported the use of prophylactic embolization using metallic coils and gelatin pledgets or gelatin pledgets alone in 6 women with AIP (1 accreta and 5 percreta) before hysterectomy, with a mean EBL of 1,767 mL (range, 300–3,000 mL) (97). Yu et al. reported 11 women with AIP (7 accreta-increta, 4 percreta) who underwent systematic uterine artery embolization with gelatin sponge (no details were given to the form) (24). The mean estimated mean blood loss was 2,279 mL (range, 1,650–3,090 mL) and 3 women had hysterectomy. Peritonitis and endometritis were observed in one woman after embolization (24).
D’Souza et al. used a technique which combined prophylactic arterial balloon occlusion and immediate post cesarean uterine artery embolization with absorbable gelatin sponge in 10 women with AIP (39). Mean EBL was 1,200 mL and 2 women only needed blood transfusion. However, 3 women had hysterectomy (39). Huang et al. reported that the 11 women with AIP who received prophylactic uterine embolization after delivery had less intraoperative blood loss [990.9±701.7 (SD) mL] than the six who did not underwent embolization [3,448.3±1,767.4 (SD) mL] (P=0.018) but found no differences in hysterectomy rate between the two groups (62). One study has reported encouraging results using prophylactic catheterization and embolization with only two women (2/25; 8%) requiring hysterectomy. However, median EBL was 2,000 mL and reached up to 9,000 mL in one woman (26).
Meller et al. proposed the use of a hybrid operating room for catheter placement, prophylactic embolization and cesarean hysterectomy in the same session and in the same room, thus avoiding a two-step procedure in different rooms and patient transfer from one room to another (98). This approach resulted in less occurrences of catheter dislodgement by comparison with the two step procedures in two different rooms (0/30, 0% vs. 10/80, 12.5%; P=0.04) (98).
Of interest, in one study, two women with prophylactic placement of catheters in the uterine arteries had severe postpartum hemorrhage requiring immediate hysterectomy and embolization was not performed (33). This outcome questions the actual role of prophylactic placement of catheters in the uterine arteries (31). Six women (6/14; 43%) underwent hysterectomy (31). Moreover, postpartum hemorrhage was reported in only 7/14 women (50%), suggesting that embolization may be actually needed in only half of women with AIP (31).
However, one concern regarding this approach is the radiation dose delivered to the fetus when performed before delivery. Niola et al. reported mean uterine radiation dose up to 15.61 mGy with a range between 8.15 and 38.18 mGy when performing prophylactic embolization with the fetus inside the uterus (44). These researchers reported adequate development in all children with a limited follow-up of 6 months 11 days to 28 months 21 days (the mean follow-up time was not given), but the long term effect of this approach is not known. In addition, it should be noted that 10/27 women with AIP required hysterectomy and that this approach was used not only for invasive placenta but also for women with noninvasive placenta previa (44).
Prophylactic balloon catheter placement in iliac arteries
Prophylactic placement of balloon catheters in the iliac arteries consists in placing one inflatable balloon catheter in each iliac artery under fluoroscopic guidance before cesarean section in women with AIP. Then, the balloons are inflated after the fetus has been delivered to control hemorrhage (99). This approach in women with AIP remains debated because of higher risks of complications than with embolization and the lack of comparative studies (22).
Some researchers have claimed a potential for prophylactic balloon catheters in the internal iliac arteries (22,27,36,37,40,42,49,55,57,58,63,100-110). Cali et al. reported the results based on a historical comparison of two groups of women with AIP (36). They found less blood loss in the group with balloon catheter placement (933 mL) than in that without catheter placement (1,507 mL) (P<0.001) only for women with placenta percreta (36). In this study, no complications related to the use of balloon catheter were reported (36). Tan et al. performed an historical comparison between 11 women with AIP who underwent cesarean section with prophylactic balloon occlusion and 14 who had cesarean section alone (22). They found reduced blood loss in the former group (2,011 mL) than in the latter one (3,316 mL) (P=0.042). Similarly, the amount of blood transfusion was decreased in the former group (1,058 mL) than in the latter (2,211 mL) (P=0.005) (22). Angstmann et al. used prophylactic balloon catheter placement in 12/22 women (55%) with AIP, of whom 8/22 (36%) had further embolization and ultimately hysterectomy (110). In these 8 women, however, blood loss was significantly lower [553±119 (SD) mL] than in those who did not have balloon catheter and embolization [4,517±711 (SD) mL] (P=0.0001) (110). Angileri et al. used prophylactic balloon catheters in the internal iliac arteries in 37 women with AIP (20 percreta, 20; increta, 3; accreta, 14) (47). Post-partum hemorrhage occurred in only 5 women (14%) and arterial thrombosis in 4 (11%) but no women had hysterectomy (47). Recently, data from the University of California Morbidly Adherent Placenta Registry allowed comparing the outcomes of women with AIP who underwent cesarean hysterectomy with aortic/internal iliac artery balloon occlusion catheters compared to those of women who underwent surgical ligation of the internal iliac arteries compared and those who had no adjunctive procedures (71). Lee et al. found that aortic and iliac artery balloon occlusion were associated with lower EBL, transfusion requirements, intensive care unit admission rates, and adverse event rates compared with women who underwent internal iliac artery ligation prior to cesarean hysterectomy or women who had no adjunctive interventions prior to cesarean hysterectomy for morbidly adherent placenta (71).
Several studies reported no benefit with the use of prophylactic balloon catheters in the internal iliac arteries with amounts of EBL, up to 16,000 mL using prophylactic balloon occlusion of the internal iliac arteries (21,23,34,43) or no benefit by comparison with historical control group (49). Bodner et al. found unfavorable results with the use of balloon-assisted occlusion of the internal iliac artery in 28 women with AIP using an historical comparison, with a rate of hysterectomy of 83% (5/6) in woman who underwent prophylactic occlusion compared to 100% (22/22) in women without occlusion (103). Only one randomized controlled trial has compared the potential of prophylactic balloon catheter placement in iliac arteries in women with prenatal diagnosis of AIP (42). In this trial, women were randomized to either preoperative prophylactic balloon catheters (n=13) or to a control group without balloon catheters (n=14). No differences were observed for the number of women with blood loss greater than 2,500 mL, number of plasma products transfused, duration of surgery, peripartum complications, and hospitalization length between the two groups (42). Although the absence of differences may be due to a small sample size, it must be noted that reversible adverse effects related to prophylactic balloon catheter insertion were observed in 2 of 13 (15.4%) women, consisting in leg pain and weakness without swelling in one woman and buttock claudication and abdominal pain in the other (42). Several complications due to the use of balloon catheters in the internal iliac arteries have been reported by several authors (17,38,41,47,54,58,59,64,65,100-105,111-113). Gagnon et al. reported left iliac artery rupture in a woman with suspected AIP in whom one balloon (Berenstein occlusion balloon catheter™ 8.5/11.5 mm) could only be placed into the distal portion of the left internal iliac artery because of tortuosity of the anterior division branches (21,111). Shrivastava et al. described 19 women with AIP who had iliac artery balloon catheter placement, resulting in 16% of balloon-related complications, including an internal iliac artery dissection that resulted in vascular occlusion and required iliofemoral bypass surgery (21). Sewell et al. reported popliteal artery thrombus after using internal iliac artery balloon catheter in one woman with AIP (accreta) without any sequellae (100). Matsueda et al. reported external iliac artery thrombosis using balloon catheter that was treated with heparin drip in one woman with AIP (accreta) with a favorable outcome (101); of interest a blood loss of 5,020 mL was reported in this woman, highlighting the questionable role the procedure and its potential risk (101). Bishop et al. reported leg ischemia and arterial dissection in a woman with placenta percreta and bladder invasion who had prophylactic bilateral internal iliac artery balloon inflation and further bilateral pseudoaneurysm of internal iliac arteries, right pseudoaneurysm rupture and permanent claudication of the right leg (102).
A literature review including 15 case reports and five studies for a total of 20 articles reported a wide variability in outcomes (17). Of most importance, several vascular complications were described, including acute limb ischemia, common and external iliac artery thrombosis, bilateral pseudoaneurysms, unilateral arterial rupture, requiring thromboembolectomy, stent placement or arterial bypass (17,100,102). Later, Peng et al. reported rupture of multiple pseudoaneurysms after common iliac artery balloon occlusion in a woman with placenta accreta (112).
In the study by Ballas et al. among 59 women with AIP who prophylactic placement of balloon catheters in the internal iliac arteries, 30 (30/59, 51%) had actually balloon inflation during delivery when excessive bleeding occurred (30). This suggests that prophylactic placement of balloon catheters in the internal iliac arteries is unnecessary in a substantial number of women with AIP. Another study, using a propensity score found that prophylactic occlusion of internal iliac arteries has no impact on maternal outcome (69).
Despite using low radiation dose techniques, fetal radiation exposure is a significant concern when considering internal iliac artery balloon occlusion (114). Although variable, Teixidor Viñas et al. reported a mean fetal radiation exposure of 4.4±3.5 (SD) mGy (range, 0.4–15.1 mGy) during prophylactic balloon catheter placement in both internal iliac arteries before cesarean section in 27 women with AIP (35). But Kai et al. reported fetal radiation doses ranging between 12.88 and 31.6 mGy using the same procedure (115). The use of low fluoroscopy rate is recommended. In this regard, Semeraro et al. showed that the use of fluoroscopy rate of 7.5 pulses per second resulted in a median fetal absorbed radiation dose of 1,713.25 µGym2 (Q1, 1,164.5; Q3, 2,274.5) compared to 660.70 µGym2 (Q1, 440.9; Q3, 1,020.9) using a fluoroscopy rate of 2 pulses per second (P=0.027) (116).
Prophylactic balloon occlusion of the abdominal aorta
Prophylactic balloon occlusion of the abdominal aorta occlusion consists in placing inflatable balloons in the abdominal aorta via a femoral access under fluoroscopic guidance (22). The balloon is positioned by an interventional radiologist prior to cesarean section and inflated after delivery of the fetus (22,99). The concept behind this approach is that internal iliac artery balloon occlusion often results in recruitment of pelvic arteries and exacerbation of the bleeding. This approach provides a more proximal occlusion, results in modest or even no recruitment of collateral vessels and a drier operative field (99).
In general, the use of prophylactic balloon occlusion in the abdominal aorta in the management of women with AIP is associated with encouraging results (18,40,48,117). A single-institution observational series of 45 women with AIP (accreta, n=22; increta, n=20; percreta, n=3) reported the use of prophylactic lower abdominal aorta balloon occlusion and suggested a reduced blood loss (mean EBL, 835 mL; range, 200–6,000 mL) resulting in the need for blood transfusion in 11/45 patients only (18). Duan et al. reported the most promising results using a combined technique that included temporary aortic balloon occlusion followed by uterine artery embolization for the treatment of 42 women with AIP, including 5 with placenta percreta (40). All women had cesarean section combined with temporary aortic balloon occlusion followed by uterine artery embolization. Forty-one women underwent successful cesarean section with conservation of the uterus. Hysterectomy was required in one (3.1%) women. There were no access-site complications after the endovascular procedure and no complications related to the intervention during follow-up (40). Similarly, Panici et al. and Luo et al. reported low EBL with no complications due to the use of prophylactic balloon occlusion in the abdominal aorta (33,55). A large study by Wu et al. reported a low EBL with no complications (45). Of interest, Liu et al. reported that prophylactic balloon occlusion of the abdominal aorta at the level of the renal artery resulted in lower EBL than when performed below the renal artery origin, particularly in women with placenta increta (117).
Only study reported no benefits in terms of EBL and hysterectomy rate with the use of prophylactic balloon occlusion of the abdominal aorta (48). In addition, in this study, uterine arterial embolization was needed in 12/38 women (32%) to stop the bleeding, highlighting that prophylactic balloon occlusion is a temporary means that needs further embolization or hysterectomy in women with severe bleeding (48).
The use of abdominal aorta balloon occlusion results in less adverse events than balloon catheter in iliac arteries However, in the study of Wei et al. one patient had lower extremity arterial thrombosis and another had ischemic injury to the femoral nerve (18). A study reported thrombotic complications in 12/121 women (10%) with AIP who underwent aortic balloon occlusion during cesarean delivery (118). Of them, 115 had the balloon inflated during surgery. One patient had venous thrombosis and 11 had arterial thrombosis involving the limb on the catheterization side. Eight women received arterial thromboembolectomy and four had conservative anticoagulation treatment (118).
Fetal radiation dose with temporary aortic balloon occlusion varies among studies. Duan et al. reported a fetal radiation dose of 4.2±2.9 (SD) mGy (40) but Nieto-Calvache reported entrance skin dose and radiation absorbed dose by the fetus of 1.31±0.96 (SD) mGy and 0.27±0.28 (SD) mGy, respectively in 10 women with AIP using C-arm (119).
Recommendations
AIP is a potentially severe condition. However, a retrospective analysis by the Maternal Health Study Group of Canadian Perinatal Surveillance System (Public Health Agency of Canada) has reported that the incidence of AIP is 14.4 (95% CI: 13.4–15.4) per 10,000 deliveries (819 women with AIP among 570,637 deliveries) whereas the incidence of AIP with postpartum hemorrhage is 7.2 (95% CI: 6.5–8.0) per 10,000 deliveries (120), indicating that only 50% of women with AIP experience postpartum hemorrhage. The same ratio was observed in other studies (30). In other words, in 50% of women with AIP no interventional radiology procedures are needed (92), thus seriously questioning a systematic approach that results in non-negligible radiation dose delivered to the fetus and conveys a non-negligible risk of serious complications.
The application of interventional procedures depends on the obstetrical approach that has been anticipated on the basis of ultrasound and MR imaging findings (121). To date, large data are available regarding the application of prophylactic catheter placement, prophylactic balloon occlusion and embolization but no well-designed comparative studies between the three approaches are available. The use of balloon occlusion catheters remains debated but evidences suggest that their use conveys high degrees of morbidity (17,122). Studies, mostly retrospective, have evaluated the role of prophylactic placement of balloon occlusion catheters to control the bleeding at the time of delivery in women with AIP, with varied results. One non-controlled study involving 230 patients reported low EBL using prophylactic balloon occlusion of the abdominal aorta (45) but another study based on randomization but with limited number of patients in the two groups did not report any advantages with the use of balloon catheter with respect to blood loss and the need for hysterectomy (42). In addition, the use of occlusive balloon may exacerbate bleeding from collateral vessels (68) so that additional intervention, such as uterine artery embolization is needed in a subset of women (57,67,123). Currently, the available evidence allows concluding that most of them result in less blood loss by comparison with women who have no prophylactic balloon occlusion (52,53,124,125).
In this systematic review, the pooled hysterectomy rate obtained from 8 studies reporting the use of uterine artery embolization was 15.5% (24/155) (9,14,25,29,31,32,46,70). This rate obtained from 22 studies that use prophylactic balloon occlusion of the internal iliac arteries was 76.5% (318/697) (21,22,27,28,34-39,47,49,54,56-58,63,65,67-69,71) and 12.1% (54/445) from 10 studies using prophylactic occlusion of the abdominal aorta (17,33,45,48,52,55,60,64,66,71).
The most effective balloon technique and also the one that results in less complications is prophylactic balloon occlusion of the abdominal aorta (51,124). Prophylactic balloon occlusion of internal iliac artery is less effective at reducing blood loos and the high incidence of adverse events it conveys should not lead to consider it as a first option (124). Another limitation to the use of prophylactic catheter placement is that they are placed before delivery raising major concerns regarding fetal radiation dose.
Conclusions
Historically arterial embolization has been performed for postpartum hemorrhage for decades (15,20,126). Large experience has been accumulated since the first description. Arterial embolization, should it be required, is performed by well-trained interventional radiologists under fluoroscopic guidance after delivery, only in woman with postpartum hemorrhage. This scenario requires dedicated centers for women with AIP where embolization is available 24 hours a day and 7 days a week (127). Pending such type of organization, arterial embolization on a case-by-case basis should be the preferred option. Well-designed randomized controlled trials are needed to truly demonstrate the safety and efficacy of occlusion balloons and prophylactic procedures and to best identify the women who should benefit of this approach. It appears that the use of prophylactic occlusion should be restricted when the endpoint is hysterectomy. On the opposite, when a conservative management is wanted to preserve future fertility, arterial embolization should be the preferred option.
Acknowledgments
Funding: None.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-548). RL serves as an unpaid Deputy Editor of Quantitative Imaging in Medicine and Surgery. OP reports relevant financial activities outside the submitted work (Consulting for Merit Medical, Terumo and Boston Scientific; Experimental study for Merit Medical). MPK reports relevant financial activities outside the submitted work (Consulting for Medtronic, Boston Scientific and Penumbra). The other authors have no conflicts of interest to declare.
References
- 1.Jha P, Pōder L, Bourgioti C, Bharwani N, Lewis S, Kamath A, Nougaret S, Soyer P, Weston M, Castillo RP, Kido A, Forstner R, Masselli G. Society of Abdominal Radiology (SAR) and European Society of Urogenital Radiology (ESUR) joint consensus statement for MR imaging of placenta accreta spectrum disorders. Eur Radiol 2020. doi: . 10.1007/s00330-019-06617-7 [DOI] [PubMed] [Google Scholar]
- 2.Silver RM, Barbour KD. Placenta accreta spectrum: accreta, increta, and percreta. Obstet Gynecol Clin North Am 2015;42:381-402. 10.1016/j.ogc.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 3.Bourgioti C, Zafeiropoulou K, Fotopoulos S, Nikolaidou ME, Theodora M, Daskalakis G, Tzavara C, Chatoupis K, Panourgias E, Antoniou A, Konstantinidou A, Moulopoulos LA. MRI prognosticators for adverse maternal and neonatal clinical outcome in patients at high risk for placenta accreta spectrum (PAS) disorders. J Magn Reson Imaging 2019;50:602-18. 10.1002/jmri.26592 [DOI] [PubMed] [Google Scholar]
- 4.Bour L, Placé V, Bendavid S, Fargeaudou Y, Portal JJ, Ricbourg A, Sebbag D, Dohan A, Vicaut E, Soyer P. Suspected invasive placenta: evaluation with magnetic resonance imaging. Eur Radiol 2014;24:3150-60. 10.1007/s00330-014-3354-z [DOI] [PubMed] [Google Scholar]
- 5.Masselli G, Gualdi G. MR imaging of the placenta: what a radiologist should know. Abdom Imaging 2013;38:573-87. 10.1007/s00261-012-9929-8 [DOI] [PubMed] [Google Scholar]
- 6.Allen L, Jauniaux E, Hobson S, Paillon-Smith J, Belfort MA, FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel FIGO consensus guidelines on placenta accreta spectrum disorders: nonconservative surgical management. Int J Gynaecol Obstet 2018;140:281-90. 10.1002/ijgo.12409 [DOI] [PubMed] [Google Scholar]
- 7.Sentilhes L, Kayem G, Chandraharan E, Palacios-Jaraquemada J, Jauniaux E, FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel FIGO consensus guidelines on placenta accreta spectrum disorders: conservative management. Int J Gynaecol Obstet 2018;140:291-8. 10.1002/ijgo.12410 [DOI] [PubMed] [Google Scholar]
- 8.Palacios-Jaraquemada JM, Fiorillo A, Hamer J, Martínez M, Bruno C. Placenta accreta spectrum: a hysterectomy can be prevented in almost 80% of cases using a resective-reconstructive technique. J Matern Fetal Neonatal Med 2020:1-8. [Epub ahead of print]. doi: . 10.1080/14767058.2020.1716715 [DOI] [PubMed] [Google Scholar]
- 9.Soyer P, Morel O, Fargeaudou Y, Sirol M, Staub F, Boudiaf M, Dahan H, Mebazaa A, Barranger E, le Dref O. Value of pelvic embolization in the management of severe postpartum hemorrhage due to placenta accreta, increta or percreta. Eur J Radiol 2011;80:729-35. 10.1016/j.ejrad.2010.07.018 [DOI] [PubMed] [Google Scholar]
- 10.Soyer P, Dohan A, Dautry R, Guerrache Y, Ricbourg A, Gayat E, Boudiaf M, Sirol M, Ledref O. Transcatheter arterial embolization for postpartum hemorrhage: indications, technique, results, and complications. Cardiovasc Intervent Radiol 2015;38:1068-81. 10.1007/s00270-015-1054-y [DOI] [PubMed] [Google Scholar]
- 11.Poujade O, Zappa M, Letendre I, Ceccaldi PF, Vilgrain V, Luton D. Predictive factors for failure of pelvic arterial embolization for postpartum hemorrhage. Int J Gynaecol Obstet 2012;117:119-23. 10.1016/j.ijgo.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 12.Sentilhes L, Gromez A, Clavier E, Resch B, Verspyck E, Marpeau L. Predictors of failed pelvic arterial embolization for severe postpartum hemorrhage. Obstet Gynecol 2009;113:992-9. 10.1097/AOG.0b013e3181a114f7 [DOI] [PubMed] [Google Scholar]
- 13.Pelage JP, Le Dref O, Jacob D, Soyer P, Rossignol M, Truc J, Payen D, Rymer R. Uterine artery embolization: anatomical and technical considerations, indications, results, and complications J Radiol 2000;81:1863-72. [PubMed] [Google Scholar]
- 14.Hwang SM, Jeon GS, Kim MD, Kim SH, Lee JT, Choi MJ. Transcatheter arterial embolisation for the management of obstetric haemorrhage associated with placental abnormality in 40 cases. Eur Radiol 2013;23:766-73. 10.1007/s00330-012-2612-1 [DOI] [PubMed] [Google Scholar]
- 15.Mitty HA, Sterling KM, Alvarez M, Gendler R. Obstetric hemorrhage: prophylactic and emergency arterial catheterization and embolotherapy. Radiology 1993;188:183-7. 10.1148/radiology.188.1.8511294 [DOI] [PubMed] [Google Scholar]
- 16.Petrov DA, Karlberg B, Singh K, Hartman M, Mittal PK. Perioperative internal iliac artery balloon occlusion, in the setting of placenta accreta and its variants: the role of the interventional radiologist. Curr Probl Diagn Radiol 2018;47:445-51. 10.1067/j.cpradiol.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 17.Dilauro MD, Dason S, Athreya S. Prophylactic balloon occlusion of internal iliac arteries in women with placenta accreta: literature review and analysis. Clin Radiol 2012;67:515-20. 10.1016/j.crad.2011.10.031 [DOI] [PubMed] [Google Scholar]
- 18.Wei X, Zhang J, Chu Q, Du Y, Xing N, Xu X, et al. Prophylactic abdominal aorta balloon occlusion during caesarean section: a retrospective case series. Int J Obstet Anesth 2016;27:3-8. 10.1016/j.ijoa.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Ballah D, Wade A, Taylor AG, Rizzuto G, Li B, Lucero J, Chen LM, Kohi MP. Uterine artery embolization following cesarean delivery but prior to hysterectomy in the management of patients with invasive placenta. J Vasc Interv Radiol 2019;30:687-91. 10.1016/j.jvir.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weston M, Soyer P, Barral M, Dohan A, Pierre S, Rabei R, Garcia-Reyes K, Kohi MP. Role of interventional procedures in obstetrics and gynecology. Radiol Clin North Am 2020;58:445-62. 10.1016/j.rcl.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 21.Shrivastava V, Nageotte M, Major C, Haydon M, Wing D. Case-control comparison of cesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters for placenta accreta. Am J Obstet Gynecol 2007;197:402.e1-5. 10.1016/j.ajog.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 22.Tan CH, Tay KH, Sheah K, Kwek K, Wong K, Tan HK, Tan BS. Perioperative endovascular internal iliac artery occlusion balloon placement in management of placenta accreta. AJR Am J Roentgenol 2007;189:1158-63. 10.2214/AJR.07.2417 [DOI] [PubMed] [Google Scholar]
- 23.Mok M, Heidemann B, Dundas K, Gillespie I, Clark V. Interventional radiology in women with suspected placenta accreta undergoing caesarean section. Int J Obstet Anesth 2008;17:255-61. 10.1016/j.ijoa.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 24.Yu PC, Ou HY, Tsang LL, Kung FT, Hsu TY, Cheng YF. Prophylactic intraoperative uterine artery embolization to control hemorrhage in abnormal placentation during late gestation. Fertil Steril 2009;91:1951-5. 10.1016/j.fertnstert.2008.02.170 [DOI] [PubMed] [Google Scholar]
- 25.Diop AN, Chabrot P, Bertrand A, Constantin JM, Cassagnes L, Storme B, Gallot D, Boyer L. Placenta accreta: management with uterine artery embolization in 17 cases. J Vasc Interv Radiol 2010;21:644-8. 10.1016/j.jvir.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 26.Sivan E, Spira M, Achiron R, Rimon U, Golan G, Mazaki-Tov S, Schiff E. Prophylactic pelvic artery catheterization and embolization in women with placenta accreta: can it prevent cesarean hysterectomy? Am J Perinatol 2010;27:455-61. 10.1055/s-0030-1247599 [DOI] [PubMed] [Google Scholar]
- 27.Carnevale FC, Kondo MM, de Oliveira Sousa W, Jr, Santos AB, da Motta Leal Filho JM, Moreira AM, Baroni RH, Francisco RP, Zugaib M. Perioperative temporary occlusion of the internal iliac arteries as prophylaxis in cesarean section at risk of hemorrhage in placenta accreta. Cardiovasc Intervent Radiol 2011;34:758-64. 10.1007/s00270-011-0166-2 [DOI] [PubMed] [Google Scholar]
- 28.Amsalem H, Kingdom JC, Farine D, Allen L, Yinon Y, D'Souza DL, Kachura J, Pantazi S, Windrim R. Planned caesarean hysterectomy versus “conserving” caesarean section in patients with placenta accreta. J Obstet Gynaecol Can 2011;33:1005-10. 10.1016/S1701-2163(16)35049-6 [DOI] [PubMed] [Google Scholar]
- 29.Jung HN, Shin SW, Choi SJ, Cho SK, Park KB, Park HS, Kang M, Choo SW, Do YS, Choo IW. Uterine artery embolization for emergent management of postpartum hemorrhage associated with placenta accreta. Acta Radiol 2011;52:638-42. 10.1258/ar.2011.100514 [DOI] [PubMed] [Google Scholar]
- 30.Ballas J, Hull AD, Saenz C, Warshak CR, Roberts AC, Resnik RR, Moore TR, Ramos GA. Preoperative intravascular balloon catheters and surgical outcomes in pregnancies complicated by placenta accreta: a management paradox. Am J Obstet Gynecol 2012;207:216.e1-216.e5. 10.1016/j.ajog.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 31.Bouvier A, Sentilhes L, Thouveny F, Bouet PE, Gillard P, Willoteaux S, Aubé C. Planned caesarean in the interventional radiology cath lab to enable immediate uterine artery embolization for the conservative treatment of placenta accreta. Clin Radiol 2012;67:1089-94. 10.1016/j.crad.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 32.Li X, Wang Z, Chen J, Shi H, Zhang X, Pan J, Liu W, Yang N, Jin Z, Lang J. Uterine artery embolization for the management of secondary postpartum haemorrhage associated with placenta accreta. Clin Radiol 2012;67:e71-76. 10.1016/j.crad.2012.07.021 [DOI] [PubMed] [Google Scholar]
- 33.Panici PB, Anceschi M, Borgia ML, Bresadola L, Masselli G, Parasassi T, Perrone G, Fetal Maternal Risk Group , Brunelli R. Intraoperative aorta balloon occlusion: fertility preservation in patients with placenta previa accreta/increta. J Matern Fetal Neonatal Med 2012;25:2512-16. 10.3109/14767058.2012.712566 [DOI] [PubMed] [Google Scholar]
- 34.Clausen C, Stensballe J, Albrechtse CK, Hanse MA, Lonn L, Langhoff-Roos J. Balloon occlusion of the internal iliac arteries in the multidisciplinary management of placenta percreta. Acta Obstet Gynecol Scand 2013;92:386-91. 10.1111/j.1600-0412.2012.01451.x [DOI] [PubMed] [Google Scholar]
- 35.Teixidor Viñas M, Chandraharan E, Moneta MV, Belli AM. The role of interventional radiology in reducing haemorrhage and hysterectomy following caesarean section for morbidly adherent placenta. Clin Radiol 2014;69:e345-51. 10.1016/j.crad.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 36.Cali G, Forlani F, Giambanco L, Amico ML, Vallone M, Puccio G, Alio L. Prophylactic use of intravascular balloon catheters in women with placenta accreta, increta and percreta. Eur J Obstet Gynecol Reprod Biol 2014;179:36-41. 10.1016/j.ejogrb.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 37.Darwish HS, Zaytoun HA, Kamel HA, Habash YH. Prophylactic preoperative balloon occlusion of hypogastric arteries in abnormal placentation: 5 years experience. Egypt J Radiol Nucl Med 2014:45:751-9. 10.1016/j.ejrnm.2014.05.018 [DOI] [Google Scholar]
- 38.Chou MM, Kung HF, Hwang JI, Chen WC, Tseng JJ. Temporary prophylactic intravascular balloon occlusion of the common iliac arteries before cesarean hysterectomy for controlling operative blood loss in abnormal placentation. Taiwan J Obstet Gynecol 2015;54:493-8. 10.1016/j.tjog.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 39.D'Souza DL, Kingdom JC, Amsalem H, Beecroft JR, Windrim RC, Kachura JR. Conservative management of invasive placenta using combined prophylactic internal iliac artery balloon occlusion and immediate postoperative uterine artery embolization. Can Assoc Radiol J 2015;66:179-84. 10.1016/j.carj.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 40.Duan XH, Wang YL, Han XW, Chen ZM, Chu QJ, Wang L, Hai DD. Caesarean section combined with temporary aortic balloon occlusion followed by uterine artery embolisation for the management of placenta accreta. Clin Radiol 2015;70:932-7. 10.1016/j.crad.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 41.Izbizky G, Meller C, Grasso M, Velazco A, Peralta O, Otaño L, Garcia-Monaco R. Feasibility and safety of prophylactic uterine artery catheterization and embolization in the management of placenta accreta. J Vasc Interv Radiol 2015;26:162-9. 10.1016/j.jvir.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 42.Salim R, Chulski A, Romano S, Garmi G, Rudin M, Shalev E. Precesarean prophylactic balloon catheters for suspected placenta accreta: a randomized controlled trial. Obstet Gynecol 2015;126:1022-8. 10.1097/AOG.0000000000001113 [DOI] [PubMed] [Google Scholar]
- 43.Omar HR, Sprenker C, Alvey E, Hoffman M, Karlnoski R, Ching YH, Cain M, Mangar D, Camporesi EM. The value of occlusive balloons in the management of abnormal placentation: a retrospective study. J Obstet Gynaecol 2016;36:333-6. 10.3109/01443615.2015.1052962 [DOI] [PubMed] [Google Scholar]
- 44.Niola R, Giurazza F, Nazzaro G, Silvestre M, Nasti G, Di Pasquale MA, Albano G, Valentino L, Sirimarco F, Maglione F. Uterine artery embolization before delivery to prevent postpartum hemorrhage. J Vasc Interv Radiol 2016;27:376-82. 10.1016/j.jvir.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 45.Wu Q, Liu Z, Zhao X, Liu C, Wang Y, Chu Q, Wang X, Chen Z. Outcome of pregnancies after balloon occlusion of the infrarenal abdominal aorta during caesarean in 230 patients with placenta praevia accreta. Cardiovasc Intervent Radiol 2016;39:1573-9. 10.1007/s00270-016-1418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Li X, Pan J, Zhang X, Shi H, Yang N, Jin Z. Uterine artery embolization for management of primary postpartum hemorrhage associated with placenta accreta. Chin Med Sci J 2016;31:228-232. 10.1016/S1001-9294(17)30005-6 [DOI] [PubMed] [Google Scholar]
- 47.Angileri SA, Mailli L, Raspanti C, Ierardi AM, Carrafiello G, Belli AM. Prophylactic occlusion balloon placement in internal iliac arteries for the prevention of postpartum haemorrhage due to morbidly adherent placenta: short term outcomes. Radiol Med 2017;122:798-806. 10.1007/s11547-017-0777-z [DOI] [PubMed] [Google Scholar]
- 48.Cui S, Zhi Y, Cheng G, Zhang K, Zhang L, Shen L. Retrospective analysis of placenta previa with abnormal placentation with and without prophylactic use of abdominal aorta balloon occlusion. Int J Gynaecol Obstet 2017;137:265-70. 10.1002/ijgo.12132 [DOI] [PubMed] [Google Scholar]
- 49.Feng S, Liao Z, Huang H. Effect of prophylactic placement of internal iliac artery balloon catheters on outcomes of women with placenta accreta: an impact study. Anaesthesia 2017;72:853-8. 10.1111/anae.13895 [DOI] [PubMed] [Google Scholar]
- 50.Pan Y, Zhou X, Yang Z, Cui S, De W, Sun L. Retrospective cohort study of prophylactic intraoperative uterine artery embolization for abnormally invasive placenta. Int J Gynaecol Obstet 2017;137:45-50. 10.1002/ijgo.12090 [DOI] [PubMed] [Google Scholar]
- 51.Wang YL, Duan XH, Han XW, Wang L, Zhao XL, Chen ZM, Chu QJ, Zhang W. Comparison of temporary abdominal aortic occlusion with internal iliac artery occlusion for patients with placenta accreta - a non-randomised prospective study. Vasa 2017;46:53-7. 10.1024/0301-1526/a000577 [DOI] [PubMed] [Google Scholar]
- 52.Wang YL, Su FM, Zhang HY, Wang F, Zhe RL, Shen XY. Aortic balloon occlusion for controlling intraoperative hemorrhage in patients with placenta previa increta/percreta. J Matern Fetal Neonatal Med 2017;30:2564-8. 10.1080/14767058.2016.1256990 [DOI] [PubMed] [Google Scholar]
- 53.Xie L, Wang Y, Luo FY, Man YC, Zhao XL. Prophylactic use of an infrarenal abdominal aorta balloon catheter in pregnancies complicated by placenta accreta. J Obstet Gynaecol 2017;37:557-61. 10.1080/01443615.2017.1291588 [DOI] [PubMed] [Google Scholar]
- 54.Al-Hadethi S, Fernando S, Hughes S, Thakorlal A, Seruga A, Scurry B. Does temproray bilateral balloon occlusion of the common iliac arteries reduce the need for intra-operative blood transfusion in cases of placenta accretism? J Med Imaging Radiat Oncol 2017;61:311-6. 10.1111/1754-9485.12560 [DOI] [PubMed] [Google Scholar]
- 55.Luo F, Xie L, Xie P, Liu S, Zhu Y. Intraoperative aortic balloon occlusion in patients with placenta previa and/or placenta accreta: a retrospective study. Taiwan J Obstet Gynecol 2017;56:147-52. 10.1016/j.tjog.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 56.Dai MJ, Jin GX, Lin JH, Zhang Y, Chen YY, Zhang XB. Pre-cesarean prophylactic balloon placement in the internal iliac artery to prevent postpartum hemorrhage among women with pernicious placenta previa. Int J Gynaecol Obstet 2018;142:315-20. 10.1002/ijgo.12559 [DOI] [PubMed] [Google Scholar]
- 57.Mei Y, Luo D, Lin Y. Clinical application of prophylactic internal iliac artery balloon occlusion combined with uterine artery embolization in patients with abnormally invasive placenta. J Matern Fetal Neonatal Med 2018;31:3287-92. 10.1080/14767058.2017.1368485 [DOI] [PubMed] [Google Scholar]
- 58.Picel AC, Wolford B, Cochran RL, Ramos GA, Roberts AC. Prophylactic internal iliac artery occlusion balloon placement to reduce operative blood loss in patients with invasive placenta. J Vasc Interv Radiol 2018;29:219-24. 10.1016/j.jvir.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 59.Ono Y, Murayama Y, Era S, Matsunaga S, Nagai T, Osada H, Takai Y, Baba K, Takeda S, Seki H. Study of the utility and problems of common iliac artery balloon occlusion for placenta previa with accreta. J Obstet Gynaecol Res 2018;44:456-62. 10.1111/jog.13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blumenthal E, Rao R, Murphy A, Gornbein J, Hong R, Moriarty JM, Kahn DA, Janzen C. Pilot study of intra-aortic balloon occlusion to limit morbidity in patients with adherent placentation undergoing cesarean hysterectomy. AJP Rep 2018;8:e57-e63. 10.1055/s-0038-1641736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li K, Zou Y, Sun J, Wen H. Prophylactic balloon occlusion of internal iliac arteries, common iliac arteries and infrarenal abdominal aorta in pregnancies complicated by placenta accreta: a retrospective cohort study. Eur Radiol 2018;28:4959-67. 10.1007/s00330-018-5527-7 [DOI] [PubMed] [Google Scholar]
- 62.Huang KL, Tsai CC, Fu HC, Cheng HH, Lai YJ, Hung HN, Tsang LL, Hsu TY. Prophylactic transcatheter arterial embolization helps intraoperative hemorrhagic control for removing invasive placenta. J Clin Med 2018;7:11. 10.3390/jcm7110460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosner-Tenerowicz A, Pomorski M, Fuchs T, Śliwa J, Pilecka K, Zimmer A, Zimmer M. Management of placenta percreta with temporary balloon occlusion of the internal iliac arteries. Eur J Obstet Gynecol Reprod Biol 2018;226:71-2. 10.1016/j.ejogrb.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 64.Sun W, Duan S, Xin G, Xiao J, Hong F, Hong H, Wu Y, Xu Y. Safety and efficacy of preoperative abdominal aortic balloon occlusion in placenta increta and/or percreta. J Surg Res 2018;222:75-84. 10.1016/j.jss.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 65.Chodraui-Filho SF, Monsignore LM, Freitas RK, Nakiri GS, de Carvalho Cavalli R, Duarte G, Abud DG. Can the combination of internal iliac temporary occlusion and uterine artery embolization reduce bleeding and the need for intraoperative blood transfusion in cases of invasive placentation? Clinics 2019;74:e946. 10.6061/clinics/2019/e946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J, Wang Y, Jiao D, Zhang W, Han X. Prophylactic occlusion balloon placement in the abdominal aorta combined with uterine or ovarian artery embolization for the prevention of cesarean hysterectomy due to placenta accreta: a retrospective study. Cardiovasc Intervent Radiol 2019;42:829-34. 10.1007/s00270-019-02170-4 [DOI] [PubMed] [Google Scholar]
- 67.Mei Y, Zhao H, Zhou H, Jing H, Lin Y. Comparison of infrarenal aortic balloon occlusion with internal iliac artery balloon occlusion for patients with placenta accreta. BMC Pregnancy Childbirth 2019;19:147. 10.1186/s12884-019-2303-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tokue H, Tokue A, Tsushima Y, Kameda T. Risk factors for massive bleeding based on angiographic findings in patients with placenta previa and accreta who underwent balloon occlusion of the internal iliac artery during cesarean section. Br J Radiol 2019;92:20190127. 10.1259/bjr.20190127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen M, Lv B, He G, Liu X. Internal iliac artery balloon occlusion during cesarean hysterectomy in women with placenta previa accreta. Int J Gynaecol Obstet 2019;145:110-15. 10.1002/ijgo.12763 [DOI] [PubMed] [Google Scholar]
- 70.Yuan Q, Jin Y, Chen L, Ling L, Bai XM. Prophylactic uterine artery embolization during cesarean delivery for placenta previa complicated by placenta accreta. Int J Gynaecol Obstet 2020;149:43-7. 10.1002/ijgo.13072 [DOI] [PubMed] [Google Scholar]
- 71.Lee AY, Ballah D, Moreno I, Dong PR, Cochran R, Pice A, Lee EW, Moriarty J, Nelson K, Kohi MP. Outcomes of balloon occlusion in the University of California Morbidly Adherent Placenta Registry. American Journal of Obstetrics & Gynecology MFM 2020;2:100065 10.1016/j.ajogmf.2019.100065 [DOI] [PubMed] [Google Scholar]
- 72.Lee HY, Shin JH, Kim J, Yoon HK, Ko GY, Won HS, Zhou Y, Zhang W. Primary postpartum hemorrhage: outcome of pelvic arterial embolization in 251 patients at a single institution. Radiology 2012;264:903-9. 10.1148/radiol.12111383 [DOI] [PubMed] [Google Scholar]
- 73.Aoki M, Tokue H, Miyazaki M, Shibuya K, Hirasawa S, Oshima K. Primary postpartum hemorrhage: outcome of uterine artery embolization. Br J Radiol 2018;91:20180132. 10.1259/bjr.20180132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lindquist JD, Vogelzang RL. Pelvic artery embolization for treatment of postpartum hemorrhage. Semin Intervent Radiol 2018;35:41-7. 10.1055/s-0038-1636520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dohan A, Soyer P, Subhani A, Hequet D, Fargeaudou Y, Morel O, Boudiaf M, Gayat E, Barranger E, Le Dref O, Sirol M. Postpartum hemorrhage resulting from pelvic pseudoaneurysm: a retrospective analysis of 588 consecutive cases treated by arterial embolization. Cardiovasc Intervent Radiol 2013;36:1247-55. 10.1007/s00270-013-0668-1 [DOI] [PubMed] [Google Scholar]
- 76.Pelage JP, Soyer P, Repiquet D, Herbreteau D, Le Dref O, Houdart E, Jacob D, Kardache M, Schurando P, Truc JB, Rymer R. Secondary postpartum hemorrhage: treatment with selective arterial embolization. Radiology 1999;212:385-9. 10.1148/radiology.212.2.r99jl05385 [DOI] [PubMed] [Google Scholar]
- 77.Soyer P, Fargeaudou Y, Morel O, Boudiaf M, Le Dref O, Rymer R. Severe postpartum haemorrhage from ruptured pseudoaneurysm: successful treatment with transcatheter arterial embolization. Eur Radiol 2008;18:1181-7. 10.1007/s00330-008-0876-2 [DOI] [PubMed] [Google Scholar]
- 78.Pellerin O, Bats AS, Di Primio M, Palomera-Ricco A, Pinot de Villechenon G, Fournier L, Pagny JY, Beyssen B, Louail B, Lécuru F, Sapoval M. Postpartum hemorrhage treated with gelfoam slurry embolization using the superselective technique: immediate results and 1-month MRI follow-up. Cardiovasc Intervent Radiol 2013;36:98-104. 10.1007/s00270-012-0355-7 [DOI] [PubMed] [Google Scholar]
- 79.Morel O, Malartic C, Muhlstein J, Gayat E, Judlin P, Soyer P, Barranger E. Pelvic arterial ligations for severe post-partum hemorrhage: indications and techniques. J Visc Surg 2011;148:e95-102. 10.1016/j.jviscsurg.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 80.Fargeaudou Y, Morel O, Soyer P, Gayat E, Sirol M, Boudiaf M, Dahan H, Barranger E, Mebazaa A, le Dref O. Persistent postpartum haemorrhage after failed arterial ligation: value of pelvic embolisation. Eur Radiol 2010;20:1777-85. 10.1007/s00330-010-1713-y [DOI] [PubMed] [Google Scholar]
- 81.Pelage JP, Le Dref O, Jacob D, Soyer P, Herbreteau D, Rymer R. Selective arterial embolization of the uterine arteries in the management of intractable post-partum hemorrhage. Acta Obstet Gynecol Scand 1999;78:698-703. [PubMed] [Google Scholar]
- 82.Pelage JP, Le Dref O, Mateo J, Soyer P, Jacob D, Kardache M, Dahan H, Repiquet D, Payen D, Truc JB, Merland JJ, Rymer R. Life-threatening primary postpartum hemorrhage: treatment with emergency selective arterial embolization. Radiology 1998;208:359-62. 10.1148/radiology.208.2.9680559 [DOI] [PubMed] [Google Scholar]
- 83.Dohan A, Pelage JP, Soyer P. How to avoid uterine necrosis after arterial embolization for post-partum hemorrhage: a proposal based on a single center experience of 600 cases. Eur J Obstet Gynecol Reprod Biol 2013;171:392-3. 10.1016/j.ejogrb.2013.09.030 [DOI] [PubMed] [Google Scholar]
- 84.Loffroy R. Glubran2®, Histoacryl® or Trufill®: which cyanoacrylate glue for endovascular use? Diagn Interv Imaging 2016;97:119. 10.1016/j.diii.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 85.Lee NJ, Shin JH, Lee SS, Park DH, Lee SK, Yoon HK. Transcatheter arterial embolization for iatrogenic bleeding after endoscopic ultrasound-guided pancreaticobiliary drainage. Diagn Interv Imaging 2018;99:717-24. 10.1016/j.diii.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 86.Griviau L, Chevallier O, Marcelin C, Nakai M, Pescatori L, Galland C, Midulla M, Falvo N, Loffroy R. Percutaneous ultrasound-guided balloon-assisted embolization of iatrogenic femoral artery pseudoaneurysms with Glubran®2 cyanoacrylate glue: safety, efficacy and outcomes. Quant Imaging Med Surg 2018;8:796-803. 10.21037/qims.2018.09.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Né R, Chevallier O, Falvo N, Facy O, Berthod PE, Galland C, Gehin S, Midulla M, Loffroy R. Embolization with ethylene vinyl alcohol copolymer (Onyx®) for peripheral hemostatic and non-hemostatic applications: a feasibility and safety study. Quant Imaging Med Surg 2018;8:280-90. 10.21037/qims.2018.04.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barral M, Dautry R, Foucher R, Guerrache Y, Dohan A, Labeyrie MA. Combined transarterial and transvenous embolization of a ruptured utero-ovarian arteriovenous malformation with ethylene vinyl alcohol copolymer (Onyx®). Diagn Interv Imaging 2018;99:417-9. 10.1016/j.diii.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 89.Perdikakis E, Fezoulidis I, Tzortzis V, Rountas C. Varicocele embolization: anatomical variations of the left internal spermatic vein and endovascular treatment with different types of coils. Diagn Interv Imaging 2018;99:599-607. 10.1016/j.diii.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 90.Mei J, Wang Y, Zou B, Hou Y, Ma T, Chen M, Xie L. Systematic review of uterus-preserving treatment modalities for abnormally invasive placenta. J Obstet Gynaecol 2015;35:777-82. 10.3109/01443615.2015.1011106 [DOI] [PubMed] [Google Scholar]
- 91.Hequet D, Morel O, Soyer P, Gayat E, Malartic C, Barranger E. Delayed hysteroscopic resection of retained tissues and uterine conservation after conservative treatment for placenta accreta. Aust N Z J Obstet Gynaecol 2013;53:580-3. 10.1111/ajo.12138 [DOI] [PubMed] [Google Scholar]
- 92.Alanis M, Hurst BS, Marshburn PB, Matthews ML. Conservative management of placenta increta with selective arterial embolization preserves future fertility and results in a favorable outcome in subsequent pregnancies. Fertil Steril 2006;86:1514.e3-7. 10.1016/j.fertnstert.2006.02.128 [DOI] [PubMed] [Google Scholar]
- 93.Soyer P, Sirol M, Fargeaudou Y, Bour L, Morel O, Dohan A, Boudiaf M, Gayat E, Hequet D, Barranger E, le Dref O. Placental vascularity and resorption delay after conservative management of invasive placenta: MR imaging evaluation. Eur Radiol 2013;23:262-71. 10.1007/s00330-012-2573-4 [DOI] [PubMed] [Google Scholar]
- 94.Kohi MP, Poder L, Thiet MP, Kerlan RK., Jr Uterine artery embolization prior to gravid hysterectomy in the setting of invasive placenta. J Vasc Interv Radiol 2017;28:1295-7. 10.1016/j.jvir.2017.03.028 [DOI] [PubMed] [Google Scholar]
- 95.Sumigama S, Itakura A, Ota T, Okada M, Kotani T, Hayakawa H, Yoshida K, Ishikawa K, Hayashi K, Kurauchi O, Yamada S, Nakamura H, Matsusawa K, Sakakibara K, Ito M, Kawai M, Kikkawa F. Placenta previa increta/percreta in Japan: a retrospective study of ultrasound findings, management and clinical course. J Obstet Gynaecol Res 2007;33:606-11. 10.1111/j.1447-0756.2007.00619.x [DOI] [PubMed] [Google Scholar]
- 96.Giurazza F, Albano G, Valentino L, Schena E, Capussela T, Di Pasquale MA, Di Pietto F, De Ritis R, Nasti G, Scognamiglio G, Niola R. Predelivery uterine arteries embolization in patients affected by placental implant anomalies. Radiol Med 2018;123:71-8. 10.1007/s11547-017-0796-9 [DOI] [PubMed] [Google Scholar]
- 97.Chou MM, Hwang JI, Tseng JJ, Ho ES. Internal iliac artery embolization before hysterectomy for placenta accreta. J Vasc Interv Radiol 2003;14:1195-9. 10.1097/01.RVI.0000086532.86489.97 [DOI] [PubMed] [Google Scholar]
- 98.Meller CH, Garcia-Monaco RD, Izbizky G, Lamm M, Jaunarena J, Peralta O, Otaño L. Non-conservative management of placenta accreta spectrum in the hybrid operating room: a retrospective cohort study. Cardiovasc Intervent Radiol 2019;42:365-70. 10.1007/s00270-018-2113-y [DOI] [PubMed] [Google Scholar]
- 99.Kaufman C, Tadros A. Endovascular interventions for the morbidly adherent placenta. J Clin Med 2018;7:E92. 10.3390/jcm7050092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sewell MF, Rosenblum D, Ehrenberg H. Arterial embolus during common iliac balloon catheterization at cesarean hysterectomy. Obstet Gynecol 2006;108:746-8. 10.1097/01.AOG.0000201992.80130.2c [DOI] [PubMed] [Google Scholar]
- 101.Matsueda S, Hidaka N, Kondo Y, Fujiwara A, Fukushima K, Kato K. External iliac artery thrombosis after common iliac artery balloon occlusion during cesarean hysterectomy for placenta accrete in cervico-isthmic pregnancy. J Obstet Gynaecol Res 2015;41:1826-30. 10.1111/jog.12777 [DOI] [PubMed] [Google Scholar]
- 102.Bishop S, Butler K, Monaghan S, Chan K, Murphy G, Edozien L. Multiple complications following the use of prophylactic internal iliac artery balloon catheterisation in a patient with placenta percreta. Int J Obstet Anesth 2011;20:70-3. 10.1016/j.ijoa.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 103.Bodner LJ, Nosher JL, Gribbin C, Siegel RL, Beale S, Scorza W. Balloon-assisted occlusion of the internal iliac arteries in patients with placenta accreta/percreta. Cardiovasc Intervent Radiol 2006;29:354-61. 10.1007/s00270-005-0023-2 [DOI] [PubMed] [Google Scholar]
- 104.Teare J, Evans E, Belli A, Wendler R. Sciatic nerve ischaemia after iliac artery occlusion balloon catheter placement for placenta percreta. Int J Obstet Anesth 2014;23:178-81. 10.1016/j.ijoa.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 105.Chouliaras S, Hickling DJ, Tuck JS. Thromboembolism of the leg following prophylactic balloon occlusion of the uterine arteries. BJOG 2009;116:1278-9. 10.1111/j.1471-0528.2009.02217.x [DOI] [PubMed] [Google Scholar]
- 106.Kidney DD, Nguye AM, Ahdoot D, Bickmore D, Deutsch LS, Majors C. Prophylactic perioperative hypogastric artery balloon occlusion in abnormal placentation. AJR Am J Roentgenol 2001;176:1521-4. 10.2214/ajr.176.6.1761521 [DOI] [PubMed] [Google Scholar]
- 107.Dubois J, Garel L, Grignon A, Lema M, Leduc L. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood losses. Am J Obstet Gynecol 1997;176:723-6. 10.1016/S0002-9378(97)70582-9 [DOI] [PubMed] [Google Scholar]
- 108.Minas V, Gul N, Shaw E, Mwenenchanya S. Prophylactic balloon occlusion of the common iliac arteries for the management of suspected placenta accreta/percreta: conclusions from a short case series. Arch Gynecol Obstet 2015;291:461-5. 10.1007/s00404-014-3436-9 [DOI] [PubMed] [Google Scholar]
- 109.Shih JC, Liu KL, Shyu MK. Temporary balloon occlusion of the common iliac artery: new approach to bleeding control during cesarean hysterectomy for placenta percreta. Am J Obstet Gynecol 2005;193:1756-8. 10.1016/j.ajog.2005.08.033 [DOI] [PubMed] [Google Scholar]
- 110.Angstmann T, Gard G, Harrington T, Ward E, Thomson A, Giles W. Surgical management of placenta accreta: a cohort series and suggested approach. Am J Obstet Gynecol 2010;202:38.e1-38.e9. 10.1016/j.ajog.2009.08.037 [DOI] [PubMed] [Google Scholar]
- 111.Gagnon J, Boucher L, Kaufman I, Brown R, Moore A. Iliac artery rupture related to balloon insertion for placenta accreta causing maternal hemorrhage and neonatal compromise. Can J Anaesth 2013;60:1212-7. 10.1007/s12630-013-0038-0 [DOI] [PubMed] [Google Scholar]
- 112.Peng Q, Zhang W. Rupture of multiple pseudoaneurysms as a rare complication of common iliac artery balloon occlusion in a patient with placenta accreta: a case report and review of literature. Medicine 2018;97:e9896. 10.1097/MD.0000000000009896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greenberg JI, Suliman A, Iranpou P, Angle N. Prophylactic balloon occlusion of the internal iliac arteries to treat abnormal placentation: a cautionary case. Am J Obstet Gynecol 2007;197:470.e1-4. 10.1016/j.ajog.2007.05.017 [DOI] [PubMed] [Google Scholar]
- 114.Patel SJ, Reede DL, Katz DS, Subramaniam R, Amorosa JK. Imaging the pregnant patient for nonobstetric conditions: algorithms and radiation dose considerations. Radiographics 2007;27:1705-22. 10.1148/rg.276075002 [DOI] [PubMed] [Google Scholar]
- 115.Kai K, Hamada T, Yuge A, Kiyosue H, Nishida Y, Nasu K, Narahara H. Estimating the radiation dose to the fetus in prophylactic internal iliac artery balloon occlusion: three cases. Case Rep Obstet Gynecol 2015;2015:170343. 10.1155/2015/170343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Semeraro V, Susac A, Morasca A, D'Antonio F, Belli AM. Foetal radiation dose during prophylactic occlusion balloon placement for morbidly adherent placenta. Cardiovasc Intervent Radiol 2015;38:1487-93. 10.1007/s00270-015-1102-7 [DOI] [PubMed] [Google Scholar]
- 117.Liu J, Xu J, Jiao D, Duan X, Han X. Comparison of the efficacy of prophylactic balloon occlusion of the abdominal aorta at or below the level of the renal artery in women with placenta accreta undergoing cesarean section. J Matern Fetal Neonatal Med 2019:1-8. [Epub ahead of print]. doi: . 10.1080/14767058.2019.1667325 [DOI] [PubMed] [Google Scholar]
- 118.Luo F, Wu Z, Mei J, Yue J, Yu X, Xie L. Thrombosis after aortic balloon occlusion during cesarean delivery for abnormally invasive placenta. Int J Obstet Anesth 2018;33:32-9. 10.1016/j.ijoa.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 119.Nieto-Calvache AJ, Salas LF, Duran EJ, Benavides SO, Ordoñez-Delgado CA, Rodriguez-Holguin F. Estimation of fetal radiation absorbed dose during the prophylactic use of aortic occlusion balloon for abnormally invasive placenta. J Matern Fetal Neonatal Med 2019:1-6. [Epub ahead of print]. doi: . 10.1080/14767058.2019.1678144 [DOI] [PubMed] [Google Scholar]
- 120.Mehrabadi A, Hutcheon JA, Liu S, Bartholomew S, Kramer MS, Liston RM, Joseph KS, Maternal Health Study Group of Canadian Perinatal Surveillance System (Public Health Agency of Canada). Contribution of placenta accreta to the incidence of postpartum hemorrhage and severe postpartum hemorrhage. Obstet Gynecol 2015;125:814-21. 10.1097/AOG.0000000000000722 [DOI] [PubMed] [Google Scholar]
- 121.Morel O, Collins SL, Uzan-Augui J, Masselli G, Duan J, Chabot-Lecoanet AC, Braun T, Langhoff-Roos J, Soyer P, Chantraine F, International Society for Abnormally Invasive Placenta (IS-AIP) A proposal for standardized magnetic resonance imaging (MRI) descriptors of abnormally invasive placenta (AIP) - From the International Society for AIP. Diagn Interv Imaging 2019;100:319-25. 10.1016/j.diii.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 122.Levine AB, Kuhlman K, Bonn J. Placenta accreta: comparison of cases managed with and without pelvic artery balloon catheters. J Matern Fetal Med 1999;8:173-6. [DOI] [PubMed] [Google Scholar]
- 123.Sentilhes L, Goffinet F, Kayem G. Management of placenta accreta. Acta Obstet Gynecol Scand 2013;92:1125-34. [DOI] [PubMed] [Google Scholar]
- 124.Shahin Y, Pang CL. Endovascular interventional modalities for haemorrhage control in abnormal placental implantation deliveries: a systematic review and meta-analysis. Eur Radiol 2018;28:2713-26. 10.1007/s00330-017-5222-0 [DOI] [PubMed] [Google Scholar]
- 125.Ordoñez CA, Manzano-Nunez R, Parra MW, Rasmussen TE, Nieto AJ, Herrera-Escobar JP, Fernandez P, Naranjo MP, García AF, Carvajal JA, Burgos JM, Rodriguez F, Escobar-Vidarte MF. Prophylactic use of resuscitative endovascular balloon occlusion of the aorta in women with abnormal placentation: a systematic review, meta-analysis, and case series. J Trauma Acute Care Surg 2018;84:809-18. 10.1097/TA.0000000000001821 [DOI] [PubMed] [Google Scholar]
- 126.Kohi MP, Spies JB. Updates on uterine artery embolization. Semin Intervent Radiol 2018;35:48-55. 10.1055/s-0038-1636521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shamshirsaz AA, Fox KA, Erfani H, Belfort MA. The role of centers of excellence with multidisciplinary teams in the management of abnormal invasive placenta. Clin Obstet Gynecol 2018;61:841-50. 10.1097/GRF.0000000000000393 [DOI] [PubMed] [Google Scholar]