Abstract
Background
Craving is a central feature of addiction. Early evidence suggests that repetitive transcranial magnetic stimulation is effective in reducing cue induced craving for patients with opioid use disorder (OUD). However, trials in large populations of patients with OUDs are lacking.
Methods
We randomly assigned 118 male heroin patients into three groups (i.e., 10 Hz rTMS, 1 Hz rTMS and a wait-list control group) from two addiction rehabilitation centers. rTMS was applied to the left dorsolateral prefrontal cortex (DLPFC) for 20 daily consecutive sessions.
Findings
Results showed that 10 Hz rTMS and 1 Hz rTMS were both effective in reducing cue-induced craving scores in heroin users when compared to the wait list group. The treatment effects lasted for up to 60 days after rTMS treatment cessation.
Interpretation
Our results suggest that rTMS applied to the DLPFC is effective in reducing craving severity in heroin use disorder patients. Our results also suggest that such treatment effects can last for up to 60 days after treatment cessation.
Keywords: Transcranial magnetic stimulation, Craving, Addiction, Heroin, Opioid
Research in Context.
Evidence before this study
Repetitive Transcranial Magnetic Stimulation (rTMS) has been tested to treat drug addiction patients (e.g. cocaine, methamphetamine, nicotine) in past decade. Yet by Jan 2020, only one pilot study in 2016 reported that high-frequency rTMS reduces cue induced craving for heroin patients, based on a small number of patients (n = 20) and without further follow-up.
Added value of this study
1. We demonstrated that rTMS applied to the DLPFC is effective in reducing craving severity in a large group of heroin use disorder patients (n = 118).
2. We showed that rTMS treatment has a lasting effect on craving (at least 60 days) following treatment cession.
3. We proved that both high and low frequency rTMS treatment are effective for craving treatment of heroin patients
Implications of all the available evidence
rTMS intervention could efficiently reduce craving for heroin patients. Future studies are required to refine the treatment protocols, to elucidate the neural circuitry mechanisms underlying the treatment effects, and to evaluate its application on relapse management in community.
Alt-text: Unlabelled box
Introduction
The opioid crisis has resulted in a significant individual morbidities, economic loss, negative impacts on family, and overdose death [1]. Opioid addiction is characterized by compulsive drug-seeking behaviors despite negative consequences [2], [3], [4], [5]. The cue-induced craving and relapse are critical therapeutic targets in the management of drug addiction. Cue induced craving increases progressively during the early stage of abstinence and remains high over an extended period [6, 7]. “Volcanic” craving can erupt suddenly into consciousness after exposure to drug cues, acting as a persistent cause for relapse [8]. In well-controlled laboratory and clinical settings, cue induced craving can reliably predict relapse in different environmental settings and across different types of addictions [6, 9, 10]. Evidence suggests that decreased craving and cue reactivity has been linked to decreased drug intake [11, 12]. Additionally, considering the fact that craving can be a very distressing symptom, targeting craving may have clinically meaningful effects for both addiction and for affective states [13].
Incubation of craving relies on formation of maladaptive neuroplasticity at cortico-striatal synapses [6, [14], [15], [16], [17]]. Neurocircuitry studies with brain imaging approaches highlighted the role of prefrontal cortex (PFC) in drug cue induced craving [6, 18, 19]. PFC hypoactivity may lead to failure in executive system and cognitive control [20], and serves as the basis of individual variability and vulnerability in transition to addiction [21]. Recent success with electrical stimulation [22] or optogenetic reversal of prefrontal cortex hypoactivity has demonstrated the possibility of modulating compulsive drug seeking behavior in rats through prefrontal brain stimulation [23, 24].
Repetitive transcranial magnetic stimulation (rTMS) can target the prefrontal cortex and activate underlying cortical regions [25, 26]. Promising evidence suggests that rTMS can be used to effectively treat different types of drug addiction (e.g. heroin, methamphetamine, cocaine, nicotine) through single or repeated sessions of high-frequency rTMS over left PFC [27], [28], [29], [30]. Additional lines of evidence suggest that both high frequency rTMS over the right PFC or low frequency rTMS over the left PFC can be effective in addiction patients (e.g. nicotine, cocaine, methamphetamine) [31], [32], [33]. Whether rTMS is effective in opioid use disorder (OUD) patients – particularly for cue-induced craving - remains to be determined.
Heroin and methamphetamine are two major drugs of abuse in China, and it is estimated that total abuse population in China affects more than 13 million people. Our two-year rehabilitation center provided a unique setting in which to evaluate rTMS as a treatment for OUDs and to track people beyond an acute treatment period. This manuscript reports the results of a randomized, two-site clinical trial designed to evaluate the effects of both high frequency and low frequency rTMS on cue-induced craving in patients with heroin use disorder. Given previous inconsistent findings of rTMS frequency on clinical treatment of addiction, it is important to understand the different clinical outcomes between high frequency (or excitatory) rTMS to low frequency (or inhibitory) rTMS. Therefore, we evaluated the effects of rTMS on cue-induced heroin craving following 20 rTMS treatments and 60 days (i.e., 2 months) after rTMS treatment cessation. This study was conducted in a controlled inpatient environment within two specialty addiction rehabilitation centers. We hypothesized that rTMS delivered to the dorsolateral prefrontal cortex (DLPFC) would reduce cue induced craving in patients with heroin use disorder.
Materials and methods
Study design and participants
The study was a randomized, two-center clinical trial. Male heroin use disorder inpatients were recruited after referral to specialty addiction rehabilitation centers - Hangzhou Gongchen Center of addiction rehabilitation (HZGC) and Longyou Shiliping Center of addiction rehabilitation (SLP) - in cities of Hangzhou and Quzhou (with no inter-center difference, Table 1).
Table 1.
Demographic Characteristics of the Study Participants.
| Variable | 10 Hz | 1 Hz | Control | F/Chi | p |
|---|---|---|---|---|---|
| Age (yrs) | 39±1.55 | 38.48±1.44 | 38.71±1.2 | 0.03 | 0.968 |
| Duration of heroin Intake in Year (yrs) | 14.63±1.04 | 13.24±1.14 | 13.43±1.31 | 2.5308 | 0.282 |
| Dosage Per Time (g) | 0.6 ± 0.07 | 0.44±0.05 | 0.64±0.07 | 2.7358 | 0.255 |
| Monthly dosage (g) | 20.07±3.18 | 15.28±1.99 | 19.06±1.55 | 5.6179 | 0.06 |
| Baseline Craving | 69.43±3.15 | 68.28±3.58 | 70.57±3.71 | 2.3901 | 0.303 |
| Duration of Abstinent Time at study entry (Days) | 88.06±9.63 | 122.38±16.71 | 92.11±10.43 | 2.230 | 0.113 |
| Center effect | |||||
| HZGC | 14(29.17%) | 13(27.08%) | 21(43.75%) | 0.242 | |
| SLP | 21(41.18%) | 16(31.37%) | 14(27.45%) |
*F-value was for Age (ANOVA test), and Chi-squared value for duration of heroin Intake in Years, Dosage Each Time, Monthly Dosage, and Baseline Craving, Abstinent Time at study entry (Day) (Kruskal-Wallis test).
We recruited adults aged between 18 and 65 years who had a recurrent history of heroin use disorder (DSM-V diagnosis, urine test positive upon admission, and in abstinence since then). Only male patients were included since male subjects dominated the local population of heroin use. Exclusion criteria include history of any other psychiatric disorders, epilepsy, cardiovascular complications and other contraindications to TMS (e.g. metal implants in the head). As part of their treatment in these rehabilitation centers, patients received routine physical exercise, psychological consultation therapy and medical education in relation to addiction. No pharmacological agents were used to treat these heroin use disorder patients over the study period. The research ethics board of Nanjing Normal University, Liaoning Normal University, and Shanghai Mental Health Center approved the study in accordance with the Declaration of Helsinki and study monitoring was provided by the local safety monitoring board (Chinese Clinical Trial Registry number, 17013610). All participants provided written informed consent and participated in the study voluntarily.
Randomization and masking
A total of 118 inpatients were recruited. 6 subjects were excluded due to mixed usage of other drugs, or with severe diseases (see Fig. 1, CONSORT flow diagram). The remaining 112 Heroin subjects were randomly assigned to either 10 Hz rTMS (n = 40), 1 Hz rTMS (n = 35) or wait list group (n = 37) using a computer generated number sequence. There were no significant differences between these three groups in relation to age, years of drug abuse, intake dosage per abuse, monthly dosage, and baseline craving, abstinent time across the three groups (demographic data in Table 1, P>0.05). There were significant correlations across these demographic variables (sTable 1, sTable 2).
Fig. 1.
Flowchart of the study. 118 heroin subjects were enrolled into experiments and assigned into three groups (10 Hz, 1 Hz, wait list). 99 heroin patients completed TMS intervention. The treatment protocol lasted for 4 weeks, and follow-up assessments were made on day 30, 60, and 90 from baseline measurement.
The treated groups were double blinded during the whole period of the study. rTMS was delivered by two independent researchers who did were not involved in craving and scale measurements. During the trial, 6 subjects (4 subjects in 10 Hz treatment, 2 in 1 Hz treatment) discontinued treatment for insomnia and headache, with an additional 5 subjects lost to first follow-up. 99 heroin disorder patients remained in the trial for its entire duration (i.e., 20 times of treatment) (Fig. 1). There was no difference in drop-out rates between the 3 groups (P>0.05).
Craving score evaluation
The primary outcome is the changes of craving score during treatment, and the evaluation was performed as previously described [28]. Patients were required to watch a video showing heroin intake for five minutes, respectively. Then the visual analogue scale (VAS) was used to evaluate the cue-induced score. The participants were asked to feel free to express themselves, and the VAS ranges from 0 (completely undesired for drug intake) to 100 (completely wanting for drug intake). The baseline craving (day 1) was evaluated prior to the rTMS treatment, and craving score was re-assessed at the end of treatment, 30 days and 60 days after last day of treatment for follow up measurements.
rTMS Treatment
Participants seated in a comfortable chair during the whole rTMS treatment procedure. The resting motor threshold (RMT) was measured individually according to 50% chance (5 out of 10 trials) of index finger muscle (FDI) contraction. 10 Hz rTMS was applied at 100% RMT, 5‑sec on, 10‑sec off for 10 min, 2000 pulses. 1 Hz rTMS was applied at 100% RMT, 10-min on, 600 pulses. Both rTMS treatments were applied to the left DLPFC, localized by the Yiruide TMS location Cap system (10–20 EEG based, corresponding to F3), with a figure-eight coil from CCY-I TMS instrument (Yiruide Co., Wuhan, China). For the coil (Type B9076), the inner diameter between the two circles was 76 mm, and the maximum output was 2.0 ± 10% Tesla. Treatment groups received 20 sessions rTMS treatments (five consecutive days on and two days off for a total of 28 days). The wait-list control group received no rTMS treatment.
Analyses and statistics
The data of 51 participants from Shiliping (SLP) rehab center and 48 from Hangzhou Gongchen (HZGC) rehab center were evaluated in the statistical analysis. Demographic characteristics and baseline craving were summarized with descriptive statistics (Table 1). The association of demographic variables with baseline craving rates were evaluated by quasi-Poisson regression models (stable 1), to confirm the balanced distribution of demographic characters across treatment groups.
To compare the efficacy of different types of treatment, the craving scores at the four time points per subject were longitudinally analyzed using the linear mixed effect model adjusted for center effect. As the scores changed over time, as shown in Fig. 1, they demonstrated a two-stage pattern, from baseline to end of treatment, and from end of treatment to 60 days after the treatment. Therefore, piecewise linear mixed effect models with a knot at Day 30 were employed to investigate the temporal feature of each treatment group. The interaction term Time∗Treatment was included in the linear mixed effect models to describe the changing rate (slope) difference between treatment groups. Pairwise comparison for craving reduction speed before and after the treatment period, and the comparisons of follow-up at 30/60 days after treatment to baseline across treatment groups, were evaluated through the piecewise linear mixed effect models.
To further specify the potential impact of demographic characteristics on craving score reduction, we dichotomized Intake/abuse Years, Dosage per Time, Monthly Dosage, and Baseline Craving by the median of each variable. By fitting mixed effect models on patients sub-grouped by demographic characteristics, the potential differences of treatment effects between particular subgroups were identified.
To illustrate if craving reduction at day 30 could predict craving changes at day 90, the Spearman's correlation coefficient of craving rate changes (Δcraving) at day 30 and 90 in each treatment group was estimated. Linear models were also employed, after confirming the normality of score changes. In addition, significant results identified were checked for consistency and robustness.
All the analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). A two-sided significance level of P<0.05 was used.
Results
Effects of rTMS treatment
After adjusted for center effect, the piecewise linear mixed effect model indicated that craving rates decreased significantly in the first 30 days in both 1 Hz (Estimate=−25.26, SE=4.15, P<0.0001) and 10 Hz (Estimate=−29.04, SE3.77, P<0.0001) treated groups, and these decreases were significantly greater that in the control group (Estimate of 1Hz-Control=−11.55, SE=5.61, P = 0.04; Estimate of 10Hz-Control=−15.32, SE=5.34, P = 0.004). Neither the active or wait-list groups had a significant change in craving rate after day 30th, further suggesting that it was active rTMS and not time that resulted in decreased craving rates.
When compared to the baseline, the craving rate of the 10 Hz (Estimate=−29.43, SE=4.11, P<0.001; Estimate=−27.57, SE=4.26, P<0.001; Estimate=−28.30, SE=4.94, P<0.001) and 1 Hz groups (Estimate=−24.82, SE=4.52, P<0.001; Estimate=−28.35, SE=4.74, P<0.0001; Estimate=−28.99, SE=5.50, P<0.001) reduced significantly at Day 30/60/90, as well as in the Control group (Estimate=−13.14, SE=4.11, P<0.001; Estimate=−15.35, SE=4.30, P<0.001; Estimate=−14.09, SE=5.08, P<0.01) (Fig. 2A). At each time point, treated groups showed significant differences compared to the control group (sTable 3). There was no significant difference in cue craving between the two treatment centres.
Fig. 2.
A: Outcomes of treatment effect within heroin groups. Cue-induced craving was assessed at four time points. Compared to baseline, there were significant differences among 3 groups at Day 30/60/90. Black line indicates for wait list group; Blue for 10 Hz group; Red for 1 Hz group.
In terms of craving reduction slopes, there was a decreased trend (marginally) in three groups before Day 30. Pairwise comparisons demonstrated that the reduction speeds of the two treated groups were faster than the control group (sTable 4, 5), further confirming the therapeutic effects of rTMS treatments relative to wait-list group.
The impacts of demographic variables on changes of craving rate
We stratified the demographic data by using the medians as cut-off points (sTable 6). The results of Linear mixed effect models of delta craving rate on subgroups are shown in Fig. 3 for Day 30 and sFig. 1 for Day 90.
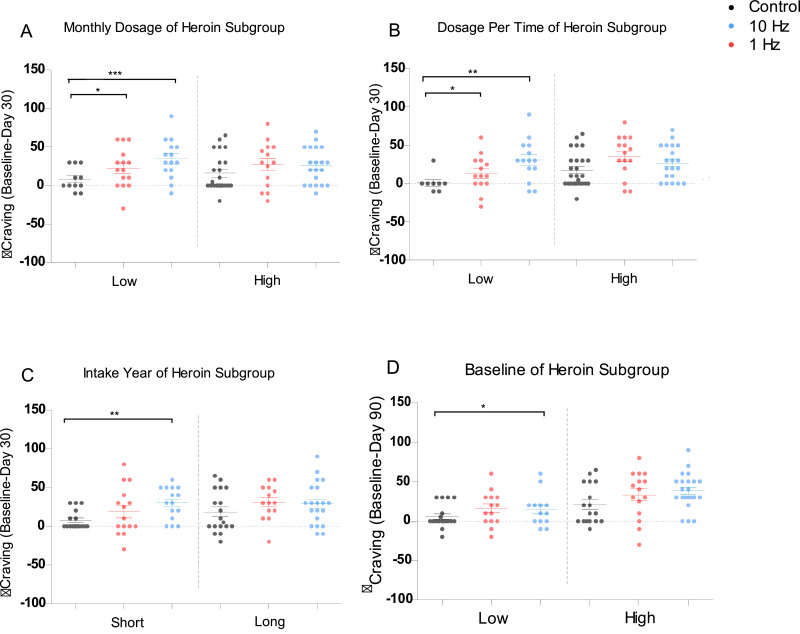
Fig. 3.
The impacts of demographic variables on delta craving rate. Figure A-D listed the association between demographic variables and reduction craving (Baseline-Day 30) in Heroin subgroups divided based on: A: Monthly dosage for heroin intake; B: Dosage per time for heroin intake; C: Duration of heroin intake in years; D: baseline Craving score for heroin patients. Black indicates for wait list group; Blue for 10 Hz group; Red for 1 Hz group.
For reduced craving rate (baseline - Day 30) in heroin patients, the 10 Hz group had larger reduction than the control group, when intake year (W = 162, P<0.01), monthly dosage (W = 224, P<0.01), dosage per time (W = 286, P<0.01); and the delta craving of 1 Hz group was higher than the control group, when monthly dosage (W = 224, P = 0.04), dosage each time (W = 286, P = 0.04) was low. However, no significant difference was observed among high level subgroups. These data further confirmed the effects of rTMS on craving reduction occurred during treatment period (Day 0 to Day 30) relative to wait-list group.
Correlations between Post-Treatment Craving changes and In-Treatment Craving changes
To identify if there was a correlation between decrease in craving at Day 30 and Day 90 as compared to baseline across all three groups, we employed linear regression analysis. Spearman's correlation coefficients were calculated, and a similar conclusion was reached via using linear regression. In heroin patients, the Spearman's Rho correlation coefficient of Post-Treatment Craving changes and In-Treatment Craving changes was 0.61 (P = 0.001) for the 10 Hz group, 0.53 for the 1 Hz group (P<0.01), and 0.63 for the control group (P = 0.001) (Fig. 4).
Fig. 4.
The correlation between delta craving (Baseline-Day 30) and delta craving (Baseline-Day 90). The size of points in figure stands for the number of observations with same level of craving rates changes. The location of points suggested that craving rate changes after treatment linearly depend on the craving rate changes during the treatment. The regression lines were plotted in respective color. Black indicates for wait list group; Blue for 10 Hz group; Red for 1 Hz group.
Side effects
There were mild side effects (dizziness, headache, neck pain, insomnia, etc.) reported. At the end of study, only 6 subjects (4 subjects in 10 Hz treatment, 2 in 1 Hz treatment) discontinued treatment for insomnia and headache, and such unpleasant symptoms disappeared by our one-month follow-up survey.
Discussion
The present study demonstrated that both low and high frequency rTMS applied over the prefrontal cortex was well tolerated and effective at reducing cue-induced craving in patients with heroin use disorder. Our results also indicate that both active treatment groups produced treatment effects over the first 30 days, relative to wait-list group, which lasted for up to 60 days after the last rTMS treatment. Collectively, these findings suggest that rTMS might produce enduring effects on craving and possibly modulate addictive behaviors.
The study demonstrated that rTMS treatment is effective in reducing craving, and as a corollary, may represent a potentially effective treatment for patients with heroin use disorder. Craving predicts relapse when measured reliably in clinical settings [34]. Negative mood and craving contribute to withdrawal symptoms, which relate to motivation of drug intake [35]. Despite limitations, self-reported craving remains the most widely accepted approach in research and clinical settings [12]. We recommend that for future studies, the inclusion of additional emotion related / implicit craving measurements (e.g. stress induced craving changes, or sub-threshold presentation of drug associated cues) should be considered. Also, the inclusion of potential biologic targets of craving - including imaging based targets – should also be included to evaluate the neural processing changes in drug associate cues following rTMS treatment.
The neural mechanisms underlying rTMS response might include: increased local metabolism, inhibitory control and plasticity at prefrontal cortex following repeated magnetic stimulation [25, 36, 37]; and potentiation of prefrontal network and modulation of monoamine concentration in cortico-striatal system (e.g. dopamine) [38]. Neurochemistry changes and hypofrontal activation of PFC during exposure to drug associated cues underlie the generation of craving [39]. Balanced dopaminergic system might contribute to improved mood status and reduce the previous learned association between drug cues and reward [39]. In future, it may be helpful to combine PET imaging to investigate the relationship between striatal dopamine changes and behavioral improvement in heroin patients receiving rTMS treatment [38, 40]. Further, rTMS may produce enhanced cognitive control together with reduced impulsivity that may contribute to a subjective reduction in craving. Notably, as both our 10 Hz and 1 Hz rTMS groups demonstrated comparable clinical effects, it is possible that simply repeated cortical stimulation is effective at reducing craving regardless of the exact frequency for stimulation. In this regard, previous studies have demonstrated differences between 10 Hz rTMS and 1 Hz rTMS in relation to excitation and inhibition. That is, it has been demonstrated that 10 Hz rTMS induces excitability and 1 Hz rTMS reduces it [41]; while some studies reported the excitatory effects of 1 Hz rTMS as well [45, 48, 49]. In addition, it has also been demonstrated that both 10 Hz rTMS and 1 Hz rTMS potentiate long acting GABA inhibitory neurotransmission (i.e., GABAB receptor mediated inhibition) [42]. Such potentiation – particularly that of increased GABAB receptor functioning - may increase inhibition in the DLPFC resulting in greater inhibitory control and possibly reduced craving. Such mechanisms have been postulated in relation to the effects of baclofen on craving [43]. Lastly, we previously reported that heroin patients exhibited impaired motor-cortical plasticity following TMS stimulation [44], indicating that there might be additional neural mechanisms other than local regional potentiation after rTMS treatment (e.g. inducing dopamine release through network connectivity [38]) that influence rTMS reductions in craving. Future studies may wish to compare the differences between 10 Hz and 1 Hz rTMS on regional brain activity and dopamine release in heroin dependent patients.
The study is limited by the outcome measurement as self-reported craving within reduced ecological environment setting. Future studies are required to track the relapse rates of these patients to evaluate the maintenance effects of rTMS on relapse prevention. The lack of a sham rTMS group is also a relative limitation. We have previously shown that sham rTMS does not have a significant effect on cue induced craving in heroin dependents, within a short period of five days treatment [28]; therefore the present study focused on the tolerability and long-term efficacy of active rTMS treatment in a large sample of heroin use disorder patients. A meta-analysis reported that placebo effects might partly contribute to the therapeutic responses to rTMS in major depression patients [46]. In addition, it is highly possible that the expectations of improvement from the patients, and additional attention provided by the rTMS treatment team may contribute to significant clinical improvements in these patients [47]. Taken together, the rTMS effects might be facilitated by regular interaction with rehabilitation center staff and increased daily surveillance. Last but not least, the study selectively focused on male patients since they represent the vast majority of heroin patients in eastern part of China.
In conclusion, the result of the present study provides evidence that a course of rTMS over left DLPFC may represent an efficacious approach to reducing craving in male heroin use disorder patients. Our current finding contributes to further add-on treatment designed for opioid cessation therapies.
Acknowledgments
We thank Chengyuan Cai, Ying Liu, Xiaoxiao Luo, Jianfei Yao, and Zhijun Yu for helps during data collection and management.
Funding
The study was supported by NSFC grants (81822017, 31771215), The Science and Technology Commission of Shanghai Municipality (18QA1403700), Shanghai Municipal Education Commission - Gaofeng Clinical Medicine Grant Support (20181715), Hundred-talent Fund from Shanghai Municipal Commission of Health (2018BR21) to TFY. The work of ZJD is supported by the Canadian Institutes of Health Research (CIHR), the National Institutes of Mental Health (NIMH), Brain Canada and the Temerty Family and Grant Family and through the center for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute.
Role of funder
The funders have no role in study design, data collection, data analysis, interpretation, and writing of the report.
Author contributions
TY and WL designed the study; XL, XZ, TL and QL performed the study; XL, XZ, LT, HZ, WL, ZJD and TY analyzed the results and wrote the paper together; all authors have read and approved the final version of the manuscript.
Competing interest
ZJD has received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc and Magventure Inc. None declared for rest authors.
Data availability
The original data is available from corresponding author upon request.
Footnotes
Funding: National Natural Science Foundation of China; The Science and Technology Commission of Shanghai Municipality; Shanghai Municipal Education Commission; Shanghai Municipal Commission of Health; Canadian Institutes of Health Research (CIHR); the National Institutes of Mental Health (NIMH); Brain Canada and the Temerty Family and Grant Family and through the center for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102809.
Contributor Information
Wenbo Luo, Email: luowb@lnnu.edu.cn.
Ti-Fei Yuan, Email: ytf0707@126.com.
Appendix. Supplementary materials
Reference
- 1.Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–574. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21(3):467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 4.Everitt BJ, Robbins TW. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- 5.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 6.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends in Neurosciences. 2011;34(8):411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43(2):107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31(3):644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 10.Fatseas M, Denis C, Massida Z, Verger M, Franques-Reneric P, Auriacombe M. Cue-induced reactivity, cortisol response and substance use outcome in treated heroin dependent individuals. Biol Psychiatry. 2011;70(8):720–727. doi: 10.1016/j.biopsych.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Addolorato G, Antonelli M, Cocciolillo F, Vassallo GA, Tarli C, Sestito L. Deep Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex in Alcohol Use Disorder Patients: Effects on Dopamine Transporter Availability and Alcohol Intake. Eur Neuropsychopharmacol. 2017;27(5):450–461. doi: 10.1016/j.euroneuro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Sayette MA. The Role of Craving in Substance Use Disorders: Theoretical and Methodological Issues. Annu Rev Clin Psychol. 2016;12:407–433. doi: 10.1146/annurev-clinpsy-021815-093351. [DOI] [PubMed] [Google Scholar]
- 13.Tiffany ST, Wray JM. The clinical significance of drug craving. Ann N Y Acad Sci. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69(4):650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17(6):351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of Cue-Induced Craving in Adults Addicted to Cocaine Measured by Electroencephalography. JAMA Psychiatry. 2016;73(11):1127–1134. doi: 10.1001/jamapsychiatry.2016.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69(7):708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35(2):232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy D, Shabatsimon M, Shalev U, Barneaygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not "natural" reinforcement. Journal of Neuroscience. 2007;27(51):179–189. doi: 10.1523/JNEUROSCI.4477-07.2007. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496(7445):359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- 24.Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci. 2017;18(11):685–693. doi: 10.1038/nrn.2017.113. [DOI] [PubMed] [Google Scholar]
- 25.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Pogarell O, Koch W, Popperl G, Tatsch K, Jakob F, Mulert C. Acute prefrontal rTMS increases striatal dopamine to a similar degree as d-amphetamine. Psychiatry Res. 2007;156(3):251–255. doi: 10.1016/j.pscychresns.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. 2013;73(8):714–720. doi: 10.1016/j.biopsych.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Y, Cao X, Tan T, Shan C, Wang Y, Pan J. 10-Hz Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex Reduces Heroin Cue Craving in Long-Term Addicts. Biol Psychiatry. 2016;80(3):e13–ee4. doi: 10.1016/j.biopsych.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 29.TerraneoA, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur Neuropsychopharmacol. 2016;26(1):37–44. doi: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104(4):653–660. doi: 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- 31.Camprodon JA, Martínezraga J, Alonsoalonso M, Shih MC, Pascualleone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86(1):91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Dieler AC, Dresler T, Joachim K, Deckert J, Herrmann MJ, Fallgatter AJ. Can intermittent theta burst stimulation as add-on to psychotherapy improve nicotine abstinence? Results from a pilot study. Eur Addict Res. 2014;20(5):248–253. doi: 10.1159/000357941. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Shen Y, Cao X, Li Y, Chen Y, Yang W. Either at left or right, both high and low frequency rTMS of dorsolateral prefrontal cortex decreases cue induced craving for methamphetamine. Am J Addict. 2017;26(8):776–779. doi: 10.1111/ajad.12638. [DOI] [PubMed] [Google Scholar]
- 34.Sayette MA, Tiffany ST. Peak provoked craving: an alternative to smoking cue-reactivity. Addiction. 2013;108(6):1019–1025. doi: 10.1111/j.1360-0443.2012.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker TB, Japuntich SJ, Hogle JM, McCarthy DE, Curtin JJ. Pharmacologic and behavioral withdrawal from addictive drugs. Curr Dir Psychol Sci. 2006;15:232–236. [Google Scholar]
- 36.Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry. 1999;46(12):1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- 37.Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- 38.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha R. The clinical neurobiology of drug craving. Curr Opin Neurobiol. 2013;23(4):649–654. doi: 10.1016/j.conb.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126(Pt 12):2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- 42.Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174(3):403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- 43.Tyacke RJ, Lingford-Hughes A, Reed LJ, Nutt DJ. GABAB receptors in addiction and its treatment. Adv Pharmacol. 2010;58:373–396. doi: 10.1016/S1054-3589(10)58014-1. [DOI] [PubMed] [Google Scholar]
- 44.Shen Y, Cao X, Shan C, Dai W, Yuan TF. Heroin Addiction Impairs Human Cortical Plasticity. Biological Psychiatry. 2017;81(7):e49–e50. doi: 10.1016/j.biopsych.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Castrillon G, Sollmann N, Kurcyus K, Razi A, Krieg SM, Riedl V. The physiological effects of noninvasive brain stimulation fundamentally differ across the human cortex. Sci Adv. 2020;6(5):eaay2739. doi: 10.1126/sciadv.aay2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razza LB, Moffa AH, Moreno ML, Carvalho AF, Padberg F, Fregni F, Brunoni AR. A systematic review and meta-analysis on placebo response to repetitive transcranial magnetic stimulation for depression trials. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:105–113. doi: 10.1016/j.pnpbp.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Yesavage JA, Fairchild JK, Mi Z, Biswas K, Davis-Karim A, Phibbs CS, Forman SD, Thase M, Williams LM, Etkin A, O'Hara R, Georgette G, Beale T, Huang GD, Noda A, George MS, VA Cooperative Studies Program Study Team Effect of Repetitive Transcranial Magnetic Stimulation on Treatment-Resistant Major Depression in US Veterans: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75(9):884–893. doi: 10.1001/jamapsychiatry.2018.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. BOLD MRI Responses to Repetitive TMS Over Human Dorsal Premotor Cortex. Neuroimage. 2005;28:22–29. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 49.Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain's intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci U S A. 2011;108(52):229–234. doi: 10.1073/pnas.1113103109. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data is available from corresponding author upon request.