Abstract
The protocols for managing intractable idiopathic epistaxis have evolved with advances in endoscopic techniques. Transnasal endoscopic sphenopalatine artery ligation (TESPAL) has been the treatment of choice for idiopathic intractable epistaxis. If TESPAL fails, transantral ligation of internal maxillary artery (IMA) used to be the dictum along with radiological interventions. Here we discuss about the role of endoscopic IMA ligation in cases of failed TESPALs. Retrospective study at a tertiary hospital was performed. 28 cases of intractable idiopathic epistaxis underwent TESPAL in our institution of which 2 cases had rebleed. We also had two referred cases of failed TESPALS. Of this 4 patients, three patients underwent endoscopic IMA ligation and one patient underwent selective embolisation. All the patients who underwent endoscopic IMA ligation for failed TESPAL had no further episodes of epistaxis. One patient who underwent selective embolization also had no further episodes of bleed but had transient facial pain and trismus. When TESPAL fails, endoscopic IMA ligation can be considered as an alternative procedure before resorting to embolization.
Keywords: Intractable idiopathic epistaxis, Transnasal endoscopic sphenopalatine artery ligation (TESPAL), Endoscopic internal maxillary artery (IMA) ligation
Introduction
Epistaxis is the most common otolaryngological emergency and accounts for 1 in 200 emergency room consultations [1]. The majority of cases are controlled with common first-line interventions such as anterior nasal packing, posterior nasal packing/nasopharyngeal tamponade, application of haemostatic agents and chemical/electrical cautery. However, there is a subset of patients who bleed despite these measures. Such patients can be termed as having intractable idiopathic epistaxis after ruling out the causes of epistaxis. These patients require more aggressive intervention. Intractable epistaxis is a challenging problem that was traditionally managed with anterior and posterior nasal packing, prolonged hospital admission and with higher health care costs and significant patient morbidity. In 1965, Chandler and Serrins described the transantral approach to ligate the internal maxillary artery in the pterygopalatine fossa [2]. Interventional radiologic techniques for percutaneous endovascular arterial occlusion to treat epistaxis were first described by Sokoloff et al. [3] in 1974 and later this technique was standardized by Lasjaunias and Berenstein [4]. These techniques were nevertheless associated with significant morbidity. In case of angiographic techniques the possible complications include stroke, hemiplegia, facial pain and paresthesia [5]. On the other hand, the complications in patients undergoing transantral maxillary artery ligation are facial swelling, tooth desensitization and oroantral fistula formation [6].With advances in endoscopic techniques, trans nasal endoscopic sphenopalatine artery ligation (TESPAL) gained popularity over the traditional approach. The studies published by Simpson et al. and Winstead point to the terminal internal maxillary artery branches as the final common pathway for posterior epistaxis [7]. Prades in 1976 introduced an endonasal approach to these vessels in his description of an endonasal approach to the sphenopalatine foramen for vidian neurectomy. This technique included the endonasal ligation of the terminal branches of the internal maxillary artery as they exit the foramen. Budrovich and Saetti [8] reported on their own experience with endonasal ligation of sphenopalatine and posterior nasal (nasopalatine) arteries. TESPAL can have a failure rate of 10–15% [9]. Here we are describing how we managed the failed TESPALs by endoscopic internal maxillary artery ligation which is a minimally invasive novel technique for managing intractable epistaxis.
Methods
From 2009 to 2018, we came across 28 cases of intractable epistaxis that continued to bleed despite initial interventions such as nasal packing and/or cauterisation. To rule out local and systemic causes all of these patients underwent a battery of investigations as follows:
Hemogram (Haemoglobin, total and differential leucocyte count, platelet count), peripheral blood smear, blood grouping.
Bleeding profile: Bleeding time, clotting time, Prothrombin time (PT), Activated partial thromboplastin time (APTT).
Liver function tests: alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin and bilirubin.
Diagnostic nasal endoscopy to rule out local causes of epistaxis.
When these investigations were found to be within normal limits and after controlling blood pressure, these patients underwent trans nasal endoscopic sphenopalatine artery ligation. Of these 28 cases, we had 2 cases of rebleed. We also had 2 referred cases of post sphenopalatine artery (SPA) cauterization re-bleed in this time period. These four patients also underwent radiological evaluation (CT scan of nose and paranasal sinuses) to rule out nasal and paranasal pathologies. 1st failed case of SPA ligation done at our institute underwent endoscopic internal maxillary artery ligation. 2nd failed case underwent maxillary artery embolization as they were not willing for a re-surgery. The 2 referred cases of failed SPA cauterization also underwent endoscopic internal maxillary artery ligation.
All cases of endoscopic internal maxillary artery ligation were undertaken under normotensive general anaesthesia. The procedure began with a wide maxillary antrostomy facilitated by widening the antrostomy posteriorly at the cost of posterior fontanelle. Posterior wall of the sinus was thinned out using drill after elevating the mucosa thereby exposing the periosteum. Following incision of the periosteum, the maxillary artery is located within the pad of fat, anterior to the neural elements of the pterygopalatine fossa. After dissecting the fat, IMA is visualized and lifted up (Fig. 1). In all cases of IMA ligation done in our institution we have applied at least two liga clips® over the IMA to make sure that the entire vessel is ligated (Fig. 2).
Fig. 1.
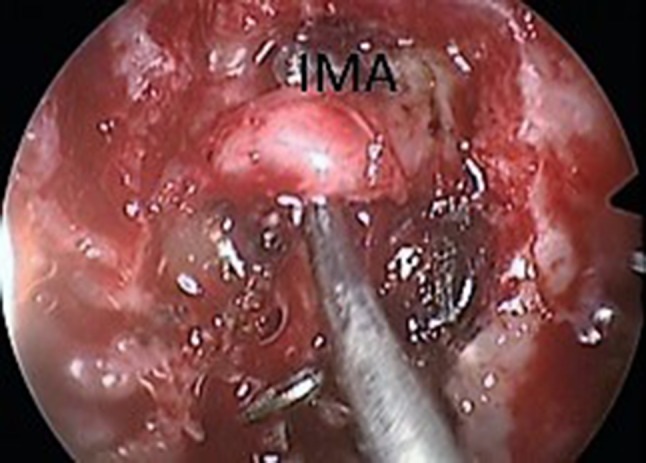
Internal maxillary artery (IMA) being identified and lifted up in the left pterygopalatine fossa
Fig. 2.
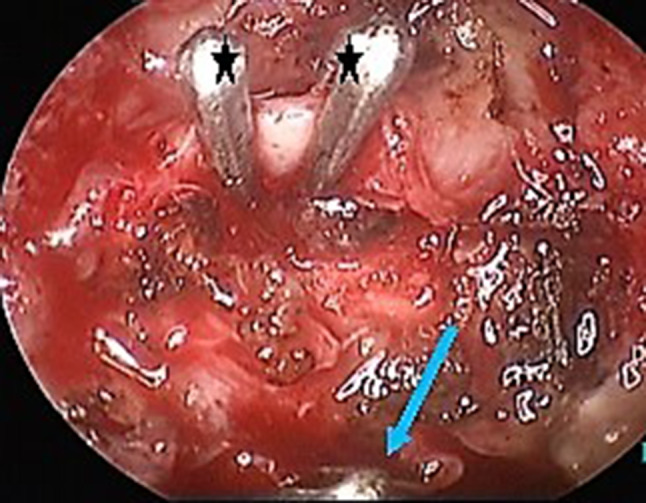
Application of liga clips ® (*) over IMA in the left side. (Liga clip® applied on the descending palatine artery can also be seen [blue arrow])
Results
Patients who have undergone endoscopic internal maxillary artery ligation ideally need follow up for a period of 6 months to 1 year. All these patients who underwent endoscopic IMA ligation in our institution are under regular follow up for 5 years and there has not been further episode of epistaxis in this time period.
Discussion
Intractable idiopathic epistaxis is defined as epistaxis of unknown cause that is refractory to first line interventional modalities to control the bleeding like nasal packing/balloon tamponade, local application of haemostatic agents or cauterization of the bleeding area. Hence to label an epistaxis as idiopathic, both local and systemic causes of nasal bleeding have to be ruled out. The common aetiologies of epistaxis can be attributed to trauma, hypertension, inflammatory diseases (CRS), tumours (benign/malignant), iatrogenic, foreign bodies, mucosal irritation, blood dyscrasias and congenital causes in descending frequencies [10].
When nasal packing/balloon tamponade fails to stop the bleeding it is required to resort to vascular ligation or embolization. Simpson et al. [7] recognized the important role of sphenopalatine artery as the final common pathway of posterior epistaxis control and also noted that access to the sphenopalatine foramen, along the medial antral wall would allow for ligation of vessels supplying virtually all blood flow to the posterior nasal cavity.
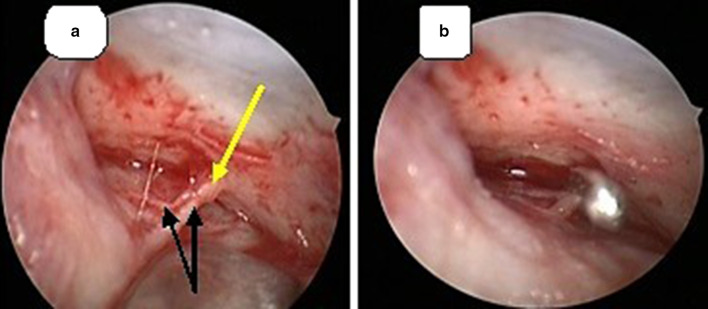
The sphenopalatine artery (SPA) is one of the terminal branches of the internal maxillary artery (IMA) which originates from the external carotid artery system. It provides 90% of the blood supply to the nasal cavity i.e. the lateral nasal wall, the turbinates and most of the nasal septum [11]. The anterior and superior nasal septum is supplied by the anterior and posterior ethmoidal arteries which originate from the ophthalmic artery (internal carotid artery system). The sphenopalatine artery is one of six terminal branches of the 3rd or pterygomaxillary portion of the internal maxillary artery along with descending palatine artery (Fig. 3). These 6 branches all originate in the ptergyopalatine fossa which is located behind the medial part of the posterior wall of the maxillary sinus. The sphenopalatine artery may have 1–10 branches, with 97% of patients having ≥ 2 branches and 67% having ≥ 3 branches. All branches arise from the SPA in the sphenopalatine foramen and enter the nasal cavity as separate blood vessels [11]. The most anterior branch is the largest and exits through the sphenopalatine foramen. This branch needs to be clipped or cauterized and cut to be able to determine the presence of 2 or more posteriorly located vessels if present. The branches of the SPA may exit posterior to the main branch or superior or inferior to the SPA foramen. An accessory artery, the posterior nasal (or nasopalatine), may appear as a separate branch within the pterygomaxillary fossa, within the foramen, or within the nasal cavity [12]. As endoscopic sinus surgery has become more common and as proficiency in endoscopic technique has increased, an endoscopic endonasal approach seems entirely reasonable. Endonasal endoscopic occlusion of the sphenopalatine artery is a much safer operation compared with other radiologic and open surgical techniques and it is more physiologically suited to addressing the problem of intractable epistaxis. But in 28 TESPAL done in our institution, we had a failure rate of 12.5%. The reason for rebleed could be probably due to failure in identifying the terminal branches of IMA or accessory branches of SPA as we encountered in one case of SPA ligation (Fig. 4), hemostatic clip dislodgements, retrograde filling or bleeding from collateral vessels. Hence it is always advisable to look for accessory branches of sphenopalatine artery after ligating the main trunk of SPA by elevating the mucoperiosteal flap of medial antral wall posteriorly up to the choana. Transecting the artery after ligation can provide access to determine the presence of more arterial branches posteriorly, superiorly and inferiorly (Fig. 5). Retrograde filling occurs when the maxillary artery anastomoses with ethmoidal, ophthalmic, intracranial, or pharyngeal vessels and permit retrograde reperfusion of the occluded vascular territory and thereby failure of the procedure. Sometimes rebleed occurs after using monopolar or bipolar cautery for SPA ligation when the artery recoils into the pterygopalatine fossa as encountered in one of our referred in cases of failed TESPAL. Arresting such bleeding within the foramen can be difficult. Hence it is better to cauterize the SPA as medial as possible.
Fig. 3.
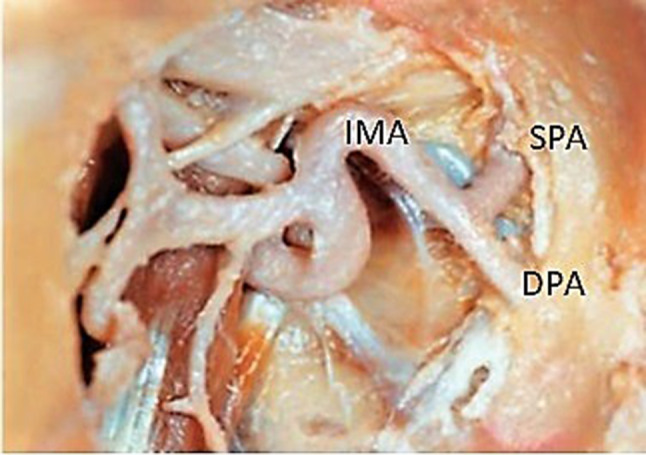
Right pterygopalatine fossa showing sphenopalatine artery (SPA) and descending palatine artery (DPA) arising from internal maxillary artery (IMA)
Fig. 4.
a Anterior and posterior branches (Black arrows) arising from SPA (yellow arrow). b The main SPA trunk being ligated using liga clip® in the left nasal cavity
Fig. 5.
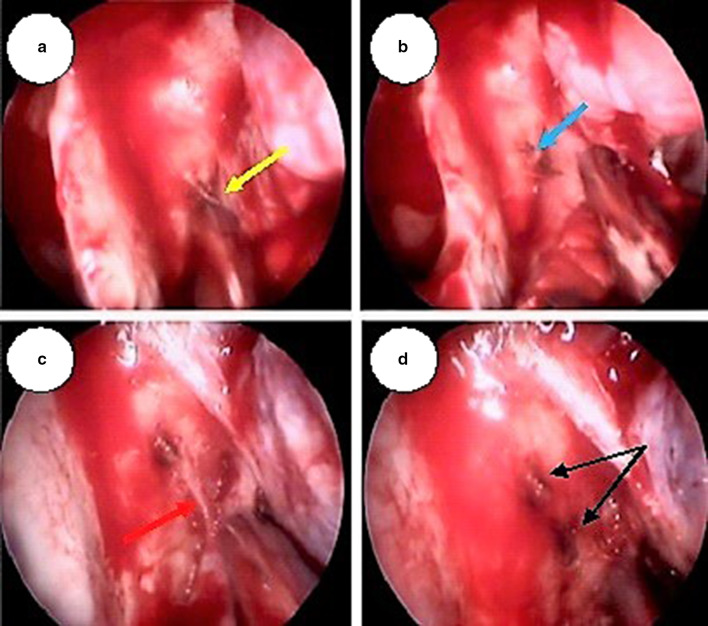
a SPA trunk (yellow arrow) in the right nasal cavity being identified and cauterized, b Cauterized Spa trunk cut (blue arrow). c A posterior branch of SPA which branched before exiting the sphenopalatine foramen (marked as blue arrow) being identified by elevating the mucoperiosteal flap posteriorly up to the choana. d Posterior branch cauterized and cut (black arrows showing cauterized and cut branches)
The terminal branching of IMA into sphenopalatine and descending palatine arteries has variations as demonstrated in a study by Kim, Cho et al. [13]. In this study the posterior wall of the maxilla was divided into 9 sections. As a result of classifying the division point of the maxillary artery where it divided into the Sphenopalatine and descending palatine arteries, the division point was placed on the superior and medial thirds of the posterior wall of the maxilla in most cases (62.0%). However, 30.0% of cases had the maxillary artery division point located on the middle and medial thirds (Fig. 6).
Fig. 6.
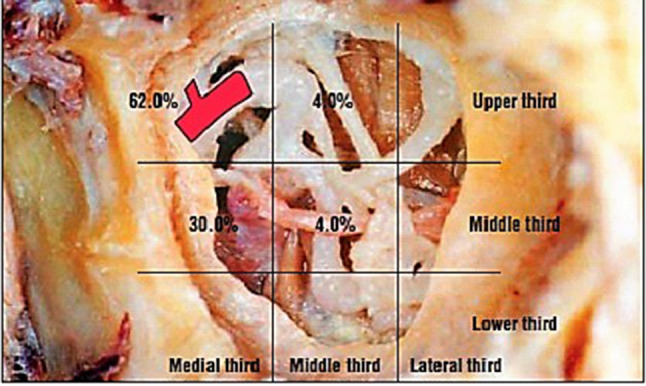
The scheme of the locations of the division point of the maxillary artery, where it divides into the descending palatine artery and the sphenopalatine artery in the pterygopalatine fossa
Hence in this era of minimally invasive surgical procedures, endoscopic ligation of internal maxillary artery is a favoured option for failed TESPALs considering the following facts. Primarily, an endonasal approach avoids all of the potential complications associated with the Caldwell-Luc transantral approach as well as extensive dissection within the pterygomaxillary fossa (blindness, decreased lacrimation, ophthalmoplegia, infection, and hematoma formation). In contrast to the average reported complication rate of 28% for transantral surgery, endonasal approach was without any morbidity. Secondly, endoscopic visualization of the pterygopalatine fossa allows for direct identification and direct access to the IMA. Extensive dissection is rarely required. Thirdly, endoscopic visualization allows for identification of the terminal branching pattern of the internal maxillary artery, with either a single sphenopalatine vessel or two separate sphenopalatine and posterior nasal branches seen. There is no uncertainty as to the vessels being ligated, and no risk of complications due to inadvertent ligation of ophthalmic or intracranial vessels. Fourthly, operative experience with endoscopic sinus surgery has led to considerable expertise and ease of access to pterygopalatine fossa thereby decreasing anaesthesia time compared to the transantral approach. Finally, this procedure, in our hands, had a 100% success rate in controlling posterior epistaxis refractory to TESPAL. On the basis of these parameters, we feel that endoscopic endonasal ligation of the internal maxillary artery has the potential to be the definitive surgical treatment for intractable posterior epistaxis in failed TESPAL [14].
Embolization on the other hand is a viable option for managing intractable idiopathic epistaxis. Selective embolization of distal IMA is done after diagnostic angiography of external and internal carotid arteries to locate the bleeding vessel as well as to rule out vascular anomalies like aneurysms and arteriovenous malformations [15]. Selective embolization can be done under local anaesthesia with shorter hospital stay and has comparable success rate with TESPAL of 80–90%. On the other hand it is a costly, specialized technique which requires expertise and has comparatively less accessibility. Possible complications include accidental embolization of facial artery, ophthalmic artery as well as internal carotid artery. The one patient who underwent selective embolization following failed TESPAL had no further rebleed but had transient facial pain.
In a study by Hervochon et al., bilateral TESPAL obviated the need for another procedure under general anaesthesia in 91.5% of cases versus 75% for unilateral TESPAL. Despite a relatively short follow-up, this study supports the rationale for bilateral rather than unilateral TESPAL in intractable unilateral spontaneous epistaxis and paves the way for further prospective studies [16]. The rationale for a bilateral approach is based on the theory of vascular transmedian anastomoses, which are not precisely described [17]. There has been concern for the risk of ischemic complications following bilateral embolization [18] and bilateral TESPAL, such as septal perforation and partial middle turbinate necrosis [19].
In conclusion, transnasal endoscopic SPA ligation is safe and effective in the management of intractable epistaxis. In earlier days when failure of TESPAL occurs due to dislodgement of haemostatic clips, failure to identify branches of IMA/SPA responsible for bleeding or due to development of collaterals, next line of treatment is either to proceed with transantral ligation of internal maxillary artery or embolization of the bleeding vessel. But in account of the complications of these approaches, endoscopic ligation of internal maxillary artery can be considered prior to embolization in controlling post SPA ligation rebleed.
Funding
None.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pallin DJ, Chng YM, McKay MP, et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46:77–81. doi: 10.1016/j.annemergmed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Chandler JR, Serrins AJ. Transantral ligation of the internal maxillary artery for epistaxis. Laryngoscope. 1965;75:1151–1159. doi: 10.1288/00005537-196507000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Sokoloff J, Wickbom I, McDonald D, Brahme F, Goergen TG, Goldberger LE. Therapeutic percutaneous embolization in intractable epistaxis. Radiology. 1974;111:285–287. doi: 10.1148/111.2.285. [DOI] [PubMed] [Google Scholar]
- 4.Elden L, Montanera W, Terbrugge K, Willinsky R, Lasjaunias P, Charles D. Angiographic embolization for the treatment of epistaxis: a review of 108 cases. Otolaryngol Head Neck Surg. 1994;111(1):44–50. doi: 10.1177/019459989411100110. [DOI] [PubMed] [Google Scholar]
- 5.Tseng EY, Narducci CA, Willing SJ, Sillers MJ. Angiographic embolization for epistaxis: a review of 114 cases. Laryngoscope. 1998;108:615–619. doi: 10.1097/00005537-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Ram B, White PS, Saleh HA, Odutoye T, Cain A. Endoscopic endonasal ligation of the sphenopalatine artery. Rhinology. 2000;38:147–149. [PubMed] [Google Scholar]
- 7.Simpson GT, Janfaza P, Becker GD. Transantral sphenopalatine artery ligation. Laryngoscope. 1982;92:1001–1005. [PubMed] [Google Scholar]
- 8.Budrovich R, Saetti R. Microscopic and endoscopic ligature of the sphenopalatine artery. Laryngoscope. 1992;102:1390–1394. doi: 10.1288/00005537-199212000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Strong EB, Bell DA, Johnson LP, Jacobs JM. Intractable epistaxis: transantral ligation versus embolization: efficacy review and cost analysis. Otolaryngol Head Neck Surg. 1995;113:674–678. doi: 10.1016/S0194-5998(95)70004-8. [DOI] [PubMed] [Google Scholar]
- 10.Gilyoma JM, Chalya PL. Etiological profile and treatment outcome of epistaxis at a tertiary care hospital in Northwestern Tanzania: a prospective review of 104 cases. BMC Ear Nose Throat Disorders. 2011;11:8. doi: 10.1186/1472-6815-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darlene Lubbe, The open access of otolaryngology, head and neck operative surgery by Johan Fagan (editor) https://vula.uct.ac.za/access/content/group/ba5fb1bd-be95-48e5-81be-586fbaeba29d/Sphenopalatine%20artery%20_SPA_%20ligation.pdf
- 12.Metson R, Hanson DG. Bilateral facial nerve paralysis following arterial embolization for epistaxis. Otolaryngol Head Neck Surg. 1983;91:299–302. doi: 10.1177/019459988309100318. [DOI] [PubMed] [Google Scholar]
- 13.Kim JK, Cho JH, Lee Y, et al. Anatomical variability of the maxillary artery: findings from 100 Asian cadaveric dissections. Arch Otolaryngol Head Neck Surg. 2010;136(8):813–818. doi: 10.1001/archoto.2010.121. [DOI] [PubMed] [Google Scholar]
- 14.Pritkin JB, Caldarelli DD, Panje WR. Endoscopic ligation of internal maxillary artery for treatment of intractable posterior epistaxis. Ann Otol Rhinol Laryngol. 1998;107:85–92. doi: 10.1177/000348949810700201. [DOI] [PubMed] [Google Scholar]
- 15.Willems PW, Farb RI, Agid R. Endovascular treatment of epistaxis. Am J Neuroradiol. 2009;30:1637–1645. doi: 10.3174/ajnr.A1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hervochon R, Khoueir N, Le Clerc N, Clément J, Kania R, Herman P, Verillaud B. Unilateral vs bilateral sphenopalatine artery ligation in adult unilateral epistaxis: a comparative retrospective study of 83 cases. Clin Otolaryngol. 2018;43:1591–1594. doi: 10.1111/coa.13183. [DOI] [PubMed] [Google Scholar]
- 17.MacArthur FJD, McGarry GW. The arterial supply of the nasal cavity. Eur Arch Oto-Rhino-Laryngol. 2017;274(2):809–815. doi: 10.1007/s00405-016-4281-1. [DOI] [PubMed] [Google Scholar]
- 18.Ntomouchtsis A, Venetis G, Zouloumis L, et al. Ischemic necrosis of nose and palate after embolization for epistaxis: a case report. Oral Maxillofac Surg. 2010;14(2):123–127. doi: 10.1007/s10006-009-0190-4. [DOI] [PubMed] [Google Scholar]
- 19.Elsheikh E, El-Anwar MW. Septal perforation and bilateral partial middle turbinate necrosis after bilateral sphenopalatine artery ligation. J Laryngol Otol. 2013;127(10):1025–1027. doi: 10.1017/S0022215113001904. [DOI] [PubMed] [Google Scholar]