Abstract
(3-aminopropyl)triethoxysilane (APTES) is a commonly used organosilane on surface functionalization of silicon oxide surfaces. However, its deposition process from solution-phase usually involves the use of toluene, which has often been identified as crucial for the formation of an aminopropylsilane monolayer. Toluene is ranked as a problematic solvent in the guide developed by a group referred to as the solvent sub-team of CHEM21. In this work, we propose a facile synthetic route for functionalizing a silicon substrate with APTES via solution-phase approach using only solvents that are classified as recommended. The influence of the APTES concentration, reaction times and different post-deposition conditions using acetic acid and methanol were studied in order to evaluate the quality and thickness of the organosilane layers.
-
•
The method uses ethanol as APTES solvent for functionalizing silicon dioxide surfaces and only uses solvents classified as recommended.
-
•
The method uses a solution phase approach, does not require complicated equipment and can be prepared at room temperature.
Keywords: Silicon dioxide functionalization, Aminosilane, Surface functionalization
Graphical abstract

Specifications Table
| Subject Area | Materials Science |
| More specific subject area | Surface Science |
| Method name | Aminosilane functionalization of silicon dioxide substrates using ethanol |
| Name and reference of original method | The method was derived from multiple methods and cannot be associated to a singular reference. |
| Resource availability | All reagents and instruments indicated are commercially available. The sources of specific components were indicated in the manuscript. |
1. Method details
1.1. Background
Organosilanes have been widely used for coatings [1], biological applications for biosensors and diagnostics [2], [3], [4], [5], [6], the recovery of carbon rich water-soluble humic acid from waste water [7], biosensing textiles [8], the preparation and functionalization of nanoparticles [9], [10], [11], [12], [13], [14], and improvement of material properties [15,16], catalysis [17,18] as well as for the fingerprinting of explosives in nanosensor arrays [19]. They have been used in the functionalization of different surfaces [20] like silicon [21], [22], [23], [24], [25], [26], titanium oxide [27], [28], [29], [30], [31] and glass [32], [33], [34]. Protocols for the functionalization of silicon substrates with an organosilane monolayer usually follow a four step process: a) a pre-cleaning of the substrates for removing organic or metal contaminants that might be on the surface; b) an activation process in order to generate hydroxyl groups on the surface of the silicon substrate making it hydrophilic; c) deposition and d) a post-deposition process to remove excessive organosilane molecules not covalently attached to the surface. The latter step usually includes a curing process with temperature to strengthen the lateral bonds.
(3-aminopropyl)triethoxysilane is one of the most applied organosilanes. Due to its amino group termination it can be used as an intermediate layer for further functionalization, for example for building nobel metal nanoparticles assemblies platforms. [35] The deposition of APTES on substrates has been performed either by solution-phase or vapor-phase methods. Yadav et al. [36] compared both methods on three different aminosilanes on SiO2 surfaces concluding that the solution-phase approaches yielded high quality silane layers with characteristics comparable to ones deposited by vapor-phase. In literature, the formation of an APTES monolayer on a silicon substrate via a solution-phase approach has been extensively studied using toluene as the organosilane solvent. [23,[37], [38], [39], [40]] According to the guide developed by the solvent sub-group of CHEM21 toluene was ranked as problematic, meaning that the solvents classified in this category can be used in the laboratory but their implementation in the pilot plant or at the production scale requires specific measures, or significant energy consumption. [41] Therefore, a detailed study of an alternative and easy to implement protocol for the preparation of APTES aminopropylsilane layers on silicon substrates via a solution-phase approach is desirable. Our aim is to present a reproducible synthetic route where only solvents classified as recommended – the ones to be preferred to be tested first in a screening exercise – [41] are used and experiments conducted at room temperature. We used ethanol as an APTES solvent and methanol in the post-deposition process. In the literature it has been described the formation of multilayers of APTES in ethanol on oxide surfaces [42] and diamond substrates [43]. Vandenberg et al. [44] describes the silanization using different solvents and the water stability of the layers on silicon substrates. Depending on the solvents used they report the formation of mono and multilayers. However, it is lacking a detailed study using APTES in ethanol addressing the effects of the aminopropylsilane concentration, the duration of deposition and different post-deposition treatments at room temperature. X-ray Photoelectron Spectroscopy (XPS) and Atomic Force Microscopy (AFM) techniques were used to determine the quality and thickness of the prepared aminopropylsilane layers.
2. Materials and methods
2.1. Chemicals
All reagents and solvents were used as received without further purification. Sodium dodecyl sulphate (for molecular biology, ≥98.5%, SDS), 3-aminopropytriethoxysilane (≥98%, APTES), acetic acid (99-100%, AcOH), hydrochloric acid (37%, p.a. grade, HCl), methanol (ACS grade, ≥99.8%, CH3OH) and toluene (ACS grade, ≥99.5%) were purchased to Sigma-Aldrich. Ethanol (absolute, p.a. grade, ≥99.5%) was purchased to Laborspirit. 2% (w/v) SDS solution was prepared with ultrapure water with a conductivity of 18.2 MΩ.cm (Milli-Q, Merck Millipore, Billerica, Massachusetts). APTES and AcOH solutions were prepared prior to each experiment. Surface functionalization was carried out on silicon wafers cut in pieces of 2.0 cm x 2.5 cm (Si [100], LG Siltron). All samples were prepared at room temperature. All reactions were carried out in glass petri dishes.
2.2. Surface characterization – XPS and AFM characterization
XPS measurements were performed using an ESCALAB 250 Xi system (Thermo Fisher Scientific, Inc.) equipped with a monochromated microfocused Al K alpha X-ray source that defined an analysis spot of ~650 × 400 µm2. All spectra were acquired in normal emission with an effective analyzer collection angle of 30°. Uniform charge neutralization was provided by beams of low-energy (≤10 eV) Ar+ ions and electrons guided by a magnetic lens; consistent charge neutralization was verified by observing adventitious C 1s peak at ~ 284.8 eV for all samples, energy at which all spectra were calibrated. For all experiments a silicon substrate freshly cleaned was used as reference sample. The results obtained for the Binding Energy (BE) are a mean of three measurements performed in three different spots in each sample.
AFM (JPK Nanowizard 3 AFM, Bruker Nano GmbH, Berlin, Germany) topographic measurements in contact mode in air were obtained with pyrex nitride probes on silicon nitride cantilevers (PNP-TR, NanoWorld AG, Switzerland) with a nominal resonance frequency of 75 KHz in air, spring constant of 0.32 N/m. The AFM instrument is on a noise vibration platform (i4, Accurion GmbH, Göttingen, Germany) and the entire set-up is mounted on an ultra-low noise floor constructed to comply with the NIST-A vibration specifications. [45] Previously, we demonstrated that we can obtain noise floor up to 75 pm. [46] Investigations have been carried out with out-of-the-box cantilevers. Image analysis and the determination of the root-mean-squared roughness Rrms were performed using the JPK Data Processing software (version 6.1.120).
2.3. Substrates preparation
2.3.1. Substrates cleaning
Substrate preparation consisted in two cycles of exposure of silicon substrates to 2% (w/v) SDS solution in an aqueous medium overnight, rinsing with ultrapure water, blow-drying with nitrogen and exposure to ultraviolet (UV) generated ozone (PSD Pro Series/digital UV Ozone System, Novascan) at 50 °C for 10 min to remove traces of organic contaminants. Cleaned substrates were immediately used for the surface activation.
2.3.2. Substrates activation
The surface activation step is important to generate -OH groups and avoid the increase of substrate roughness. Protocols using strong acids and bases such as piranha and sulfuric acid with potassium dichromate solutions have been evaluated and it was concluded that the most effective, i.e. the one that has not increased the surface roughness, was the one that uses a mixture of CH3OH/HCl (1:1) at room temperature. [42] We selected this protocol as the standard activating procedure for our experiments. Immediately after the last exposure to UV generated ozone, Si substrates were immersed in a freshly prepared solution of CH3OH/HCl (1:1) mixture for 30 min at room temperature.
2.3.3. Substrates functionalization
Si substrate samples were thoroughly washed with methanol and immersed for 5 min in ethanol in a glass petri dish. After that, samples were immersed in APTES solution on ethanol for the different concentrations tested (1% (v/v), 5% (v/v) and 10% (v/v)) and for different reaction times (20 min and 60 min). For testing toluene as a solvent, a Si substrate sample was immersed in APTES 1% (v/v) for 20 min and the sample rinsed in the same solvent.
2.3.4. Substrates post-deposition
Post-deposition processes using acetic acid or a combination of methanol and acetic acid were tested using different conditions. Using only acetic acid, samples were immersed in 6% (v/v) and 40% (v/v) for 20 min or 60 min; combining, substrates were immersed 20 min or 48 hour in methanol prior to immersion in acetic acid. All samples were gently blow dried with N2 and kept in a desiccator under vacuum until further analysis with XPS or AFM.
3. Method validation
In order to validate the functionalization route proposed, the chemistry interface between the organosilane and the silicon substrate was studied by XPS for different samples: [1] a blank Si substrate; [2] a Si substrate functionalized with APTES in toluene 2% (v/v); [3] a Si substrate functionalized with APTES 5% (v/v) in ethanol without any post-deposition washing process (Fig. 1). The comparison of the XPS survey (left) and Si 2p (right) core level peaks of these three samples is presented in Fig. 1. Spectra were normalized to the maximum intensity of the signal. The analysis of the survey correspondent to the blank Si substrate, Fig. 1a evidenced the presence of silicon and oxygen from the substrate, as well as carbon arising from contamination through air exposure. When the Si substrate is functionalized, nitrogen can be detected in all cases, irrespective of the functionalization treatment (Fig. 1b,c). This is a clear evidence of APTES functionalization. However, there are differences depending on the solvent used. When the sample is treated with APTES 5% (v/v) in ethanol, Fig. 1c, the presence of nitrogen is three times larger than the treatment in toluene - Fig. 1b, which suggests the presence of larger amounts of APTES on the Si surface. In addition, the Si content decreases three times, in comparison to the Si substrate, compared to the 1.3 ratio with the toluene treatment, which is also indicative of a larger presence of APTES on the surface with the treatment in ethanol. Furthermore, the increase in the carbon content, which is almost double after this treatment, and the modification of the background of these samples treated with APTES 5% (v/v) in ethanol - see the region below 300 eV, close to the Si 2s and Si 2p - suggests the presence of more than one monolayer of APTES on the Si surface when it is treated with APTES 5% (v/v) in ethanol. All these evidences are in accordance with Young-Jong Kim et al. [47], who made a detailed study of organosilane self-assembled multilayers, precisely controlling the number of layers. According to their work, an increasing layer number resulted in an enhancement of the amount of carbon detected, while the silicon at 99.6 eV (99.5 eV in our case, see below), which is mainly due to the Si substrate underneath, decreased. All this supports the presence of a multilayer in our case. Detailed analysis of the silicon core level peaks are depicted in Fig. 1d-f plots. In the case of the blank substrate, the Si 2p (Fig. 1d) presents 3 components: the ones at a BE of 99.5 eV and 99.9 eV corresponds to the Si02p3/2 and Si02p1/2 of elemental silicon. The component at 103.4 eV corresponds to the superficial oxide layer of SiO2. When the silicon is functionalized with APTES in toluene, the Si 2p still presents 3 components: the Si° components reveal that elemental Si is still detectable, which is indicative for the absence of a thick layer of APTES on top of the Si substrate. However, the oxide component is shifted towards lower binding energies (102.8 eV) in comparison to blank Si, close to the one of Si3+. Silane monolayers usually present a BE of 101.7 eV. [48] According to Dietrich et al. [49], the BE we found could arise from a multilayer of aminopropylsilane. However, in our case the substrate is still clearly detectable which is representative of the absence of a thick multilayer. This displacement is correlated with a shift of 0.8 eV in the O 1s core level (Fig. S1). Both facts are evidences of the presence of silane as the major contribution to these peaks. Also, as there was no treatment to remove superficial oxide from the Si substrate. The amount of SiO2 is much lower than elemental Si and the oxide component can include both, the presence of Si oxide and the Si from the siloxane. When the Si substrate is functionalized with APTES 5% (v/v) in ethanol, elemental Si is hardly detectable. The BE of the maximum appeared at 102.4 eV, an energy that has been reported as aminopropylsilane (Si-O) on the silicon oxide. [26]
Fig. 1.
XPS survey and Si 2p spectra: blank Si substrate [a,d], APTES in toluene [b,e], APTES in ethanol without any post-deposition process [c,f].
3.1. APTES deposition concentrations and reaction times
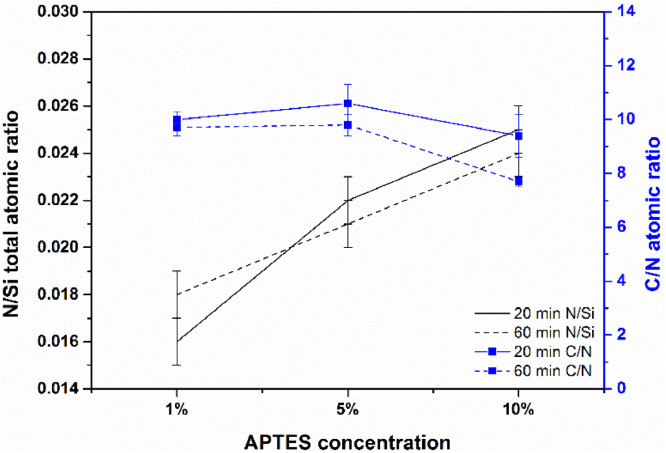
Different concentrations (1% (v/v), 5% (v/v) and 10% (v/v)) of the organosilane were tested to assess their influence on the functionalization of the Si substrate for 20 min and 60 min reaction times. Fig. 2 depicts the results obtained for N/Si total and C/N atomic concentration ratio under the conditions mentioned. The N/Si total atomic ratio follows the same trend for both immersion times tested. Comparing the values we observed an increase of 64% of the atomic ratio from the APTES 1% (v/v) to 10% (v/v) while for 60 min we observed an increase of 75%. Regarding the C/N atomic ratio a different trend is observed. For the set of samples of 20 min, taking into account the experimental uncertainty, the values are constant ranging from 10.6 ± 0.7 pm to 9.4 ± 0.8 pm. For the set of 60 min reaction time the C/N ratio decreased from 9.7 ± 0.3 pm to 7.7 ± 0.2 pm. Values obtained for the C/N ratios suggest that the three ethoxy groups were not replaced by siloxane linkages. C/N ratio values also suggest that up to 60 min reaction time and APTES 10% (v/v) the organosilane layers were not thicker. Namely it was reported [38] that C/N ratios of 9/1 are related to APTES unreacted form and ratios of C/N of 3/1 suggest perfectly hydrolysed APTES molecules. However, the higher C/N values can also be due to adventitious carbon indicating that increasing APTES concentration and reaction time on the time scale studied (up to 60 min) contrary to the expected the organosilanes layers were not thicker or had an increased polymerization. Furthermore, the atomic percentage of nitrogen remains constant when increasing the reaction times (Table S1). The results obtained are comparable to ones reported in literature using (3-aminopropyl)-trimethoxysilane (APTMS) in ethanol and changing temperature, not the reaction duration. This phenomena has been related to the polar protic nature of ethanol that can solvolyze the Si-O-Si bonds faster than the condensation reaction resulting in lower polymerization. [26]
Fig. 2.
Comparison of the N/Si total and C/N atomic concentration ratio for Si substrate functionalized with APTES 1% (v/v), 5% (v/v) and 10% (v/v) for different reaction times.
3.2. Post-deposition conditions variation
After substrate functionalization there are organosilanes molecules that have been deposited but not covalently bound to the silicon surface. Therefore it is important to proceed with a post-deposition process that removes the unbond organosilane molecules without affecting the aminopropylsilane layer formed. To this end, solvents like methanol and AcOH have been reported in the literature: methanol has been tested through soxhlet extraction and was found to help to remove the excess silanes deposited on the surface [50] while AcOH is a complexing agent of silanes molecules [42]. In this work we tested AcOH and a combination of AcOH and methanol at room temperature.
With respect to the use of AcOH, the aim was to find out if higher concentrations and longer immersion times modified the effectiveness on the removal of non-covalently attached APTES molecules. To this end, we tested different concentrations (6% (v/v) and 40% (v/v)) and immersion times of (20 min and 60 min) of AcOH. Fig. 3 shows a representative AFM topography image of a silicon substrate after the cleaning protocol and a silicon substrate modified with APTES 5% (v/v) in ethanol and treated with AcOH 6% (v/v) for 20 min. The cleaned silicon substrate (Fig. 3) exhibits a Rrms of 112 pm while the functionalized silicon substrate (Fig. 3) has a Rrms of 382 pm and depicts smaller islands on the aminopropylsilane layer. These islands have height values from 1 to 10 nm and an average width of 65 ± 10 nm. The occurrence of such islands in organosilanes layers was reported previously in silicon wafer substrates functionalized for three hours at 25 °C with (3-aminopropyl)trimethoxysilane in several organic solvents (ethanol, acetone, N,N-dimethylformamide, toluene and acetonitrile) [26] and in silicon wafers functionalized for six hours at 25 °C using (3‐aminopropyl)diethoxymethylsilane and (3‐aminopropyl)ethoxydimethylsilane in toluene. [48] Their formation was related to an organosilane polymerization process occurring in solution prior to the functionalization of the substrates. Despite the fact we are using different experimental conditions it can be inferred that the protruding features we observe are due to an APTES polymerization process. Fig. S2 depicts an AFM image with an incomplete functionalized surface area. We determined the depth of the area that showed no functionalization. The value obtained for the depth was 681 pm which is in accordance to what has been reported in the literature for a monolayer of APTES obtained via solution approach. [44,50,51]
Fig. 3.
AFM contact mode images: (left) cleaned silicon substrate, (right) APTES 5% (v/v) in ethanol for 20 min at room temperature.
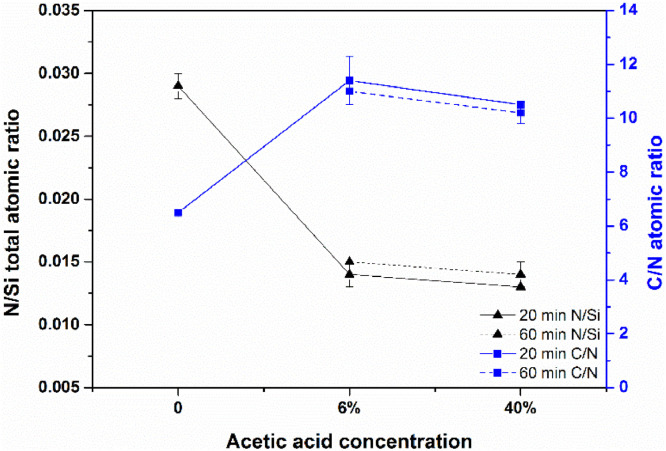
Fig. 4 depicts the results obtained for N/Si total and C/N ratios on substrates functionalized with APTES 5% (v/v) in ethanol treated with 6% (v/v) and 40% (v/v) of AcOH. When using AcOH the N/Si total decreases approximately 50 % in atomic ratio suggesting the removal of APTES molecules non covalently attached to the Si substrate. The non-relevant difference between 6% (v/v) and 40% (v/v) for the N/Si total and the C/N atomic ratios indicates that a small concentration of AcOH is enough to remove any clumps that might exist. Furthermore, the increase of C/N ratio is indicative of AcOH detection on the sample surface. Consequently the C/N ratio cannot be taken as a single characterization parameter to indicate an ideal APTES film. A combination of AcOH 6% (v/v) and methanol was further tested with the intention to check if methanol was able to act as a removal agent at room temperature. For this purpose two samples functionalized with APTES 5% (v/v) were immersed in methanol; one for 20 min and another for 48 hour. These samples were imaged with AFM in contact mode (Fig. S3) and the root-mean-squared roughness Rrms of the samples obtained on an area of 5 µm x 5 µm. For the sample immersed for 20 min in methanol the Rrms increased to 541 pm while for the substrate immersed for 48 hour in methanol the Rrms was 118 pm. Comparing AFM and XPS results in Table 1, Rrms decrease is correlated with an increase of the atomic ratio of N/Si total and N/Si bulk. The results obtained for the C/N atomic ratios are in the same range of the ones obtained in Fig. 2 indicating that APTES is not fully hydrolysed. Given we did not apply temperature higher than room temperature, methanol revealed to be a good solvent for removing non covalent attached APTES molecules for periods of 48 hour.
Fig. 4.
Comparison of the N/Si total and C/N atomic ratios for Si substrate grafted with APTES 5% (v/v) in ethanol using different AcOH concentrations on the post-deposition washing process.
Table 1.
Atomic ratio of the elemental composition of APTES 5% (v/v) grafted on a Si surface using different post-deposition processes at room temperature: no methanol (only AcOH 6% (v/v)) and a combined process with AcOH and methanol for 20 min and 48 hour.
| AcOH 6% (v/v) | AcOH 6% (v/v) and methanol |
||
|---|---|---|---|
| 20 min | 48 h | ||
| N/Si total | 0.022 | 0.029 | 0.037 |
| N/Si bulk | 0.028 | 0.038 | 0.047 |
| C/N | 11.3 | 9.2 | 9.9 |
Fig. 5 shows the survey spectrum (Fig. 5a) as well as Si 2p (Fig. 5b), O 1s (Fig. 5c), N 1s (Fig. 5d) and C 1s (Fig. 5e) core level spectra of the sample functionalized with APTES 5% (v/v) in ethanol and a post-treatment combined with AcOH and methanol for 2 days. The analysis of the survey spectra (Fig. 5a) revealed the presence of nitrogen and more carbon than the blank Si surface, indicative of an effective functionalization. However, as we already discussed in Figure 1, the clear detection of Si0 at around 99.5 eV arising from the substrate (see Fig. 5e) is indicative of a thinner APTES layer [47] than the one depicted in Fig. 1e before the AcOH treatment. Therefore, the treatment with AcOH effectively removed the multilayer. Furthermore, the background of this sample is similar to the blank substrate suggesting a thinner layer than the ones presented in Fig. 5c,d. In general terms, this functionalization resulted in similar results as the treatment with APTES in toluene (Fig. 5b). These similarities could be also observed in a detailed analysis of the core level spectra (Fig. 5b-e). The Si 2p (Fig. 5b) evidenced the detection of Si° from the substrate at 99.5 eV and 99.9 eV as well as an oxide component at 102.8 eV. The O 1s also presents the same trend as the treatment in toluene, with an oxygen contribution at 531.8 eV (Fig. S1). The analysis of the N 1s core level revealed the presence of two components: one at 399.6 eV from free amine group -NH2 and another at 401.6 eV from the protonated amine -NH3+. [52] The C 1s peak presents three contributions typical from organic compounds: the main component at 285 eV from C-C / C-H bonds, another component at higher BE 286.6 eV from C-O / C-N bonds and a third component at 288.9 eV from carboxyl groups. [37] The presence of amide groups O=C-N presents the XPS energies at 288.1 eV in the C 1s; 399.8 eV in the N 1s. Therefore, the energy of one of the components on the N 1s peak is compatible with this O=C-N group, but a difference of 0.8 eV in the C 1s spectrum is too big to assign the component at 288.9 eV to the O=C-N group. Therefore the presence of amide groups in the functionalized substrate has been excluded.
Fig. 5.
a) Survey, (b) Si 2p, (c) O 1s, (d) N 1s and (e) C 1s XPS spectra characterizing the APTES/Si interface chemistry in detail of a Si substrate functionalized with APTES 5% (v/v) in ethanol and a post-deposition treatment combined with AcOH 6% (v/v) and methanol for 2 days.
4. Conclusions
In this method paper, we demonstrated that using a solution-phase approach an aminopropylsilane layer of APTES can be formed on top of a silicon substrate following a reproducible and easy-to-implement protocol. In the future, the protocol presented will be extended to further study the functionalization with organosilane with different number of reactive groups on silicon dioxide substrates and its stability under aqueous medium
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
AM and PDB acknowledge Norte's Regional Operational Programme 2014-2020-Norte2020 (NORTE-01-0145-FEDER-000019). LM acknowledges the ERC-synergy program funding (grant ERC-2013-SYG-610256 NANOCOSMOS), Comunidad de Madrid and European Structural Funds for their financial support to FotoArt-CM project (S2018/NMT-4367). The authors thank Dr. Jérôme Borme for providing high quality suitable-sized silicon substrates and Dr. Dmitri Petrovykh for assistance with XPS data acquisition.
Author contributions
AM: Conceptualization, Methodology, Formal Analysis, Investigation, Visualization, Writing – original draft preparation. LM: Formal Analysis, Visualization. PDB: Supervision, Funding acquisition. All authors contributed to the writing, reviewing and editing of the manuscript and gave final approval for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2020.100931.
Appendix. Supplementary materials
References
- 1.Witucki G.L. A Silane Primer: Chemistry and Applications of Alkoxy Silanes. J Coatings Technol. 1993;65(822):57–60. [Google Scholar]
- 2.Dixon D V., Hosseinidoust Z., Tufenkji N. Effects of environmental and clinical interferents on the host capture efficiency of immobilized bacteriophages. Langmuir. 2014;30(11):3184–3190. doi: 10.1021/la500059u. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y.J., Korkmaz N., Nam C.H., Yj K.Phage Assembly Using APTES-Conjugation of Major Coat p8 Protein for Possible Scaffolds . 2012;4494 (49):1–7.
- 4.Santini G.C., Potrich C., Lunelli L., Pasquardini L., Vaghi V., Pederzolli C. Innovative microRNA purification based on surface properties modulation. Colloids Surfaces B Biointerfaces. 2014;116:160–168. doi: 10.1016/j.colsurfb.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 5.Vashist S.K., Lam E., Hrapovic S., Male K.B., Luong J.H.T. Immobilization of antibodies and enzymes on 3-aminopropyltriethoxysilane-functionalized bioanalytical platforms for biosensors and diagnostics. Chem Rev. 2014;114(21):11083–11130. doi: 10.1021/cr5000943. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S., Huda S., Kilpatrick P.K., Velev O.D. Characterization and optimization of gold nanoparticle-based silver-enhanced immunoassays. Anal Chem. 2007;79(10):3810–3820. doi: 10.1021/ac062341m. [DOI] [PubMed] [Google Scholar]
- 7.Zhou T., Huang S., Niu D., Su L., Zhen G., Zhao Y. Efficient Separation of Water-Soluble Humic Acid Using (3-Aminopropyl)triethoxysilane (APTES) for Carbon Resource Recovery from Wastewater. ACS Sustain Chem Eng. 2018;6(5):5981–5989. [Google Scholar]
- 8.Pasche S., Schyrr B., Wenger B., Scolan E., Ischer R., Voirin G. Smart Textiles with Biosensing Capabilities. Advances in Science and Technology. 2012;80:129–135. [Google Scholar]
- 9.Xu Z., Liu Q., Finch J.A. Silanation and stability of 3-aminopropyl triethoxy silane on nanosized superparamagnetic particles: I. Direct silanation. Appl Surf Sci. 1997;120(3):269–278. [Google Scholar]
- 10.Kollbe Ahn B., Wang H., Robinson S., Shrestha T.B., Troyer D.L., Bossmann S.H. Ring opening of epoxidized methyl oleate using a novel acid-functionalized iron nanoparticle catalyst. Green Chem. 2012;14(1):136–142. [Google Scholar]
- 11.Usrey M.L., Strano M.S. Controlling single-walled carbon nanotube surface adsorption with covalent and noncovalent functionalization. J Phys Chem C. 2009;113(28):12443–12453. [Google Scholar]
- 12.Phonthammachai N., Kah J.C.Y., Jun G., Sheppard C.J.R., Olivo M.C., Mhaisalkar S.G. Synthesis of contiguous silica-gold core-shell structures: Critical parameters and processes. Langmuir. 2008;24(9):5109–5112. doi: 10.1021/la703580r. [DOI] [PubMed] [Google Scholar]
- 13.Ding S., Qian W., Tan Y., Wang Y. In-situ incorporation of gold nanoparticles of desired sizes into three-dimensional macroporous matrixes. Langmuir. 2006;22(17):7105–7108. doi: 10.1021/la060273t. [DOI] [PubMed] [Google Scholar]
- 14.Mieszawska A.J., Slawinski G.W., Zamborini F.P. Directing the growth of highly aligned gold nanorods through a surface chemical amidation reaction. J Am Chem Soc. 2006;128(17):5622–5623. doi: 10.1021/ja061046g. [DOI] [PubMed] [Google Scholar]
- 15.Yang X., Li Q., Li Z., Xu X., Liu H., Shang S. Preparation and Characterization of Room-Temperature-Vulcanized Silicone Rubber Using Acrylpimaric Acid-Modified Aminopropyltriethoxysilane as a Cross-Linking Agent. ACS Sustain Chem Eng. 2019;7(5):4964–4974. 7. [Google Scholar]
- 16.Meng X., Nguyen N.A., Tekinalp H., Lara-Curzio E., Ozcan S. Supertough PLA-Silane Nanohybrids by in Situ Condensation and Grafting. ACS Sustain Chem Eng. 2018;6(1):1289–1298. [Google Scholar]
- 17.Cheng T., Zhao Q., Zhang D., Liu G. Transition-metal-functionalized ordered mesoporous silicas: An overview of sustainable chiral catalysts for enantioselective transformations. Green Chem. 2015;17(4):2100–2122. [Google Scholar]
- 18.Sharma R.K., Sharma S., Dutta S., Zboril R., Gawande M.B. Silica-nanosphere-based organic-inorganic hybrid nanomaterials: Synthesis, functionalization and applications in catalysis. Green Chem. 2015;17(6):3207–3230. [Google Scholar]
- 19.Lichtenstein A., Havivi E., Shacham R., Hahamy E., Leibovich R., Pevzner A. Supersensitive fingerprinting of explosives by chemically modified nanosensors arrays. Nat Commun. 2014;5(May):1–12. doi: 10.1038/ncomms5195. [DOI] [PubMed] [Google Scholar]
- 20.Pujari S.P., Scheres L., Marcelis A.T.M., Zuilhof H. Covalent surface modification of oxide surfaces. Angew Chemie - Int Ed. 2014;53(25):6322–6356. doi: 10.1002/anie.201306709. [DOI] [PubMed] [Google Scholar]
- 21.Onclin S., Ravoo B.J., Reinhoudt D.N. Engineering Silicon Oxide Surfaces Using Self-Assembled Monolayers. Angew Chemie Int Ed. 2005;44(39):6282–6304. doi: 10.1002/anie.200500633. [DOI] [PubMed] [Google Scholar]
- 22.Herzer N., Haensch C., Hoeppener S., Schubert U.S. Orthogonal Functionalization of Silicon Substrates Using Self-Assembled Monolayers. Langmuir. 2010;26(11):8358–8365. doi: 10.1021/la9047837. [DOI] [PubMed] [Google Scholar]
- 23.Zhu M., Lerum M.Z., Chen W. How To Prepare Reproducible, Homogeneous, and Hydrolytically Stable Aminosilane-Derived Layers on Silica. Langmuir. 2012;28(1):416–423. doi: 10.1021/la203638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aissaoui N., Bergaoui L., Boujday S., Lambert J.F., Méthivier C., Landoulsi J. Enzyme immobilization on silane-modified surface through short linkers: Fate of interfacial phases and impact on catalytic activity. Langmuir. 2014;30(14):4066–4077. doi: 10.1021/la404935q. [DOI] [PubMed] [Google Scholar]
- 25.Kannan B., Higgins D.A., Collinson M.M. Chelation gradients for investigation of metal ion binding at silica surfaces. Langmuir. 2014;30(33):10019–10027. doi: 10.1021/la502088k. [DOI] [PubMed] [Google Scholar]
- 26.Jakša G., Štefane B., Kovač J. Influence of different solvents on the morphology of APTMS-modified silicon surfaces. Appl Surf Sci. 2014;315(1):516–522. [Google Scholar]
- 27.Xiao S.J., Textor M., Spencer N.D., Wieland M., Keller B., Sigrist H. Immobilization of the cell-adhesive peptide Arg–Gly–Asp–Cys (RGDC) on titanium surfaces by covalent chemical attachment. J Mater Sci Mater Med. 1997 Dec;8(12):867–872. doi: 10.1023/a:1018501804943. [DOI] [PubMed] [Google Scholar]
- 28.Song Y.Y., Hildebrand H., Schmuki P. Optimized monolayer grafting of 3-aminopropyltriethoxysilane onto amorphous, anatase and rutile TiO2. Surf Sci. 2010;604(3–4):346–353. [Google Scholar]
- 29.Bao N., Wu G., Niu J., Zhang Q., He S., Wang J. Wide spectral response and enhanced photocatalytic activity of TiO 2 continuous fibers modified with aminosilane coupling agents. J Alloys Compd. 2014;599:40–48. [Google Scholar]
- 30.Cheng F., Sajedin S.M., Kelly S.M., Lee A.F., Kornherr A. UV-stable paper coated with APTES-modified P25 TiO2 nanoparticles. Carbohydr Polym. 2014;114:246–252. doi: 10.1016/j.carbpol.2014.07.076. [DOI] [PubMed] [Google Scholar]
- 31.Meroni D., Lo Presti L., Di Liberto G., Ceotto M., Acres R.G., Prince K.C. A close look at the structure of the TiO2-APTES interface in hybrid nanomaterials and its degradation pathway: An experimental and theoretical study. J Phys Chem C. 2017;121(1):430–440. doi: 10.1021/acs.jpcc.6b10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halliwell C.M., Cass A.E.G. A Factorial Analysis of Silanization Conditions for the Immobilization of Oligonucleotides on Glass Surfaces. Anal Chem. 2001;73(11):2476–2483. doi: 10.1021/ac0010633. [DOI] [PubMed] [Google Scholar]
- 33.Metwalli E., Haines D., Becker O., Conzone S., Pantano C.G. Surface characterizations of mono-, di-, and tri-aminosilane treated glass substrates. J Colloid Interface Sci. 2006;298(2):825–831. doi: 10.1016/j.jcis.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 34.Wayment J.R., Harris J.M. Controlling binding site densities on glass surfaces. Anal Chem. 2006;78(22):7841–7849. doi: 10.1021/ac061392g. [DOI] [PubMed] [Google Scholar]
- 35.Klug J., Pérez L.A., Coronado E.A., Lacconi G.I. Chemical and Electrochemical Oxidation of Silicon Surfaces Functionalized with APTES: The Role of Surface Roughness in the AuNPs Anchoring Kinetics. J Phys Chem C. 2013;117(21):11317–11327. [Google Scholar]
- 36.Yadav A.R., Sriram R., Carter J.A., Miller B.L. Comparative study of solution–phase and vapor–phase deposition of aminosilanes on silicon dioxide surfaces. Mater Sci Eng C. 2014;35:283–290. doi: 10.1016/j.msec.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acres R.G., Ellis A.V., Alvino J., Lenahan C.E., Khodakov D.A., Metha G.F. Molecular structure of 3-aminopropyltriethoxysilane layers formed on silanol-terminated silicon surfaces. J Phys Chem C. 2012;116(10):6289–6297. [Google Scholar]
- 38.Howarter J.A., Youngblood J.P. Optimization of silica silanization by 3-aminopropyltriethoxysilane. Langmuir. 2006;22(26):11142–11147. doi: 10.1021/la061240g. [DOI] [PubMed] [Google Scholar]
- 39.Pasternack R.M., Rivillon Amy S., Chabal Y.J. Attachment of 3- (Aminopropyl)triethoxysilane on Silicon Oxide Surfaces: Dependence on Solution Temperature. Langmuir. 2008;24(22):12963–12971. doi: 10.1021/la8024827. [DOI] [PubMed] [Google Scholar]
- 40.Siqueira Petri D.F., Wenz G., Schunk P., Schimmel T. An Improved Method for the Assembly of Amino-Terminated Monolayers on SiO2 and the Vapor Deposition of Gold Layers. Langmuir. 1999;15(13):4520–4523. [Google Scholar]
- 41.Prat D., Wells A., Hayler J., Sneddon H., McElroy C.R., Abou-Shehada S. Correction: CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2015;17(10) 4848–4848. [Google Scholar]
- 42.Han Y., Mayer D., Offenhäusser A., Ingebrandt S. Surface activation of thin silicon oxides by wet cleaning and silanization. Thin Solid Films. 2006;510(1–2):175–180. [Google Scholar]
- 43.Amemiya Y., Hatakeyama A., Shimamoto N. Aminosilane multilayer formed on a single-crystalline diamond surface with controlled nanoscopic hardness and bioactivity by a wet process. Langmuir. 2009;25(1):203–209. doi: 10.1021/la801556x. [DOI] [PubMed] [Google Scholar]
- 44.Vandenberg E.T., Bertilsson L., Liedberg B., Uvdal K., Erlandsson R., Elwing H. Structure of 3-aminopropyl triethoxy silane on silicon oxide. Journal of Colloid And Interface Science. 1991;147:103–118. [Google Scholar]
- 45.Amick H., Gendreau M., Busch T., Gordon C. Evolving criteria for research facilities: vibration. In: Amick H, editor. Buildings for Nanoscale Research and Beyond. SPIE; 2005. pp. 16–28. [Google Scholar]
- 46.Miranda A., Martins M., De Beule P.A.A. Simultaneous differential spinning disk fluorescence optical sectioning microscopy and nanomechanical mapping atomic force microscopy. Rev Sci Instrum. 2015;86(9) doi: 10.1063/1.4931064. [DOI] [PubMed] [Google Scholar]
- 47.Kim Y.-J., Han J., Sano H., Lee K.-H., Noda K., Ichii T. Organosilane self-assembled multilayer formation based on activation of methyl-terminated surface with reactive oxygen species generated by vacuum ultra-violet excitation of atmospheric oxygen molecules. Appl Surf Sci. 2009;256(5):1507–1513. [Google Scholar]
- 48.Jakša G., Štefane B., Kovač J. XPS and AFM characterization of aminosilanes with different numbers of bonding sites on a silicon wafer. Surf Interface Anal. 2013 Nov;45(11–12):1709–1713. [Google Scholar]
- 49.Dietrich P.M., Glamsch S., Ehlert C., Lippitz A., Kulak N., Unger W.E.S. Synchrotron-radiation XPS analysis of ultra-thin silane films: Specifying the organic silicon. Appl Surf Sci. 2016;363:406–411. [Google Scholar]
- 50.Simon A., Cohen-Bouhacina T., Porté M.C., Aimé J.P., Baquey C. Study of Two Grafting Methods for Obtaining a 3-Aminopropyltriethoxysilane Monolayer on Silica Surface. J Colloid Interface Sci. 2002 Jul;251(2):278–283. doi: 10.1006/jcis.2002.8385. Available from. [DOI] [PubMed] [Google Scholar]
- 51.Crampton N., Bonass W.A., Kirkham J., Thomson N.H. Formation of aminosilane-functionalized mica for atomic force microscopy imaging of DNA. Langmuir. 2005;21(17):7884–7891. doi: 10.1021/la050972q. [DOI] [PubMed] [Google Scholar]
- 52.Maria Chong A.S., Zhao X.S. Functionalization of SBA-15 with APTES and Characterization of Functionalized Materials. J Phys Chem B. 2003;107(46):12650–12657. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.