Abstract
Nutritional and microbiological psychiatry, especially the contribution of the gut microbiota to depression, has become a promising research field over the past several decades. An imbalance in the “microbiota-gut-brain axis”, which reflects the constant bidirectional communication between the central nervous system and the gastrointestinal tract, has been used as a hypothesis to interpret the pathogenesis of depression. Alterations in gut microbiota composition could increase the permeability of the gut barrier, activate systemic inflammation and immune responses, regulate the release and efficacy of monoamine neurotransmitters, alter the activity and function of the hypothalamic-pituitary-adrenal (HPA) axis, and modify the abundance of brain-derived neurotrophic factor (BDNF), eventually leading to depression. In this article, we review changes in gut microbiota in depressive states, the association between these changes and depression-like behavior, the potential mechanism linking gut microbiota disruptions and depression, and preliminary attempts at using gut microbiota intervention for the treatment of depression. In summary, although the link between gut microbiota and depression and the potential mechanism have been discussed, a more detailed mechanistic understanding is needed to fully realize the importance of the microbiota-gut-brain axis in depression. Future efforts should aim to determine the potential causative mechanisms, which will require further animal and clinical research as well as the development of analytical approaches.
Keywords: Neuroscience, Immunology, Neurology, Psychiatry, Microbiota-gut-brain axis, Depression, Gut microbiota, Probiotics
1. Introduction
Depression, a prevalent neuropsychiatric disease with a high recurrence rate that affects over 350 million people worldwide, exerts heavy public health and economic burdens [1]. As the 4th leading cause of disability [2], depression reportedly represent the largest share of the total disease burden, accounting for 10.3% of the total disease burden and causing 76.4 million years lost to disability (YLD) worldwide [3]. However, most of the currently available antidepressants, which aim to improve the levels of monoamine neurotransmitters, do not meet therapeutic requirements; an inadequate response occurs in approximately 30% patients [4]. Although the identification of the robust and rapid-onset antidepressant effects of ketamine has shifted the direction of antidepressant research and development [5], its long-term efficacy and adverse effects must be taken into account. Thus, deeply exploring the pathogenesis of depression and develop potential therapeutic targets are both imperative and necessary [6].
Since Nobel laureate Elie Metchnikoff proposed that lactic acid bacteria are beneficial to human health over 100 years ago, increasing attention has been paid to the relationship between the microbiota and human health. Apart from its classical role in regulating gastrointestinal (GI) function [7], the gut microbiota reportedly plays roles in regulating mood and cognition. The tight association between the brain and microbiota was first clearly identified. This is based on the favorable improvement of symptoms in patients suffering from hepatic encephalopathy (HE) as a result of treatment with nonabsorbable oral antibiotics [8]. Indeed, accumulating evidence has revealed that the brain may impact the gut microbiota, which may, in turn, shape the brain via the microbiota-gut-brain axis [9]. Dysfunction of the microbiota-gut-brain axis may play an important role in the pathogenesis of depression [10]. The mechanism may involve the hypothalamic-pituitary-adrenal (HPA) axis, the central nervous system (CNS), the enteric nervous system (ENS), the immune system, several neurotransmitters and neural regulators, the intestinal mucosal barrier and blood-brain barrier pathways [11]. This review aims to elucidate the physiological and pathological effects of the intestinal microbiota and discuss the potential role of the microbiota-gut-brain axis in the pathogenesis of depression.
2. Gut microbiota and the microbiota-gut-brain axis
The gut microbial flora constitutes the collective genome of the enormous number of human gut cells and reflects evolutionary selection pressures at both the host and microbial cell levels [12]. Microbiota represents all microorganisms living on/in the human body of which there are up to 1014 microbe cells in the human GI tract [13]. The robust human gut is inhabited by two dominant phyla, Bacteroidetes and Firmicutes, which account for about 70–75% of the microbiota [14]. The phyla Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia, are present at relatively low abundances [15]. These symbiotic microorganisms communicate with each other and with the host, playing an important role in human health. The gut microbial composition could be changed with infant delivery mode and breast-feeding [16, 17]. Moreover, age, geography [18], diet [19], and the use of medication [20] could influence the microbiota composition [21].
In recent years, attention has been paid to the bidirectional signaling pathway between the brain and the intestinal microbiota. This interaction could result in a change of human physiological and pathological behaviors, including the function of both the GI tract and the CNS. Moreover, based on accumulating evidence that gut microbes could communicate with the CNS via interacting channels involving nervous, endocrine, and immune signaling mechanisms, a model called the microbiota-gut-brain axis has been introduced. Dysfunction of the microbiota-gut-brain axis has been demonstrated to play an essential role in the pathogenesis of depression.
3. Alterations in the gut microbiota and depression
Human studies have demonstrated differences between patients with neurological disorders and healthy controls. In healthy individuals, 16S rRNA analysis of fecal microbiota showed that bacteria from the phyla Firmicutes and Bacteroidetes comprised a comparatively large share of the microbiota, while those from the phyla Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia accounted for smaller proportions [22].
Using cross-sectional microbial analysis, Bajaj and colleagues demonstrated a close relationship between specific microbial families and decreased cognition and altered behavior in HE patients [23]. A disrupted gut microbiota was found in HE patients, characterized by relatively lower abundances of Lachnospiraceae and Ruminococcaceae and substantially higher abundances of potentially pathogenic bacteria, including Enterobacteriaceae, Fusobacteriaceae, Alcaligenaceae, Lactobacillaceae, and Leuconostocaceae. This microbial profile is associated with cognitive impairment and systemic inflammation, which can predict negative outcomes [24]. Accordingly, targeted treatment of microbiota that can supplement microbial deficiencies may be promising. Similar findings were reported in patients with autism spectrum disorder (ASD) [25], and these findings again suggest the role of gut microbiota in the regulation of brain function.
Similarly, gut microbial abundance and diversity in patients with depression are reportedly reduced compared with those in controls. The abundances of the Lachnospiraceae and Ruminococcaceae families are decreased in depressive patients. Regarding the diversity of genera, the abundances of Faecalibacterium and Ruminococcus are significantly decreased [26]. In addition, the abundances of Lactobacillus and Bifidobacterium are also decreased in depressed subjects [27]. Although the sample size is small, a higher proportion of Bacteroidales and significantly lower levels of Lachnospiraceae have been reportedly associated with depression [28]. In line with these findings, Jiang et al. reported an underrepresentation of Firmicutes but an overrepresentation of Bacteroidetes, Proteobacteria, and Actinobacteria in fecal samples from depressed patients [27].
Consistently, the diversity of the gut microbiota between animal models of depression and controls has also been demonstrated [29]. For instance, a rat model of chronic variable stress (CVS)-induced depression exhibits decreased relative abundances of the bacterial genera Corynebacterium, Psychrobacter, Lactobacillus, Peptostreptococcaceae incertae sedis, Clostridiales, and Coprococcus [30]. In the bilateral olfactory bulbectomy model (OBx), a common rodent model of depression, the relative abundance of bacterial phyla was reported to be redistributed within the intestinal flora of the OBx group [31] (Table 1). Moreover, changes in the abundance and diversity of gut microbiota have also been demonstrated in other animal models of depression including the maternal separation model [32], social disruption model [33] and chronic restraint stress model [34]. Accordingly, a probable link between gut microbiota and behavior has been established.
Table 1.
Studies investigating the link between the microbiota-gut-brain axis and depression.
| Evidence | Species | Reference |
|---|---|---|
| Changes in gut microbiota abundance and diversity in depression | Human, rat and mouse | [26, 27, 28, 29, 30, 31] |
| Alteration of depression-like behavior by gut microbiota | Rat and mouse | [41, 42, 44, 45, 47, 48] |
| Modulation of 5-HT, DA and GABA signaling systems by intestinal flora in depression | Mouse | [54, 73, 74, 80, 81, 83, 85] |
| Regulation of the activity and function of the HPA axis via the intestinal microbiome in depression | Human and mouse | [34, 45, 50, 84, 93, 94, 95] |
| Amelioration of inflammation and the immune response by gut microbiota in depression | Mouse | [49, 108, 109, 113] |
| Increased expression of BDNF via the regulation of gut microbiota in depression | Human, rat and mouse | [39, 84, 113, 128] |
| FMT from depressed patients could induce depression-like behavior in germ-free rodents | Rat and mouse | [42, 44] |
| Diet-associated microbiota may influence depression-like phenotypes | Mouse | [62, 63] |
| Antibiotics could ameliorate stress-induced depressive-like behavior via gut microbiota | Mouse | [41, 73] |
| Probiotic and prebiotic administration may contribute to the treatment of depression | Rat and mouse | [45, 46, 50, 51, 52, 53, 57, 58, 59] |
Abbreviations: 5-HT – 5-hydroxytryptamine; DA – dopamine; GABA – γ-aminobutyric acid; HPA – hypothalamic-pituitary-adrenal; BDNF – brain-derived neurotrophic factor; FMT – fecal microbiota transplantation.
Moreover, the linkage of gut microbiota and depression seems to be involved with the liver function. Gut bacteria and their metabolites such as LPS and alkaline phosphatase (AP) are common biochemical signals that occurred on the gut-liver-brain axis [35]. They can directly enter the liver through the portal vein, resulting in inflammation in the liver [36]. In addition to the direct induction and promotion of liver damage progression, the bacteria and their products could induce inflammation in the brain through the blood circulation and via a cytokine cascade, which in turn, modulates several brain processes impacting mood and behavior [37]. Furthermore, gut microbiota-triggered inflammation and its implications in shifting the tryptophan metabolism towards kynurenine biosynthesis will disrupt the serotonergic signaling [38], resulting in mental health problems including depressive symptoms [39].
4. The microbiota-gut-brain axis and depression-like behavior
Anhedonia, a core symptom of depression, is represented by the percentage of sucrose/saccharin solution intake in the sucrose/saccharin preference test (SPT). In the SPT, animals were always exposed to water and 1% or 2% sucrose/saccharin solution for 48 h, followed by 24 h of water and food deprivation and a 24-h exposure to two identical bottles, one is water, and another is 1% or 2% sucrose/saccharin solution. The bottles containing water and sucrose/saccharin were weighed before and at the end of this period and the sucrose/saccharin preference was determined. Less amounts of sucrose/saccharin consumption are used to indicate a lower sucrose/saccharin preference (SP) and greater degree of anhedonia [40]. Treatment with antibiotics could reportedly decrease the SP in mice, which may suggest the important role of gut microbiota in depression-like phenotypes because over 90% of gut microbiota could be killed by antibiotics. Moreover, fecal microbiota transplantation from anhedonia-susceptible rats could significantly worsen the depression-like phenotypes in pseudogerm-free mice, as indicated by a decreased sucrose/saccharin preference index (SPI) [41]. Similarly, when fecal microbiota from depressed patients transplanted into the microbiota-depleted rats, depression-like behaviors can be observed in the recipient animals [42].
Despair behavior is another symptom of depression and could be tested in rodents using the forced-swim test (FST) and the tail suspension test (TST). For example, in the FST tested by an automated forced-swim apparatus YH-FST (Yihong Co., Ltd., Wuhan, China), the mice were placed individually in a cylinder (diameter: 25 cm; height: 35 cm) containing 20 cm of water, maintained at 23 ± 1 °C. The immobility time for mouse was recorded for 5 min. In the TST, a small piece of adhesive tape placed approximately 2 cm from the tip of the tail for mouse. A single hole was punched in the tape and mice were hung individually on a hook. The immobility time was recorded by camera for 10 min [41]. The length of immobility time in the FST and TST is considered an index of despair behavior [43]. The role of gut microbiota in regulating despair behavior was demonstrated, as the immobility time in the FST and TST of germ-free (GF) mice was shorter than that of specific pathogen free (SPF) mice and healthy controls [44, 45]. However, when GF mice harbor microbiota from patients with major depressive disorder (MDD), they show an increased immobility time in the FST and TST and a reduced distance traveled in the center of the open field test (OFT) [44].
Stress, along with the consequential hyperactivity of the HPA axis, is one of the greatest risk factors for depression. Rodent models of chronic unpredictable mild stress (CUMS) [46], which exhibit depression-like behaviors including anhedonia, despair behavior, and decreased exploration and locomotion, as demonstrated by decreased SPI, exhibit decreased locomotion and rearing in the OFT and increased immobility in both the FST and the TST [47]. In a CUMS mouse model, Bifidobacterium longum subsp. infantis E41 and Bifidobacterium breve M2CF22M7 have an antidepressant effect in mice, and the mechanism of action was partly related to microbial regulation. The result showed a decreased immobility time in the FST and increased sucrose preference in the SPT [48]. Increased immobility has also been found in GF rats exposed to maternal separation stress, which is another animal model of depression. Furthermore, the administration of Bifidobacterium infantis has been shown to normalize the immune response, reverse behavioral deficits, and restore basal noradrenaline (NA) concentrations. These findings imply that the microbiota plays an important role in modulating depression-like behavior [49].
Based on these findings, various deliberate interventions, including the use of probiotics [50], and prebiotics [51], fecal microbiota transplantation (FMT) [42], and diet change [52], have been used to investigate the impact of the microbiota on depression. Probiotic treatment appears to have therapeutic value [53]. Mice fed L. rhamnosus (JB-1) exhibited less immobility than those fed broth in the FST [54], and combination treatment with L. helveticus R0052 and B. longum R0175 could also reduce the duration of immobility in rats in the FST [55]. Moreover, probiotics treatment consisting of eight bacterial strains, including Bifidobacterium bifidum, markedly reduces the immobility time in the FST by 34%, and the underlying mechanism may be associated with the HPA axis, the immune system and microbial tryptophan metabolism [56]. Similarly, prebiotics, such as galactooligosaccharide (GOS), polydextrose (PDX) and the glycoprotein lactoferrin (LAC), also appear to regulate gut microbiota and improve behavior through the microbiota-gut-brain axis [57]. A prolonged immobility time in the FST has been reported in rats fed a low-n-3 PUFA diet without probiotics, and this could be reversed by a high-n-3 PUFA diet and/or probiotics treatment [58]. The mechanism may involve the beneficial effects of n-3 PUFA on the gut microbiota composition, as it may elevate the abundances of fecal Bifidobacterium and Lactobacillus [59]. These results suggest that a high-PUFA n-3 diet or the administration of probiotics may alleviate depression-like behavior [60].
FMT is another strategy for modulating gut microbiota. Microbiota-depleted rats have demonstrated anhedonia-like behaviors after receiving FMT from depressed subjects [42]. Similarly, compared with control mice with healthy microbiota, GF mice showed increased immobility time in both the FST and TST and a decreased distance traveled in the center of the OFT after FMT from depressed patients [44].
Diet has also been suggested to impact both the gut microbiota and depression-like behavior [61]. A high-fat diet could reportedly induce depression-like phenotypes, including decreased sociability and sucrose preference in mice. These changes are correlated with changes in the intestinal microbiota, as indicated by a decreased abundance of Bacteroidetes and increased abundances of Firmicutes and Cyanobacteria in the caecum [62]. Additionally, a magnesium (Mg)-deficient diet has also been demonstrated to alter the gut microbiota in mice and increased their immobility time in the FST [63]. These findings support the link between diet-associated microbiota and depression-like behavior [64, 65] and suggest that altering gut microbiota composition may have a beneficial impact on depression via the microbiota-gut-brain axis [66].
5. Potential mechanisms underlying the involvement of microbiota-gut-brain axis in depression
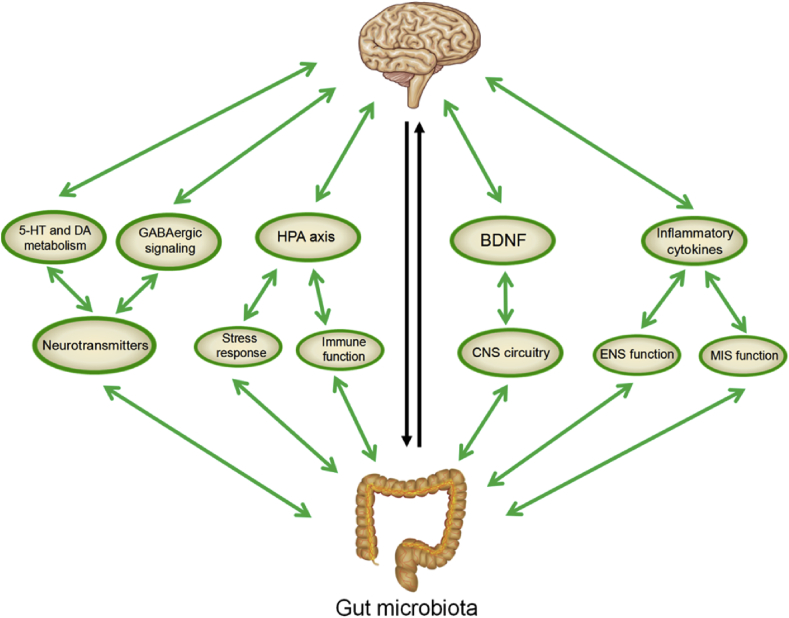
Although the mechanism underlying the effect of the microbiota-gut-brain axis on depression remains unclear, increasing evidence indicates the important role of neural, endocrine, immune, and metabolic pathways in the communication between gut microbes and the brain [67] (Figure 1).
Figure 1.
Potential mechanisms underlying the involvement of the microbiota-gut-brain axis in depression.
As reviewed in the article, the bidirectional communication between gut microbes and the central nervous system via interacting channels involving nervous, endocrine, and immune signaling mechanisms can influence mood and depressive disorders. The liver coordinates and participates in important immunological functions due to its anatomical location and vascular supply. In the chemical signaling pathway, the expression of monoamine neurotransmitters, particularly 5-HT, DA and GABA, is modulated by intestinal flora to regulate the development a plasticity of neural circuits involved in mood and behavior. The hyperactivity of the HPA axis as indicated by the increased secretion of cortisol, adrenocorticotropin (ACTH) and corticotropin-releasing hormone (CRH), can be prevented or normalized by altering the function of the microbiota-gut-brain axis via gut microbiota. In the immune pathway, the mucosal system is the first line of immune defense. The basic functions of the mucosal immune system (MIS) include protection from various pathogens, preventing penetration of various foreign antigens, inducing oral tolerance to various antigens and maintaining mucosal homeostasis. Gut-derived bacteria and their products directly enter the liver through the portal vein, and the liver also plays a vital role in the need for an effective innate immune response. Alterations in gut microbiota are helpful for improving symptoms in depressed patients by modulating anti- and pro-inflammatory cytokines, such as IL-6, IL-1β, and TNF-α. Changes in gut microbiota have the potential to increase the levels of BDNF expression and thus influence the development of depressive-like behavior. Each of these mechanisms is implicated in the pathophysiology of depression.
5.1. Modulating the release and efficacy of monoamine neurotransmitters
The exhaustion of monoamine neurotransmitters is considered the greatest risk factor for depression, and most the current antidepressants aim to increase the levels of monoamine neurotransmitters in the synapse. Serotonin (5-hydroxytryptamine, 5-HT), dopamine (DA) and γ-aminobutyric acid (GABA) are the three main monoamine neurotransmitters. They play pivotal roles in maintaining homeostasis in the entire human body and regulating the development and plasticity of neural circuits involved in mood disorders such as depression [68].
5-HT, a paracrine messenger utilized by enterochromaffin (EC) cells and a system of long descending myenteric interneurons, is causally implicated in multiple central nervous facets of mood control [69]. In the pathogenesis of mood disorders, the serotonergic neural system is considered a main biological substrate [70]. More than 90% of the body's 5-HT is synthesized in the gut [69], wherein intestinal 5-HT plays an essential role in activating the ENS and GI function [71]. Compared with SPF controls, GF mice present not only significantly increased plasma concentrations of the 5-HT precursor tryptophan, but also increased mRNA expression levels of the 5-hydroxytrytamine 1A (5HT1A) receptor and the 5HT2A receptor in the hippocampus [72]. Microbiota depletion induced by antibiotics was demonstrated to increase circulating tryptophan availability, resulting in elevated hippocampal 5-HT concentrations [73]. Moreover, bifidobacteria treatment may have antidepressant properties by reducing 5-hyroxyindole acetic acid (5-HIAA) concentration in the frontal cortex and dihydroxyphenylacetic acid (DOPAC) in the amygdaloid cortex in Sprague-Dawley rats [74]. Furthermore, the processing of substances controlling emotion may subtly alter the control of 5-HT function [75]; For example, in the intestinal microbial community, tryptophan decarboxylases play a role in increasing the ability of microbes to extract tryptophan from the diet by converting it into tryptophan amine, thus regulating mood and behavior by reducing the production of 5-HT in the brain [76]. However, the detailed molecular mechanisms underlying the manipulation of 5-HT metabolism in the gut are not yet fully understood [69].
DA and the dopaminergic system are very important for regulating anhedonia, a core feature of MDD [77]. Significantly lower DA transporter (DAT) binding [78] and reduced striatal dopaminergic activity [79] have been described in depressed patients compared with those in healthy individuals, and these changes are associated with anhedonia based on PET imaging results. Interestingly, DA also impacts intestinal motility and intestinal secretion [80], and the DA system is reportedly involved in the function of the microbiota-gut-brain axis. E. faecium, a recognized natural member of the GI flora, was demonstrated to modulate the immune system and influence the host via dopaminergic pathways [81], and mice treated with Bifidobacterium long-term were shown to exhibit increased DA and 5-HT levels and improved depression-like behaviors [53].
GABA is known as the main inhibitory neurotransmitter in the brain and is involved in multiple physiological and psychological processes; dysfunction of the GABA system is associated with a variety of neuropsychiatric disorders, including depression [82]. The Lactobacillus brevis and Bifidobacterium dentium strains, which are particularly efficient GABA producers, are able to metabolize optimal monosodium glutamate (MSG) to produce GABA, again indicating that microbiota modulation could be considered a promising therapeutic approach for depression [83]. The numbers of Bifidobacterium and Lactobacillus species in the intestinal tracts of depressed patients are reportedly significantly reduced [54], and probiotics may be implicated in the reduced mRNA expression of GABA receptors associated with depressive disorders [84]. Of note, L. rhamnosus (JB-1) is known to reduce stress-induced corticosterone (CORT) levels in mice and ameliorate depression-like behavior in the FST [54]. Moreover, alterations in GABAAα2 and GABAB1b receptor mRNA expression have also been found in the thalamic nucleus, amygdale, and hippocampal regions of mice treated with L. rhamnosus [85]. N-acetyl aspartate + N-acetyl aspartyl glutamic acid and glutamate, two clinically validated biomarkers of brain activity and function, are also increased [86]. Furthermore, Hughes et al. revealed that the exposure of enterohaemorrhagic E. coli (EHEC) to adrenaline and NA increases its toxicity [87]. These findings again indicate that monoamine neurotransmitters may be involved in the functionality of the microbiota-gut-brain axis, bridging the brain and the intestinal microecology.
5.2. Altering the activity and function of the HPA axis
The HPA axis is an important part of the neuroendocrine system that could be activated by both psychological and physiological stress [88]. The hyperactivity of the HPA axis, as indicated by the increased secretion of cortisol, adrenocorticotropin (ACTH) and corticotropin-releasing hormone (CRH), has been demonstrated to play a core role in triggering depressive episodes [89, 90, 91].
Extensive studies have elucidated a direct link between microbiota and the HPA axis. Follow-up studies have demonstrated the essential role of microbiota in the organization of the HPA axis during the early phases of life and the stress response throughout life [92, 93]. GF mice also reportedly exhibit elevated levels of CORT and ACTH compared with those of SPF controls in response to mild restraint stress. However, an exaggerated HPA stress response could be counteracted and restored to normal levels in a time-dependent manner in GF mice by the administration of Bifidobacterium infantis [45].
Probiotic treatment has also been reported to regulate the activity of the HPA axis. In 2007, Gareau and colleagues found that CORT levels were elevated in mice exposed to maternal separation stress but were normalized following concurrent treatment with probiotics (Lactobacillus sp.) during the early stress stage [93]. Consistently, mice treated with a combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 present not only improvements in depression-like behavior, but also significantly decreased plasma levels of CORT, adrenaline and noradrenaline [84]. Treatment with the probiotics L. farciminis or Lactobacillus helveticus NS8 could weaken the activity of the HPA axis in rodents, as indicated by decreased plasma concentrations of ACTH and CORT and decreased hypothalamic corticotropin-releasing factor (CRF) expression [34, 94]. The administration of Bifidobacterium pseudocatenulatum CECT 7765 was shown to partially ameliorate the exaggerated HPA axis response caused by maternal separation in juvenile mice [95].
Messaoudi et al. investigated the effect of Bifidobacterium longum R0175 and Lactobacillus helveticus R0052 in combination on neuropsychiatric behavior in healthy human volunteers. Consistent with the results of animal studies, lower urine-free cortisol levels and improved mood were found in the subjects, indicating attenuation of the HPA axis in response to stress [50]. These findings suggest that probiotics positively affect depression-like behavior via altering the activity of the HPA axis. Indeed, depression is often accompanied by overactivation of the HPA axis, and increased concentrations of CRH in the cerebrospinal fluid could allow access to intestinal epithelial cells [96], leading to increased gut permeability. As a result, the HPA axis provides a pathway for microbiota to cross the epithelial barrier and act on immune cells and neurocytes directly through the intestinal mucosa, linking gut microbiota to brain function [97]. Obviously, the hyperactivity of the HPA axis and consequential depressive disorders are likely preventable by altering the function of the microbiota-gut-brain axis via gut microbiota.
5.3. Activating inflammation and the immune response
Inflammation and accompanying alterations in immune function have long been considered important pathogenic factors of depression [98]. The discovery of the role of gut microbiota in the immune system and its bidirectional communication with the CNS has aroused increasing interest in the reciprocal interplay among inflammation, microbiota and depression. Recent studies have suggested that the pathogenesis of many immune-mediated diseases, as well as the responses to immune-based treatments such as anti PD-1 and anti-CTLA4 therapy, are closely related to gut microbiota [99]. Importantly, higher levels of inflammation have been reported to increase the risk of developing de novo depression [100], and higher levels of inflammatory cytokines including +interleukin-6 (IL-6), IL-1β, and TNF-α have been observed in depressed subjects [101, 102]. Indeed, cytokines could cause depressive-like behaviors by disrupting neurotransmitter synthesis and signal transduction [103]. Moreover, the levels of IL-6, IL-12p70, and IL-17A are significantly associated with sucrose consumption and memory [104].
Extensive evidence supports the role of gut microbiota in altering immune activity related to the severity of depression symptoms [105]. For instance, differences in the numbers of lymphocytes and CD4+ cells among ovarian cancer patients with mild depression, moderate depression and severe depression are statistically significant. The proportions of CD3+, CD4+ and natural killer (NK) cells as well as immune activity are decreased in patients with severe depression. Thus, a strong correlation has been suggested between various depressive states and immune activity [106]. Dantzer et al. observed a positive association between the caecal microbiota composition and the serum levels of IL-1α and IFN-γ, which are known to be positively correlated with depression-like behavior and trigger “sickness behavior” [107]. Treatment with B. infantis and L. reuteri could also decrease TNF-α, IL-6, and MCP-1 levels in mice [108]. Alterations in gut microbiota are helpful for improving symptoms in depressed patients by modulating the expression of anti- and proinflammatory cytokines [109]. Minocycline, a broad-spectrum antibiotic, could ameliorate stress-induced depressive-like behavior in mice in addition to exerting its basic effect on the gut microbiota by increasing the relative abundances of Akkermansia spp. and Blautia spp [110]. Rats with maternal separation-induced depression display not only depression-like behavior but also increased proinflammatory cytokine levels. However, these alterations are restored to normal levels following treatment with the probiotic Bifidobacterium infantis [49]. These results further establish the intimate relationship between depressive disorders and intestinal flora via immune signaling.
Parallel studies in human subjects have shown that Faecalibacterium, which has anti-inflammatory activity in the gut, is relatively less abundant in depressed subjects [27], and a significant correlation between the enrichment of Alistipes and gut inflammation has also been found in depressed patients [28]. Although the mechanism is not clear, these factors may be partially ascribed to depression-related increases in blood-brain barrier (BBB) and intestinal barrier permeability. This allows microflora metabolites, luminal antigens, and toxins to more easily penetrate into the systemic circulation and the CNS [111] and thus triggering an exaggerated immune inflammatory response [112]. Importantly, the gut microbiota in healthy humans and animals could produce lipopolysaccharide (LPS), which activates the biosynthesis of TNF-α via the NF-κB signaling pathway [113]. Under normal circumstances, immune cells are separated from intestinal gram-negative bacteria in the gut [114]. The work of Cani has shown that under stressful condition, intestinal permeability changes and barrier integrity is disrupted. This can result in translocation of certain gram-negative bacteria such as Enterobacteriaceae from the gut, ultimately to the systemic circulation. The endotoxins produced by bacteria known as LPS would lead to inflammation [115, 116, 117, 118]. However, feeding mice with prebiotics changes gut microbiota towards an increased number of intestinal bifidobacteria, a group of bacteria that has been shown to reduce intestinal LPS levels and to improve the mucosal barrier function in mice [119, 120, 121]. Apart from a lower plasma LPS and cytokines, prebiotic-treated mice exhibited a decreased hepatic expression of inflammatory and oxidative stress markers [122]. Moreover, inflammation also links depression to other peripheral diseases. For example, the comorbidity of depression and chronic fibromyalgia is well documented, and the mechanism reportedly involves proinflammatory cytokines, chemokines, and chemical mediators [123].
Based on these findings, it seems plausible that modulating inflammation and the immune response is an important mechanism that orchestrates microbiota-gut-brain interactions [124].
5.4. Regulating the abundance of brain-derived neurotrophic factor
Brain-derived neurotrophic factor (BDNF), a neurotrophin family member, has been implicated in multiple processes, including cell differentiation, neuronal survival, synapses formation, and neuroplasticity development [125]. Insufficient BDNF is a risk factor for impaired neuroplasticity and the development of depressive symptoms [126]. Numerous studies have reported that depressed patients possess relatively lower serum BDNF levels than healthy controls [127]. Antidepressant therapies such as ketamine can increase BDNF activity via mTOR signaling and improve mood and cognition in correlation with depression [128].
Similarly, probiotic treatment has shown promise as a preventative or therapeutic approach for counteracting behavioral deficits by inducing changes in the gut microbiota and BDNF expression [14, 129]. Bercik et al. demonstrated that SPF mice treated with oral antimicrobials show transiently perturbed gut microbiota, leading to increased hippocampal BDNF concentrations and the subsequent prevention of depression [113]. Moreover, pretreatment with probiotics (a combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) for two weeks was shown to increase the hippocampal expression of BDNF in stressed mice, thus reversing the synaptic dysfunction and ultimately improving performance in learning and memory tasks [84]. Additionally, supplementation with Lactobacillus helveticus NS8 could improve both the behavior performance and hippocampal BDNF mRNA expression in a rat model of chronic stress-induced depression [34]. Thus, the gut microbiota has the potential to regulate the abundance of BDNF and thus influence the development of depressive-like behavior.
6. Conclusions and prospective
Depression is a leading cause of disability, morbidity and mortality worldwide, and gut microbiota have been demonstrated to be involved in its pathogenesis. Although the mechanism underlying this association remains unclear, it may be related to the modulation of monoamine neurotransmitters release and efficacy, alterations in the activity and function of the HPA axis, activation of the inflammatory and immune responses, and regulation of BDNF expression. Based on these findings, targeting the microbiota-gut-brain axis to treat depression has increasingly been attempted, and some strategies have shown promising beneficial effects. Moreover, many advanced tools based on the role of the microbiota-gut-brain axis, including consumer apps, wearable sensors, and electronic health records, have been developed.
In summary, targeting the microbiota-gut-brain axis may provide new insight for antidepressant research and development. To better understand the underlying mechanism, additional animal and clinical studies and the development of analytical approaches are crucially needed.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the National Natural Science Foundation of China (81401122, 81870403) and the Foundation of Innovation and Entrepreneurship Education and Training for Colleges by Anhui Province (201810366116).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank the editor and reviewers very much for their constructive guidance that significantly increased the scientific value of the article.
References
- 1.Ledford H. Medical research: if depression were cancer. Nature. 2014;515(7526):182–184. doi: 10.1038/515182a. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich M.J. Depression is the leading cause of disability around the world. J. Am. Med. Assoc. 2017;317(15):1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 3.Zalar B., Haslberger A., Peterlin B. The role of microbiota in depression - a brief review. Psychiatr. Danub. 2018;30(2):136–141. doi: 10.24869/psyd.2018.136. [DOI] [PubMed] [Google Scholar]
- 4.Peng W., Jia Z., Gong Q. Current progress in neuroimaging research on treatment resistant depression. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2018;35(5):794–798. doi: 10.7507/1001-5515.201705086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhir A. Investigational drugs for treating major depressive disorder. Expet Opin. Invest. Drugs. 2017;26(1):9–24. doi: 10.1080/13543784.2017.1267727. [DOI] [PubMed] [Google Scholar]
- 6.Shafiee M. Saffron in the treatment of depression, anxiety and other mental disorders: current evidence and potential mechanisms of action. J. Affect. Disord. 2018;227:330–337. doi: 10.1016/j.jad.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Petra A.I. Gut-microbiota-brain Axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin. Therapeut. 2015;37(5):984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattarai Y. Microbiota-gut-brain axis: interaction of gut microbes and their metabolites with host epithelial barriers. Neuro Gastroenterol. Motil. 2018;30(6):e13366. doi: 10.1111/nmo.13366. [DOI] [PubMed] [Google Scholar]
- 9.Farzi A. Diabesity and mood disorders: multiple links through the microbiota-gut-brain axis. Mol. Aspect. Med. 2018 doi: 10.1016/j.mam.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Evrensel A., Ceylan M.E. The gut-brain Axis: the missing link in depression. Clin. Psychopharmacol. Neurosci. 2015;13(3):239–244. doi: 10.9758/cpn.2015.13.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kundu P. Our gut microbiome: the evolving inner self. Cell. 2017;171(7):1481–1493. doi: 10.1016/j.cell.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Ley R.E., Peterson D.A., Gordon J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Sekirov I. Gut microbiota in health and disease. Physiol. Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 14.Foster J.A., Neufeld K.A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson F.H. A closer look at bacteroides: phylogenetic relationship and genomic implications of a life in the human gut. Microb. Ecol. 2011;61(3):473–485. doi: 10.1007/s00248-010-9796-1. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez-Bello M.G. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U. S. A. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fallani M. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010;51(1):77–84. doi: 10.1097/MPG.0b013e3181d1b11e. [DOI] [PubMed] [Google Scholar]
- 18.Yatsunenko T. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claesson M.J. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 20.Davey K.J. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl. Psychiatry. 2013;3:e309. doi: 10.1038/tp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dash S. The gut microbiome and diet in psychiatry: focus on depression. Curr. Opin. Psychiatr. 2015;28(1):1–6. doi: 10.1097/YCO.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 22.Eckburg P.B. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajaj J.S. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302(1):G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajaj J.S. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66(6):1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams J.B. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly J.R. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017;61:50–59. doi: 10.1016/j.bbi.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Naseribafrouei A. Correlation between the human fecal microbiota and depression. Neuro Gastroenterol. Motil. 2014;26(8):1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 29.Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 30.Yu M. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharmaceut. Biomed. Anal. 2017;138:231–239. doi: 10.1016/j.jpba.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Park A.J. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neuro Gastroenterol. Motil. 2013;25(9) doi: 10.1111/nmo.12153. p. 733-e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Mahony S.M. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatr. 2009;65(3):263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 33.Bharwani A. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017;15(1):7. doi: 10.1186/s12916-016-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang S. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Lu J. Herbal formula Fo shou san attenuates alzheimer's disease-related pathologies via the gut-liver-brain Axis in APP/PS1 mouse model of alzheimer's disease. Evid. Based Complement. Alternat. Med. 2019;2019:8302950. doi: 10.1155/2019/8302950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel V.C. Clinical science workshop: targeting the gut-liver-brain axis. Metab. Brain Dis. 2016;31(6):1327–1337. doi: 10.1007/s11011-015-9743-4. [DOI] [PubMed] [Google Scholar]
- 37.Federico A. Gut microbiota and the liver. Minerva Gastroenterol. Dietol. 2017;63(4):385–398. doi: 10.23736/S1121-421X.17.02375-3. [DOI] [PubMed] [Google Scholar]
- 38.Waclawikova B., El Aidy S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals (Basel) 2018;11(3) doi: 10.3390/ph11030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cervenka I., Agudelo L.Z., Ruas J.L. Kynurenines: tryptophan's metabolites in exercise, inflammation, and mental health. Science. 2017;357(6349) doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 40.Rizvi S.J. Assessing anhedonia in depression: potentials and pitfalls. Neurosci. Biobehav. Rev. 2016;65:21–35. doi: 10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang C. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry. 2019;9(1):57. doi: 10.1038/s41398-019-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly J.R. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Bangsgaard Bendtsen K.M. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PloS One. 2012;7(10) doi: 10.1371/journal.pone.0046231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol. Psychiatr. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 45.Sudo N. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su W.J. NLRP3 gene knockout blocks NF-kappaB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav. Brain Res. 2017;322(Pt A):1–8. doi: 10.1016/j.bbr.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 47.Han Y.X. BDNF-related imbalance of copine 6 and synaptic plasticity markers couples with depression-like behavior and immune activation in CUMS rats. Front. Neurosci. 2018;12:731. doi: 10.3389/fnins.2018.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian P. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019;66:43–51. doi: 10.1016/j.jnutbio.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Desbonnet L. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Messaoudi M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105(5):755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 51.Gibson G.R. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 52.Carmody R.N. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17(1):72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kennedy P.J. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112:399–412. doi: 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Bravo J.A. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arseneault-Breard J. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012;107(12):1793–1799. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- 56.Abildgaard A. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology. 2017;79:40–48. doi: 10.1016/j.psyneuen.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Mika A. Early life diets with prebiotics and bioactive milk fractions attenuate the impact of stress on learned helplessness behaviours and alter gene expression within neural circuits important for stress resistance. Eur. J. Neurosci. 2017;45(3):342–357. doi: 10.1111/ejn.13444. [DOI] [PubMed] [Google Scholar]
- 58.Gilbert K. Attenuation of post-myocardial infarction depression in rats by n-3 fatty acids or probiotics starting after the onset of reperfusion. Br. J. Nutr. 2013;109(1):50–56. doi: 10.1017/S0007114512003807. [DOI] [PubMed] [Google Scholar]
- 59.Robertson R.C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017;59:21–37. doi: 10.1016/j.bbi.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 60.Liu X., Cao S., Zhang X. Modulation of gut microbiota-brain Axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 2015;63(36):7885–7895. doi: 10.1021/acs.jafc.5b02404. [DOI] [PubMed] [Google Scholar]
- 61.Sharma S., Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. 2013;37(3):382–389. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- 62.Hassan A.M. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr. Neurosci. 2018:1–17. doi: 10.1080/1028415X.2018.1465713. [DOI] [PubMed] [Google Scholar]
- 63.Winther G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015;27(3):168–176. doi: 10.1017/neu.2015.7. [DOI] [PubMed] [Google Scholar]
- 64.Sandhu K.V. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl. Res. 2017;179:223–244. doi: 10.1016/j.trsl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Del Rosario A., McDermott M.M., Panee J. Effects of a high-fat diet and bamboo extract supplement on anxiety- and depression-like neurobehaviours in mice. Br. J. Nutr. 2012;108(7):1143–1149. doi: 10.1017/S0007114511006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schachter J. Effects of obesity on depression: a role for inflammation and the gut microbiota. Brain Behav. Immun. 2018;69:1–8. doi: 10.1016/j.bbi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 67.Grenham S. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mittal R. Neurotransmitters: the critical modulators regulating gut-brain Axis. J. Cell. Physiol. 2017;232(9):2359–2372. doi: 10.1002/jcp.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yano J.M. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lucki I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatr. 1998;44(3):151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 71.Gershon M.D., Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Clarke G. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatr. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 73.Desbonnet L. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav. Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Desbonnet L. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008;43(2):164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 75.Harmer C.J. Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacology (Berlin) 2003;167(4):411–417. doi: 10.1007/s00213-003-1401-6. [DOI] [PubMed] [Google Scholar]
- 76.Williams B.B. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16(4):495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belujon P., Grace A.A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 2017;20(12):1036–1046. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyer J.H. Lower dopamine transporter binding potential in striatum during depression. Neuroreport. 2001;12(18):4121–4125. doi: 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- 79.Pruessner J.C. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J. Neurosci. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walker J.K. Mice lacking the dopamine transporter display altered regulation of distal colonic motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279(2):G311–G318. doi: 10.1152/ajpgi.2000.279.2.G311. [DOI] [PubMed] [Google Scholar]
- 81.Villageliu D., Lyte M. Dopamine production in Enterococcus faecium: a microbial endocrinology-based mechanism for the selection of probiotics based on neurochemical-producing potential. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0207038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cryan J.F., Kaupmann K. Don't worry 'B' happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol. Sci. 2005;26(1):36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Barrett E. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012;113(2):411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 84.Ait-Belgnaoui A. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neuro Gastroenterol. Motil. 2014;26(4):510–520. doi: 10.1111/nmo.12295. [DOI] [PubMed] [Google Scholar]
- 85.Dinan T.G., Cryan J.F. Melancholic microbes: a link between gut microbiota and depression? Neuro Gastroenterol. Motil. 2013;25(9):713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 86.Janik R. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage. 2016;125:988–995. doi: 10.1016/j.neuroimage.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 87.Hughes D.T., Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat. Rev. Microbiol. 2008;6(2):111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J. Psychiatry Neurosci. 2004;29(3):185–193. [PMC free article] [PubMed] [Google Scholar]
- 89.Swaab D.F., Bao A.M., Lucassen P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005;4(2):141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Bao A.M. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128(Pt 6):1301–1313. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- 91.Wang S.S. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol. Psychiatr. 2008;13(8):786–799. doi: 10.1038/mp.2008.38. 741. [DOI] [PubMed] [Google Scholar]
- 92.Bailey M.T., Coe C.L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 1999;35(2):146–155. [PubMed] [Google Scholar]
- 93.Gareau M.G. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56(11):1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ait-Belgnaoui A. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37(11):1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 95.Moya-Perez A. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav. Immun. 2017;65:43–56. doi: 10.1016/j.bbi.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 96.Nemeroff C.B. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 97.Teitelbaum A.A. Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295(3):G452–G459. doi: 10.1152/ajpgi.90210.2008. [DOI] [PubMed] [Google Scholar]
- 98.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 99.Franza L. Gut microbiota and immunity in common variable immunodeficiency: crosstalk with pro-inflammatory cytokines. J. Biol. Regul. Homeost. Agents. 2019;33(2):315–319. [PubMed] [Google Scholar]
- 100.Pasco J.A. Association of high-sensitivity C-reactive protein with de novo major depression. Br. J. Psychiatry. 2010;197(5):372–377. doi: 10.1192/bjp.bp.109.076430. [DOI] [PubMed] [Google Scholar]
- 101.Dowlati Y. A meta-analysis of cytokines in major depression. Biol. Psychiatr. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 102.Connor T.J., Leonard B.E. Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sci. 1998;62(7):583–606. doi: 10.1016/s0024-3205(97)00990-9. [DOI] [PubMed] [Google Scholar]
- 103.Reichenberg A. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatr. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 104.Pyndt Jorgensen B. A possible link between food and mood: dietary impact on gut microbiota and behavior in BALB/c mice. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0103398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wium-Andersen I.K. Anti-inflammatory treatment and risk for depression after first-time stroke in a cohort of 147 487 Danish patients. J. Psychiatry Neurosci. 2017;42(5):320–330. doi: 10.1503/jpn160244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Su J.P. Effects of different degrees of depression on inflammatory response and immune function in patients with ovarian cancer. J. Biol. Regul. Homeost. Agents. 2018;32(5):1225–1230. [PubMed] [Google Scholar]
- 107.Dantzer R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 2009;29(2):247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van der Kleij H. Protective effects of Lactobacillus rhamnosus [corrected] and Bifidobacterium infantis in murine models for colitis do not involve the vagus nerve. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295(4):R1131–R1137. doi: 10.1152/ajpregu.90434.2008. [DOI] [PubMed] [Google Scholar]
- 109.Rea K., Dinan T.G., Cryan J.F. The microbiome: a key regulator of stress and neuroinflammation. Neurobiol. Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wong M.L. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatr. 2016;21(6):797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santos J. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48(5):630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Theoharides T.C. Mast cells and inflammation. Biochim. Biophys. Acta. 2012;1822(1):21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bercik P. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. doi: 10.1053/j.gastro.2011.04.052. 609 e1-3. [DOI] [PubMed] [Google Scholar]
- 114.Wiest R., Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41(3):422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 115.Cani P.D. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 116.Cani P.D. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 117.Cai D. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005;11(2):183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cani P.D. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 119.Griffiths E.A. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig. Dis. Sci. 2004;49(4):579–589. doi: 10.1023/b:ddas.0000026302.92898.ae. [DOI] [PubMed] [Google Scholar]
- 120.Wang Z.T. Risk factors of development of gut-derived bacterial translocation in thermally injured rats. World J. Gastroenterol. 2004;10(11):1619–1624. doi: 10.3748/wjg.v10.i11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ruan X. Encapsulated Bifidobacteria reduced bacterial translocation in rats following hemorrhagic shock and resuscitation. Nutrition. 2007;23(10):754–761. doi: 10.1016/j.nut.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 122.Cani P.D. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mastrangelo F. Low-grade chronic inflammation mediated by mast cells in fibromyalgia: role of IL-37. J. Biol. Regul. Homeost. Agents. 2018;32(2):195–198. [PubMed] [Google Scholar]
- 124.Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017;20(2):145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Greenberg M.E. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 2009;29(41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gerhard D.M., Wohleb E.S., Duman R.S. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov. Today. 2016;21(3):454–464. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Karege F. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatr. Res. 2002;109(2):143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 128.Duman R.S. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luna R.A., Foster J.A. Gut brain axis: diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015;32:35–41. doi: 10.1016/j.copbio.2014.10.007. [DOI] [PubMed] [Google Scholar]