Highlights
-
•
BRCA immunohistochemistry is simple, less expensive and widely available.
-
•
Negative BRCA1 and/or BRCA2 expression was found in 34 patients (32.4%).
-
•
BRCA immunohistochemistry has high negative predictive value of 96%.
-
•
It may be useful to exclude patients without BRCA dysfunction.
Keywords: BRCA mutation, Immunohistochemistry, Ovarian cancer
Abstract
To evaluate BRCA1/2 immunohistochemistry (IHC) as a screening test for germline BRCA1/2 in epithelial ovarian cancer (EOC), tumor tissue from 105 EOC patients who had germline BRCA mutations, including 9 BRCA1 mutations, 6 BRCA2 mutations and 90 no BRCA mutations, were studied. Paraffin-embedded tissue blocks were stained for BRCA1 and BRCA2. Tumors were indicated as a loss of BRCA expression when neoplastic nuclear stained less than 10%. Loss of BRCA1 and/or BRCA2 expression was found in 36 patients (34.3%). BRCA1 IHC loss was found in 21 patients (20%) while 24 patients (22.9%) had BRCA2 IHC loss. There were no significant differences in patient characteristics between both groups. Loss of BRCA1 expression had 66.7% sensitivity, 84.3% specificity, 28.6% positive predictive value (PPV), and 96.4% negative predictive value (NPV) for detection of germline BRCA1 mutation. Meanwhile, loss of BRCA2 expression had 50% sensitivity, 78.8% specificity, 12.5% PPV, and 96.3% NPV for detection of germline BRCA2 mutation. There was no significant difference in survival outcomes between both groups.
Based on high NPV, BRCA IHC may be useful to exclude patients without BRCA dysfunction if IHC showed intact expression. Only patients with BRCA IHC loss should be offered further genetic testing.
1. Introduction
At least 10% of epithelial ovarian cancer (EOC) is caused by genetic alteration (Arts-de Jong et al., 2016) and about 80% of the alteration are BRCA1 or BRCA2 mutations. (Norquist et al., 2016) It has been reported that BRCA mutations are the highest, up to 20%, in the high grade serous subtype. (Ledermann et al, 2016) Our previous study reported that BRCA mutation was detected in 25% of high grade serous carcinoma. (Manchana et al., 2019a) BRCA mutation occurred less than 10% in endometrioid subtype and very low frequency in clear cell carcinoma and the other subtypes. (Arts-de Jong et al., 2016) EOC patients with BRCA mutation usually present with platinum sensitivity and have better progression free and overall survival. (Bolton et al., 2012) Moreover, poly (ADP-ribose) polymerase (PARP) inhibitors have been shown to be a promising targeted therapy in EOC patients with BRCA dysfunction. It has been approved for maintenance treatment following platinum sensitive recurrent EOC, including fallopian tube and primary peritoneal cancers. Recently, it has also been approved as maintenance treatment in advanced stage, high grade serous or endometrioid carcinoma following primary surgical treatment and first line platinum-based chemotherapy. (Vanacker et al., 2019) Therefore, various guidelines by the American College of Obstetricians and Gynecologists (ACOG), Society of Gynecologic Oncologists (SGO), and National Comprehensive Cancer Network (NCCN) have recommended universal genetic testing in all EOC patients. In Thailand, major obstacles to follow this guideline include high costs, limited geneticists, lack of testing services, and no coverage by the Thai Universal Coverage Scheme.
Immunohistochemistry (IHC) for BRCA is simple, less expensive and has widespread service in almost all pathological laboratories across the nation. Loss of BRCA expression can be used as a screening tool for BRCA dysfunction which includes germline, somatic mutations and methylation. It showed high sensitivity and specificity of about 80–90% and has a very high negative predictive value of up to 95%. (Garg et al., 2013, Meisel et al., 2014) This study was conducted to evaluate the potential of using IHC for BRCA as a screening test for EOC patients in Thailand.
2. Methods
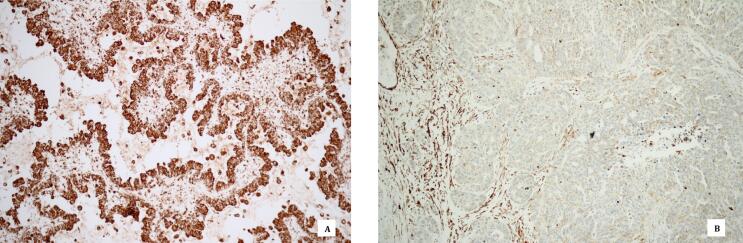
Subjects in this study were non-mucinous EOC patients including fallopian tube and primary peritoneal cancer patients who received genetic testing with multi-gene panels and next generation sequencing at King Chulalongkorn Memorial Hospital from November 2015 to July 2017. This study was approved by Institutional Review Board, Faculty of Medicine, Chulalongkorn University (IRB No.141/59). Firstly, formalin fixed paraffin-embedded tissues of the patients were obtained from the hospital. Patients were excluded if the specimen or clinical data were not available. The paraffin-embedded tissue blocks were selected by gynecologic pathologist (P.T.) and were subjected to immunohistochemical staining for BRCA1 and BRCA2. The tissue sections (2-µm-thick) were cut, mounted, deparaffinized and pretreated with standard cell conditioning 1 (CC1) in Ventana Benchmark XT. Samples were stained and incubated for 60 min with BRCA1 mouse monoclonal antibody (Novus biological Inc., USA) and BRCA2 rabbit polyclonal antibody (Novus biological Inc., USA) at a dilution ratio of 1:100. Optiview DAB IHC Detection Kit was used to visualize the staining of primary antibodies in tissue sections. Counterstaining was performed with hematoxylin. Immunoreactivity was evaluated using light microscope by two gynecologic pathologists (P.T. and N.P.) who were blinded to the BRCA mutation status. If there were disagreements, the slides were reviewed to achieve consensus agreement. The regions with greatest immunostaining were selected for cell count. The percentage of BRCA immunostaining was calculated. Tumors were considered as loss of BRCA expression (BRCA IHC loss) when neoplastic nuclear staining was less than 10% and retained BRCA expression (BRCA IHC intact) when staining was more than 10% (Fig. 1). (Thrall et al., 2006) Tumors with known BRCA1 or BRCA2 mutation status were used as a positive control and nuclear staining of stromal cells were used as an internal positive control. Slides without the primary antibody were used as negative control. The association between BRCA expression and clinical variables were analyzed by standard Chi-square tests or Fisher’s exact test when appropriate. Kaplan–Meier method was used to estimate progression free survival (PFS) and overall survival (OS), and the log-rank test was used to assess differences between groups. A p-value of less than 0.05 was considered statistically significant.
Fig. 1.
BRCA immunohistochemistry in serous ovarian carcinoma. Retained BRCA expression in tumor cell nuclei (A) and loss of BRCA expression in tumor cell nuclei with positive internal control in stromal cells (B).
3. Results
One hundred and five patients with BRCA mutation status were studied. From 105 patients, 9 patients (8.6%) had germline BRCA1 mutation, 6 patients (5.7%) had BRCA2 mutation, and 90 patients had no BRCA 1/2 mutation. The mean age was 52.7 years (SD 10.9 years) and the median parity was 1 (range 0–5) and 22.9% of the patients had family history of breast and or ovarian cancer. Most patients were diagnosed with EOC (91.4%) followed by peritoneal cancer (5.7%), and fallopian tube cancer (2.9%). Fifty-one patients (48.6%) were advanced stage (stage 3) while 31.4% and 20% of the patients were at stage 1 and 2, respectively. Forty-five patients (42.8%) had high grade serous carcinoma, 15 (14.3%) had high grade endometrioid carcinoma, 24 (22.8%) low grade endometrioid carcinoma, 12 (11.4%) clear cell carcinoma, and 9 (8.6%) low grade serous carcinoma. Ninety-three patients (88.6%) had optimal cytoreduction but only 11.4% had suboptimal cytoreduction. During the study period with a median follow up time of 44 months (10–59 months), 24 patients (22.9%) were lost to follow up, 48 patients (47.1%) had developed recurrence of disease and 6 patients (5.7%) had died. Thirty-six recurrent patients (75%) were platinum sensitive and 25% were platinum resistant.
Loss of BRCA1 and/or BRCA2 expression was found in 36 patients (34.3%) where 12 patients (11.4%) had BRCA1 IHC loss, 15 patients (14.3%) had BRCA2 IHC loss, and 9 patients (8.6%) had both BRCA1 and BRCA2 IHC loss. Overall, BRCA1 IHC loss was found in 21 patients (20%) and 24 patients (22.9%) had BRCA2 IHC loss. There was no statistical significance in patient characteristics between both groups as shown in Table 1.
Table 1.
Patient characteristics according to BRCA1/2 immunohistochemistry (IHC).
| Characteristic | BRCA1/2 IHC loss (N = 36) | BRCA1/2 IHC intact (N = 69) | p value |
|---|---|---|---|
| Age at diagnosis (years) | 55.6 ± 12.2 | 51.3 ± 10 | 0.06 |
| Family history of cancers | 9 (25%) | 15 (21.7%) | 0.81 |
| Tumor site | |||
| Ovary | 33 (91.7%) | 63 (91.3%) | 0.33 |
| Fallopian tube | 0 | 3 (4.3%) | |
| Peritoneum | 3 (8.3%) | 3 (4.3%) | |
| Histology | |||
| High grade serous carcinoma | 15 (41.7%) | 30 (43.5%) | 0.94 |
| Low grade serous carcinoma | 3 (8.3%) | 6 (8.7%) | |
| High grade endometrioid carcinoma | 6 (16.7%) | 9 (13%) | |
| Low grade endometrioid carcinoma | 9 (25%) | 15 (21.7%) | |
| Clear cell carcinoma | 3 (8.3%) | 9 (13%) | |
| Stage | |||
| 1 | 9 (25%) | 24 (34.8%) | 0.05 |
| 2 | 12 (33.3%) | 9 (13%) | |
| 3 | 15 (41.7%) | 36 (52.2%) | |
| Cytoreduction | |||
| Optimal | 30 (83.3%) | 63 (91.3%) | 0.33 |
| Suboptimal | 6 (16.7%) | 6 (8.7%) | |
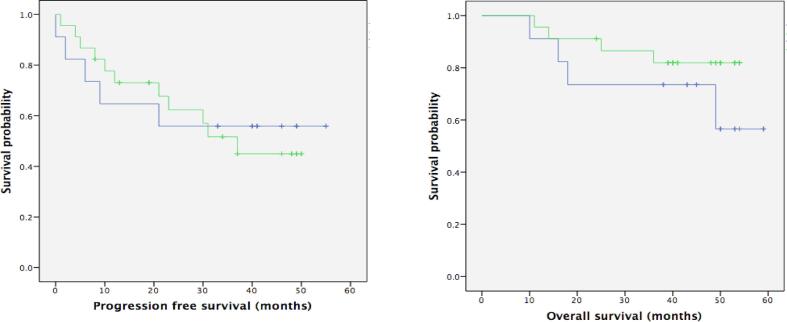
The association between BRCA IHC and BRCA mutation status are shown in Table 2, Table 3. BRCA1 IHC loss for detection of germline BRCA1 mutation had 66.7% sensitivity, 84.3% specificity, 28.6% positive predictive value (PPV), and 96.4% negative predictive value (NPV). BRCA2 IHC loss for detection of germline BRCA2 mutation had 50% sensitivity, 78.8% specificity, 12.5% PPV, and 96.3% NPV (Table 4). There was no significant difference in progression free survival (PFS) and overall survival (OS) between patients with BRCA IHC loss and intact (Fig. 2).
Table 2.
Performance of BRCA1 immunohistochemistry (IHC) and BRCA mutation status.
| BRCA1 mutation (N = 9) | BRCA2 mutation (N = 6) | No BRCA1/2 mutation (N = 90) | |
|---|---|---|---|
| BRCA1 IHC loss (N = 21) | 6 (66.7%) | 0 | 15 (16.7%) |
| BRCA1 IHC intact (N = 84) | 3 (33.3%) | 6 (100%) | 75 (83.3%) |
Table 3.
Performance of BRCA2 immunohistochemistry (IHC) and BRCA mutation status.
| BRCA1 mutation (N = 9) | BRCA2 mutation (N = 6) | No BRCA1/2 mutation (N = 90) | |
|---|---|---|---|
| BRCA2 IHC loss (N = 24) | 6 (66.7%) | 3 (50%) | 15 (16.7%) |
| BRCA2 IHC intact (N = 81) | 3 (33.3%) | 3 (50%) | 75 (83.3%) |
Table 4.
Performance of BRCA 1/2 immunohistochemistry (IHC) for detection of germline BRCA1/2 mutation.
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| BRCA1 IHC loss for detection of germline BRCA1 mutation | 66.7% | 84.3% | 28.6% | 96.4% |
| BRCA2 IHC loss for detection of germline BRCA2 mutation | 50% | 78.8% | 12.5% | 96.3% |
PPV: Positive predictive value, NPV: Negative predictive value
Fig. 2.
Progression free survival and overall survival by BRCA immunohistochemistry (IHC).
4. Discussion
Loss of BRCA1 and/or BRCA2 expression was found in 36 patients (34.3%); 20% had BRCA1 IHC loss and 22.9% had BRCA2 IHC loss. The incidence of BRCA1 IHC loss in EOC tissue was reported to vary about 21–56%. (Carser et al., 2011, Garg et al., 2013, Hjortkjær et al., 2017, Lesnock et al., 2013, Meisel et al., 2014, Thrall et al., 2006) There is no standard scoring protocol for BRCA IHC and the cut-off values of 10% of tumor staining are simple and commonly used by general pathologists in Thailand. The current study also used this cut-off value to classify IHC loss or intact, which is similar to previous published articles. Loss of BRCA expression was found more commonly in high-grade serous carcinoma and it has been reported that the expression may reach up to 36% in the previous study. (Meisel et al., 2014) This is comparable to our findings where 41.7% of high-grade serous carcinoma showed BRCA IHC loss. However, overall loss of BRCA expression in our study was lower than previous studies, which reported about 41–54%. (Carser et al., 2011, Lesnock et al., 2013, Thrall et al., 2006) Different common histological subtype in Thailand is a possible hypothesis. High-grade serous subtype is the most common subtype worldwide but it is less common in Thais. Endometrioid carcinoma is the most common subtype (32%), followed by serous carcinoma (25%), clear cell carcinoma (23%) and mucinous carcinoma (19%). (Manchana and Kobwitaya, 2018)
BRCA IHC may be a potential biomarker for BRCA dysfunction in EOC, especially high-grade serous ovarian cancer. (Meisel et al., 2014) Germline BRCA mutation is the most common BRCA dysfunction but somatic BRCA mutation and epigenetic change are other mechanisms. In ovarian cancer tissue, somatic BRCA mutation and promoter methylation has been reported at 5–7% and 10–15%, respectively. (Cancer Genome Atlas Research Network, 2011, Moschetta et al., 2016) Both germline and somatic mutations have prognostic and therapeutic significance where it can increase sensitivity to platinum-based chemotherapy, have better survival rates, and more susceptibility to PARP inhibitors. (Sun et al., 2014) However, the clinical impact of BRCA hypermethylation on oncological outcome is still inconclusive. (Moschetta et al., 2016) BRCA1 IHC loss to indicate BRCA1 mutation with 80% sensitivity, 93% specificity, and an estimated PPV of 73% was reported by Skytte et al. (2011) Another study also reported that BRCA1 IHC had sensitivity of 86%, specificity of 78%, NPV of 95% but low PPV of 52% for identifying germline BRCA1 mutation. However, PPV rose to 87% if somatic BRCA1 mutation and promotor hypermethylation were included. (Meisel et al., 2014) Garg et al. reported similar results where the sensitivity, specificity, PPV and NPV were 89%, 88%, 83%, and 92%, respectively. (Garg et al., 2013) In contrast, our study showed BRCA1 IHC loss had lower sensitivity (68%) and PPV (29%) for screening of germline BRCA1 mutation. Somatic BRCA mutation and promoter hypermethylation were not tested in the current study and less prevalence of the germline BRCA mutation in Thais might be a possible explanation. Worldwide incidence of germline BRCA mutation is reported at about 10–15% but it increased to about 20–30% in high grade serous ovarian cancer. (Cancer Genome Atlas Research Network, 2011, Mafficini et al., 2016). Our previous study reported similar incidence of germline BRCA mutation in high grade serous carcinoma (25.6%). (Manchana et al., 2019a) However, as previously mentioned, high grade serous carcinoma is not common in Thais. It is estimated that BRCA mutations in unselected Thai patients might be less than 10% (Manchana et al., 2019b).
BRCA IHC for prediction of BRCA mutation has been extensively studied for BRCA1 compared to BRCA2 IHC. BRCA2 IHC has poorer sensitivity and specificity, which are 50% and 38%, respectively. It is not significantly correlated with BRCA2 carrier status. (Vaz et al., 2007) This finding is comparable to our results where BRCA2 IHC loss for detection of BRCA2 mutation had 50% sensitivity, 78.8% specificity, 12.5% PPV and 96.3% NPV.
Loss of BRCA1 expression had been reported to have better clinical response to chemotherapy and improves the survival of sporadic EOC patients who may have other BRCA defect mechanisms such as somatic mutation and epigenetic alteration. (Carser et al., 2011, Hjortkjær et al., 2017, Lesnock et al., 2013, Weberpals et al., 2011) However, the current study did not show prognostic significance of BRCA IHC loss. The small number of patients in this study is probably insufficient to detect statistical significance and it also cannot be stratified by BRCA genotype. A large cohort study should be conducted to detect this difference compared with sporadic ovarian cancer. Another limitation is that the germline BRCA mutation was tested as BRCA dysfunction in this study, somatic BRCA mutation and promoter methylation were not included. The performance for BRCA IHC for detection of somatic BRCA mutation and/or promoter methylation is unknown.
BRCA IHC is simple, inexpensive and a potential biomarker for BRCA dysfunction in EOC patients. The sensitivity of BRCA1 IHC for detection of germline BRCA1 mutation is less than 90% of BRCA1 patients (Garg et al., 2013, Meisel et al., 2014) and less than 70% in our study. However, in the current study, BRCA IHC had very high NPV, 96.4% and 96.3% for BRCA1 and BRCA2 mutation, respectively. This finding is comparable to previous studies which reported the NPV of 92–95% for prediction of BRCA dysfunction. (Garg et al., 2013, Meisel et al., 2014) This means that if tests show retained BRCA expression, it can exclude patients with BRCA dysfunction especially in areas with low frequency of BRCA mutation. Only those who have loss of BRCA expression are candidates for further genetic testing. This application may be suitable in limited resource countries, which still have limitations in genetic counseling and genetic testing.
In conclusion, based on high NPV of BRCA IHC, it may be useful to exclude patients with BRCA dysfunction if IHC shows retained expression. Only one-third of patients in this study showed BRCA IHC loss and should be further investigated for germline/somatic BRCA mutation or promoter methylation. This strategy may be more cost-effective than universal BRCA testing, especially in low resource settings or where there are limited genetic counseling and testing facilities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This study was funded by the RatchadapisekSompoch Fund (R1038/59), Chulalongkorn University, Bangkok, Thailand.
Author Contribution section
TM did conception and study design, acquisition of data, analysis, interpretation of data, drafting and revision of the manuscript.
PT and NP did pathological revision and revision of the manuscript.
References
- Arts-de Jong M., de Bock G.H., van Asperen C.J., Mourits M.J., de Hullu J.A., Kets C.M. Germline BRCA1/2 mutation testing is indicated in every patient with epithelial ovarian cancer: A systematic review. Eur J Cancer. 2016;61:137–145. doi: 10.1016/j.ejca.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Bolton K.L., Chenevix-Trench G., Goh C., Sadetzki S., Ramus S.J., Karlan B.Y. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carser J.E., Quinn J.E., Michie C.O., O’Brien E.J., McCluggage W.G., Maxwell P. BRCA1 is both a prognostic and predictive biomarker of response to chemotherapy in sporadic epithelial ovarian cancer. Gynecol. Oncol. 2011;123:492–498. doi: 10.1016/j.ygyno.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Garg K., Levine D.A., Olvera N., Dao F., Bisogna M., Secord A.A. BRCA immunohistochemistry in a molecularly characterized cohort of ovarian high-grade serous carcinomas. Am. J. Surg. Pathol. 2013;37:138–146. doi: 10.1097/PAS.0b013e31826cabbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortkjær M., Waldstrøm M., Jakobsen A., Kanstrup H., Søgaard-Andersen E., Steffensen K.D. The Prognostic value of BRCA1 and PARP expression in epithelial ovarian carcinoma: Immunohistochemical detection. Int. J. Gynecol. Pathol. 2017;36:180–189. doi: 10.1097/PGP.0000000000000310. [DOI] [PubMed] [Google Scholar]
- Ledermann J.A., Drew Y., Kristeleit R.S. Homologous recombination deficiency and ovarian cancer. Eur. J. Cancer. 2016;60:49–58. doi: 10.1016/j.ejca.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Lesnock J.L., Darcy K.M., Tian C., Deloia J.A., Thrall M.M., Zahn C. BRCA1 expression and improved survival in ovarian cancer patients treated with intraperitoneal cisplatin and paclitaxel: a Gynecologic Oncology Group Study. Br. J. Cancer. 2013;108:1231–1237. doi: 10.1038/bjc.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafficini A., Simbolo M., Parisi A., Rusev B., Luchini C., Cataldo I. BRCA somatic and germline mutation detection in paraffin embedded ovarian cancers by next-generation sequencing. Oncotarget. 2016;7:1076–1083. doi: 10.18632/oncotarget.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchana T., Kobwitaya K. Survival outcomes for different subtypes of epithelial ovarian cancer. Clin. Res. Obstet. Gynecol. 2018;1:1–7. [Google Scholar]
- Manchana T., Phoolcharoen N., Tantbirojn P. BRCA mutation in high grade epithelial ovarian cancers. Gynecol. Oncol. Rep. 2019;29:102–105. doi: 10.1016/j.gore.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchana T., Phowthongkum P., Teerapakpinyo C. Germline mutations in Thai patients with non-mucinous epithelial ovarian cancer. World J. Clin. Oncol. 2019;10:358–368. doi: 10.5306/wjco.v10.i11.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel J.L., Hyman D.M., Garg K., Zhou Q., Dao F., Bisogna M. The performance of BRCA1 immunohistochemistry for detecting germline, somatic, and epigenetic BRCA1 loss in high-grade serous ovarian cancer. Ann. Oncol. 2014;25:2372–2378. doi: 10.1093/annonc/mdu461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschetta M., George A., Kaye S.B., Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann. Oncol. 2016;27:1449–1455. doi: 10.1093/annonc/mdw142. [DOI] [PubMed] [Google Scholar]
- Norquist B.M., Harrell M.I., Brady M.F., Walsh T., Lee M.K., Gulsuner S. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482–490. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skytte A.B., Waldstrøm M., Rasmussen A.A., Crüger D., Woodward E.R., Kølvraa S. Identification of BRCA1-deficient ovarian cancers. Acta. Obstet. Gynecol. Scand. 2011;90:593–599. doi: 10.1111/j.1600-0412.2011.01121.x. [DOI] [PubMed] [Google Scholar]
- Sun C., Li N., Ding D., Weng D., Meng L., Chen G., Ma D. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0095285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall M., Gallion H.H., Kryscio R., Kapali M., Armstrong D.K., Deloia J.A. BRCA1 expression in a large series of sporadic ovarian carcinomas: a Gynecologic Oncology Group Study. Int. J. Gynecol. Cancer. 2006;16:166–171. doi: 10.1111/j.1525-1438.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- Vanacker H., Romeo C., Ray-Coquard I. Current role of poly(ADP-ribose) polymerase inhibitors: which poly(ADP-ribose) polymerase inhibitor and when? Curr. Opin. Oncol. 2019;31:394–403. doi: 10.1097/CCO.0000000000000557. [DOI] [PubMed] [Google Scholar]
- Vaz F.H., Machado P.M., Brandão R.D., Laranjeira C.T., Eugénio J.S., Fernandes A.H. Familial breast/ovarian cancer and BRCA1/2 genetic screening: the role of immunohistochemistry as an additional method in the selection of patients. J. Histochem. Cytochem. 2007;55:1105–1113. doi: 10.1369/jhc.7A7209.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weberpals J.I., Tu D., Squire J.A., Amin M.S., Islam S., Pelletier L.B. Breast cancer 1 (BRCA1) protein expression as a prognostic marker in sporadic epithelial ovarian carcinoma: an NCIC CTG OV.16 correlative study. Ann. Oncol. 2011;22:2403–2410. doi: 10.1093/annonc/mdq770. [DOI] [PubMed] [Google Scholar]