Abstract
Niclosamide has been known to inhibit a number of pH-dependent viruses via the neutralization of endosomal acidic pH. It has also been shown to disrupt the mTORC1 signaling pathway. The replication of many viruses requires mTORC1 activation. Here, we investigated the inhibitory activity of niclosamide against HIV-1, and determined whether mTORC1 inhibition was involved. The cytotoxicity and anti-HIV-1 activity of niclosamide were tested in TZM-bl and SupT1 cells. Niclosamide showed a dose- and time-dependent inhibitory activity against HIV-1 replication, but the inhibition did not involve the reverse transcription and transcription steps. The mechanism of mTORC1 inhibition was explored by using MHY1485, an mTORC1 activator, to reverse the mTORC1 inhibition, which could partially restore HIV-1 replication. In addition, niclosamide was found to downregulate mTORC1 via AMPK activation, resulting in a decreased phosphorylation of the downstream substrates of S6K and 4EBP1. Niclosamide could also reduce the synthesis of HIV-1 p24 protein. Likewise, MHY-1485 could partially reverse the inhibitory effect of niclosamide by increasing the phosphorylation in the mTORC1 pathway and HIV-1 viral protein synthesis. Our findings, therefore, demonstrated the antiviral mechanism of niclosamide is via the AMPK-mTORC1 pathway, which could be a common therapeutic target for various viruses.
Keywords: Microbiology, Cell biology, Virology, Molecular biology, Biomolecules, Niclosamide, HIV-1, mTORC1, AMPK
Microbiology; Cell biology; Virology; Molecular biology; Biomolecules; Niclosamide; HIV-1; mTorC1; AMPK
1. Introduction
Niclosamide, an FDA-approved anthelmintic drug, has been repurposed to treat various diseases, including viral and microbial infections, diabetes, Parkinson's disease, and various types of cancer (Chen et al., 2018; Kadri et al., 2018). It has been well recognized that the protonophoric activity of niclosamide can inhibit the replication of pH-dependent viruses at the entry stage (Jurgeit et al., 2012; Kao et al., 2018). This activity leads to cytoplasmic acidification, which has a potential role in downregulating the mechanistic (formerly “mammalian”) target of rapamycin complex 1 or the mTORC1 signaling pathway (Fonseca et al., 2012). A number of viruses employ this signaling cascade for replication (Sage et al., 2016); therefore, niclosamide may have a broad-spectrum antiviral activity beyond anti-pH dependent viruses. Huang et al. (2017) revealed the novel antiviral mechanism of niclosamide to inhibit the Epstein-Barr virus (EBV) through disrupting mTORC1 activation. This suggested a therapeutic potential of niclosamide for the treatment of pH-independent viruses.
It was previously shown that HIV-1 uses a viral envelope protein to activate the mTORC1 pathway and that this activation contributes to the pathogenesis of HIV-1-associated malignancies (Nicoletti et al., 2011; Sage et al., 2016). It is possible that mTORC1 activation may be important for HIV-1 replication and that the disruption of mTORC1 may inhibit the replication and pathogenesis.
In the present study, we investigated the anti-HIV-1 mechanism of niclosamide through mTORC1 inhibition. Since mTORC1 activation has been shown to facilitate the replication of various viruses (Sage et al., 2016), our findings suggest that mTORC1 inhibition may contribute to the broad-spectrum inhibitory activity of niclosamide against viruses, including pH-independent ones.
2. Materials and methods
2.1. Cells and culture condition
TZM-bl cells (NIH AIDS Reagent Program) were maintained in DMEM (Gibco). SupT1 cells were grown in RPMI 1640 medium (Gibco). Human embryonic kidney 293T (HEK-293T) cells were cultured in DMEM. All the culture media were supplemented with 10% fetal bovine serum (FBS) (Gibco) and antibiotics. All the cell maintenance and cell-based in vitro assays were carried out in a humidified 37 °C incubator with 5% CO2.
2.2. Reagents
Niclosamide (N3510, Sigma), temsirolimus (PZ0020, Sigma), Zidovudine (HY-17413, MedchemExpress) and mTOR activator-MHY1485 (B0795, MedchemExpress) were dissolved in culture-grade 100% DMSO (Sigma) to a final stock concentration of 10 mM and kept at -20 °C before use. All the reagents were diluted to working concentrations using a growth medium. The final concentration of DMSO in all the experiments was lower than 0.5% as this did not affect cell viability and viral replication.
2.3. Plasmid, production of virus stock, and virus titration
Molecular clone plasmid DNA of pNL4-3 containing a full-length NL4-3 strain of HIV-1 (Adachi et al., 1986), provided by the NIH AIDS Reagent Program, was transfected into HEK-293T cells using DEMRIE-C transfection reagent (Invitrogen). The transfected cells were cultured for 2–3 days, then the virus-containing supernatants were harvested and frozen at -80 °C until further use.
The virus stock was titrated by an ELISA specific to viral core p24 protein (Abcam) or by luciferase assay. For the luciferase assay, briefly, undiluted and tenfold dilutions of the virus stock were transduced into TZM-bl cells in the presence of 10% DMEM supplemented with DEAE-dextran hydrochloride (D9885, Sigma). After 2 days, the transduced TZM-bl cells were assessed using Steadylite plus reagent (PerkinElmer) according to the manufacturer's instructions. The luciferase signal was reported in relative luciferase units (RLUs).
2.4. Cell viability assay
TZM-bl cells were seeded in 96-well plates, then the cells were treated with the desired concentrations of drugs for 48 h in 10% DMEM supplemented with DEAE. MTT dye (Invitrogen) was used to measure the conversion of the 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide to MTT formazan. DMSO was added to dissolve the precipitates in the cells, and the absorbance was read at 570 nm.
A SupT1 cell suspension was grown on 96-well plates. The cells were then incubated with the indicated concentrations of drugs for 144 h in 10% RPMI 1640 supplemented with DEAE. Trypan blue was used to stain the cells and to differentiate between living and dead cells. The percentage cell viability was then determined by counting the number of living cells.
Cells treated with DMSO (final concentration ≤0.5%) were used as the positive control (100% cell viability). The 50% cytotoxic concentration (CC50) was calculated and analyzed with GraphPad Prism version 5.01 (GraphPad Software, Inc.).
2.5. Activity-based assay in cell culture
Anti-HIV-1 activity was evaluated under a time-of-additional niclosamide study. Cells were treated with niclosamide at concentration of 0.625 μM in TZM-bl cells and of 0.312 μM in SupT1 cells under indicated time points, including before (pre-treatment at 1, 3, and 6 h), during (co-treatment at 0 h), and after (post-treatment at 1, 3, and 6 h) virus infection.
For the TZM-bl cells, 2 × 104 cells/well were grown in 96-well plates overnight. An HIV-1 inoculum dose/well of 600 pg of p24 or 4,800 RLU was used. Upon reaching confluence, the culture medium was removed, and the cells were added and incubated with niclosamide, a mixture of niclosamide and HIV-1, or HIV-1 depending on the specified times and conditions. To the cells were then added HIV-1 (pre-treatment) and niclosamide (post-treatment), and they were then incubated for 2 days. The virus-infected TZM-bl cells were titrated directly by luciferase assay.
For the SupT1 cells, 3 × 105 cells/well were seeded into 6-well plates containing niclosamide, a mixture of niclosamide and HIV-1, or HIV-1 in medium depending on the specified conditions, and incubated for the indicated time points. An HIV-1 inoculum dose/well of 4 ng of p24 or 32,000 RLU was used. After incubation, HIV-1 was added to the pre-treatment wells and absorbed for 2 h. Then, the cells were washed twice with PBS and resuspended with niclosamide. In addition, the co- and post-treatment wells containing HIV-1 were washed and resuspended with niclosamide. The cells were incubated for 6 days and the cytopathic effect (CPE) observed daily. The supernatant was then transferred to quantitate the luciferase expression in fresh TZM-bl cells.
The percentage of relative luciferase units was calculated by comparing the results to the virus-infected cells as a positive control (100% luciferase expression).
2.6. Dose-dependent study
Cells were seeded as described above. Cells were tested with twofold dilutions of niclosamide against the virus in triplicate experiments. Luciferase expression was assessed, and non-linear regression analysis of the dose-dependent response graph was performed to determine the effective concentration 50 (EC50) by GraphPad Prism version 5.01.
2.7. Real-time PCR
Samples from niclosamide pretreatment in HIV-1-infected TZM-bl cells, HIV-1-infected TZM-bl cells (positive control), and DMSO-treated TZM-bl cells (negative control) were used to extract DNA or RNA at indicated time points (3, 24, or 48 h). Total DNA was isolated by using the QIAamp DNA mini kit (QIAGEN), and RNA was extracted by TRIzol reagent and converted to cDNA by using a conventional system for reverse transcription using AMV reverse transcriptase (Promega). 100 ng of DNA and 5 μl of cDNA of the samples were used to amplify the target products in HIV-1 reverse transcription and proviral transcription, respectively, using specific primers as previously described in Table 1, with human GAPDH used as an internal control for the normalization. Real-time PCR amplification was performed using the SYBR Green Luna®universal qPCR Master Mix (BioLabs) in a LightCycler 480 instrument (Roche).
Table 1.
Sequence primers for detection of the reverse transcription products and HIV-1 mRNA.
| Targets | Primer sequences | References |
|---|---|---|
| Early product (R5/U5) | Early F:5′-GCTCTCTGGCTAACTAGGGAAC-3′ Early R: 5′-TGACTAAAAGGGTCTGAGGGAT-3′ |
Heredia et al. (2015) |
| Late product (U5/PBS/Ψ) | MH531 F: 5′-TGTGTGCCCGTCTGTTGTGT-3′ MH532 R 5′-GAGTCCTGCGTCGAGAGATC-3′ |
Mbisa et al. (2009) |
| 2-LTR circles | MH535 F:5′-AACTAGGGAACCCACTGCTTAAG-3′ MH536 R: 5′-TCCACAGATCAAGGATATCTTGTC-3′ |
Mbisa et al. (2009) |
| Integrated provirus |
Alu-based nested PCR First round PCR: Alu F:5′GCCTCCCAAAGTGCTGGGATTACAG-3′ Gag R: 5′-GCTCTCGCACCCATCTCTCTCC-3′ Second round PCR: LTR-R-F: 5′-GCCTCAATAAAGCTTGCCTTGA-3′ LTR-U5-R: 5′-TCCACACTGACTAAAAGGGTCTGA-3′ |
Heredia et al. (2015) |
| Cellular HIV mRNA (Full length) | US.1a -F: 5′-GCTTGCTGAAGCGCGCACGG-3′ US.2a-R: 5′-CGTTCTAGCTCCCTGCTTGC-3′ |
Hermankova et al. (2003) |
The reaction for the detection of early-, late-, 2LTR-products, and GAPDH involved denaturing at 95 °C for 2 min, followed by 45 amplification cycles (95 °C for 15 s and 56–60 °C for 60 s). For the detection of the integrated provirus, Alu-based nested real-time PCR was used as previously described (Heredia et al., 2015). The first-round PCR was done for 25 cycles (pre-denatured at 95 °C for 2 min, followed by 95 °C for 15 s, 61 °C for 15 s, and 72 °C for 2.30 min). Next, 1/20th of the amplified products was used as a template for the second-round PCR for 45 amplification cycles (pre-denatured at 95 °C for 2 min, followed by 95 °C for 15 s, 61 °C for 30 s, and 72 °C for 1 min). To detect cellular HIV-1 mRNA, the reaction was done for 32 cycles (pre-denatured at 95 °C for 2 min, followed by 95 °C for 15 s, 65 °C for 30 s, and 68 °C for 1 min). The specificity of the targets was determined by the value of the melting temperature (Tm).
To analyze the results, the threshold cycle (Ct) from each amplified target was normalized with GAPDH. The relative expression or fold change was determined by comparing it with the positive control.
2.8. Reversion of mTORC1 inhibition
MHY1485 was used to reverse mTORC1 inhibition. Cells were incubated with a mixture of MHY1485 and niclosamide in an indicated condition, and the luciferase signal was then analyzed.
2.9. Intracellular ATP measurement
Cells were measured for their intracellular ATP level using a commercial ATPlite luminescence ATP detection assay system (PerkinElmer) according to the manufacturer's instructions.
2.10. Western blotting
Cells were washed with PBS and harvested in RIPA buffer. Lysates were centrifuged at 14,000 g for 10 min at 4 °C and protein extracts were measured by Bradford assay. Next, 30 μg of protein was subjected to SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked with 3% not-fat dry milk in Tris-buffered saline containing 0.01% Tween 20 (TBST) buffer and then incubated with the indicated antibodies overnight at 4 °C.
The antibodies for the mTORC1 signaling pathway were purchased from Cell Signaling Technology as follows: anti-phospho AMPK (#2535), anti- AMPK (#5831), anti-phospho Thr389 p70-S6K1 (#9234), anti- p70-S6K1 (#9202), anti-phospho Thr37/46 4EBP1 (#2855), anti- 4EBP1 (#9644), anti-phospho mTOR (#2971), and anti-mTOR (#2983). Anti-HIV-1 p24 antibody (ab9071) was purchased from Abcam. Additionally, the anti-GAPDH antibody (sc-47724) used as an internal control was purchased from Santa Cruz Biotechnology Inc. Specific bands were detected using HRP-labeled anti-mouse or anti-rabbit IgG, and the reactions were developed using the enhanced chemiluminescence system (Bio-Rad). The protein band density was quantified using ImageJ and normalized to the GAPDH control.
2.11. Statistical analysis
All the results were obtained from at least three independent experiments. Data are described herein as the mean ± SD. Statistical analysis was performed by using unpaired Student's t-tests for comparing the differences between groups. Significance was accepted when p < 0.05.
2.12. Biosafety
This study was approved by the Siriraj Safety Risk Management Taskforce, Mahidol University (approval no. SI 2019-007).
3. Results
3.1. Cellular toxicity of niclosamide
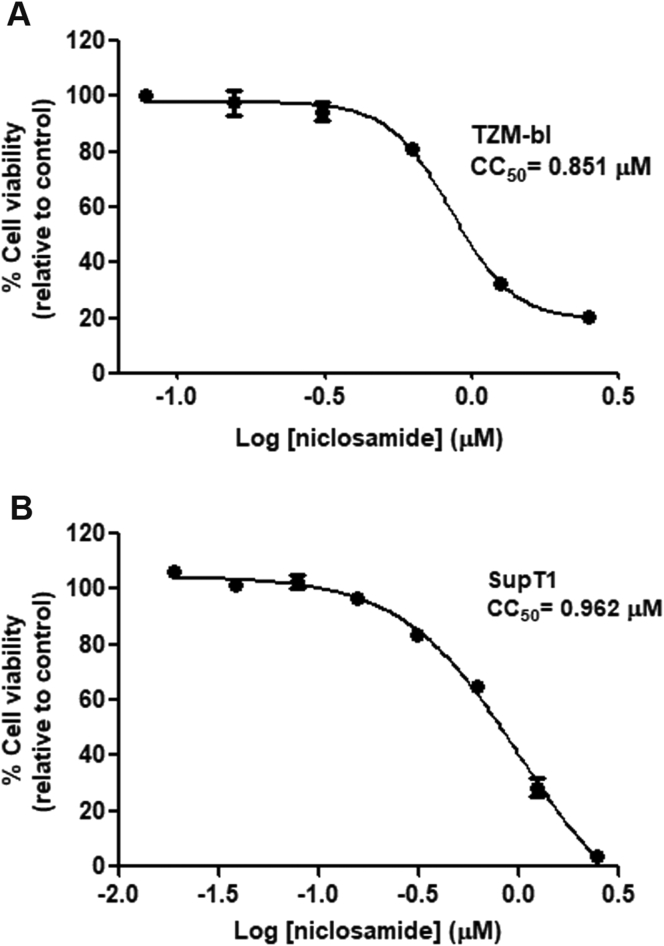
TZM-bl and SupT1 cells, which are susceptible to HIV-1 infection, were evaluated for the cytotoxicity of niclosamide (Figure 1A and B). Both cell types were sensitive to niclosamide, and the concentrations that reduced the proliferation of cells by 50% (CC50) were 0.851 μM in TZM-bl cells and 0.962 μM in SupT1 cells. The selected nontoxic concentrations (≥80% cell viability) for all subsequent experiments in TZM-bl and SupT1 cells were 0.625 μM and 0.312 μM, respectively.
Figure 1.
Cellular cytotoxicity of niclosamide. Cells were treated with niclosamide at indicated concentrations (μM) for 48 h in (A) TZM-bl cells and 144 h in (B) SupT1 cells. Cell viability and CC50 of niclosamide were determined. Data are mean with SD from triplicate experiments.
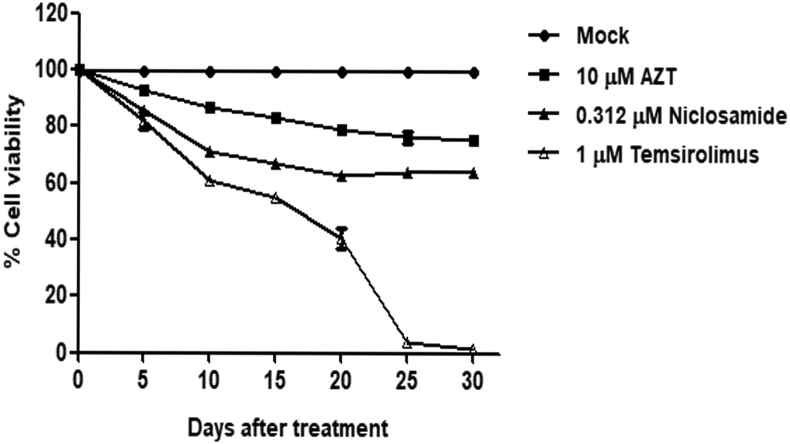
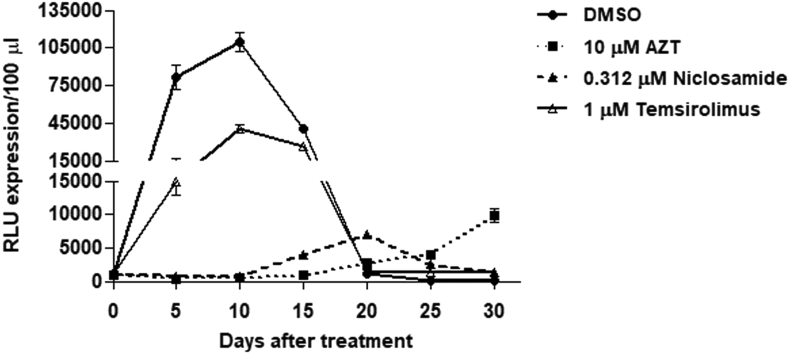
In addition, SupT1 cells were evaluated for long-term cytotoxicity of niclosamide treatment for 30 days by comparing with DMSO-, temsirolimus-, and nucleoside reverse transcriptase inhibitor (NRTI) zidovudine (AZT) used as reference drug (Figure 2). After an early treatment for 5 days, niclosamide and other drugs showed the percentage of cell viability above 80%. Among these, AZT-treated cells had the lowest cytotoxicity. However, more cytotoxicity from all drugs was then observed after day 5, especially in temsirolimus-treated cells causing a marked decrease of cell survival. Both AZT- and niclosamide-treated cells demonstrated a similar trend of cytotoxic results.
Figure 2.
Niclosamide cytotoxicity in a long-term treatment. SupT1 cells were treated with niclosamide or other drugs as indicated for 30 days. Every 5 days, cells were passaged in the presence of indicated drugs and (%) cell viability was determined by trypan blue staining. Data are mean with SD from duplicate experiments.
3.2. Effect of time-of-addition for the activity of niclosamide on HIV-1 replication
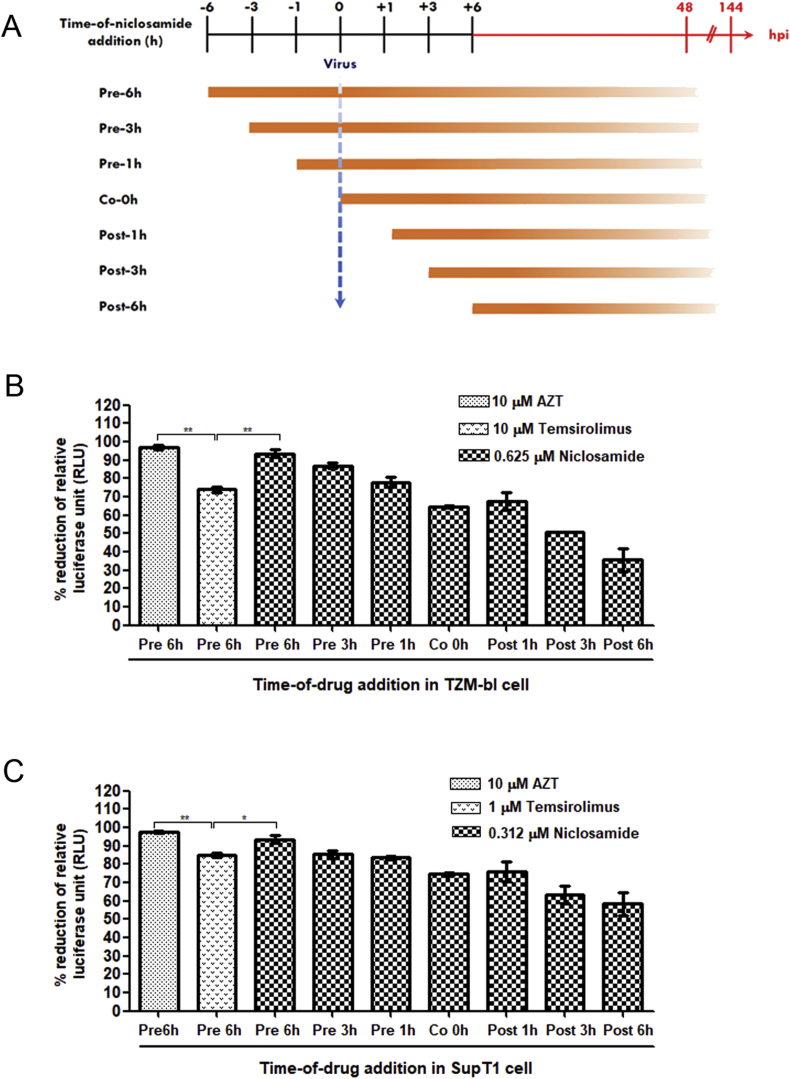
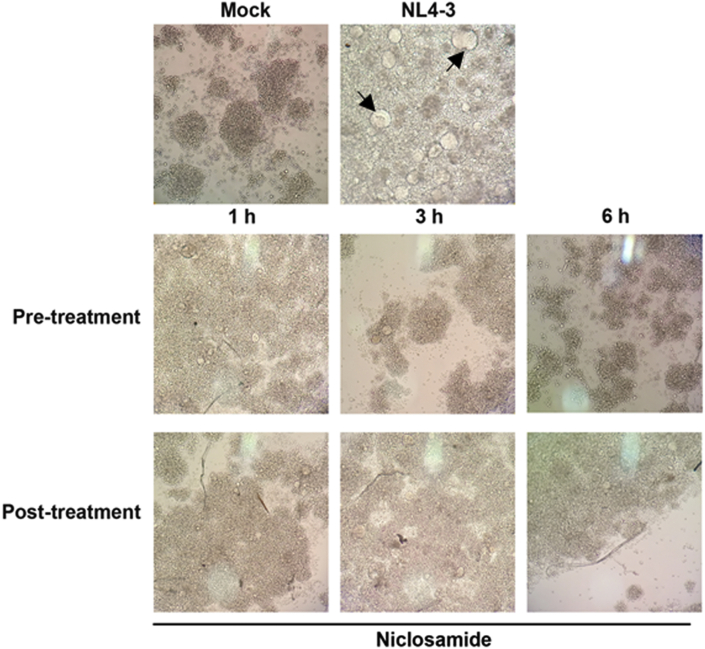
To investigate the effect of the time of addition on HIV-1 replication, measurements were taken with adding the drug at 1, 3, and 6 h before (pre-treatment), 0 h during (co-treatment), and 1, 3, and 6 h after (post-treatment) of HIV-1-infection (Figure 3A). The percentage reduction in relative luciferase units (RLUs) compared to mock-treated HIV-1-infected cells was calculated (Figure 3B and C). We found that pretreatment with niclosamide had more inhibitory effect on HIV-1 replication than during co- or post-treatment conditions. Notably, the pretreatment with niclosamide for 6 h showed the highest reduction of luciferase expressions: 94% in TZM-bl cells and 92% in SupT1 cells. The inhibitory effect of niclosamide was also observed through a reduction of HIV-1-produced CPE in SupT1 cells. As shown in Figure 4, CPE of HIV-1 decreased in a time-dependent manner. Cells pretreated with niclosamide for 6 h most efficiently suppressed HIV-1 infection. Taken together, this would seem to emphasize that niclosamide requires a suitable pre-incubation time for its optimal anti-HIV-1 activity. Thus, pretreatment with niclosamide for 6 h was used for all subsequent experiments.
Figure 3.
Effect of the time-of-addition niclosamide study. (A) Schematic representation of the time-of-addition study. Cells were treated with niclosamide at indicated time points before infection (Pre-1, -3, and -6 h), during infection (Co 0 h), and after infection (Post-1, -3, and -6 h). The virus titer was determined by luciferase assay after 48 h for TZM-bl cells and 144 h for SupT1 cells. (B) TZM-bl cells and (C) SupT1 cells were treated with niclosamide or other drugs as indicated under specified times and conditions. The percentage of reduction of RLU was calculated by comparing with the virus-infected cells with DMSO treatment. Data are mean with SD from three independent experiments. Statistical significance was determined with unpaired t-test. ∗p < 0.05 and ∗∗p < 0.01.
Figure 4.
Effect of niclosamide on the formation of HIV-1-induced CPE. SupT1 cells were treated with 0.312 μM niclosamide under specified times and conditions. The presence of syncytial formation (indicated by arrows) was daily observed under a light microscope (10X).
In Figure 3B and C, furthermore, the anti-HIV-1 activity of niclosamide was compared to AZT and temsirolimus mTORC1 inhibitor, which was reported to show HIV inhibition (Nicoletti et al., 2011). AZT at 10 μM efficiently inhibited HIV-1 infection showing 97% and 98% inhibition in TZM-bl cells and SupT1 cells, respectively. However, inhibitory effect of temsilorimus was significantly lower than that of niclosamide and AZT. Temsilorimus decreased HIV-1 expression by 74% and 85% in TZM-bl and SupT1 cells, respectively.
Similarly, the inhibitory effect against HIV-1 replication in prolonged treatment with niclosamide was also verified in SupT1 cells for 30 days (Figure 5). A luciferase activity from viral culture supernatants was monitored in every passage. Treatment with niclosamide and AZT showed a greater inhibitory efficacy against HIV-1 infection than temsirolimus treatment, in which majority of cells were infected at day 10 resulting in subsequent cell death. After day 10–15, low level of viral replication was observed in niclosamide – and AZT-treated cells suggesting emergence of resistant viruses, which caused a gradual cell death in niclosamide-treated cells.
Figure 5.
Inhibitory effect of niclosamide in a long-term treatment. HIV-1-infected SupT1 cells were treated with niclosamide or other drugs as indicated for 30 days. Every 5 days, cells were passaged in the presence of indicated drugs and viral supernatants were measured the luciferase activity as expressed in RLUs/100 μl. Data are mean with SD from two independent experiments.
3.3. Effect of dose-dependent study on HIV-1 replication
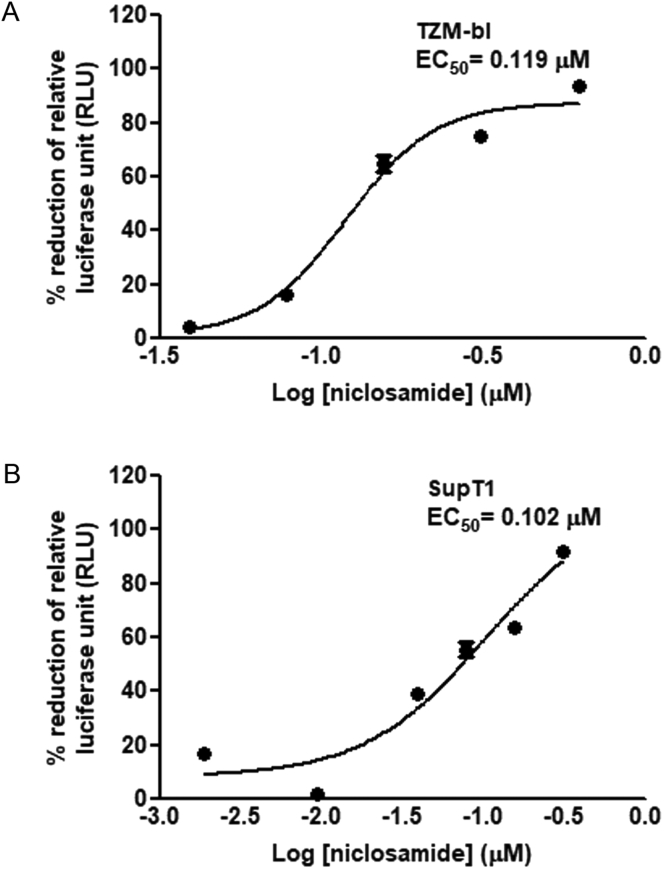
Beside the time-dependent study, the inhibitory effect from varying niclosamide concentrations to treat HIV-1 was also determined. As shown in Figure 6A and B, anti-HIV-1 activity of niclosamide was dose-dependent in both cell types with a 50% effective concentration (EC50) values of 0.119 μM in TZM-bl cells and 0.102 μM in SupT1 cells. A ratio of CC50/EC50 was translated to a cell-based therapeutic index (TI) of 7.1 in TZM-bl cells and 9.4 in SupT1 cells.
Figure 6.
Dose-dependent inhibition of niclosamide. (A) TZM-bl cells and (B) SupT1 cells were pretreated for 6 h with twofold dilutions of niclosamide as indicated before HIV-1 infection. The percentage of reduction of RLUs and EC50 were calculated after the incubation time. Data are mean with SD from three independent experiments.
3.4. Effect of niclosamide on reverse transcription
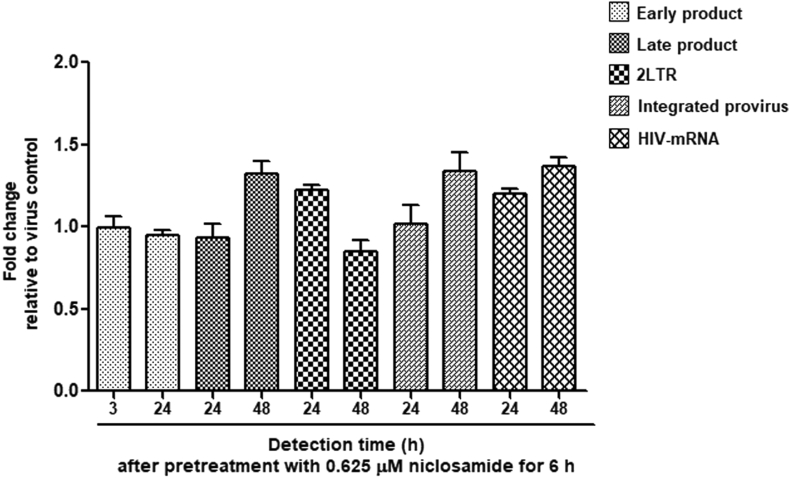
To determine whether the mechanism of niclosamide against HIV-1 affects the early phase of viral replication, we used real-time PCR to detect the relative expression of HIV-1 viral DNA products from each step in reverse transcription and of cellular HIV-1 mRNA by comparing between the samples of niclosamide- and mock-treated HIV-1 infected TZM-bl cells. Fold changes of early reverse transcription products, late reverse transcription products, 2-LTR circle unintegrated products, integrated provirus products, and cellular HIV-1 mRNA were analyzed, as shown in Figure 7. Pretreatment with niclosamide for 6 h in HIV-1-infected cells did not disrupt the steps in HIV-1 reverse transcription or in transcription of the provirus. Thus, niclosamide may inhibit HIV-1 through post-transcription steps.
Figure 7.
Effect of niclosamide on reverse transcription and proviral transcription. Real-time PCR was used to detect gene expression from the products of reverse transcription (early-, late-, 2LTR, and integrated provirus) and transcription (HIV-1 mRNA) by specific primers, and the expression was normalized by GAPDH. The fold change of expression was compared between niclosamide treated-HIV-1 infected cells and HIV-1 infected cells at different time points. Data are mean with SD from three independent experiments.
3.5. Reversion of niclosamide activity by mTOR activator
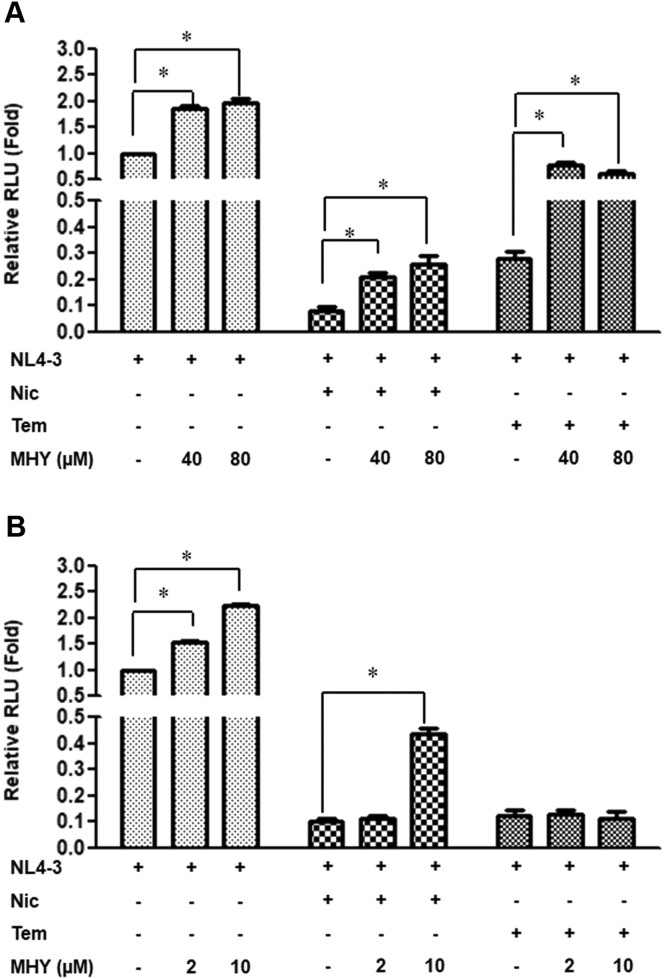
Mazzon et al. (2019) in their study suggested that the antiviral activity of niclosamide was likely due to the inhibition of viral protein translation. We hypothesized that disruption of the mTORC1 pathway by niclosamide may involve this process. To investigate whether the inhibition of mTORC1 was involved in HIV-1 suppression, the MHY1485 (MHY)-mTORC1 activator was used to neutralize mTOR inhibitory activity of niclosamide by upregulating p-mTOR via a downstream feedback of autophagy inhibition (Choi et al., 2012). It was found that MHY significantly increased HIV-1 replication in HIV-1-infected cells with DMSO treatment (Figure 8A and B). In TZM-bl cells, 40 and 80 μM of MHY could partially reverse HIV-1 inhibition by both niclosamide and temsirolimus (Figure 8A). However, we could not test MHY in SupT1 cells at as high concentrations as that tested in TZM-bl cells because this cell was more sensitive to the cytotoxic effect of MHY. Indeed, 10 μM of MHY was the highest concentration that could be used, which could only partially neutralize niclosamide activity (Figure 8B).
Figure 8.
Reversion of niclosamide activity by MHY1485. DMSO-, niclosamide-, and temsirolimus-treated virus-infected cells were co-incubated with or without MHY1485 for 48 h in (A) TZM-bl cells and 144 h in (B) SupT1 cells. The fold change of RLU expression was determined. Data are mean with SD from three independent experiments. Statistical significance was determined with unpaired t-test. ∗p < 0.05.
3.6. Expression of the mTORC1 signaling pathway and HIV-1 protein
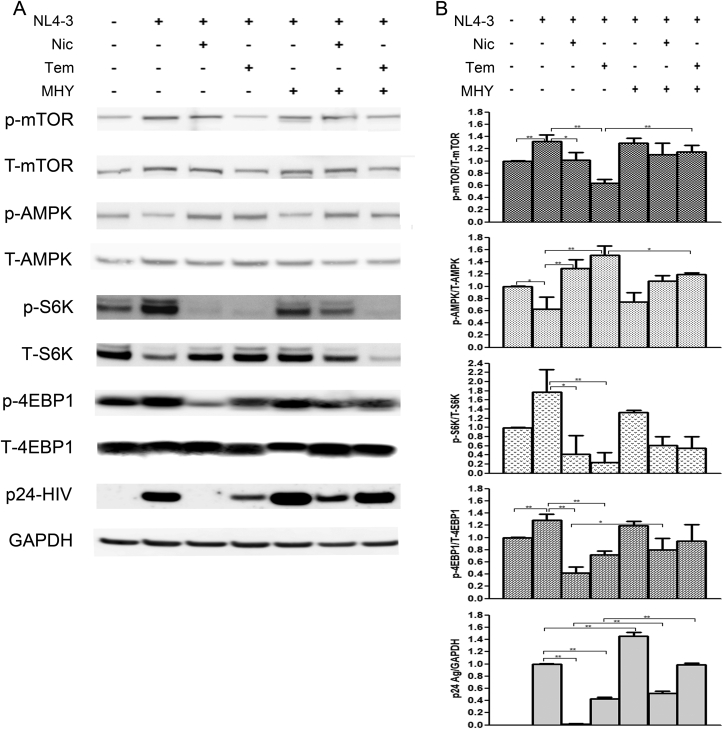
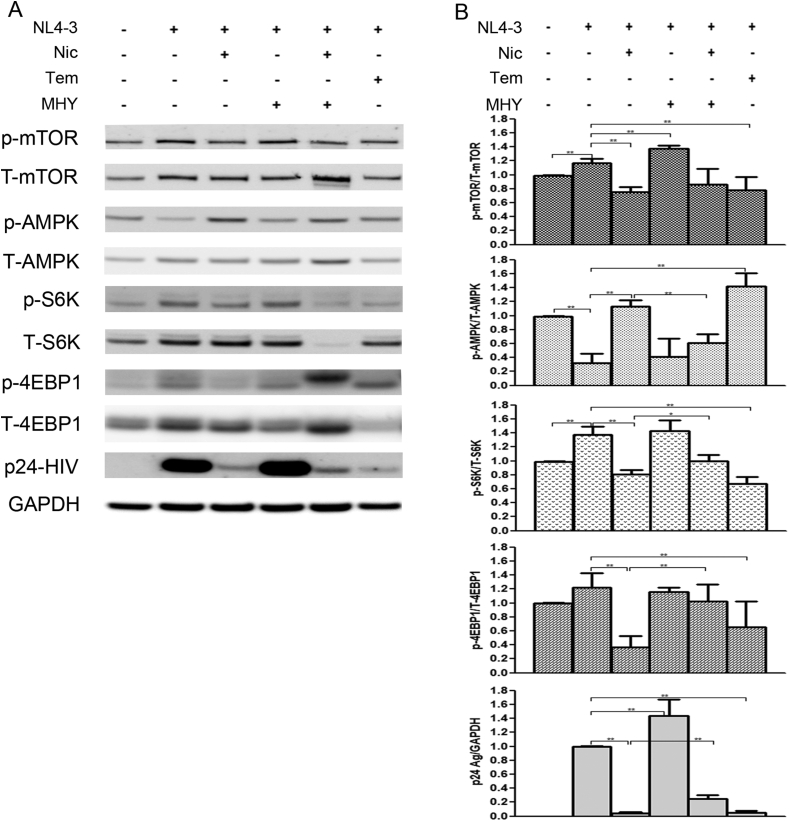
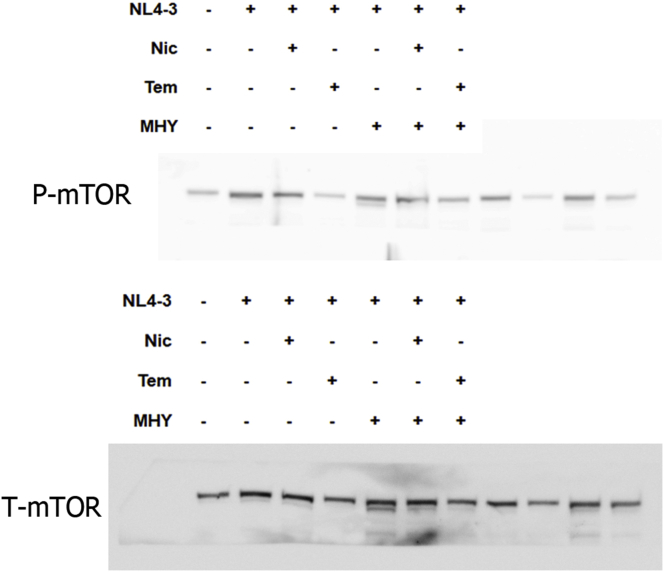
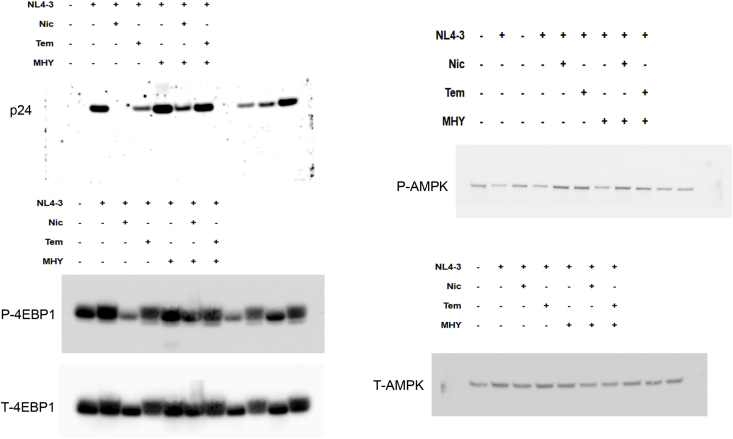
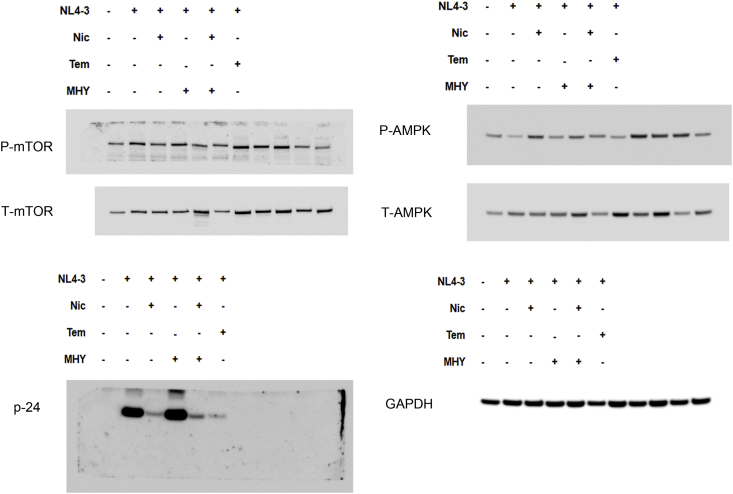
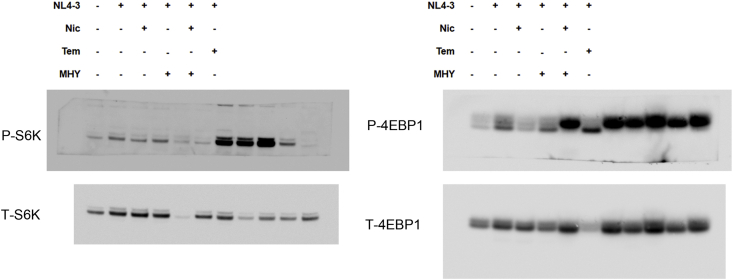
To gain an insight into the anti-HIV-1 mechanism of niclosamide through mTORC1 inhibition, changes of target protein expression levels in the mTORC1 pathway and HIV-1 p24 protein were examined (Figures 9 and 10). HIV-1-infection in TZM-bl cells and SupT1 cells could activate the mTORC1 pathway by increasing mTOR and the phosphorylation of its downstream substrates, including p70-S6K1 and eIF4E-binding protein 1 (4EBP1). This activation was reduced when treated with either niclosamide or temsirolimus, correlating with a decrease in p24-HIV-1 protein. Treatment with MHY mTORC1 activator could increase HIV-1-viral protein production and partially reverse mTORC1 inhibition by niclosamide and temsirolimus showing increased phosphorylation of p-4EBP1 in niclosamide-treated cells and increased phosphorylation of P-mTOR and P-S6K in temsirolimus-treated cells. The co-occurrence of suppression and partial reversion in both HIV-1 protein and mTORC1 activation levels by niclosamide/temsirolimus and MHY, respectively, indicated a mechanistic link between mTORC1 suppression and HIV-1 inhibition by niclosamide and temsirolimus.
Figure 9.
Western blotting of niclosamide treatment on HIV-1 infection in TZM-bl cells. (A) Mock cells or HIV-1-infected cells were treated with or without DMSO, niclosamide, temsirolimus, or MHY1485 as indicated for 48 h. Proteins were then extracted from cells to perform western blotting to detect protein expressions between phosphorylated- and total forms of mTOR, AMPK, S6K, 4EBP1, HIV-1 p24 Ag, and GAPDH as loading control. Full pictures of the western blot membranes are provided in supplementary material files (S1–S3 Figures). (B) Target proteins were quantitated and the relative expressions were determined. Data are mean with SD from three independent experiments. Statistical significance was determined with unpaired t-test. ∗p < 0.05 and ∗∗p < 0.01.
Figure 10.
Western blotting of niclosamide treatment on HIV-1 infection in SupT1 cells. (A) Mock cells or HIV-1-infected cells were treated with or without DMSO, niclosamide, temsirolimus, or MHY1485 as indicated for 144 h. Proteins were then extracted from cells to perform western blotting to detect protein expressions between phosphorylated- and total forms of mTOR, AMPK, S6K, 4EBP1, HIV-1 p24 Ag, and GAPDH as loading control. Full pictures of the western blot membranes are provided in supplementary material files (S4–S5 Figures). (B) Target proteins were quantitated and the relative expressions were determined. Data are mean with SD from three independent experiments. Statistical significance was determined with unpaired t-test. ∗p < 0.05 and ∗∗p < 0.01.
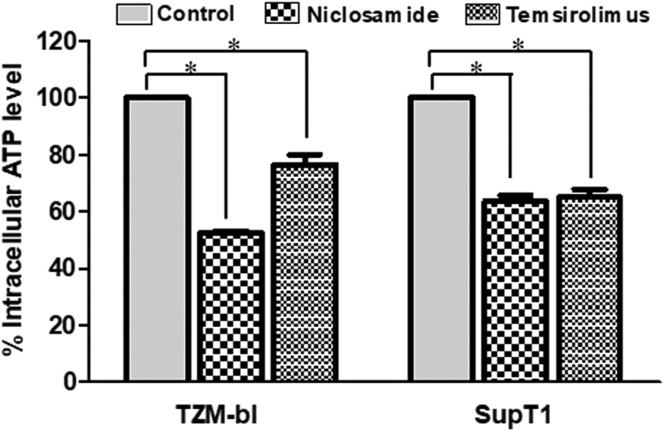
The mitochondrial uncoupling activity of niclosamide can reduce intracellular ATP (Hamilton and Rath, 2017). Here, we revealed that niclosamide- and temsirolimus-treated cells depleted intracellular ATP levels (Figure 11). This may lead to mTORC1 inhibition via the activation of AMP-activated protein kinase (AMPK). We then examined AMPK expression and found a significant increase in AMPK phosphorylation (Figures 9 and 10). This suggests that the anti-HIV-1 mechanism of niclosamide could occur through the AMPK-mTORC1 pathway.
Figure 11.
Measurement of intracellular ATP. Cells were treated with DMSO control solvent, niclosamide or temsirolimus. After the incubation time, cells were lysed to measure the ATP level. The percentage of intracellular ATP was calculated by comparing with mock-treated cell control. Data are mean with SD from three independent experiments. Statistical significance was determined with unpaired t-test. ∗p < 0.05.
4. Discussion
The protonophoric activity of niclosamide plays a key role in inhibiting the replication of pH-dependent viruses that require low-pH within the endosome in the uncoating process; however, the consequence of endosomal neutralization causes mTORC1 disruption. Huang et al. (2017) were the first to reveal the anti-pH independent viral activity of niclosamide, which can inhibit EBV replication via mTORC1 inhibition. Likewise, HIV-1 is a pH-independent virus and requires the mTORC1 pathway for viral replication. We showed here that niclosamide effectively inhibited HIV-1 through mTORC1 inhibition without disruption at the early replication phase of reverse transcription and proviral transcription. This finding could support the recent studies that presented the promising anti-HIV-1 activity of niclosamide (Fan et al., 2019; Mazzon et al., 2019).
Niclosamide has been used to treat parasitic infections in humans for over 50 years with a good safety profile (Chen et al., 2018). Because niclosamide inhibits HIV-1 through a cellular target inhibition, its cell-based therapeutic index is lower than that of antiretroviral drugs designing to act on viral proteins. As the study of Kobayashi et al. (2011), a next-generation HIV integrase inhibitor of S/GSK1349572 showed a therapeutic index of at least 9,400. Nevertheless, long-term toxicity is still a problem in current antiretroviral drugs (Anderson et al., 2004; Kakuda, 2000). There are no data on long-term use of niclosamide in humans and toxicity may be a major concern.
As mentioned above, niclosamide is recognized as a broad-spectrum entry inhibitor of pH-dependent viruses under submicrololar level (Jung et al., 2019; Jurgeit et al., 2012; Wang et al., 2016; Xu et al., 2016). Time-of-niclosamide addition in previous studies indicated that niclosamide exerts its antiviral activity at early stage of replication by showing a greater inhibitory effect at co- or early post-treatments (Jung et al., 2019; Jurgeit et al., 2012; Kao et al., 2018). However, its activity against pH-independent viruses is expected to use different mechanisms, such as mTORC1 inhibition. Based on the study of Fonseca et al. (2012), niclosamide required 1–4 h for the mTORC1 inhibition. This is in agreement with our data that pretreatment for 6 h showed the greatest efficacy as the pretreatment time allowed niclosamide to reach maximum effect on mTORC1 by the time of infection. Having to apply the drug prior to infection may seem impractical for treatment, but in a chronic infection situation as in HIV-1 infection, this requirement is irrelevant since there will always be new infected cells occurring at a roughly constant rate and whether a drug takes effect immediately or 6 h afterward should make little difference.
Our data and other (Kobayashi et al., 2011), however, found that using a single drug to treat HIV-1 in long-term treatment could induce drug-resistant viruses resulting in therapeutic failure. Although the problems of cytotoxicity and occurrence of resistant-viruses in the long-term treatment by niclosamide are main obstacle to successful repurposing, a combination therapy by using the synergism of two or three drugs with different mechanisms of action (Sun et al., 2016) may help minimize cytotoxicity and maximize efficacy.
In part of the mechanism, our data indicated that the HIV-1 inhibition occurred after transcription, which is consistent with the study of Mazzon et al. (2019), who did not find a reduction of HIV-1 mRNA transcription after treatment with niclosamide. However, it is in disagreement with the recent study of Fan et al. (2019). They reported the inhibitory effect of niclosamide against HIV-1 occurring at the transcription process. Our data revealed that HIV-1 was probaby inhibited by niclosamide through the mTORC1 inhibition, which had a deleterious effect on viral protein synthesis. Niclosamide treatment showed a compatible result to temsirolimus, a rapamycin analog-classical mTORC1 inhibitor, used as a control. However, niclosamide had a higher antiviral activity than temsirolimus that may due to lack of ability to inhibit all the signaling cascade mediated by mTORC1 (Gingras et al., 2001). Additionally, using MHY-mTOR activator in co-treatment with both niclosamide and temsirolimus seemed to support the mechanism of anti-HIV-1 through the mTORC1 inhibition, although in SupT1 cells, MHY could not neutralize temsirolimus activity. This may be because temsirolimus required a high concentration of MHY, which could not be used in SupT1 cells, to neutralize the high-affinity binding to an allosteric site at mTOR.
Downregulation in the mTORC1 pathway and HIV-1 p24 protein was detected after niclosamide treatment, and a partial upregulation was also found under neutralization by MHY. The energy sensor AMPK, an upstream negative regulator in the mTORC1 pathway, was activated. The treatment with niclosamide caused a depletion of intracellular ATP, which occured through interfering with the mitochondrial oxidative phosphorylation (Hamilton and Rath, 2017); thus, causing a direct effect on AMPK activation. AMPK can directly inhibit the mTORC1 through the phosphorylation of mTOR protein at the Thr 2446 site. It can also indirectly inhibit mTORC1 by suppressing the formation of mTORC1 via increasing the phosphorylation of other mTORC1-regulatory proteins, especially tuberous sclerosis complex (TSC), a negative regulator of mTORC1, and Raptor (Ke et al., 2018). Besides, we found that treatment with temsirolimus could reduce intracellular ATP as well. This may result from an inhibition of glycolysis, a downstream process that is controlled by mTORC1 (Saxton and Sabatini, 2017).
In summary, this in vitro study revealed a new mechanism of niclosamide anti-HIV-1 activity via the energy stress-activated AMPK-mTORC1 pathway that affected viral protein synthesis.
Declarations
Author contribution statement
Nattamon Niyomdecha: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ornpreya Suptawiwat: Performed the experiments; Analyzed and interpreted the data.
Chompunuch Boonarkart, Kunlakunya Jitobaom: Contributed reagents, materials, analysis tools or data.
Prasert Auewarakul: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by the Thailand Research Fund [Grant no. IRN60W0002] and Thammasat University, Thailand [Grant no. 006, the fiscal year 2017].
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
S1 Figure.tif.
S2 Figure.tif.
S3 Figure.tif.
S4 Figure.tif.
S5 Figure.tif.
References
- Adachi A., Gendelman H.E., Koenig S., Folks T., Willey R., Rabson A., Martin M.A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC253077/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P.L., Kakuda T.N., Lichtenstein K.A. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin. Infect. Dis. 2004;38:743–753. doi: 10.1086/381678. [DOI] [PubMed] [Google Scholar]
- Chen W., Mook R.A., Jr., Premont R.T., Wang J. Niclosamide: beyond an antihelminthic drug. Cell. Signal. 2018;41:89–96. doi: 10.1016/j.cellsig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.J., Park Y.J., Park J.Y., Jeong H.O., Kim D.H., Ha Y.M., Kim J.M., Song Y.M., Heo H.S., Yu B.P., Chun P., Moon H.R., Chung H.Y. Inhibitory effect of mTOR activator MHY1485 on autophagy: suppression of lysosomal fusion. PloS One. 2012;7:1–10. doi: 10.1371/journal.pone.0043418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Xu J., Files M., Cirillo J.D., Endsley J.J., Zhou J., Endsley M.A. Dual activity of niclosamide to suppress replication of integrated HIV-1 and Mycobacterium tuberculosis (Beijing) Tuberculosis. 2019;116:S28–S33. doi: 10.1016/j.tube.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca B.D., Diering G.H., Bidinosti M.A., Dalal K., Alain T., Galgi A.D., Forestieri R., Nodwell M., Rajadurai C.V., Gunaratnam C., Tee A.R., Duong F., Andersen R.J., Orlowski J., Numata M., Sonenberg N., Roberge M. Structure-activity analysis of niclosamide reveals a potential role for cytoplasmic pH in the control of mammalian target of rapamycin complex 1 (mTORC1) signaling. J. Biol. Chem. 2012;1–26 doi: 10.1074/jbc.M112.359638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A.C., Raught B., Gygi S.P., Niedzwiecka A., Miron M., Burley S.K., Polakiewicz R.D., Wyslouch-Cieszynska A., Aebersold R., Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton G., Rath B. Repurposing of anthelminthics as anticancer drugs. Oncomedicine. 2017;2:142–149. [Google Scholar]
- Heredia A., Le N., Gartenhaus R.B., Sausville E., Medina-Monero S., Zapata J.C., Davis C., Gallo R.C., Redfield R.R. Targeting of mTOR catalytic site inhibits multiple steps of the HIV-1 lifecycle and suppresses HIV-1 viremia in humanized mice. Proc. Natl. Acad. Sci. U.S.A. 2015;112:9412–9417. doi: 10.1073/pnas.1511144112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermankova M., Siliciano J.D., Zhou Y., Monie D., Chadwick K., Margolick J.B., Quinn T.C., Siliciano R.F. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J. Virol. 2003;77:7383–7392. doi: 10.1128/JVI.77.13.7383-7392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Yang M., Yuan Y., Li X., Kuang E. Niclosamide inhibits lytic replication of Epstein-Barr virus by disrupting mTOR activation. Antivir. Res. 2017;138:68–78. doi: 10.1016/j.antiviral.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Jung E., Nam S., Oh H., Jun S., Ro H.J., Kim B., Kim M., Go Y.Y. Neutralization of acidic intracellular vesicles by niclosamide inhibits multiple steps of the dengue virus life cycle in vitro. 2019;9:1–12. doi: 10.1038/s41598-019-45095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgeit A., McDowell R., Moese S., Meldrum E., Schwendener, Greber U.F. Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects. PLoS Pathog. 2012;8:1–14. doi: 10.1371/journal.ppat.1002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadri H., Lambourne O.A., Mehellou Y. Niclosamide, a drug with many (re)purposes. ChemMedChem. 2018;13:1088–1091. doi: 10.1002/cmdc.201800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J.C., HuangFu W.C., Tsai T.T., Ho M.R., Jhan M.K., Shen T.J., Tseng P.C., Wang Y.T., Lin C.F. The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR. PLoS. Negl. Trop. Dis. 2018;12:1–16. doi: 10.1371/journal.pntd.0006715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda T.N. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Therapeut. 2000;22:685–708. doi: 10.1016/S0149-2918(00)90004-3. [DOI] [PubMed] [Google Scholar]
- Ke R., Xu Q., Li C., Luo L., Huang D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2018;42:384–392. doi: 10.1002/cbin.10915. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Yoshinaga T., Seki T., Wakasa-Morimoto C., Brown K.,W.,, Ferris R., Foster S.A., Hazen R.J., Miki S., Suyama-Kagitani A., Kawauchi-Miki S., Taishi T., Kawasuji T., Johns B.A., Underwood M.R., Garvey E.P., Sato A., Fujiwara T. In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob. Agents Chemother. 2011;55:813–821. doi: 10.1128/AAC.01209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzon M., Ortega-Prieto A.M., Imrie D., Luft C., Hess L., Czieso S., Grove J., Skelton J.K., Farleigh L., Bugert J.J., Wright E., Temperton N., Angell R., Oxenford S., Jacobs M., Ketteler R., Dorner M., Marsh M. Identification of broad-spectrum antiviral compounds by targeting viral entry. Viruses. 2019;11:1–26. doi: 10.3390/v11020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbisa J.L., Delviks-Frankenberry K.A., Thomas J.A., Gorelick R.J., Pathak V.K. HIV Protocols. Humana Press; 2009. Real-time PCR analysis of HIV-1 replication post-entry events; pp. 55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Fagone P., Meroni P., McCubrey J., Bendtzen K. mTOR as a multifunctional therapeutic target in HIV infection. Drug Discov. Today. 2011;16:715–721. doi: 10.1016/j.drudis.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Sage V.L., Cinti A., Amorim R., Mouland A.J. Adapting the stress response: viral subversion of the mTOR signaling pathway. Viruses. 2016;8:1–19. doi: 10.3390/v8060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Sanderson P.E., Zheng W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today. 2016;21:1189–1195. doi: 10.1016/j.drudis.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.M., Lu J.W., Lin C.C., Chin Y.F., Wu T.Y., Lin L.I., Lai Z.Z., Kuo S.C., Ho Y.J. Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission. Antivir. Res. 2016;135:81–90. doi: 10.1016/j.antiviral.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Lee E.M., Wen Z., Cheng Y., Huang W.K., Qian X., Tcw J., Kouznetsova J., Ogden S.C., Hammack C., Jacob F., Nguyen H.N., Itkin M., Hanna C., Shinn P., Allen C., Michael S.G., Simeonov A., Huang W., Christian K.M., Goate A., Brennand K.J., Huang R., Xia M., Ming G.L., Zheng W., Song H., Tang H. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016;22:1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.