ABSTRACT
Thoracic surgery has evolved drastically in recent years. Although thoracic surgeons mainly deal with tumorous lesion in the lungs, mediastinum, and pleura, they also perform lung transplantation surgery in patients with end-stage lung disease. Herein, we introduce various major current topics in thoracic surgery. Minimally invasive surgical procedures include robot-assisted thoracic surgery and uniportal video-assisted thoracic surgery. Novel techniques for sublobar resection include virtual-assisted lung mapping, image-guided video-assisted thoracic surgery, and segmentectomy using indocyanine green. Three-dimensional (3D) computed tomography (CT) simulation consists of surgeon-friendly 3D-CT image analysis systems and new-generation, dynamic 3D-CT imaging systems. Updates in cadaveric lung transplantation include use of marginal donors, including donation after circulatory death, and ex vivo lung perfusion for such donors. Topics in living donor lobar lung transplantation include size matching, donor issues, and new surgical techniques. During routine clinical practice, thoracic surgeons encounter various pivotal topics related to thoracic surgery, which are described in this report.
Key Words: lung transplantation, robotic surgery, three-dimensional computed tomography, uniportal surgery, video-assisted thoracic surgery
INTRODUCTION
Recently, drastic changes in thoracic surgery have been noted. In the past, thoracic surgeons would treat infectious diseases such as tuberculosis. In more recent years, thoracic surgeons mainly treat tumorous chest lesions, such as cancerous pulmonary tumors; mediastinal tumors, including thymomas; and pleural tumors, including malignant pleural mesotheliomas. Some thoracic surgeons perform surgery for esophageal lesions, whereas others, such as those in Japan, do not. Furthermore, lung transplantation has also been performed by an increasing number of thoracic surgeons. Herein, we identify major current worldwide topics in thoracic surgery.
Various Minimally Invasive Surgical Procedures
Robot-assisted thoracic surgery. In the last three decades, thoracic surgeons have pursued the concept of minimally invasive techniques. Thoracoscopic surgery is less invasive than thoracotomy and generally has better outcomes.1 In fact, video-assisted thoracic surgery (VATS) has been recognized worldwide as a standard surgical procedure for the treatment of lung cancer. In Japan, approximately 70% of surgeries for lung cancer are performed by VATS.
Technological developments led to the introduction of robotic surgery. Currently, robot-assisted thoracic surgery (RATS) is used, especially in Western countries. In Japan, RATS was first used approximately 10 years ago (Figure 1) and has been performed with acceptable outcomes.2 In 2018, RATS was finally recognized by the government as a surgical procedure that could be used for malignant pulmonary and mediastinal tumors. Since then, many surgeons have used RATS and VATS.
Fig. 1.
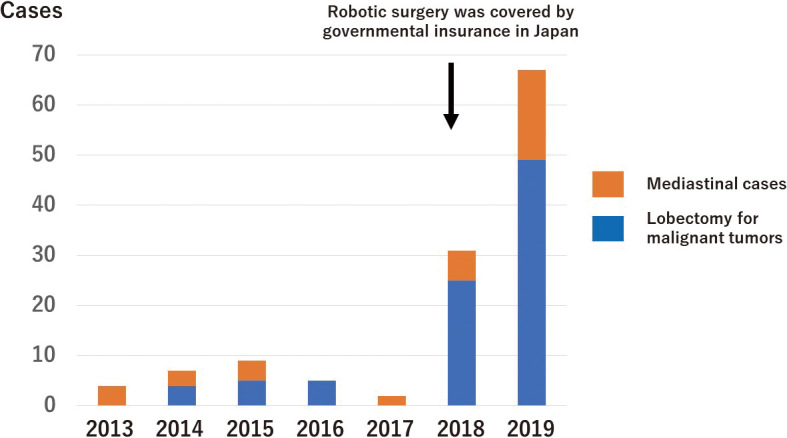
The trend of robot-assisted thoracic surgery (RATS) at Nagoya University, where RATS were started as one of the institutions with a spirit of enterprise in 2013
The operability of a surgical robot is advantageous and supplements the disadvantages of conventional endoscopic surgery. The biggest advantage is the markedly free movement of joint-equipped robotic forceps under three-dimensional (3D) vision. Based on an analysis of its perioperative results, RATS lobectomy is comparable to VATS lobectomy in terms of its safety and efficacy. RATS lobectomy is also superior to VATS lobectomy in terms of its operability and steeper learning curve.3 However, the relatively high cost and longer operation time are the concerns. Furthermore, because RATS has only been performed in early-stage lung cancer cases in most institutions, more research is necessary. It is of interest to explore how RATS and VATS would be performed for specific patients in the near future.4,5
Uniportal video-assisted thoracic surgery. Minimally invasive thoracic surgery has pursued fewer ports, and uniportal VATS has been developed and is used regularly in certain countries in Europe and Southeast Asia. Unfortunately, no studies have reported the superiority of uniportal VATS when compared with multiportal VATS; however, further studies are warranted. The early period of uniportal VATS development was focused on minor procedures. In the early 2010s, VATS was adopted in major pulmonary resection. Currently, experts in this technique use uniportal VATS to complete complex procedures, including bronchial sleeves, vascular reconstruction, carinal resection, and a range of nonintubated techniques.6 Now, many thoracic surgeons believe that uniportal RATS could be the next promising procedure in thoracic surgery.
Novel Technique for Sublobar Resection
Virtual-assisted lung mapping as a preoperative marker. Due to radiological developments such as thin-slice computed tomography (CT), an increasing number of small peripheral nodules have been detected. Thoracic surgeons are likely to encounter referrals of patients with small nodules. In these cases, pre- or intraoperative markings are needed because the nodules are invisible and unpalpable during surgery. Various techniques have been developed to achieve minimal invasiveness. For example, the Kyoto University group developed a unique preoperative dye-marking system using a bronchoscope known as virtual-assisted lung mapping (VAL-MAP).7 This technique uses indigo carmine, which is bronchoscopically injected under the pleura near to the tumor prior to surgery. VAL-MAP helps surgeons to perform surgical diagnoses and complete resections successfully, even in cases where small tumors are invisible or unpalpable (Figure 2).
Fig. 2.
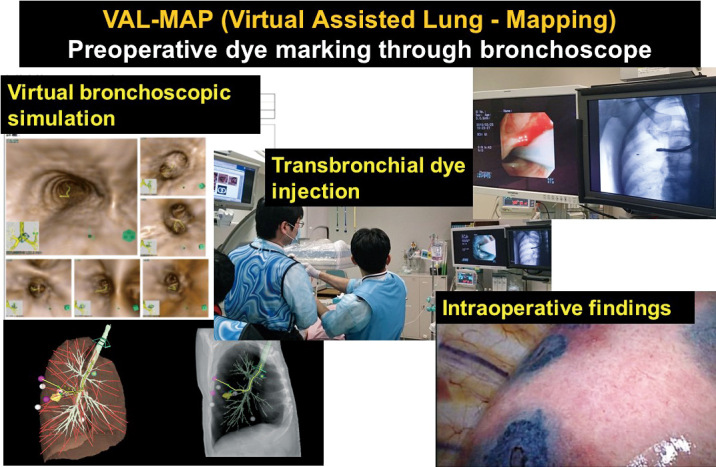
Concept of virtual-assisted lung mapping (VAL-MAP)
Image-guided video-assisted thoracic surgery. The precise preoperative localization of small pulmonary nodules is a key prerequisite to successful excision. With the advent of hybrid operating rooms, a patient-tailored approach, which encompasses simultaneous localization and removal of small pulmonary nodules, is feasible. This method is known as image-guided VATS (iVATS) because surgical resection is performed using the guidance of CT images taken intraoperatively using a C-arm CT scan in a hybrid operating room.8 The number of institutions performing iVATS has increased worldwide.
Segmentectomy using indocyanine green. Lobectomy has long been the standard therapy for lung cancer; however, sublobar resection has emerged as a less invasive alternative since smaller nodules have recently been detected and targeted for surgical resection. Indocyanine green (ICG) is a fluorescent dye used for hepatic segmentectomies. Intravenous ICG injections aid in pulmonary segmentectomies.9 In Japan in 2018, ICG was introduced as a technique to detect organ blood flow. This approach helps surgeons to perform pulmonary segmentectomies with more confidence. Also, some surgeons use transbronchial instillation of ICG to mark tumors in addition to sublobar resection.10,11
Three-Dimensional Computed Tomography Simulation
Clinical necessity of three-dimensional computed tomography images. Multidetector CT constructs 3D images and has been used widely in various clinical situations including thoracic surgery.12,13 In fact, 3D images of anatomical structures have become feasible for decision making and preoperative simulations of various surgical procedures.14,15
To date, several software programs have been used in the field of thoracic surgery; however, image quality and the time required for image acquisition are key issues that limit wider use of this technology.14 In addition, complexity in manipulating the software is another major obstacle to its widespread use.
Surgeon-friendly three-dimensional computed tomography image analysis systems. 3D-CT technology has recently been developed and introduced into the field of thoracic surgery.14 In Japan, a recently developed, high-speed, high-quality 3D image analysis system (Synapse Vincent, Fuji Film Co., Ltd., Tokyo, Japan) has been widely used, and many surgeons have used this system to obtain 3D images of pulmonary vessels and the tracheobronchial tree. This system automatically extracts information on the pulmonary structures and displays 3D images in just a few clicks. This allows thoracic surgeons to independently create 3D images in their spare time.
Because of technological advances in software, the equipment is mostly automated, and it takes less than 10 minutes for a surgeon to obtain suitable 3D images of pulmonary structures for surgical simulation without guidance from a radiology expert.15,16 Furthermore, the constructed images can be rotated freely and visualized from any angle with an interactive interface, providing information on the 3D relationship between pulmonary structures. The results of preoperative assessment of variations in pulmonary vessel branching patterns, as well as short-term surgical outcomes, have been satisfactory.17
Development of dynamic three-dimensional computed tomography images. These newly developed systems allow thoracic surgeons to construct 3D images without having to consult radiologists or technicians, which represents a considerable advantage.15,18 Nonetheless, surgeons still rely on their experience as these systems only provide static images, which is a significant limitation for surgical simulation.
Recently, another novel simulation system was developed, which generates dynamic images based on patient-specific CT data and reflects the intraoperative deformation of the lung.19 This system was developed based on the novel technology, deformable resection process mapping (RPM), which was originally developed for liver resection.20 The clinical application of RPM to regular thoracic surgery is an ongoing project. Furthermore, these novel dynamic 3D-CT images can be used to educate surgical residents and medical students, in addition to providing real clinical simulations for certified thoracic surgeons (Figure 3).
Fig. 3.
Resection process map (RPM)
Update in Cadaveric Lung Transplantation
Use of marginal donors including donation after circulatory death. Lung transplantation has become an established treatment option for end-stage respiratory diseases; however, a severe donor shortage remains one of the biggest universal problems.21,22 To overcome this issue, lung transplantation from donors after circulatory death (DCD) and marginal donors has been performed extensively worldwide.23 The international DCD Registry Report with a five-year follow up demonstrated favorable long-term survival in DCD and cadaveric lung donor recipients at 22 centers globally. The data indicates that more extensive use of DCD lung transplantation would increase donor organ availability and may reduce waiting list mortality.23
Ex vivo lung perfusion for marginal donors. Ex vivo lung perfusion (EVLP) was used to investigate lung physiology, such as pulmonary edema and pulmonary ischemia–reperfusion injuries (Figure 4).21,22,24 In 2001, Steen et al successfully performed the first lung transplantation from an uncontrolled DCD donor after an EVLP system evaluated the lungs from DCD.25 Thereafter, EVLP became widely used to assess marginal donors, including DCD, and has been modified over time in clinical situations.25-27 According to the Toronto team, EVLP-treated lungs increased in the number of patients undergoing transplantation with comparable long-term outcomes (Figure 5).28 EVLP is currently used in many countries and will be formally introduced in Japan in the near future.
Fig. 4.
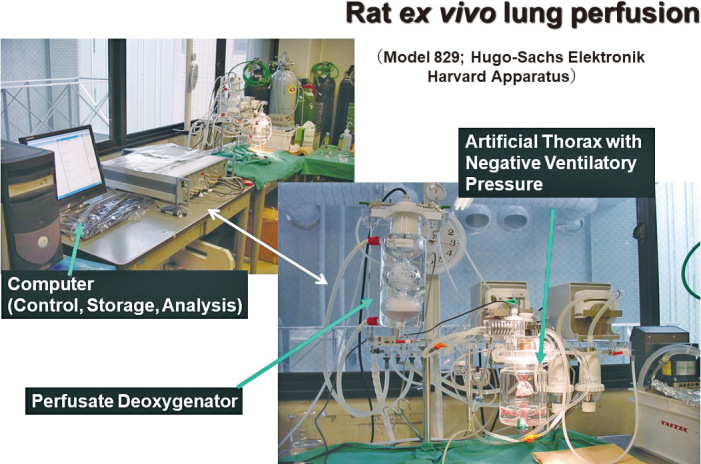
Rat ex-vivo lung perfusion (EVLP) system
Fig. 5.
Human ex-vivo lung perfusion (EVLP) system
Updates in Living Donor Lobar Lung Transplantation
The current worldwide situation of living donor lobar lung transplantation. Living donor lobar lung transplantation (LDLLT) was developed at the University of Southern California in the 1990s.29,30 This procedure had been performed with acceptable results as one of the realistic solutions for the severe cadaveric donor shortage in the United States until the lung allocation score (LAS) system was introduced.31 After the LAS system was implemented, LDLLT has rarely been performed.32 Now, LDLLT is mostly performed in Japan.33 In fact, because of a severe donor shortage, LDLLT is essential in Japan34-36 and has been performed successfully to save the lives of rapidly deteriorating and critically ill patients.37-39
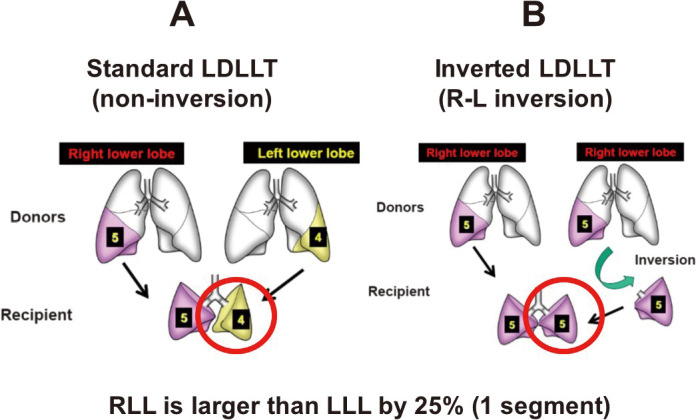
Donor issues with living donor lobar lung transplantation. Usually, two separate donors are required for each recipient in an LDLLT (Figure 6A). The recipient and the two donors are at risk of morbidity; however, only the recipient can benefit from the procedure. Therefore, the success of LDLLT is largely dependent on the donor’s outcome.40 To prevent perioperative complications, it is essential for surgeons to evaluate CT findings in every LDLLT case using 3D CT angiography.18,41,42 Of note, small branches of the pulmonary artery were sacrificed to obtain an adequate arterial cuff for safe implantation in approximately half of donors.43 Furthermore, pulmonary arterioplasties were sometimes conducted with autopericardial patches or an end-to-end anastomosis in living donor lobectomies.
Fig. 6.
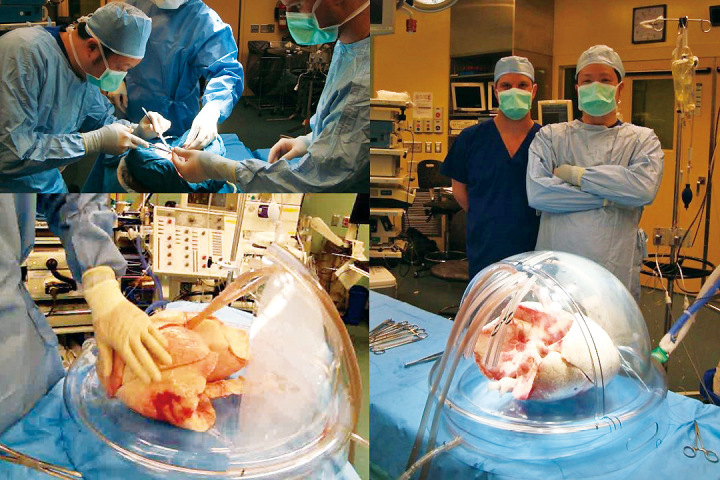
Living-donor lobar lung transplantation (LDLLT)
Size matching using three-dimensional computed tomography in living donor lobar lung transplantation. An appropriately size-matched recipient can be selected from a specific donor in cadaveric lung transplantation. However, in LDLLT, ideal size matching between the donor and recipient is difficult because of the limited number of potential donors. As a result, there are size discrepancies between the donor and recipient in cases of LDLLT.44 For example, some donor lungs might be too small, necessitating native lung-sparing surgical procedures,45,46 and others might be too large, requiring volume reduction of donor lungs.47 Thus, size matching between the donor and recipient is particularly important to complete LDLLT with good outcomes.
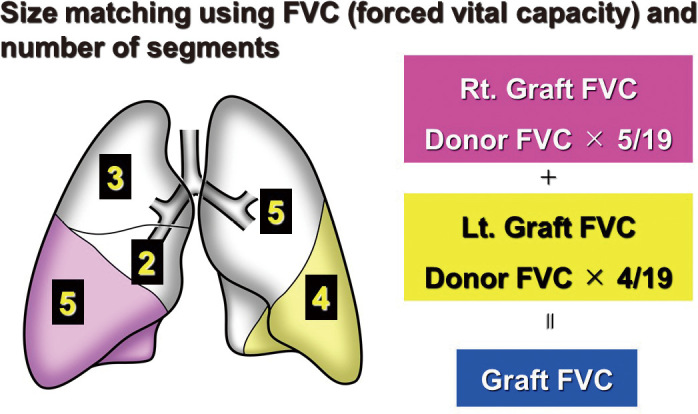
The segment-based estimation formula of the graft forced vital capacity (FVC) has been used as a size-matching method for LDLLT.38,48,49 Given that the right lower lobe (RLL) consists of five segments, the left lower lobe (LLL) consists of four, and the whole lung consists of nineteen, we estimated the graft FVC using the following equation: graft FVC = measured FVC of the right-side donor × 5/19 + measured FVC of the left-side donor × 4/19 (Figure 7). When the graft FVC is greater than 45% of the predicted FVC of the recipient (calculated according to height, age, and sex), the size disparity is generally accepted irrespective of the recipient’s diagnosis.
Fig. 7.
Size matching in living-donor lobar lung transplantation (LDLLT)
Recently, 3D-CT volumetric data has been utilized for size matching in LDLLT.50-52 This anatomical size matching is useful when considering relatively large donor lungs being used for LDLLT in a small child or adult recipient. In the former case, delayed chest closure and/or volume reduction of the donor lungs might be necessary.50,53 In the latter case, native lung-sparing techniques might be a treatment option.45,47
In LDLLT with oversized donors, although various case reports have been performed, it is still uncertain how large a donor lung could be for successful implantation into a recipient’s small thoracic cage.50,51,53 Furthermore, postoperative graft size, measured using 3D-CT volumetry after LDLLT, revealed that the donor lungs accommodated well to the thoracic cage of the recipient and functioned well, despite the wide size range.44
New surgical procedures in living donor lobar lung transplantation. Small-for-size donor grafts are often an impediment in adult LDLLT. To rescue patients with such small lobar grafts, LDLLT sparing the native upper lobes can be performed. This strategy is used when the upper lobes or segments are less impaired preoperatively.45,46
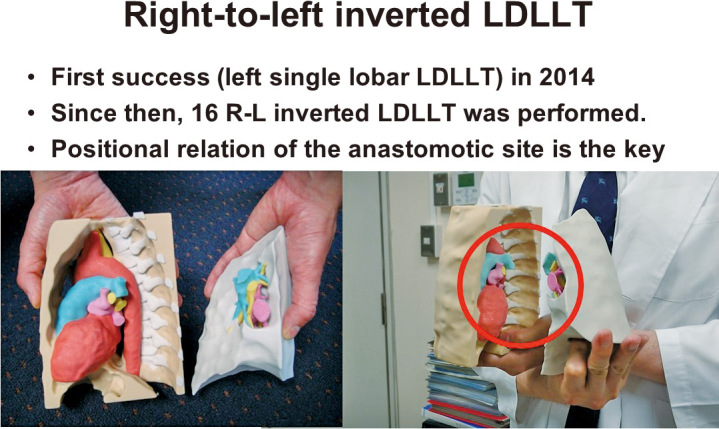
Another method is the right-to-left inverted LDLLT (Figure 6B). In general, the RLL is approximately 125% larger than the LLL. If an RLL is transplanted into the left thorax instead of an LLL, a larger donor graft can be transplanted into the recipient, which might potentially solve the issue of small-for-size donor grafts. With this concept, a novel surgical technique was developed, in which an inverted RLL graft can be transplanted into the left thorax. This procedure was performed for the first time after accurate surgical simulation of the positional relationship of the anastomotic structures was conducted using a 3D model (Figure 8).54 Because of the initial success, this procedure was performed in more than 15 additional patients.55 Furthermore, the first LDLLT using the middle lobe from a donor was successfully conducted.56
Fig. 8.
Preoperative simulation using 3D models
In cadaveric lung transplantation, 3D-CT images were also used for preoperative planning and postoperative evaluation in a rare case of lung transplantation in a patient with Kartagener syndrome57 and a patient with a giant pulmonary arterial aneurysm.58
Three-Dimensional Computed Tomography Images in Lung Transplantation
Quantitative CT has been used to predict postoperative lung function in patients with lung cancer.59,60 Although there are a limited number of reports on this topic,44,50-52,61 interesting results were reported in data collected from LDLLT donors and recipients.62 In brief, FVC and total lung capacity were significantly correlated with the total lung volume measured by 3D-CT volumetry in healthy subjects. It was also reported that the current method of calculating FVC using the number of segments was acceptable for size accommodation in LDLLT. Moreover, various studies have been conducted using CT data, including 3D-CT volumetry, to reveal compensatory lung growth.63,64 The data clearly show the existence of compensatory lung growth in adult lungs, which was thought to be impossible.65 In lung transplantation with a flat chest, 3D-CT volumetry clearly shows that a flat chest significantly recovers after lung transplantation.66
Internationally, the annual rate of bilateral lung transplantation has increased because of its advantages when compared with single lung transplantation, including improved prognosis and the absence of native lung complications.67,68 However, in Japan, because of the severe donor shortage, single lung transplantation is the first choice of treatment in cadaveric lung transplantation, unless it is contraindicated.69 3D-CT volumetry was reportedly useful in detecting native lung complications, such as native lung hyperinflation, after a single lung transplantation for a patient with chronic obstructive pulmonary disease.70
Major causes of death more than one year after lung transplantation include chronic lung allograft dysfunction (CLAD), infection, and malignancy; half of lung transplant patients died of CLAD.67 CLAD is classified into two subtypes: bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome.71 Early detection of BOS was possible using ventilatory scintigraphy,72 which is not currently available worldwide. Furthermore, several studies reported that 3D-CT volumetry might also detect CLAD and could be used to classify its subtypes.73-75 Lung volumes also predicted survival in patients with CLAD.76
Airway complications are serious complications that can be encountered after lung transplantation. 3D-CT images, such as virtual bronchoscopies, could detect airway complications such as stricture, necrosis, and dehiscence.77
In conclusion, thoracic surgeons mainly deal with tumorous lesion in the chest, including lung cancer, mediastinal tumors, and pleural tumors. Thoracic surgeons also perform lung transplantation for patients with end-stage pulmonary disease. 3D-CT images have been used as a simulation tool and as a tool for various other types of evaluation. In addition to a busy clinical practice on a daily basis, thoracic surgeons can stay up to date on various pivotal topics related to thoracic surgery, as described in the present report.
ACKNOWLEDGEMENT
None.
CONFLICT OF INTEREST
All the authors have declared no competing interest.
Abbreviations
- BOS
bronchiolitis obliterans syndrome
- CLAD
chronic lung allograft dysfunction
- CT
computed tomography
- DCD
donation after circulatory death
- EVLP
ex vivo lung perfusion
- FVC
forced vital capacity
- iVATS
image-guided VATS
- ICG
indocyanine green
- LLL
left lower lobe
- LDLLT
living donor lobar lung transplantation
- LAS
lung allocation score
- RPM
resection process map
- RLL
right lower lobe
- RATS
robot-assisted thoracic surgery
- 3D
three-dimensional
- VAL-MAP
virtual-assisted lung mapping
- VATS
video-assisted thoracic surgery
REFERENCES
- 1.Mun M, Nakao M, Matsuura Y, Ichinose J, Nakagawa K, Okumura S. Video-assisted thoracoscopic surgery lobectomy for non-small cell lung cancer. Gen Thorac Cardiovasc Surg. 2018;66(11):626–631. [DOI] [PubMed]
- 2.Nakamura H Taniguchi Y. Robot-assisted thoracoscopic surgery: current status and prospects. Gen Thorac Cardiovasc Surg. 2013;61(3):127–132. [DOI] [PubMed]
- 3.Nakamura H. Systematic review of published studies on safety and efficacy of thoracoscopic and robot-assisted lobectomy for lung cancer. Ann Thorac Cardiovasc Surg. 2014;20(2):93–98. [DOI] [PubMed]
- 4.Suda T. Transition from video-assisted thoracic surgery to robotic pulmonary surgery. J Vis Surg. 2017;3:55. [DOI] [PMC free article] [PubMed]
- 5.Feczko AF, Wang H, Nishimura K, et al. Proficiency of robotic lobectomy based on prior surgical technique in The Society of Thoracic Surgeons General Thoracic Database. Ann Thorac Surg. 2019;108(4):1013–1020. [DOI] [PubMed]
- 6.Gonzalez-Rivas D. Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg. 2016;5(2):85–91. [DOI] [PMC free article] [PubMed]
- 7.Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multi-spot dyemarking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg. 2014;147(6):1813–1819. [DOI] [PubMed]
- 8.Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS) – phase I–II clinical trial. J Surg Oncol. 2015;112(1):18–25. [DOI] [PMC free article] [PubMed]
- 9.Kasai Y, Tarumi S, Chang SS, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2- and 1-wavelength methods. Eur J Cardiothorac Surg. 2013;44(6):1103–1107. [DOI] [PubMed]
- 10.Sekine Y, Itoh T, Toyoda T, et al. Precise anatomical sublobar resection using a 3D medical image analyzer and fluorescence-guided surgery with transbronchial instillation of indocyanine green. Semin Thorac Cardiovasc Surg. 2019;31(3):595–602. [DOI] [PubMed]
- 11.Anayama T, Qiu J, Chan H, et al. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann Thorac Surg. 2015;99(1):224–230. [DOI] [PubMed]
- 12.Hemminger BM, Molina PL, Egan TM, et al. Assessment of real-time 3D visualization for cardiothoracic diagnostic evaluation and surgery planning. J Digit Imaging. 2005;18(2):145–153. [DOI] [PMC free article] [PubMed]
- 13.Umakoshi H, Iwano S, Inoue T, Li Y, Nakamura K, Naganawa S. Quantitative follow-up assessment of patients with interstitial lung disease by 3D-curved high-resolution CT imaging parallel to the chest wall. Nagoya J Med Sci. 2019;81(1):41–53. [DOI] [PMC free article] [PubMed]
- 14.Ikeda N, Yoshimura A, Hagiwara M, Akata S, Saji H. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg. 2013;19(1):1–5. [DOI] [PubMed]
- 15.Chen-Yoshikawa TF, Date H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J Thorac Dis. 2016;8(Suppl 3):S295–301. [DOI] [PMC free article] [PubMed]
- 16.Mochizuki K, Takatsuki M, Soyama A, et al. The usefulness of a high-speed 3D-image analysis system in pediatric living donor liver transplantation. Ann Transplant. 2012;17(1):31–34. [DOI] [PubMed]
- 17.Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery. Eur J Cardiothorac Surg. 2014;46(6):e120–126. [DOI] [PubMed]
- 18.Chen-Yoshikawa TF, Date H. Three-dimensional image in lung transplantation. Gen Thorac Cardiovasc Surg. 2018;66(1):19–26. [DOI] [PubMed]
- 19.Tokuno J, Chen-Yoshikawa TF, Nakao M, Matsuda T, Date H. Resection process map: a novel dynamic simulation system for pulmonary resection [published online ahead of print September 13, 2019]. J Thorac Cardiovasc Surg. 2020;159(3):1130–1138. doi: 10.1016/j.jtcvs.2019.07.136. [DOI] [PubMed]
- 20.Nakao M, Taura K, Matsuda T. Deformable resection process map for intraoperative cutting guides. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:2554–2557. [DOI] [PubMed]
- 21.Chen F, Nakamura T, Wada H. Development of new organ preservation solutions in Kyoto University. Yonsei Med J. 2004;45(6):1107–1114. [DOI] [PubMed]
- 22.Chen F, Date H. Update on ischemia-reperfusion injury in lung transplantation. Curr Opin Organ Transplant. 2015;20(5):515–520. [DOI] [PubMed]
- 23.Van Raemdonck D, Keshavjee S, Levvey B, et al. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT Registry. J Heart Lung Transplant. 2019;38(12):1235–1245. [DOI] [PubMed]
- 24.Chen F, Nakamura T, Fujinaga T, et al. Protective effect of a nebulized β2-adrenoreceptor agonist in warm ischemic–reperfused rat lungs. Ann Thorac Surg. 2006;82(2):465–471. [DOI] [PubMed]
- 25.Steen S, Sjöberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357(9259):825–829. [DOI] [PubMed]
- 26.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364(15):1431–1440. [DOI] [PubMed]
- 27.Loor G, Warnecke G, Villavicencio MA, et al. Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND): a single-arm, pivotal trial. Lancet Respir Med. 2019;7(11):975–984. [DOI] [PubMed]
- 28.Divithotawela C, Cypel M, Martinu T, et al. Long-term outcomes of lung transplant with ex vivo lung perfusion [published online ahead of print October 9, 2019]. JAMA Surg. doi: 10.1001/jamasurg.2019.4079. [DOI] [PMC free article] [PubMed]
- 29.Cohen RG, Barr ML, Schenkel FA, DeMeester TR, Wells WJ, Starnes VA. Living-related donor lobectomy for bilateral lobar transplantation in patients with cystic fibrosis. Ann Thorac Surg. 1994;57(6):1423–1428. [DOI] [PubMed]
- 30.Barr ML, Schenkel FA, Cohen RG, et al. Bilateral lobar transplantation utilizing living related donors. Artif Organs. 1996;20(10):1110–1111. [DOI] [PubMed]
- 31.Starnes VA, Bowdish ME, Woo MS, et al. A decade of living lobar lung transplantation: recipient outcomes. J Thorac Cardiovasc Surg. 2004;127(1):114–122. [DOI] [PubMed]
- 32.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Lung. Am J Transplant. 2019;19(Suppl 2):404–484. [DOI] [PubMed]
- 33.Date H. Living-related lung transplantation. J Thorac Dis. 2017;9(9):3362–3371. [DOI] [PMC free article] [PubMed]
- 34.Date H, Sato M, Aoyama A, et al. Living-donor lobar lung transplantation provides similar survival to cadaveric lung transplantation even for sicker patients. Eur J Cardiothorac Surg. 2015;47(6):967–972. [DOI] [PubMed]
- 35.Chen F, Yamane M, Inoue M, et al. Less maintenance immunosuppression in lung transplantation following hematopoietic stem cell transplantation from the same living donor. Am J Transplant. 2011;11(7):1509–1516. [DOI] [PubMed]
- 36.Date H. Current status and problems of lung transplantation in Japan. J Thorac Dis. 2016;8(Suppl 8):S631–636. [DOI] [PMC free article] [PubMed]
- 37.Starnes VA, Barr ML, Cohen RG, et al. Living-donor lobar lung transplantation experience: intermediate results. J Thorac Cardiovasc Surg. 1996;112(5):1284–1291. [DOI] [PubMed]
- 38.Date H, Aoe M, Nagahiro I, et al. Living-donor lobar lung transplantation for various lung diseases. J Heart Lung Transplant. 2003:126(2):476–481. [DOI] [PubMed]
- 39.Starnes VA, Barr ML, Schenkel FA, et al. Experience with living-donor lobar transplantation for indications other than cystic fibrosis. J Thorac Cardiovasc Surg. 1997;114(6):917–922. [DOI] [PubMed]
- 40.Barr ML, Belghiti J, Villamil FG, et al. A report of the Vancouver Forum on the care of the live organ donor: lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006;81(10):1373–1385. [DOI] [PubMed]
- 41.Duong PT, Ferson PF, Fuhrman CR, McCurry KR, Lacomis JM. 3D-multidetector CT angiography in the evaluation of potential donors forliving donor lung transplantation. J Thorac Imaging. 2005;20(1):17–23. [DOI] [PubMed]
- 42.Chen F, Yamada T, Sato M, et al. Postoperative pulmonary function and complications in living-donor lobectomy. J Heart Lung Transplant. 2015;34(8):1089–1094. [DOI] [PubMed]
- 43.Kayawake H, Chen-Yoshikawa TF, Aoyama A, et al. Excellent outcome of donor lobectomy with various surgical techniques for the interlobar artery. Eur J Cardiothorac Surg. 2017;51(2):279–283. [DOI] [PubMed]
- 44.Chen F, Kubo T, Yamada T, et al. Adaptation over a wide range of donor graft lung size discrepancies in living-donor lobar lung transplantation. Am J Transplant. 2013;13(5):1336–1342. [DOI] [PubMed]
- 45.Aoyama A, Chen F, Minakata K, et al. Sparing native upper lobes in living-donor lobar lung transplantation: Five cases from a single center. Am J Transplant. 2015;15(12):3202–3207. [DOI] [PubMed]
- 46.Fujinaga T, Bando T, Nakajima D, et al. Living-donor lobar lung transplantation with sparing of bilateral native upper lobes: a novel strategy. J Heart Lung Transplant. 2011;30(3):351–353. [DOI] [PubMed]
- 47.Chen F, Fujinaga T, Bando T, Date H. Pulmonary function of individual lung lobes after complex living-donor lobar lung transplantation using inspiratory and expiratory three-dimensional computed tomographic volumetry. Interact Cardiovasc Thorac Surg. 2012;15(6):1077–1079. [DOI] [PMC free article] [PubMed]
- 48.Date H, Aoe M, Nagahiro I, et al. How to predict forced vital capacity after living-donor lobar-lung transplantation. J Heart Lung Transplant. 2004;23(5):547–551. [DOI] [PubMed]
- 49.Kojima K, Kato K, Oto T, et al. Preoperative graft volume assessment with 3D-CT volumetry in living-donor lobar lung transplantations. Acta Med Okayama. 2011;65(4):265–268. [DOI] [PubMed]
- 50.Chen F, Fujinaga T, Shoji T, et al. Perioperative assessment of oversized lobar graft downsizing in living-donor lobar lung transplantation using three-dimensional computed tomographic volumetry. Transpl Int. 2010;23(9):e41–44. [DOI] [PubMed]
- 51.Shiraishi T, Hiratsuka M, Munakata M, et al. Living-donor single-lobe lung transplantation for bronchiolitis obliterans in a 4-year-old boy. J Thorac Cardiovasc Surg. 2007;134(4):1092–1093. [DOI] [PubMed]
- 52.Camargo JJP, Irion KL, Marchiori E, et al. Computed tomography measurement of lung volume in preoperative assessment for living donor lung transplantation: volume calculation using 3D surface rendering in the determination of size compatibility. Pediatr Transplantation. 2009;13(4):429–439. [DOI] [PubMed]
- 53.Takahashi K, Chen F, Ikeda T, et al. Single-lobe lung transplantation for rapidly deteriorating pulmonary veno-occlusive disease Ann Thorac Surg. 2013;95(2):689–691. [DOI] [PubMed]
- 54.Chen F, Miyamoto E, Takemoto M, et al. Right and left inverted lobar lung transplantation. Am J Transplant. 2015;15(6):1716–1721. [DOI] [PubMed]
- 55.Chen-Yoshikawa TF, Tanaka S, Yamada Y, et al. Intermediate outcomes of right-to-left inverted living-donor lobar lung transplantation. Eur J Cardiothorac Surg. 2019;56(6):1046–1053. [DOI] [PubMed]
- 56.Oto T, Miyoshi K, Sugimoto S, Yamane M. Living related donor middle lobe lung transplant in a pediatric patient. J Thorac Cardiovasc Surg. 2015;149(3):e42–44. [DOI] [PubMed]
- 57.Schertler T, Lardinois D, Boehm T, Weder W, Wildermuth S, Alkadhi H. Lung transplantation in Kartagenersyndrome and situs inversus: potential of multidetector row computed tomography and three-dimensional postprocessing. J Thorac Cardiovasc Surg. 2007;134(3):814–815. [DOI] [PubMed]
- 58.Oda H, Hamaji M, Motoyama H, et al. Use of a three-dimensional model in lung transplantation for a patient with giant pulmonary aneurysm [published online ahead of print August 22, 2019]. Ann Thorac Surg. 2020;109(3):e183–185. doi: 10.1016/j.athoracsur.2019.06.092. [DOI] [PubMed]
- 59.Hiroshige S, Shimada M, Harada N, et al. Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography. Transplantation. 2003;75(9):1561–1564. [DOI] [PubMed]
- 60.Ueda K, Tanaka T, Hayashi M, Li TS, Tanaka N, Hamano K. Computed tomography-defined functional lung volume after segmentectomy versus lobectomy. Eur J Cardio-thorac Surg. 2010;37(6):1433–1437. [DOI] [PubMed]
- 61.Konheim JA, Kon ZN, Pasrija C, et al. Predictive equations for lung volumes from computed tomography for size matching in pulmonary transplantation. J Thorac Cardiovasc Surg. 2016;151(4):1163–1169. [DOI] [PubMed]
- 62.Chen F, Kubo T, Shoji T, Fujinaga Bando T, Date H. Comparison of pulmonary function test and computed tomography volumetry in living lung donors. J Heart Lung Transplant. 2011;30(5):572–575. [DOI] [PubMed]
- 63.Mizobuchi T, Chen F, Yoshino I, et al. Radiologic evaluation for volume and weight of remnant lung in living lung donors. J Thorac Cardiovasc Surg. 2013;146(5):1253–1258. [DOI] [PubMed]
- 64.Mizobuchi T, Wada H, Sakairi Y, et al. Spirometric and radiological evaluation of the remnant lung long after major pulmonary resection: can compensatory phenomena be recognized in clinical cases? Surg Today. 2014;44(9):1735–1743. [DOI] [PubMed]
- 65.Butler JP, Loring SH, Patz S, Tsuda A, Yablonskiy DA, Mentzer SJ. Evidence for adult lung growth in humans. N Engl J Med. 2012;367(3):244–247. [DOI] [PMC free article] [PubMed]
- 66.Miyoshi R, Chen-Yoshikawa TF, Takahagi A, et al. Pulmonary function and exercise capacity in patients with flat chests after lung transplantation. Ann Thorac Surg. 2017;104(5):1695–1701. [DOI] [PubMed]
- 67.Chambers DC, Cherikh WS, Harhay MO, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult lung and heart-lung transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1042–1055. [DOI] [PMC free article] [PubMed]
- 68.Kondo T, Okumura N, Adachi S, Murohara T. Pulmonary hypertension: diagnosis, management, and treatment. Nagoya J Med Sci. 2019;81(1):19–30. [DOI] [PMC free article] [PubMed]
- 69.Miyoshi R, Chen-Yoshikawa TF, Hijiya K, et al. Significance of single lung transplantation in the current situation of severe donor shortage in Japan. Gen Thorac Cardiovasc Surg. 2016;64(2):93–97. [DOI] [PubMed]
- 70.Motoyama H, Chen F, Ohsumi A, et al. Quantitative evaluation of native lung hyperinflation after single lung transplantation for emphysema using three-dimensional computed tomography volumetry. Transplant Proc. 2014;46(3):941–943. [DOI] [PubMed]
- 71.Verleden GM, Raghu C, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33(2):127–133. [DOI] [PubMed]
- 72.Miyamoto E, Chen F, Aoyama A, Sato M, Yamada T, Date H. Unilateral chronic lung allograft dysfunction is a characteristic of bilateral living-donor lobar lung transplantation. Eur J Cardiothorac Surg. 2015;48(3):463–469. [DOI] [PubMed]
- 73.Saito T, Horie M, Sato M, et al. Low-dose computed tomography volumetry for subtyping chronic lung allograft dysfunction. J Heart Lung Transplant. 2016;35(1):59–66. [DOI] [PubMed]
- 74.Dettmer S, Shin HO, Vogel-Claussen J, et al. CT at onset of chronic lung allograft dysfunction in lung transplant patients predicts development of the restrictive phenotype and survival. Eur J Radiol. 2017;94:78–84. [DOI] [PubMed]
- 75.Saito M, Chen-Yoshikawa TF, Nakamoto Y, et al. Unilateral chronic lung allograft dysfunction assessed by biphasic computed tomographic volumetry in bilateral living-donor lobar lung transplantation. Transplant Direct. 2018;4(11):e398. [DOI] [PMC free article] [PubMed]
- 76.Kneidinger N, Milger K, Janitza S, et al. Lung volumes predict survival in patients with chronic lung allograft dysfunction. Eur Respir J. 2017;49(4):1601315. doi: 10.1183/13993003.01315-2016. [DOI] [PubMed]
- 77.Miyamoto E, Chen-Yoshikawa TF, Higuchi H, Aoyama A, Date H. Stenosis of the segmental bronchus is a characteristic airway complication in living-donor lobar lung transplantation. J Heart Lung Transplant. 2016;35(3):389–392. [DOI] [PubMed]


