ABSTRACT
Diet plays an important role in the regulation of chronic inflammation, which is linked to cardiovascular disease (CVD) and several cancers. The dietary inflammatory index (DII®) was developed to estimate the inflammatory potential of an individual’s diet. We examined the association between DII scores and serum high-sensitivity C-reactive protein (hs-CRP) concentrations using the baseline data from the Japan Collaborative Cohort Study (JACC Study). Data were from 1176 control subjects (650 men and 526 women) in a nested case-control study of several cancers and CVD in the JACC Study who were free of cancer and CVD at baseline. DII scores were calculated from 26 food parameters that were derived from a validated food frequency questionnaire administered at the baseline. Energy-adjusted DII scores were calculated using the residual method. Serum hs-CRP concentrations were measured by latex-enhanced nephelometry or enzyme-immunoassay. In multivariable logistic regression analysis adjusting for potential confounders including sex, age, smoking habits, drinking habits, body mass index, and history of hypertension, the odds ratio (OR) and 95% confidence intervals (CI) for high serum hs-CRP concentrations (>1.0 mg/L) was significantly higher in the highest versus the lowest DII quartile (ORQuartile4vs1 = 1.32, 95% CI = 1.01 to 2.52). Likewise, a 1-point increase in DII score was associated with a 14% increased risk of high serum hs-CRP concentrations (ORContinuous = 1.09, 95%CI = 1.01 to 1.19). A pro-inflammatory diet, as represented by high DII scores, was associated with high serum hs-CRP concentrations in this Japanese population.
Key Words: dietary inflammatory index, serum hs-CRP concentrations, Japanese population, inflammation, cross-sectional study
INTRODUCTION
Diet is well known to play a major role in regulating chronic inflammation1,2 that is involved in the etiology and progression of most chronic diseases.3 The Western dietary pattern, characterized by high intakes of red and processed meat, refined grains, and high-fat dairy products, has consistently been associated with increased markers of inflammation.4,5 On the other hand, a diet rich in vegetables and fruits, including the Mediterranean diet, have been shown to reduce inflammation.6,7 Specific nutrients such as fiber,8 vitamin C,9 beta-carotene,9 and n-3 PUFAs10 also have been shown to be associated with lower concentrations of inflammation.
Shivappa et al11 developed the dietary inflammatory index (DII®), a literature-derived, population-based dietary score summarizing the effect of dietary parameters on six inflammatory biomarkers (IL-1β, IL-6, TNF-α or C-reactive protein (CRP), IL-4 and IL-10). According to a comprehensive review of the literature published from 1950 to 2010 a total of 45 dietary parameters, including micro and macro nutrients, flavonoids, and individual food items, were associated with the six inflammatory biomarkers. The DII can be used to estimate the inflammatory potential of diet as estimated by a variety of assessment methods, and has been shown to be associated with serum inflammation markers such as CRP and IL-6 in Western and Middle Eastern countries.12-17 Hence, the DII appears to be a useful tool for assessing the inflammatory potential of the diet that can be applied to any population-based study anywhere in the world.
Japanese have lower concentrations of CRP than their Western counterparts, in part because they have lower concentrations of adiposity and their BMI is lower.18 Japanese also have lower mortality from coronary heart disease (CHD).19 To date, there are few studies that have examined the association between DII scores and serum CRP concentrations in a Japanese population. Given what has been observed in other countries, it would be interesting to explore the association between DII scores and inflammation markers in Japanese adults. To examine the association between DII scores and serum high-sensitivity C-reactive protein (hs-CRP) concentrations in Japanese, we conducted a cross-sectional study using the data from the Japan Collaborative Cohort Study (JACC Study).
MATERIALS AND METHODS
Study subjects
The baseline survey of the JACC Study, which was conducted from 1988 to 1990, involved subjects aged 40 to 79 years, living in 45 communities across Japan. Methodologic details have been described elsewhere.20,21 A total of 39,242 participants were administered a questionnaire on life-style and donated serum samples at baseline. Of these, 818 cases and 1552 controls were chosen to examine the associations between serum hs-CRP concentrations and the risk for lung, stomach, colorectal22 and gallbladder cancers, and cardiovascular disease (CVD).23 Of the 1552 control subjects, we excluded the following subjects; 179 with a self-reported history of cancer, CHD, cerebrovascular disease, kidney disease, and diabetes mellitus at baseline that may affect the outcome; 180 subjects missing any of the measurements including DII scores; and 17 subjects with serum hs-CRP > 10mg/L. A total of 1176 control subjects (650 men and 526 women) were eligible for the present analysis. There was no subject with implausibly high or low total energy intakes (<500 or >3,500 kcal/d) among study subjects. Informed consent was obtained individually from most subjects, except in a few study areas where informed consent was provided at the group level, and data confidentiality was explained to community leaders. The study protocol was approved by the Ethics Committees of Hokkaido University (approval number; 14-044), which is where the central secretariat of the JACC study is located.
Data collection
Information on lifestyle at baseline was collected using a self-administered questionnaire, that covered age, sex, height, weight, medical history of cancer (yes or no), CHD (yes or no), cerebrovascular disease (yes or no), hypertension (yes or no), kidney disease (yes or no), and diabetes mellitus (yes or no), smoking habits (never, ex-smoker, and current smoker), drinking habits (nondrinker, Ex-drinker, and current drinker). A food frequency questionnaire (FFQ)24 was used to ascertain the average intake frequency of 40 food items in the past year. For 33 foods or dishes, we asked about the average intake frequency without specifying portion size. We used the following 5 response choices: almost never; 1–2 times a month; 1–2 times a week; 3–4 times a week; and almost every day. For rice and miso soup, we asked about the number of bowls or cups consumed each day. Non-alcoholic beverages (green tea, black tea, oolong tea, and coffee) were assessed using five frequency categories: almost never; 1–2 cups/month; 1–2 cups/week; 3–4 cups/week; and almost every day. For alcoholic beverages, we inquired about the frequency of consumption (<1 time/week, 1–2 times/week, 3–4 times/week, and almost daily) and the usual amount consumed on each occasion. The FFQ was validated by comparing to four 3-day weighted dietary records over a 1-year period as a standard.25 The daily intake of nutrients was calculated by multiplying the intake frequency of each item by its nutrient content per serving and totaling the nutrient intake for all food items queried on the FFQ.25
The DII was developed to assess the inflammatory potential of an individual’s overall diet.11 The nutrients data derived from the FFQ was used to calculate the DII scores for all study subjects. The details the design and development of the DII scoring algorithm have been described elsewhere.26 The food parameters were scored as follows: “+1” was assigned if the effects were pro-inflammatory (significantly increased IL-1β, IL-6, TNF-α, or CRP, or decreased IL-4 or IL-10); ‘–1’ was assigned if the effects were anti-inflammatory (significantly decreased IL-1β, IL-6, TNF-α or CRP, or increased IL-4 or IL-10); and ‘0’ was assigned if no significant association was observed between the food parameter and the above inflammatory markers.11 These scores were weighted based on study design. To avoid the arbitrariness resulting from simply using raw consumption amounts, intakes of foods and nutrition were standardized to a representative range of dietary intake based on actual human consumption in 11 populations living in different countries across the world that provided an estimate of a mean and standard deviation for each parameter. These values were converted to a proportion (with values from 0 to 1). Each proportion was doubled, and then 1 was subtracted to achieve a symmetrical distribution around a mean of ≈0. Each of these values was then multiplied by an overall food parameter-specific inflammation score. All the food parameter-specific DII scores were summed to create the overall DII scores for each subject. Higher DII scores indicate more pro-inflammatory diets, while lower DII scores represent anti-inflammatory diets. In this study, data were available for a total of 26 food parameters (carbohydrate, protein, total fat, alcohol, fiber, cholesterol, saturated fatty acid, monounsaturated fatty acid, polyunsaturated fatty acid, omega-3 fatty acid, omega-6 fatty acid, vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, niacin, folic acid, beta-carotene, iron, magnesium, zinc, and tea) to calculate the DII scores.
Serum samples were stored at -80 degrees centigrade until biochemical analysis. Serum hs-CRP concentrations were measured by latex-enhanced nephelometry (BN Prospec nephrometer; Dade Behring, Tokyo, Japan) in the nested case-control study of CVD or enzyme-immunoassay (High sensitivity C-reactive protein enzyme immunoassay test kit, Diagnostic Automation Inc., USA) in nested case-control study of lung, stomach, colorectal, and gallbladder cancers. The inter-assay and intra-assay coefficients of variations (CVs) for hs-CRP of each kit were less than 10%, respectively.23,27 The correlation between the enzyme-immunoassay and nephelometry assay according to the attached manual of ELISA assay kit was 0.96. These were satisfactory according to the CDC/AHA scientific criterion.28 Based on previous work in a Japanese population, we defined more than 1.0 mg/L as high serum hs-CRP concentrations, which is recommended as a relevant cut-off point for high risk of future development of CVD in Japanese.18
Statistical analysis
Energy adjustment was performed using the residual method. Analysis of variance (ANOVA) was used to compare the mean values of continuous variable across quartiles of the DII scores and t-tests were used to compare the mean values of continuous variables between men and women. Because hs-CRP values had a lognormal distribution, the log-transformed value of hs-CRP was used for both simple t-tests and ANOVA. The chi-square test was used to compare percentages of categorical variables such as smoking habits, drinking habits, and hypertension across quartiles of the DII scores. We used multiple linear regression analysis to examine the association between DII scores and serum hs-CRP concentrations. Multivariable logistic regression analysis was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for high serum hs-CRP concentrations (>1.0 mg/L). The distribution of DII scores was divided into quartiles and the ORs for high serum hs-CRP concentrations were compared by DII quartile [DII(quartiles)], using the lowest quartile as a reference. ORs and 95%CIs were also estimated using continuous DII scores [DII(continuous)]. We used sex, age, smoking habits, drinking habits, body mass index (BMI), total energy intake and history of hypertension as covariates as they may influence both dietary intake and inflammation and may either modify effects or cause spurious associations by confounding the effect of diet. A p value of <0.05 was considered statistically significant. The statistical software JMP ver. 12.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
RESULTS
The mean DII score was +0.83 (standard error: 0.04) and scores ranged from –3.61 (maximally anti-inflammatory) to +5.07 (maximally pro-inflammatory) among study subjects. Figure shows the distributions of DII score and serum hs-CRP concentration by sex. The DII scores were significantly higher in women than in men (mean ± standard deviation: 0.74±1.69 in men and 0.94±1.67 in women, p = 0.04). Men had significantly higher serum hs-CRP concentrations, smoking rates, and drinking rates compared to women (median and 25 – 75th percentiles: 0.50 (0.22–1.31) in men and 0.40 (0.16–0.85) in women, p<0.001). The percentages of serum hs-CRP concentrations of > 1.0 mg/L were 32.2% (n = 209) in men and 20.5% (n = 108) in women. Table 1 shows the characteristics of the study subjects across quartiles of the DII scores. The percentage of subjects with high serum hs-CRP concentrations (>1.0 mg/L) was significantly higher in the highest quartile of the DII scores, though no significant differences in serum hs-CRP concentrations were observed across quartiles of DII scores. Dietary intakes of nutrients, except for carbohydrate and alcohol, significantly decreased with increase in DII scores. Dietary intakes of carbohydrate and alcohol were higher in the subjects with high DII scores. There also were no significant differences in age, BMI and percentages of men across quartiles of DII scores. In the multivariable linear regression analyses (Table 2), DII scores were positively associated with log serum hs-CRP concentrations (β = 0.048, 95%CI = 0.008 to 0.087), indicating that pro-inflammatory diet was associated with high serum hs-CRP. [Table 2] Similar results were obtained in men (β = 0.085, 95%CI = 0.002 to 0.106); however, despite a positive regression coefficient, no significant association was observed in women (β = 0.041, 95%CI = –0.002 to 0.103). Table 3 shows ORs and 95% CI for high serum hs-CRP concentrations (>1.0 mg/L) according to the DII scores. [Table 3] A significantly higher OR was observed in the highest quartile of DII scores (ORQuartile4vs1 = 1.32, 95%CI = 1.01 to 2.52) than in the lowest quartile in all subjects. A 1-point increase in DII scores [DII score (continuous)] was associated with a 9% increased risk of high hs-CRP concentrations (OR = 1.09, 95%CI = 1.01 to 1.19). In sex-stratified analyses, men had a significantly increased risk OR and 95%CI was observed in the highest quartile of DII scores compared to the lowest quartile (ORQuartile4vs1 = 1.76, 95%CI = 1.07 to 2.92). We obtained results of similar magnitude in women; however, they were not statistically significant. DII scores calculated by regressing separately by sex produced results similar to those obtained in multiple linear regression analysis and logistic regression analysis with data from men and women combined.
Fig. 1.
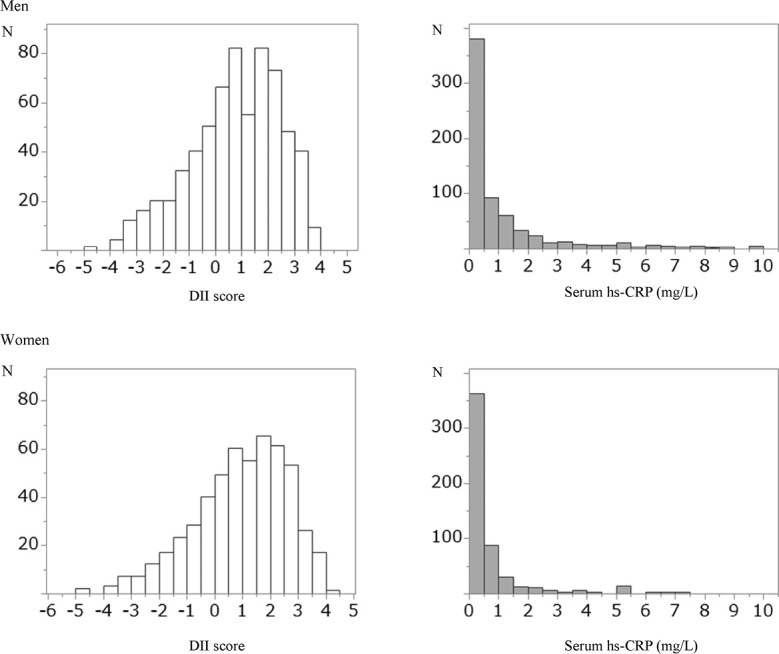
Distributions of DII score and serum hs-CRP concentrations by sex
N: number, DII: dietary inflammatory index, hs-CRP: high sensitivity- C-reactive protein.
Table 1.
Characteristics of the study subjects across quartiles of DII score
| DII score | ||||||
| Q1 | Q2 | Q3 | Q4 | p | ||
| n | 294 | 294 | 294 | 294 | ||
| DII score range | < 0.01 | 0.02 to 0.90 | 0.91 to 1.70 | ≧ 1.71 | ||
| DII score | –0.87 (0.67) | 0.48 (0.26) | 1.29 (0.23) | 2.44 (0.60) | < 0.001 | 1 |
| Median | –0.81 | 0.48 | 1.28 | 2.28 | ||
| Men (%) | 122 (41.5) | 155 (52.7) | 153 (52.0) | 220 (74.8) | < 0.001 | 2 |
| Age (y) | 64.3 (8.2) | 64.0 (9.3) | 63.6 (8.5) | 61.8 (9.7) | 0.01 | 1 |
| BMI (kg/m2) | 22.9 (3.0) | 22.5 (2.8) | 22.5 (2.8) | 22.6 (2.8) | 0.20 | 1 |
| hs-CRP (mg/L) | 0.40 (0.17, 1.00) | 0.47 (0.18, 0.96) | 0.48 (0.22, 1.12) | 0.49 (0.20, 1.25) | 0.19 | 3 |
| >1.0 mg/L n (%) | 72 (24.5) | 71 (24.2) | 80 (27.2) | 94 (29.7) | 0.04 | 4 |
| Current smoker n (%) | 60 (20.4) | 61 (20.8) | 79 (26.9) | 116 (39.5) | < 0.001 | 2 |
| Current drinker n (%) | 97 (33.0) | 127 (43.2) | 127 (43.2) | 200 (68.0) | < 0.001 | 2 |
| Hypertension n (%) | 77 (26.6) | 76 (27.0) | 73 (25.7) | 70 (24.7) | 0.93 | 2 |
| Dietary intake | ||||||
| Energy (kcal) | 1717.4 (426.6) | 1578.3 (450.7) | 1490.2 (427.8) | 1711.2 (470.8) | < 0.001 | 1 |
| Protein (g) | 77.3 (19.6) | 58.4 (17.3) | 46.3 (16.5) | 37.5 (16.3) | < 0.001 | 1 |
| (% energy) | 14.8 (2.2) | 14.1 (2.4) | 13.5 (2.6) | 12.5 (2.5) | < 0.001 | 1 |
| Fat (g) | 47.4 (17.5) | 34.9 (14.3) | 26.8 (13.0) | 20.3 (14.7) | < 0.001 | 1 |
| (% energy) | 19.9 (4.3) | 18.6 (4.6) | 17.9 (4.9) | 16.9 (5.2) | < 0.001 | 1 |
| Carbohydrate (g) | 248.6 (87.1) | 232.6 (92.4) | 233.2 (88.5) | 271.5 (111.4) | < 0.001 | 1 |
| (% energy) | 59.0 (7.1) | 60.6 (7.9) | 60.9 (8.9) | 63.0 (9.4) | < 0.001 | 1 |
| Alcohol (g) | 1.6 (0, 5.7) | 3.3 (0.9, 9.9) | 4.0 (2.2, 8.6) | 6.2 (1.8, 17.8) | < 0.001 | 3 |
| Tea (g) | 4.6 (3.4) | 4.2 (3.5) | 3.5 (2.9) | 2.4 (2.7) | < 0.001 | 1 |
| Zinc (mg) | 9.4 (2.2) | 7.7 (2.3) | 6.8 (2.2) | 6.6 (2.3) | < 0.001 | 1 |
| Magnesium (mg) | 363.4 (80.9) | 292.7 (82.8) | 246.3 (83.2) | 222.1 (86.9) | < 0.001 | 1 |
| Iron (mg) | 11.7 (3.1) | 8.5 (2.5) | 6.3 (2.7) | 4.1 (2.7) | < 0.001 | 1 |
| β Carotene equivalent (μg) | 5960.8 (1836.1) | 3731.2 (1574.0) | 2129.7 (1477.0) | 834.7 (1491.3) | < 0.001 | 1 |
| Retinol equivalent (μg) | 1529.1 (1983.0) | 795.3 (1136.6) | 464.6 (880.5) | 150.0 (648.1) | < 0.001 | 1 |
| Vitamin B1 (mg) | 1.3 (0.3) | 1.0 (0.3) | 0.9 (0.3) | 0.8 (0.3) | < 0.001 | 1 |
| Vitamin B2 (mg) | 1.5 (0.6) | 1.1 (0.4) | 0.8 (0.4) | 0.5 (0.4) | < 0.001 | 1 |
| Vitamin B6 (mg) | 1.8 (0.3) | 1.3 (0.3) | 1.0 (0.3) | 0.8 (0.3) | < 0.001 | 1 |
| Vitamin B12 (mg) | 12.6 (7.4) | 7.8 (4.8) | 5.3 (4.0) | 2.5 (3.6) | < 0.001 | 1 |
| Vitamin C (mg) | 179.5 (51.6) | 115.5 (44.7) | 75.4 (41.9) | 34.4 (45.2) | < 0.001 | 1 |
| Vitamin D (μg) | 12.2 (5.6) | 7.9 (5.5) | 4.9 (4.5) | 2.1 (4.2) | < 0.001 | 1 |
| Vitamin E (mg) | 18.5 (3.5) | 15.4 (2.9) | 13.6 (3.1) | 11.0 (3.7) | < 0.001 | 1 |
| Folic acid (μg) | 554.3 (207.1) | 362.3 (124.6) | 242.0 (113.3) | 135.9 (109.1) | < 0.001 | 1 |
| Niacin (mg) | 24.2 (5.8) | 19.0 (6.1) | 16.1 (5.3) | 14.9 (5.7) | < 0.001 | 1 |
| SFA (g) | 13.2 (6.2) | 10.3 (5.6) | 8.2 (5.1) | 6.6 (5.8) | < 0.001 | 1 |
| MUFA (g) | 15.5 (6.5) | 11.1 (5.1) | 8.4 (4.6) | 6.3 (5.2) | < 0.001 | 1 |
| PUFA (g) | 12.0 (4.2) | 8.6 (3.3) | 6.6 (3.5) | 5.0 (3.4) | < 0.001 | 1 |
| ω-3 PUFA (g) | 2.1 (0.4) | 1.7 (0.4) | 1.4 (0.3) | 1.1 (0.3) | < 0.001 | 1 |
| ω-6 PUFA (g) | 7.7 (1.5) | 6.7 (1.3) | 6.1 (1.3) | 5.2 (1.5) | < 0.001 | 1 |
| Cholesterol (mg) | 353.2 (148.2) | 259.6 (136.0) | 191.1 (134.8) | 114.8 (137.8) | < 0.001 | 1 |
| Fiber (g) | 18.7 (5.0) | 13.7 (4.3) | 10.7 (4.6) | 8.7 (4.6) | < 0.001 | 1 |
DII: dietary inflammatory index, BMI: body mass index, hs-CRP: high-sensitivity C-reactive protein.
DII score, age, body mass index, and dietary intakes, excluding alcohol, are expressed as mean value (standard deviation).
Hs-CRP and alcohol are expressed as median and 25 – 75th percentiles in parentheses.
Dietary intakes of nutrients and DII excluding energy were adjusted by energy intake using the residual method.
1 Analysis of variance (ANOVA), 2 Chi-square test, 3 Kruskal-Wallis test, 4 Cochran-armitage trend test.
Table 2.
Relation of DII score with serum C-reactive protein levels
| β (95%CI)1 | p | |
| All | 0.048 (0.008, 0.087) | 0.02 |
| Men | 0.085 (0.002, 0.106) | 0.04 |
| Women | 0.041 (–0.02, 0.103) | 0.20 |
β: regression coefficient.
1Adjusted variables were sex, age, smoking habits, drinking habits, history of hypertension, total energy intake, and body mass index.
Table 3.
Multivariable adjusted odds ratios for high serum C-reactive protein levels according to DII score
| DII score | OR (95%CI)1 | |
| All | DII score(quartiles) | |
| Q1 | 1 | |
| Q2 | 1.05 (0.60, 1.84) | |
| Q3 | 1.05 (0.62, 1.77) | |
| Q4 | 1.32 (1.01, 2.52) | |
| DII score (continuous) | 1.09 (1.01, 1.19) | |
| Men | DII score (quartiles) | |
| Q1 | 1 | |
| Q2 | 1.18 (0.72, 1.94) | |
| Q3 | 1.26 (0.76, 2.08) | |
| Q4 | 1.76 (1.07, 2.92) | |
| DII score (continuous) | 1.10 (1.01, 1.27) | |
| Women | DII score (quartiles) | |
| Q1 | 1 | |
| Q2 | 0.84 (0.36, 1.95) | |
| Q3 | 0.92 (0.43, 1.96) | |
| Q4 | 1.09 (0.95, 1.25) | |
| DII score (continuous) | 1.05 (0.52, 2.12) |
DII: dietary inflammatory index.
OR(95%CI) : Odds ratio and 95% confidence intervals.
1Adjusted variables were sex, age, smoking habits, drinking habits, history of hypertension, total energy intake, and BMI.
DISCUSSION
In this cross-sectional study within the Japan Collaborative Cohort study, we found that a pro-inflammatory diet, as represented as high DII scores, was associated with higher serum hs-CRP concentrations. The results of this study are consistent with the hypothesis that diet modulates inflammation status in the body. Several cross-sectional studies in Western populations16,29,30 have shown that higher DII scores are associated with high serum hs-CRP concentrations (≥3.0 mg/L). Our results are consistent with these findings in that pro-inflammatory diet, as evidenced by higher DII scores, was associated with increasing circulating hs-CRP concentrations. Even though Japanese have lower serum hs-CRP concentrations than Westerners, the DII scores may be a useful tool for evaluating the total inflammatory potential of diet in Japanese.
It is generally accepted that serum CRP concentrations are higher in men than in women. However, previous studies have not reported on sex differences in the association between the DII score and inflammation markers. In the present study, the DII score was significantly and positively associated with serum hs-CRP concentrations only in men. Results of similar magnitude were observed in women, but they were not statistically significant. The relatively low number of women with high serum hs-CRP concentrations was the likely explanation for this null finding.
Some studies have reported that overall diets and specific nutrients regulate inflammation. Nanri et al31 have shown that the healthy dietary pattern (high in vegetables and fruit) is associated with suppressed inflammation using the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study). Specific nutrients contained in vegetables and fruits, such as vitamin C,6 fiber,8 and vitamin E,32 have consistently been shown to be negatively associated with serum CRP concentrations. On the other hand, intakes of high-fat33 or high saturated-fat foods34 have been reported to be associated with increased concentrations of inflammation markers such as IL-6, TNF-α, and CRP. In this study, we observed that subjects with low DII scores tended to have higher intakes of most nutrients, including fat, compared to those with high DII score. These findings are identical to the results of another study using a health examinee cohort.35 The subjects who consumed high levels of fat also may have consumed more anti-inflammatory/antioxidant foods. This trend implies that the subjects with lower DII scores may have taken various foods to have achieved a nutritionally balanced diet; i.e., without excessive caloric intakes.
The DII score tended to be higher in this study than in previous studies such as the National Health and Nutrition Examination Survey (NHANES)29 and the SEASONS.12 Because the method of collecting dietary data is different in each study, the difference in food parameters used for analysis may influence the mean DII score. For calculating DII score, 28 food parameters were used in the NHANES study and 28 food parameters for 7DDR and 44 food parameters for 24HR were available in the SEASONS. Inter-population differences exist on available food parameters. For example, the NHANES study included selenium and caffeine as anti-inflammatory food parameter to perform the DII score calculation; however, we have no data on those parameters in this study. Moreover, some anti-inflammatory food parameters are fairly universal missing including turmeric, flavones, isoflavones, flavonols, ginger, flavan-3-ol, onions, and others. Because these food parameters are typically consumed in small amounts they probably would not have had a large impact on the scoring. Still, the missing information may underestimate the association. The difference between this study and other studies DII scores may be due to those missing food parameters, especially anti-inflammatory food parameters.
There are several limitations in this study. First, serum CRP concentrations of ≥3.0 mg/L is recommended as a relevant clinical cut-off point for identifying individuals at high CVD risk in Westerners.36 However, Japanese people tend to have lower serum CRP concentrations compared to those of Western people. Among these study subjects, the percentages of those with serum CRP concentrations of ≥3.0 mg/L were 9.7% (n = 63) in men and 6.5% (n = 34) in women. Unlike in Western populations,12,13 there are too few subjects with serum hs-CRP concentrations of ≥3.0 mg/L to analyze using that value as a clinical cut-off point. We used serum hs-CRP concentrations of 1.0 mg/L as a cut-off point in logistic regression analysis, with reference to the previous study that analyzed hs-CRP and the risk of CHD in a Japanese population.18 Second, serum hs-CRP concentrations were measured using two different measurement methods (latex-enhanced nephelometry and enzyme-immunoassay). Because the correlation coefficient between enzyme-immunoassay and latex-enhanced nephelometry is very high (r = 0.96), the measurement method did not have a materially relevant effect on the results. Third, the FFQ used in this study provided estimates for only 40 foods and did not include options for food portion size. In general, the fewer parameters available, the higher DII scores; so, the high scores may have been somewhat artefactual.37 Additionally, energy intake may have been underestimated because some foods simply are not covered by the FFQs. Moreover, there was the non-availability of the remaining 18 food parameters that could be used to calculate the DII score, because only 26 food parameters were available in this study. Comparison of DII scores across studies using different FFQs should be carefully considered as the available food parameters may be different. Future studies in Japanese populations using more detailed FFQs will be needed to confirm the association between DII score and inflammation markers.
In conclusion, we showed that more pro-inflammatory DII scores were associated with elevated serum hs-CRP concentrations in Japanese subjects with generally lower serum hs-CRP concentrations than their Western counterparts.12,13 These results, which demonstrate that lower DII scores are associated with higher intakes of nutrients associated with chronic diseases, underline the need for prospective studies to elucidate the causal link between the DII scores and individual inflammatory status in Japanese and other Asian populations that have generally lower levels of chronic, systemic inflammation.
ACKNOWLEDGEMENT
The authors sincerely express their appreciation to Drs. Kunio Aoki and Yoshiyuki Ohno, professors emeritus of the Nagoya University School of Medicine and former chairpersons of the JACC Study, for their encouragement and support during this study. We are also greatly indebted to Dr. Haruo Sugano, former director of the Cancer Institute, Tokyo, who made substantial contributions to the initiation of the JACC Study; Dr. Tomoyuki Kitagawa, director emeritus of the Cancer Institute of the Japanese Foundation for Cancer Research and former project leader of the Grant-in-Aid for Scientific Research on Priority Areas of Cancer; and Dr. Kazuo Tajima of Aichi Cancer Center, who was the previous project leader of the Grant-in-Aid for Scientific Research on Priority Areas of Cancer Epidemiology. We thank Dr. Chigusa Date for contributing to the validation of FFQ and estimation of nutrient intake in the JACC study.
CONFLICT OF INTEREST
All authors declare no conflicts of interests.
SOURCES OF FUNDING
The JACC Study has been supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT); Grants-in-Aid for Scientific Research on Priority Areas of Cancer; Grants-in-Aid for Scientific Research on Priority Areas of Cancer Epidemiology from MEXT (Nos. 61010076, 62010074, 63010074, 1010068, 2151065, 3151064, 4151063, 5151069, 6279102, 11181101, 17015022, 18014011, 20014026, 20390156, and 16H06277).
DISCLOSURE
Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company that has licensed the right to his invention of the dietary inflammatory index (DII®) from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI.
MEMBER OF THE JAPAN COLLABORATIVE COHORT STUDY GROUP
The present members of the JACC Study Group who co-authored this paper include Dr. Akiko Tamakoshi (present chairperson of the study group), Hokkaido University Faculty of Medicine; Dr. Mitsuru Mori, Hokkaido Chitose College of Rehabilitation; Dr. Yoshihiro Kaneko, Japan Support Center for Suicide Countermeasures; Dr. Ichiro Tsuji, Tohoku University Graduate School of Medicine; Dr. Yosikazu Nakamura, Jichi Medical School; Dr. Hiroyasu Iso, Osaka University Graduate School of Medicine; Dr. Kazumasa Yamagishi, Faculty of Medicine, University of Tsukuba; Dr. Haruo Mikami, Cancer Prevention Center, Chiba Cancer Center Research Institute; Dr. Michiko Kurosawa, Juntendo University Faculty of Medicine; Dr. Yoshiharu Hoshiyama, Yokohama Soei University; Dr. Naohito Tanabe, University of Niigata Prefecture; Dr. Koji Tamakoshi, Nagoya University Graduate School of Health Sciences; Dr. Kenji Wakai, Nagoya University Graduate School of Medicine; Dr. Masahiko Ando, Nagoya University Hospital; Dr. Koji Suzuki, Fujita Health University School of Health Sciences; Dr. Shuji Hashimoto, Fujita Health University School of Medicine; Dr. Hiroshi Yatsuya, Fujita Health University School of Medicine; Dr. Shogo Kikuchi, Aichi Medical University School of Medicine; Dr. Yasuhiko Wada, Wakayama Prefecture Tanabe Public Health Center; Dr. Takashi Kawamura, Kyoto University Health Service; Dr. Yoshiyuki Watanabe, Graduate School of Medical Science, Kyoto Prefectural University of Medicine; Dr. Kotaro Ozasa, Radiation Effects Research Foundation; Dr. Kazuya Mikami, Japanese Red Cross Kyoto Daiichi Hospital; Dr. Kiyomi Sakata, Iwate Medical University; Dr. Yoichi Kurozawa, Tottori University Faculty of Medicine; Dr. Yoshihisa Fujino, University of Occupational and Environmental Health, Japan; and Dr. Akira Shibata, Kurume University.
Abbreviations
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- DII
dietary inflammatory index
- FFQ
food frequency questionnaire
- hs-CRP
high-sensitivity C-reactive protein
- IL
interleukin
- JACC Study
Japan Collaborative Cohort Study
- OR
odds ratios
REFERENCES
- 1.Connaughton RM, McMorrow AM, McGillicuddy FC, Lithander FE, Roche HM. Impact of anti-inflammatory nutrients on obesity-associated metabolic-inflammation from childhood through to adulthood. Proc Nutr Soc. 2016;75(2):115–124. doi: 10.1017/S0029665116000070. [DOI] [PubMed]
- 2.Kirwan AM, Lenighan YM, O’Reilly ME, McGillicuddy FC, Roche HM. Nutritional modulation of metabolic inflammation. Biochem Soc Trans. 2017;45(4):979–985. doi: 10.1042/BST20160465. [DOI] [PubMed]
- 3.Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012;54(Suppl):S29–37. doi: 10.1016/j.ypmed.2011.11.011. [DOI] [PMC free article] [PubMed]
- 4.Fung TT, Rimm EB, Spiegelman D, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73(1):61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed]
- 5.Nettleton JA, Steffen LM, Mayer-Davis EJ, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2006;83(6):1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed]
- 6.Wannamethee SG, Lowe GD, Rumley A, Bruckdorfer KR, Whincup PH. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr. 2006;83(3):567–574;quiz 726–567. doi: 10.1093/ajcn.83.3.567. [DOI] [PubMed]
- 7.Bonaccio M, Cerletti C, Iacoviello L, de Gaetano G. Mediterranean diet and low-grade subclinical inflammation: the Moli-sani study. Endocr Metab Immune Disord Drug Targets. 2015;15(1):18–24. [DOI] [PubMed]
- 8.Ma Y, Griffith JA, Chasan-Taber L, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83(4):760–766. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed]
- 9.Suarez EC, Schramm-Sapyta NL. Race differences in the relation of vitamins A, C, E, and beta-carotene to metabolic and inflammatory biomarkers. Nutr Res. 2014;34(1):1–10. doi: 10.1016/j.nutres.2013.10.001. [DOI] [PMC free article] [PubMed]
- 10.Micallef MA, Munro IA, Garg ML. An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur J Clin Nutr. 2009;63(9):1154–1156. doi: 10.1038/ejcn.2009.20. [DOI] [PubMed]
- 11.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed]
- 12.Shivappa N, Steck SE, Hurley TG, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014;17(8):1825–1833. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed]
- 13.Shivappa N, Hebert JR, Rietzschel ER, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. 2015;113(4):665–671. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed]
- 14.Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy. 2015;45(1):177–183. doi: 10.1111/cea.12323. [DOI] [PMC free article] [PubMed]
- 15.Shivappa N, Bonaccio M, Hebert JR, et al. Association of proinflammatory diet with low-grade inflammation: results from the Moli-sani study. Nutrition. 2018;54:182–188. doi: 10.1016/j.nut.2018.04.004. [DOI] [PMC free article] [PubMed]
- 16.Phillips CM, Shivappa N, Hebert JR, Perry IJ. Dietary inflammatory index and biomarkers of lipoprotein metabolism, inflammation and glucose homeostasis in adults. Nutrients. 2018;10(8):1033. doi: 10.3390/nu10081033. [DOI] [PMC free article] [PubMed]
- 17.Vahid F, Shivappa N, Faghfoori Z, et al. Validation of a dietary inflammatory index (DII) and association with risk of gastric cancer: a case-control study. Asian Pac J Cancer Prev. 2018;19(6):1471–1477. doi: 10.22034/APJCP.2018.19.6.1471. [DOI] [PMC free article] [PubMed]
- 18.Arima H, Kubo M, Yonemoto K, et al. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: the Hisayama study. Arterioscler Thromb Vasc Biol. 2008;28(7):1385–1391. doi: 10.1161/ATVBAHA.107.157164. [DOI] [PubMed]
- 19.Saito I, Folsom AR, Aono H, Ozawa H, Ikebe T, Yamashita T. Comparison of fatal coronary heart disease occurrence based on population surveys in Japan and the USA. Int J Epidemiol. 2000;29(5):837–844. [DOI] [PubMed]
- 20.Tamakoshi A, Yoshimura T, Inaba Y, et al. Profile of the JACC study. J Epidemiol. 2005;15(Suppl 1):S4–8. [DOI] [PMC free article] [PubMed]
- 21.Tamakoshi A, Ozasa K, Fujino Y, et al. Cohort profile of the Japan Collaborative Cohort Study at final follow-up. J Epidemiol. 2013;23(3):227–232. [DOI] [PMC free article] [PubMed]
- 22.Ito Y, Suzuki K, Tamakoshi K, et al. Colorectal cancer and serum C-reactive protein levels: a case-control study nested in the JACC Study. J Epidemiol. 2005;15(Suppl 2):S185–189. [DOI] [PMC free article] [PubMed]
- 23.Iso H, Cui R, Date C, Kikuchi S, Tamakoshi A, Group JS. C-reactive protein levels and risk of mortality from cardiovascular disease in Japanese: the JACC Study. Atherosclerosis. 2009;207(1):291–297. doi: 10.1016/j.atherosclerosis.2009.04.020. [DOI] [PubMed]
- 24.Iso H, Date C, Noda H, Yoshimura T, Tamakoshi A; Group JS. Frequency of food intake and estimated nutrient intake among men and women: the JACC Study. J Epidemiol. 2005;15(Suppl 1):S24–42. [DOI] [PMC free article] [PubMed]
- 25.Date C, Fukui M, Yamamoto A, et al. Reproducibility and validity of a self-administered food frequency questionnaire used in the JACC study. J Epidemiol. 2005;15(Suppl 1):S9–23. [DOI] [PMC free article] [PubMed]
- 26.Cavicchia PP, Steck SE, Hurley TG, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139(12):2365–2372. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed]
- 27.Suzuki K, Ito Y, Ochiai J, et al. Relationship between obesity and serum markers of oxidative stress and inflammation in Japanese. Asian Pac J Cancer Prev. 2003;4(3):259–266. [PubMed]
- 28.Nakamura M, Sato S, Shimamoto T. Establishment of external quality control program for hs-CRP and three-year follow-up of the performance for precision and accuracy. J Atheroscler Thromb. 2007;14(6):287–293. [DOI] [PubMed]
- 29.Shivappa N, Wirth MD, Hurley TG, Hebert JR. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999-2002. Mol Nutr Food Res. 2017;61(4):1600630. doi: 10.1002/mnfr.201600630. [DOI] [PMC free article] [PubMed]
- 30.Wirth MD, Burch J, Shivappa N, et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med. 2014;56(9):986–989. doi: 10.1097/JOM.0000000000000213. [DOI] [PMC free article] [PubMed]
- 31.Nanri H, Nakamura K, Hara M, et al. Association between dietary pattern and serum C-reactive protein in Japanese men and women. J Epidemiol. 2011;21(2):122–131. [DOI] [PMC free article] [PubMed]
- 32.Bertran N, Camps J, Fernandez-Ballart J, et al. Diet and lifestyle are associated with serum C-reactive protein concentrations in a population-based study. J Lab Clin Med. 2005;145(1):41–46. doi: 10.1016/j.lab.2004.11.002. [DOI] [PubMed]
- 33.Nappo F, Esposito K, Cioffi M, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39(7):1145–1150. [DOI] [PubMed]
- 34.King DE, Egan BM, Geesey ME. Relation of dietary fat and fiber to elevation of C-reactive protein. Am J Cardiol. 2003;92(11):1335–1339. [DOI] [PubMed]
- 35.Na W, Kim M, Sohn C. Dietary inflammatory index and its relationship with high-sensitivity C-reactive protein in Korean: data from the health examinee cohort. J Clin Biochem Nutr. 2018;62(1):83–88. doi: 10.3164/jcbn.17-22. [DOI] [PMC free article] [PubMed]
- 36.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. [DOI] [PubMed]
- 37.Hebert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG. Perspective: the dietary inflammatory index (DII)-lessons learned, improvements made, and future directions. Adv Nutr. 2019;10(2):185–195. doi: 10.1093/advances/nmy071. [DOI] [PMC free article] [PubMed]


