ABSTRACT
High-flow nasal cannula (HFNC) oxygen is a therapy that has demonstrated survival benefits in acute respiratory failure (ARF). However, the role of HFNC in ARF due to interstitial pneumonia (IP) is unknown. The aim of this study was to compare the effects of HFNC therapy and non-invasive positive pressure ventilation (NPPV) in ARF due to IP. This retrospective observational study included 32 patients with ARF due to IP who were treated with HFNC (n = 13) or NPPV (n = 19). The clinical characteristics, intubation rate and 30-day mortality were analyzed and compared between the HFNC group and the NPPV group. Predictors of 30-day mortality were evaluated using a logistic regression model. HFNC group showed higher mean arterial blood pressure (median 92 mmHg; HFNC group vs 74 mmHg; NPPV group) and lower APACHEII score (median 22; HFNC group vs 27; NPPV group) than NPPV group. There was no significant difference in the intubation rate at day 30 between the HFNC group and the NPPV group (8% vs 37%: p = 0.069); the mortality rate at 30 days was 23% and 63%, respectively. HFNC therapy was a significant determinant of 30-day mortality in univariate analysis, and was confirmed to be an independent significant determinant of 30-day mortality in multivariate analysis (odds ratio, 0.148; 95% confidence interval, 0.025–0.880; p = 0.036). Our findings suggest that HFNC therapy can be a possible option for respiratory management in ARF due to IP. The results observed here warrant further investigation of HFNC therapy in randomized control trials.
Key Words: critical care, pulmonary fibrosis, respiratory failure, nasal cannula
INTRODUCTION
Interstitial pneumonia (IP) is a progressive inflammatory interstitial lung disease characterized by relative unresponsiveness to therapy and a poor prognosis. Some patients experience acute respiratory failure (ARF) during the courses of the disease. ARF has also been reported to occur in patients with idiopathic IP,1 including idiopathic pulmonary fibrosis (IPF),2,3 and non-specific interstitial pneumonia (NSIP),4,5 collagen vascular disease-associated IP,3,6 and chronic hypersensitivity pneumonitis.7 For these patients, non-invasive positive pressure ventilation (NPPV) can be considered as a therapeutic option. Several retrospective studies have investigated the impact of NPPV on the mortality in patients with ARF due to IP, however its clinical benefit has not seemed to be promising.8,9 Therefore, how to manage ARF in patients with IP is a clinically important yet common, unresolved problem.
High flow nasal cannula (HFNC) therapy is a novel approach to oxygen and noninvasive respiratory support that can deliver heated and humidified oxygen via nasal cannula at high flow rates. These high flow rates generate low levels of positive pressure in the upper airways, and the fraction of inspired oxygen (FIO2) can be adjusted by changing the fraction of oxygen in the driving gas.10-12
HFNC is increasingly used for noninvasive respiratory support in intensive care units (ICUs). This is because several studies have found that HFNC therapy improves oxygenation, survival, tolerance and comfort in patients with ARF.10,13,14 Most of those studies have been performed in patients with pneumonia; however, ARF in patients with IP has etiologies that are different from those of pneumonia, has no effective treatment, and heralds a poor prognosis, especially in patients with acute exacerbation of IPF and clinically amyopathic dermatomyositis related IP. The effectiveness of HFNC therapy in these patients is unknown. The aim of this study was to compare the effectiveness of HFNC therapy with that of NPPV in patients with ARF due to IP in the ICU setting.
MATERIALS AND METHODS
Study subjects
This was a retrospective observational study of patients admitted for episodes of ARF due to IP to emergency & medical ICU at Nagoya University Hospital, Aichi, Japan. From April 2011 to June 2017, 46 consecutive patients were diagnosed with ARF due to IP and 14 patients were excluded because they were treated with mechanical ventilation (N = 13) or conventional oxygen therapy (N = 1). The remaining 32 patients were categorized in two groups: 13 patients treated with HFNC (HFNC group) and 19 patients with treated with NPPV (NPPV group; Fig. 1).
Fig. 1.
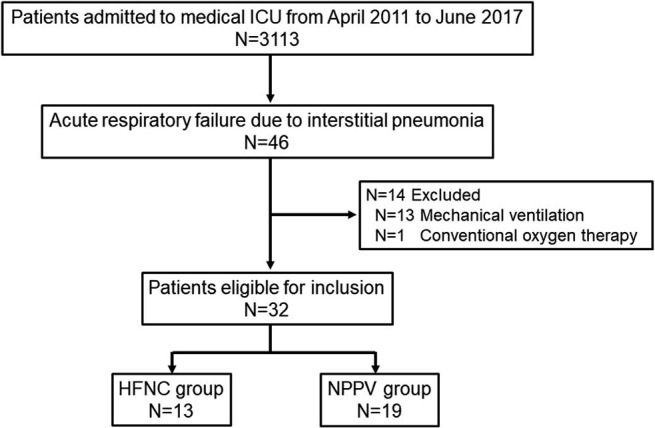
Patient flow.
ICU: intensive care unit, HFNC: high-flow nasal cannula, NPPV: non-invasive positive pressure ventilation.
Eligible patients had a clinical diagnosis of chronic fibrosing IP, including IPF and fibrosing NSIP, collagen vascular disease-associated IP, chronic hypersensitivity pneumonitis or drug-induced lung injury. IPF and NSIP were defined by consensus criteria.15 Collagen vascular disease-associated IP was diagnosed according to established criteria.6,16-20 Chronic hypersensitivity pneumonitis or drug-induced lung injury were defined as previously described.21,22
ARF due to IP was defined using the following criteria with a slight modification for adaptation for various underlying diseases: (1) exacerbation of dyspnea within 1 month; (2) newly-developed diffuse pulmonary opacities on chest computed tomography and/or chest radiography; (3) hypoxia; a PaO2/ FIO2 ratio < 300; (4) absence of heart failure or infectious lung diseases.23,24 These assessments were determined for each subject with the aid of clinical, radiological, hemodynamic, and pathology results obtained from the medical records.
This study was approved by the Nagoya University Hospital Institutional Review Board (No. 2017-0315). The requirement for informed consent from the patients of this study was waived due to the retrospective nature, and any personal information from the data were removed beforehand.
Data collection
The following data were collected at initial admission to the medical ICU (day0). The variables used to assess comparability between the two groups were age, sex, body temperature, mean arterial blood pressure (MAP), respiratory rate, acute physiology and chronic health evaluation (APACHE) II score, white blood cell count, serologic tests (C-reactive protein [CRP], lactate dehydrogenase [LDH], and Krebs von den Lungen-6 [KL-6] levels), and arterial blood gases. The causes of IP, underlying disease, treatments, ventilator settings, length of stay in the ICU, intubation rate and 30-day mortality were retrospectively reviewed.
Interventions
In the HFNC group, HFNC therapy was started when patients fulfilled the criteria for ARF. Oxygen was passed through a heated humidifier (MR850, Fisher and Paykel Healthcare) and applied continuously through large-bore binasal prongs, with a gas flow rate of 40–50 liters per minute and an FIO2 of 1.0 at initiation (Optiflow, Fisher and Paykel Healthcare).
In the NPPV group, noninvasive ventilation was delivered to the patient through a face mask (Respironics Inc, Murrysville, PA, USA) connected to a ventilator (BiPAP Vision/V60; Respironics Inc, Murrysville, PA, USA). The initial setting for NPPV was continuous positive airway pressure mode. Pressure support was given if high respiratory frequency or respiratory acidosis was found.
In both groups, the FIO2 was set at the lowest value to keep the PaO2 at more than 60 mmHg. HFNC therapy or NPPV was applied for at least 2 calendar days; thereafter it could be stopped and the patient switched to standard oxygen therapy. Sedation and pain management in the medical ICU were assessed using the Richmond Agitation-Sedation Scale (RASS) and the numeric rating scale (NRS), respectively. The target sedation level was a RASS score of –1 and the target pain level was a NRS score of ≤ 2.
Statistical analysis
Categorical data were summarized as frequencies in percentage and continuous data as median with interquartile range. The chi-squared test or Fisher’s exact test was used for categorical data, and the Mann–Whitney U test was used for continuous data. A multivariate logistic regression analysis was performed to compare the 30-day mortality between the treatment groups, with adjustment for disease severity, in particular, PaO2/FIO2 ratio (< 100). Univariate logistic regressions were also performed for other factors to assess their possible effects on 30-day mortality, including age (≥ 70 years), sex (male), cause of interstitial pneumonia (acute exacerbation of IPF), APACHE II score (≥ 20), LDH level (≥ 280), KL-6 level (≥ 1000), and respiratory management (HFNC therapy).
Candidate factors were determined a priori referring to those published in previous reports.9,23,25,26,27 Odds ratios and their 95% confidence intervals were calculated. Survival curves were compared using the log-rank test, and Kaplan-Meier survival curves were plotted. All statistical analyses were performed using a statistical software package (SPSS for Windows version 23.0; SPSS, Inc.; Chicago). A p-value < 0.05 was considered to be statistically significant.
RESULTS
Patient characteristics
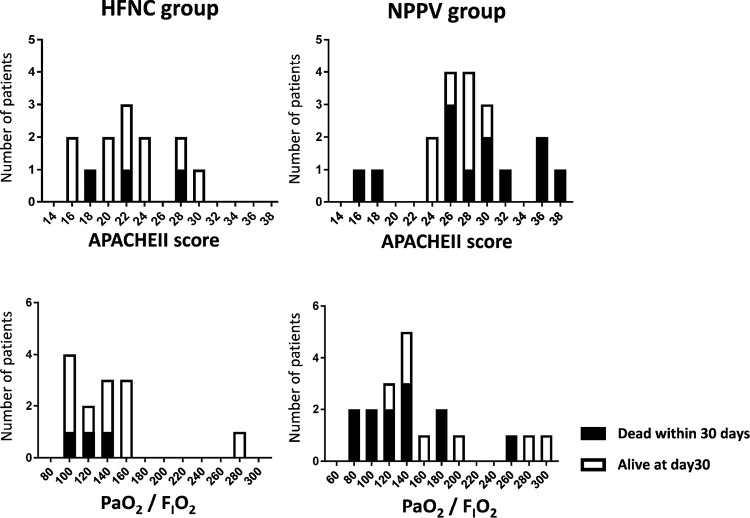
The baseline characteristics are shown in Table 1. The median age was 73 years (interquartile range, 67–76) in the HFNC group and 73 years (interquartile range, 58–77) in the NPPV group. The HFNC group showed higher MAP (median 92 mmHg; HFNC group vs 74 mmHg; NPPV group) and lower APACHEII score (median 22; HFNC group vs 27; NPPV group) than the NPPV group. There were no significant differences in age, sex, PaO2 / FIO2, respiratory rate, WBC count, CRP, LDH, and KL-6 levels. The distribution of APACHEII score, PaO2 / FIO2 and cases died within 30 days in both the HFNC group and the NPPV group are shown in Figure 2.
Table 1.
Patient characteristics
| HFNC | NPPV | p | |
| Patients, number | 13 | 19 | |
| Age, year, median (range) | 73 (67–76) | 73 (58–77) | 0.66 |
| Male sex, number (%) | 10 (77) | 16 (80) | 0.58 |
| Cause of interstitial pneumonia | |||
| Idiopathic interstitial pneumonia, number | 8 | 12 | |
| Acute exacerbation of IPF | 7 | 11 | |
| Idiopathic NSIP | 1 | 1 | |
| Connective tissue disease, number | 4 | 4 | |
| Others, number | 1 | 3 | |
| Underlying disease | |||
| Diabetes mellitus, number (%) | 5 | 8 | 0.84 |
| Chronic kidney disease, number (%) | 1 | 0 | 0.41 |
| Chronic heart disease, number (%) | 1 | 5 | 0.20 |
| Liver disease, number (%) | 0 | 0 | NA |
| Malignancy, number (%) | 8 | 8 | 0.28 |
| Physiological data | |||
| Tempreture, °C | 37.5 (36.6–38.1) | 36.8 (36.6–37.4) | 0.058 |
| Mean BP, mmHg | 92 (89–98) | 74 (65–94) | 0.013 |
| Respiratory rate per min | 25 (20–27) | 24 (21–33) | 0.31 |
| Laboratory Findings | |||
| WBC per mm3 | 13100 (9400–15500) | 11200 (6200–16700) | 0.52 |
| CRP, mg/dL | 12.2 (6.3–16.7) | 12.0 (8.0–16.1) | 0.80 |
| LDH, IU/L | 391 (265–498) | 414(358–541) | 0.51 |
| KL-6, U/mL | 682 (542–1485) | 1083 (575–1398) | 0.84 |
| Arterial blood gas | |||
| PaO2, mmHg | 62 (56–75) | 74 (68–88) | NA |
| PaCO2, mmHg | 34 (31–36) | 38 (34–40) | 0.12 |
| PaO2/FIO2 ratio | 133 (105–158) | 144 (114–191) | 0.43 |
| APACHE II score, median (range) | 22 (18–26) | 27 (25–30) | 0.006 |
| Treatment | |||
| Steroid pulse, number (%) | 12 | 18 | |
| Steroid, number (%) | 13 | 18 | |
| Immunosupressant, number (%) | 6 | 6 | |
| IVCY, number (%) | 1 | 0 |
Data are presented as No. (%), or median (interquartile range) unless otherwise noted.
AE: acute exacerbation, APACHE: acute physiology and chronic health evaluation, BP: blood pressure, CRP: C-reactive protein, IPF: idiopathic pulmonary fibrosis, IVCY: intravenous cyclophosphamide, KL-6: Krebs von der Lungen-6, LDH: lactate dehydrogenase, NSIP: non-specific interstitial pneumonia, HFNC: high-flow nasal cannula, NPPV: non-invasive positive pressure ventilation.
Fig. 2.
Distribution of APACHEII and PiO2/FIO2 ratio in the HFNC group and the NPPV group
White bar indicates cases alive at day30 and black bar indicates cases died within 30 days.
HFNC: high-flow nasal cannula, NPPV: non-invasive positive pressure ventilation.
As for therapeutic intervention, 12 patients in the HFNC group and 18 patients in the NPPV group received high-dose intravenous corticosteroids (methylprednisolone 1000 mg/day) for 3 days. Corticosteroid therapy was followed by a tapered dosage.
Ventilator setting and sedation
The initial ventilator settings were as follows: in the HFNC group, a median gas flow rate of 50 liters per minute (interquartile range, 40–50), yielding a median FIO2 of 0.45 (interquartile range, 0.40–0.63); and in the NPPV group, a median positive end-expiratory pressure (PEEP) of 6 cm H2O (interquartile range, 4–8) with a median FIO2 of 0.50 (interquartile range, 0.40–0.70) and median pressure support of 2 cm H2O (interquartile range, 0–4.0). (Table 2).
Table 2.
Ventilator setting and sedation
| HFNC | NPPV | p-value | |
| Setting, HFNC | |||
| Flow, L/min | 50 (40–50) | – | |
| FiO2 | 0.45 (0.40–0.63) | – | |
| Setting, NPPV | |||
| PEEP, cmH2O | – | 6.0 (4.0–8.0) | |
| PS, cmH2O | 2.0 (0–4.0) | ||
| FIO2 | – | 0.50 (0.40–0.70) | |
| Sedation | |||
| Dexmedetomidine, number (%) | 10 (77) | 19 (100) | 0.17 |
| Midazoram, number (%) | 1 (8) | 1 (5) | 0.66 |
| Fentanyl, number (%) | 1 (8) | 4 (21) | 0.31 |
| Morphine, number (%) | 0 | 2 (11) | 0.35 |
Data are presented as No. (%), or median (interquartile range) unless otherwise noted.
HFNC: high-flow nasal cannula, NPPV: non-invasive positive pressure ventilation, PEEP: positive end-expiratory pressure, PS: pressure support.
After the initiation of respiratory care, dexmedetomidine was administered for sedation in 10 patients (77%) in the HFNC group and 19 patients (100%) in the NPPV group.
Outcome
The major outcomes in this study were shown in Table 3. The 30-day mortality was 23% in the HFNC group and 63% in the NPPV group. There was no significant difference in the numbers of patients with do-not-intubate orders between the group (p = 0.53). There was no significant difference in the intubation rate at day 30 (8% in the HFNC group and 37% in the NPPV group, p = 0.069). If patients with a do-not-intubate order were excluded, there was a significant difference in the intubation rate (9% vs 47%, p = 0.049). There was no significant difference in the length of stay in the ICU (7 days in the HFNC group and 8 days in the NPPV group, p = 0.81).
Table 3.
Outcome
| HFNC | NPPV | p-value | |
| N | 13 | 19 | |
| Mortality | |||
| 30-days, number of death (%) | 3 (23) | 12 (63) | 0.026 |
| Intubation | |||
| Intubation, number (%) | 1 (8) | 7 (37) | 0.069 |
| Do-not intubation, number (%) | 2 (15) | 4 (21) | 0.53 |
| Intubation rate excluding DNI cases, % | 9 | 47 | 0.049 |
| ICU length of stay, days | 7 (5–12) | 8 (6–16) | 0.36 |
Data are presented as No. (%), or median (interquartile range) unless otherwise noted.
DNI: do-not intubation, HFNC: high-flow nasal cannula, NPPV: non-invasive positive pressure ventilation.
Univariate and Multivariate Analysis of Predictors of 30-days Mortality
In univariate analysis, HFNC therapy was a significant predictor for 30-day mortality (Table 4). The results of the multivariate analysis are shown in Table 4. HFNC therapy was independently associated with 30-day mortality (odds ratio, 0.148; 95% confidence interval, 0.025–0.880; p = 0.036). Figure 3 shows the Kaplan-Meier curves at 30 days in the HFNC and NPPV groups. We applied different cutoff points for APACHEII score (20, 22, 24 and 26), and APACHEII score had no significant impact on mortality in both univariate and multivariate analysis (Table 5).
Table 4.
Univariate and multivariate analysis of 30-days mortality
| Variables | OR | 95% CI | p-value |
| Univariate analysis of predictors of mortality | |||
| Respiratory care, HFNC | 0.175 | 0.036–0.860 | 0.032 |
| Age, ≧ 75 | 2.095 | 0.506–8.674 | 0.31 |
| Sex, male | 0.267 | 0.043–1.653 | 0.16 |
| Cause of interstitial lung disease, AE of IPF | 0.364 | 0.087–1.526 | 0.17 |
| PaO2/FIO2 ratio, < 100 | 8.000 | 0.812–78.825 | 0.075 |
| APACHE II score, ≧ 20 | 0.857 | 0.148–5.064 | 0.86 |
| LDH, IU/L, ≧ 280 | 4.308 | 0.424–43.733 | 0.22 |
| KL-6, U/mL, ≧ 1000 | 0.984 | 0.245–3.958 | 0.98 |
| Multivariate analysis of predictors of mortality | |||
| Respiratory care, HFNC | 0.148 | 0.025–0.880 | 0.036 |
| PaO2/FIO2 ratio, < 100 | 10.072 | 0.806–125.896 | 0.073 |
See Table 1 legend for expansion of abbreviations.
Fig. 3.
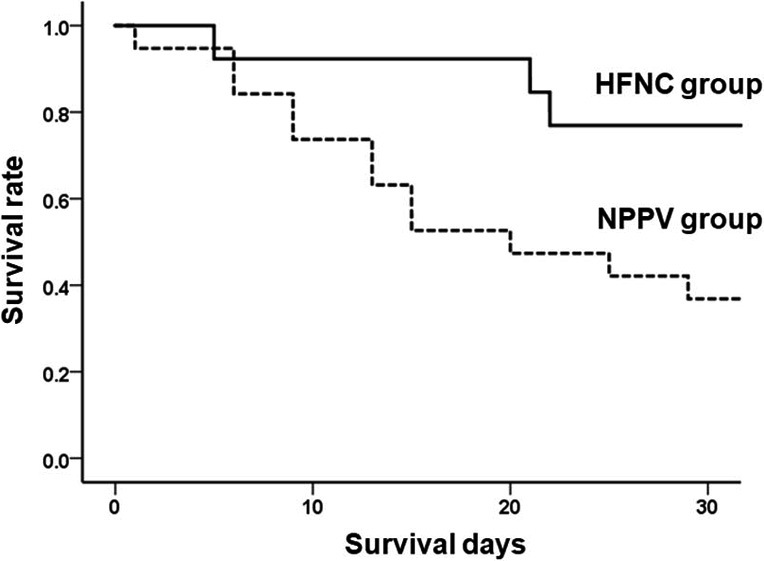
Kaplan-Meier distribution for the probability of survival
The p-value was calculated using the log-rank test. The solid line represents patients in the HFNC group, and the dotted line represents patients in the NPPV group. Survival was significantly better in the HFNC group than the NPPV group (p = 0.029).
HFNC: high-flow nasal cannula, NPPV: non-invasive positive pressure ventilation.
Table 5.
Univariate and multivariate analysis of 30-days mortality by using different cutoff points for APACHEII score
| Variables | OR | 95% CI | p-value |
| Univariate analysis of predictors of mortality | |||
| APACHE II score, ≧ 20 | 0.857 | 0.148–5.064 | 0.86 |
| APACHE II score, ≧ 22 | 1.667 | 0.323–8.590 | 0.54 |
| APACHE II score, ≧ 24 | 1.925 | 0.431–8.606 | 0.39 |
| APACHE II score, ≧ 26 | 1.778 | 0.423–7.467 | 0.43 |
| Multivariate analysis of predictors of mortality - 1 | |||
| APACHE II score, ≧ 20 | 0.399 | 0.047–3.401 | 0.40 |
| Respiratory care, HFNC | 0.137 | 0.023–0.801 | 0.027 |
| Multivariate analysis of predictors of mortality - 2 | |||
| APACHE II score, ≧ 22 | 0.696 | 0.093–5.196 | 0.72 |
| Respiratory care, HFNC | 0.152 | 0.025–0.921 | 0.040 |
| Multivariate analysis of predictors of mortality - 3 | |||
| APACHE II score, ≧ 24 | 0.792 | 0.121–5.194 | 0.81 |
| Respiratory care, HFNC | 0.157 | 0.025–0.993 | 0.049 |
| Multivariate analysis of predictors of mortality - 4 | |||
| APACHE II score, ≧ 26 | 0.716 | 0.114–4.485 | 0.72 |
| Respiratory care, HFNC | 0.148 | 0.023–0.962 | 0.045 |
See Table 1 legend for expansion of abbreviations.
DISCUSSION
This is the observational report to investigate the efficacy of HFNC therapy for ARF due to IP. Although the retrospective study with small number of patients has several limitations, the primary selection of HFNC therapy was associated with a better outcome in patients with ARF due to IP in the equally severe respiratory condition of PaO2/FIO2 ratio. In addition, there was a tendency for a decreased intubation rate in the HFNC group more than in the NPPV group.
Several advantages of HFNC therapy could account for the decreased risk of short-term mortality in patients with ARF due to IP. First, HFNC therapy is more comfortable than NPPV, and has the advantages of allowing the patients to eat, drink and talk.13 Other beneficial aspects of HFNC therapy, including heated humidification, flushing of the anatomical dead space, and reduction of airway resistance, add further to the patient’s comfort.28,29 These advantages account for the good tolerance of HFNC therapy, and therefore improved short-term mortality.30 In the previous literature, poor tolerance for ventilatory support was the reason for intubation in patients with ARF and these patients subsequently exhibited high mortality.31,32
Second, the relatively low PEEP in the HFNC group is suitable for patients with ARF due to IP. HFNC therapy can deliver a relatively low PEEP that corelates with the gas flow rate.11 In our study, the median gas flow rate in the HFNC group was 50 L/min, which provides a mean airway pressure of 1.7–3.3 cmH2O. In comparison, the NPPV group received a median PEEP of 6 cmH2O. A previous study showed that high PEEP settings failed to improve oxygenation and were associated with worse prognosis in patients with ARF due to IP.33 This might be because high PEEP promotes lung overdistension and ventilator interaction lung injury in patients with IP. In addition, a significant association has been reported between a PEEP of ≥ 5 cmH2O and increased short-term mortality.33 These data support that the relatively low PEEP in HFNC therapy might improve short-term mortality.
Third, the tendency for a lower intubation rate in the HFNC group might have contributed to the reduced short-term mortality. Patients with ARF due to IP who initially receive NPPV treatment and subsequently require invasive mechanical ventilation have been reported to have a rather poor prognosis.8,9 Other studies have reported that patients with ARF due to IP who required mechanical ventilation showed a high in-hospital mortality (81–87%).34,35 Therefore, a lower intubation rate is associated with a lower mortality in these patients.
Fourth, in terms of breathing efficacy and respiratory workload, HFNC therapy might have more favorable effect than NPPV. HFNC therapy was reported to be associated with a lower respiratory rate than NPPV in patients with ARF.36 In addition, several studies reported that higher respiratory rate was associated with increased mortality in patients with ARF due to IP.37,38 A possible explanation for this finding is that the rapid respiratory rate observed in IP is thought to occur secondary to increased inspiratory elastic loading on the respiratory muscles.39 This beneficial aspect of HFNC therapy might decrease breathing efficacy and respiratory workload, thereby improved mortality. Overall, our study findings suggested that HFNC therapy might be a better strategy for respiratory support in patients with ARF due to IP.
In our study, the difference in intubation rate between the HFNC and NPPV groups was not statistically significant but there was a borderline trend towards a lower intubation rate in the HFNC group. Moreover, if we excluded patients with do-not-intubate order, lower intubation rate was seen in the HFNC group. Several studies have reported that the intubation rate was not different between patients with ARF who receive HFNC therapy and those who receive NPPV therapy.13,14,40 However, in the subgroup of patients with a PaO2/ FIO2 ≤200 mmHg, the intubation rate was significantly lower in the HFNC group than in the NPPV group.13 Most of the patients (28/32 patients) in our study represented severe hypoxia with a PaO2/ FIO2 ≤200 mmHg. These data suggest that HFNC therapy reduces the need for intubation in ARF patients due to IP. Moreover, a post-hoc power analysis (one-sided, 5% alpha) to detect a significant between-group difference in the intubation rate revealed a power of 0.46, indicating that our study was significantly underpowered with risk of a type two error. Additional prospective studies with large sample sizes are warranted to confirm our results.
We recognize that there are some limitations to this study. First this is a retrospective observational study, not a randomized control study, and it is possible that this might somehow have biased results. Patients in the NPPV group tended to be more severe conditions such as lower MAP and higher APACHEII scores than those in the HFNC group. In this study, APACHEII score had no effect on 30-day mortality in univariate logistic regression analysis though we applied several cutoff points for APACHEII score. In addition, even if we included the APACHEII score in multivariate logistic regression analysis, HFNC therapy remained a significant determinant of 30-day mortality. However, these data do not confirm that APACHEII score is not associated with mortality in this cohort because of small sample size. Therefore, we could not eliminate the possibility that less severe conditions in the HFNC group results in favorable outcome. Second, we could not include too many covariates in multivariate logistic regression analysis because of the small number of patients in our study. Many studies have reported that the PaO2/FIO2 ratio was associated with mortality in patients with ARF due to IP.23,25,41 Therefore, we used the PaO2/FIO2 ratio for adjustment to clarify whether HFNC therapy did or did not improve 30-day mortality in patients with ARF due to IP. Third, this study included various causes of IP such as acute exacerbation of IPF, NSIP and collagen vascular related IP. Acute exacerbation of IPF is the most fatal disease condition, but is not associated with 30-day mortality. Two patients with collagen vascular disease related IP were clinically amyopathic dermatomyositis related IP, which is also fatal disease and therefore might affect the outcome.
CONCLUSION
HFNC therapy might have a favorable effect on 30-day mortality and the intubation rate. This study provides preliminary data with several limitations and therefore we could not establish the effectiveness of HFNC therapy on ARF due to IP. However, our findings suggest that HFNC therapy can be one of the option strategies for respiratory management in patients with ARF due to IP. The results observed here warrant the need for further investigation of HFNC therapy in randomized control trials.
ACKNOWLEDGEMENT
None.
CONFLICT OF INTEREST
All of the authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
AVAILABILITY OF DATA AND MATERIALS
Not applicable in this retrospective evaluation of clinical data.
AUTHORS’ CONTRIBUTIONS
NO and NM contributed to study design, data collection, analysis of data, review of manuscript, and submission of this manuscript. NH contributed to study design and interpretation. KS, AA, MH, and YN contributed to review of this manuscript. KN and SM contributed to review of this manuscript, and data analysis. MN and YH contributed to study design and review of this manuscript. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable; see the Materials and methods section of the main text.
CONSENT FOR PUBLICATION
Not applicable; see the Materials and methods of the main text.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- ARF
Acute respiratory failure
- FIO2
Fraction of inspired oxygen
- HFNC
High-flow nasal cannula oxygen
- ICU
Intensive care unit
- IP
Interstitial pneumonia
- IPF
Idiopathic pulmonary fibrosis
- MAP
Mean arterial blood pressure
- NPPV
Non-invasive positive pressure ventilation
- NSIP
Non-specific interstitial pneumonia
REFERENCES
- 1.Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132(1):214–220. [DOI] [PubMed]
- 2.Kondoh Y, Taniguchi H, Kawabata Y, et al. Acute exacerbation in idiopathic pulmonary fibrosis: analysis of clinical and pathologic findings in three cases. Chest. 1993;103(6):1808–1812. [DOI] [PubMed]
- 3.Martinez FJ, Safrin S, Weycker D, et al; IPF Study Group. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;21;142:963–967. [DOI] [PubMed]
- 4.Silva CI, Müller NL, Fujimoto K, et al. Acute exacerbation of chronic interstitial pneumonia: high-resolution computed tomography and pathologic findings. J Thorac Imaging. 2007;22(3):221–229. [DOI] [PubMed]
- 5.Churg A, Muller NL, Silva CI, et al. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol. 2007;31(2):277–284. [DOI] [PubMed]
- 6.Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med. 2009;103(6):846–853. [DOI] [PMC free article] [PubMed]
- 7.Olson AL, Huie TJ, Groshong SD, et al. Acute exacerbations of fibrotic hypersensitivity pneumonitis. Chest. 2008;134(4):844–850. [DOI] [PubMed]
- 8.Yokoyama T, Kondoh Y, Taniguchi H, et al. Noninvasive ventilation in acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2010;49(15):1509–1514. [DOI] [PubMed]
- 9.Güngör G, Tatar D, Saltürk C, et al. Why do patients with interstitial lung diseases fail in the ICU? A 2-center cohort study. Respir Care. 2013;58(3):5255–5231. [DOI] [PubMed]
- 10.Chanques G, Riboulet F, Molinari N, et al. Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol. 2013;79:1344–1355. [PubMed]
- 11.Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011;56:1151–1155. [DOI] [PubMed]
- 12.Dysart K, Miller TL, Wolfson MR, et al. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400–1405. [DOI] [PubMed]
- 13.Frat JP, Thille AW, Mercat A, et al; FLORALI Study Group; REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. [DOI] [PubMed]
- 14.Zhao H, Wang H, Sun F, et al. High-flow nasal cannula oxygen therapy is superior to conventional oxygen therapy but not to noninvasive mechanical ventilation on intubation rate: a systematic review and meta-analysis. Crit Care. 2017;21(1):184. [DOI] [PMC free article] [PubMed]
- 15.Travis WD, Costabel U, Hansell DM, et al; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–738. [DOI] [PMC free article] [PubMed]
- 16.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. [DOI] [PubMed]
- 17.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581-590. [DOI] [PubMed]
- 18.Smolen JS, Steiner G. Mixed connective tissue disease: to be or not to be? Arthritis Rheum. 1998;41:768–777. [DOI] [PubMed]
- 19.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. [DOI] [PubMed]
- 20.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344–347. [DOI] [PubMed]
- 21.Ohtani Y, Saiki S, Kitaichi M, et al. Chronic bird fancier’s lung: histopathological and clinical correlation: an application of the 2002 ATS/ERS consensus classification of the idiopathic interstitial pneumonias. Thorax. 2005;60:665–671. [DOI] [PMC free article] [PubMed]
- 22.Camus P, Fanton A, Bonniaud P, et al. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71(4):301–326. [DOI] [PubMed]
- 23.Tachikawa R, Tomii K, Ueda H, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration. 2012;83(1):20–27. [DOI] [PubMed]
- 24.Collard HR, Moore BB, Flaherty KR, et al; Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–643. [DOI] [PMC free article] [PubMed]
- 25.Kishaba T, Tamaki H, Shimaoka Y, et al. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung. 2014;192(1):141–149. [DOI] [PubMed]
- 26.Roca O, Sacanell J, Laborda C, et al. Cohort study on incidence of ARDS in patients admitted to the ICU and prognostic factors of mortality. Med Intensiva. 2006;30(1):6–12. [DOI] [PubMed]
- 27.Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–363. [DOI] [PubMed]
- 28.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61(4):529–541. [DOI] [PubMed]
- 29.Goligher EC, Slutsky AS. Not just oxygen? Mechanisms of benefit from high-flow nasal cannula in hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1128–1131. [DOI] [PubMed]
- 30.Frat JP, Brugiere B, Ragot S, et al. Sequential application of oxygen therapy via high-flow nasal cannula and noninvasive ventilation in acute respiratory failure: an observational pilot study. Respir Care. 2015;60(2):170–178. [DOI] [PubMed]
- 31.Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med. 2001;27(11):1718–1728. [DOI] [PubMed]
- 32.Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284(18):2352–2360. [DOI] [PubMed]
- 33.Fernández-Pérez ER, Yilmaz M, Jenad H, et al. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest. 2008;133(5):1113–1119. [DOI] [PMC free article] [PubMed]
- 34.Mallick S. Outcome of patients with idiopathic pulmonary fibrosis (IPF) ventilated in intensive care unit. Respir Med. 2008;102(10):1355–1359. [DOI] [PubMed]
- 35.Gaudry S, Vincent F, Rabbat A, et al. Invasive mechanical ventilation in patients with fibrosing interstitial pneumonia. J Thorac Cardiovasc Surg. 2014;147(1):47–53. [DOI] [PubMed]
- 36.Ou X, Hua Y, Liu J, et al. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017;189:E260–267. [DOI] [PMC free article] [PubMed]
- 37.Aliberti S, Messinesi G, Gamberini S, et al. Non-invasive mechanical ventilation in patients with diffuse interstitial lung diseases. BMC Pulm Med. 2014;14:194. [DOI] [PMC free article] [PubMed]
- 38.Abe M, Tsushima K, Matsumura T, et al. Efficacy of thrombomodulin for acute exacerbation of idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia: a nonrandomized prospective study. Drug Des Devel Ther. 2015;9:5755-5562. [DOI] [PMC free article] [PubMed]
- 39.Burdon JG, Killian KJ, Jones NL. Pattern of breathing during exercise in patients with interstitial lung disease. Thorax. 1983;38:778–784. [DOI] [PMC free article] [PubMed]
- 40.Maitra S, Som A, Bhattacharjee S, et al. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: a meta-analysis and systematic review. J Crit Care. 2016;35:138–144. [DOI] [PubMed]
- 41.Oishi K, Aoe K, Mimura Y, et al. Survival from an acute exacerbation of idiopathic pulmonary fibrosis with or without direct hemoperfusion with a Ppolymyxin B-immobilized fiber column: a retrospective analysis. Intern Med. 2016;55(24):3551–3559. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable in this retrospective evaluation of clinical data.