Abstract
The last five decades of molecular and systems biology research have provided unprecedented insights into the molecular and genetic basis of many cellular processes. Despite these insights, however, it is arguable that there is still only limited predictive understanding of cell behaviours. In particular, the basis of heterogeneity in single-cell behaviour and the initiation of many different metabolic, transcriptional or mechanical responses to environmental stimuli remain largely unexplained. To go beyond the status quo, the understanding of cell behaviours emerging from molecular genetics must be complemented with physical and physiological ones, focusing on the intracellular and extracellular conditions within and around cells. Here, we argue that such a combination of genetics, physics and physiology can be grounded on a bioelectrical conceptualization of cells. We motivate the reasoning behind such a proposal and describe examples where a bioelectrical view has been shown to, or can, provide predictive biological understanding. In addition, we discuss how this view opens up novel ways to control cell behaviours by electrical and electrochemical means, setting the stage for the emergence of bioelectrical engineering.
Keywords: bioelectricity, bioelectrical cell biology, cell physiology, cell biophysics, electrochemistry
1. Introduction
Biological electrical phenomena were recognized by Luigi Galvani and his contemporaries in the eighteenth century through the study of animal muscles and the nervous system [1]. These early studies have led to the development of major fields, especially neuroscience and cardiology. Outside of these fields, however, studies of bioelectricity, i.e. electrical and electrochemical processes in cellular systems, have remained fragmented. While it was discussed as early as the 1970s that bioelectricity may be fundamental to understanding various cellular behaviours [2], the electrical investigations of cells were only focused on cellular bioenergetics [3,4]. The bioelectrical view of cells as a more general concept has remained confined to the fringes of biological research for five decades, during which molecular biology has made astonishing advancements in our understanding of cells and our ability to manipulate genes.
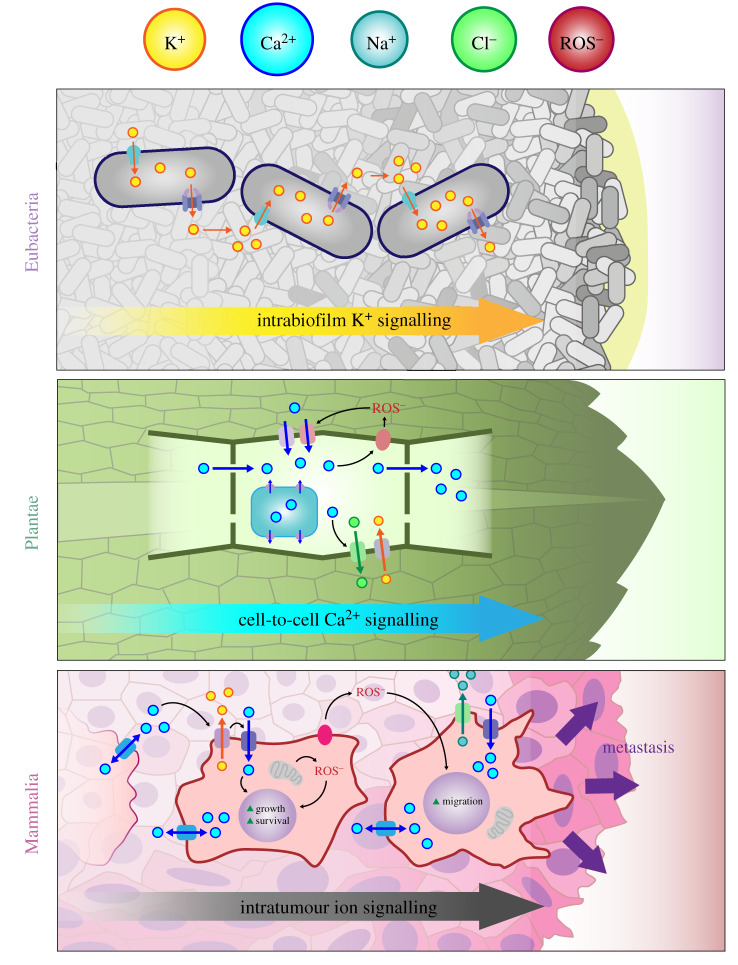
Interestingly, the resulting findings from molecular studies highlight once again the importance of bioelectricity, and now there is a revival of a bioelectrical view of biological systems. Studies in diverse systems show that bioelectrical signals are at the heart of cell–cell communication in microorganisms, plants and animals (figure 1). Bioelectricity can underpin efficient growth and antibiotic resistance in bacterial biofilms [5,6] and organization, morphogenesis and regeneration in mammalian and plant tissues [7–9]. These findings, together with the realization that externally applied electrical fields can modulate multicellular processes such as regeneration in plant and vertebrate tissue [10–12], have resulted in the recent proposition that multicellular organization, and development more broadly, can, and should, be studied as a bioelectrical paradigm [13–15].
Figure 1.
Recent research shows that both prokaryotes and eukaryotes use ion- and redox-based electrochemical signals for communication. It has been shown that such communication enables the organization of growth and developmental processes across multiple length scales.
We argue here that bioelectricity can lead to a fundamental understanding of single-cell behaviours, beyond its roles in the multicellular context. By cell behaviours, we refer to high-level processes such as proliferation, dormancy and differentiation that are underpinned by dynamic changes in gene expression programmes, metabolic flux switching and mechanical cell properties. Notably, these changes are ultimately linked to the integrated physio-chemical properties of the electrochemically active cell–microenvironment interface. This motivates a bioelectrical view of the cell, the development of which can lead to a predictive understanding of cell behaviour.
2. Bioelectrical nature of the cell
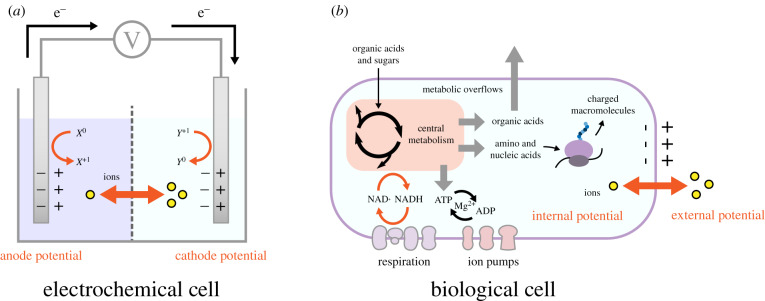
The bioelectrical conceptualization of cell behaviour can be illustrated with an analogy between a biological cell and a battery, both of which use redox reactions and ion movements (figure 2). In the case of the biological cell, its enclosed structure composed of multiple membranes partitions ions and charged and uncharged molecules (including macromolecules) across cellular compartments and within and outside the cell. The partitioning of charged molecules and ions gives rise to electrical and chemical potential differences across cellular membranes, and electrochemical gradients (ion motive forces, IMFs). A membrane potential (MP) arises from the combined electrical potential differences across a given membrane due to all charged molecules.
Figure 2.
The basis for a bioelectrical view of cells can be motivated by drawing an analogy between a battery (a) and a biological cell (b). Both systems rely on ion flows and redox reactions across interfaces.
The maintenance of the MP and the coupling of IMFs to membrane-bound chemical reactions are key components of cellular bioenergetics and physiology, as recognized in the chemi-osmotic theory of respiration [3,4]. While the specific mechanisms of MP and IMF can be different across different membranes (e.g. mitochondrial versus plasma membrane in mammalian cells or the inner versus outer membranes in a bacterial cell [16]), their generation always involves electro-static and electro-dynamic processes [4]. IMFs can arise from selective transport or differential permeability to charged molecules, or can be generated through membrane-bound redox reactions of the respiratory chain [3,4]. A key point to recognize is that IMF and MP are coupled to each other and further to cell volume and metabolism [17,18]. The connection to volume arises from the distribution of ions and charged molecules (essential for the formation of MP), determining also the osmotic forces on cells [17]. The connection to metabolism is achieved by four general mechanisms (figure 2b). First, the steady-state concentrations of key metabolic redox and energy carrier pairs (NADH/NAD+ and ATP/ADP), which can determine metabolic pathway fluxes, can be altered by membrane-bound dehydrogenases and ATPases that can either use IMF to convert these pairs or use their conversions to sustain/generate it. Second, membrane transporters can couple the transport of metabolites, in particular organic acids and sugars, with the transport of ions, thereby linking this metabolically central process to IMF generation [2,19]. Third, several ‘master’ compounds within central metabolism—such as glutamate, which is involved in nitrogen assimilation and the synthesis of many other amino acids—can also act as gating molecules to control the state of ion channels, thereby influencing IMFs via MP [20]. Finally, the well-described excretions of metabolites and proteins from cells, as well as membrane-bound enzymatic processes, can influence the electrical and chemical potential of the cell microenvironment either directly or through redox reactions. In this context, it is worth noting that extracellular matrix polymers, such as collagen, chitin and cellulose, are shown to be piezoelectric [21].
3. Bioelectricity as a holistic approach to understand diverse cell behaviours
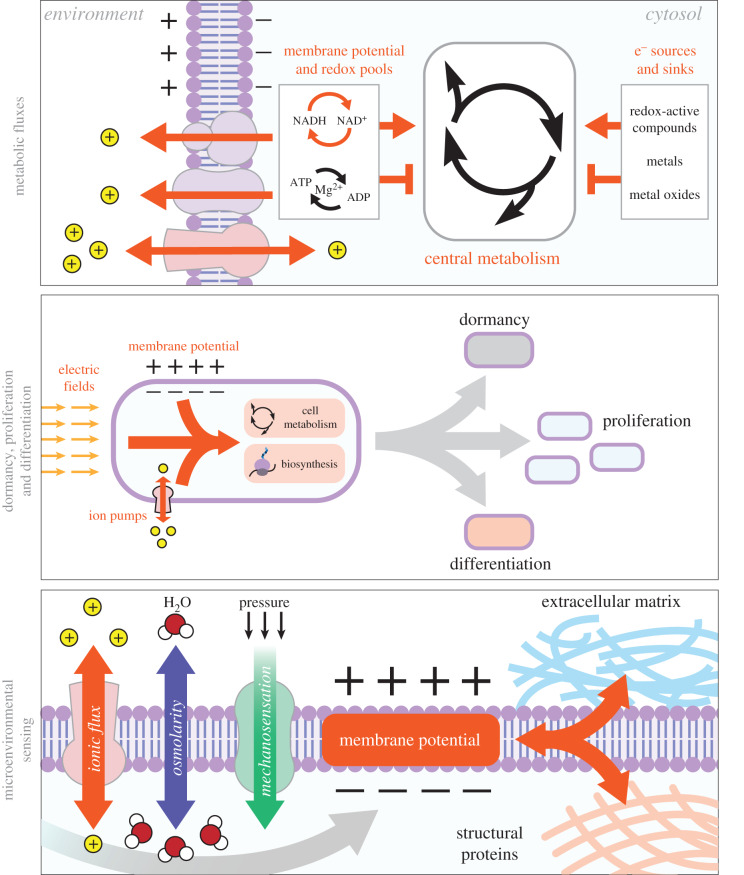
The electrochemical nature of the cells and their microenvironments gives rise to a coupling between cell physiology and bioelectricity (i.e. MP and IMF) (figure 3). This bioelectrical conceptualization of the cell provides not only plausible explanations for many cell behaviours, but also a new framework to re-formulate much of the existing knowledge in cell physiology. To illustrate this point, we discuss here a few example cell behaviours in the bioelectrical context.
Figure 3.
Cartoon illustration of the coupling between the bioelectrical nature of the cell, in particular MP and IMF, and higher level cellular behaviours.
3.1. Metabolic fluxes
A key physiological response known as ‘the Warburg effect’ or ‘overflow metabolism’ is seen in many cell types including bacteria and is shown to underpin the behaviour of cancer cells and fermentative yeasts [22]. This effect involves the metabolic switching of cells from respiration to respiration and fermentation (i.e. respiro-fermentation), despite the presence of oxygen [22]. Respiration allows cells to generate mitochondrial IMFs through NADH oxidation/oxygen reduction and then harvest these for ATP synthesis [3,4]. This process releases more energy per carbon source than fermentation, which involves the reduction of NAD+. It is therefore puzzling that cells switch to respiro-fermentation in the presence of oxygen, when they could theoretically still respire and extract more energy per carbon. One explanation put forward to explain the Warburg effect is that it is a cellular strategy to maintain ‘optimal’ growth rates under limited enzyme capacity that has to be invested between biosynthesis, cell maintenance and fermentative/respiratory metabolism [23]. While this theory provides a plausible rationalization of the Warburg effect, protein allocation measurements across different growth conditions do not show major alterations to respiratory and fermentative enzyme allocations [24]. An alternative, simpler explanation can be proposed in the context of a bioelectrical framework: increasing respiration rates would result in an increased ATP/ADP ratio and a decreased NADH/NAD+ ratio, thereby reducing the thermodynamic feasibility of IMF generation and harvesting, which require NADH and ADP as substrates, respectively [3,4]. In other words, the respiration to respiro-fermentation switch could be underpinned by the thermodynamic feasibility of these two processes under a given MP value and based on the constrained NADH/NAD+ and ATP/ADP ratios. In line with this bioelectrical view, it has been indicated that cancer cells, known for their overflow metabolism, have altered MP levels [25] and that influencing the NADH/NAD+ ratio through engineered redox reactions directly alters the initiation point of the Warburg effect in bacteria and yeast [26,27]. While further work will ascertain the validity of this bioelectrical viewpoint to explain the Warburg effect, we note that this viewpoint is experimentally testable and offers a novel means to manipulate cellular metabolism through redox and MP manipulations.
Metabolism, more broadly, can be conceptualized as a bioelectrical process in its own right, as a coupled redox process in which electrons are transferred from an electron donor to an electron acceptor [18,28]. To facilitate this process, cells use a variety of electron sources and sinks, including redox-active compounds, metals and their oxides [29]. This opens up the possibility of using such compounds, or even electrode surfaces, to withdraw, or introduce, electrons into cellular metabolism [30]. While this possibility has already been realized in biotechnological research (e.g. cell-mediated bioelectrosynthesis or waste-to-electricity generation using microorganisms [29,30]), its true potential is in opening up new routes to study metabolism and its links to cell physiology through bioelectrical interfacing in mammalian and microbial cells. For example, redox-active, cell-permeable compounds have been used together with external electrodes to achieve an added, controllable redox cycling between cell metabolism and electrodes [31,32], thereby allowing the control of cellular physiological processes, such as the circadian clock [33] and gene expression [34].
3.2. Dormancy, proliferation and differentiation
The nature of the bioelectricity–metabolism coupling and its possible links to enabling different cell states are now being elucidated. In bacteria, it was recently shown that proliferating versus non-proliferating bacterial cells respond differently to electric fields [35]. In addition, it has been shown that MP responses are different when bacterial cell metabolism or biosynthesis is perturbed by carbon starvation or antibiotic treatment, respectively [36]. Other studies have also found that ionic fluxes can influence MP and physiological outputs such as the generation of metabolically dormant bacterial cells [37].
In mammalian cells, proliferating cancer cells are suggested to have altered plasma or mitochondrial MP (reviewed in [25]). Furthermore, MP is shown to be an indicator for stem-cell differentiation potential, alongside metabolic markers [38]. Hyperpolarization of the plasma MP is sufficient to induce differentiation [39], and, conversely, depolarization of MP in a differentiated cell can induce reversal to a multi-potent progenitor [40]. In this context, it is interesting that electrical fields can induce intracellular calcium oscillations in certain stem cells [41], possibly offering a route to control MP and differentiation in these cells.
3.3. Microenvironmental sensing
A coupling between bioelectricity and physiology is also evident in cellular sensing of internal and external conditions across all cell types. Osmoregulation of cell size can be achieved through both the production and consumption of key metabolites (e.g. glutamate) or through the alteration of ionic fluxes across the membrane [17]. Both processes can alter MP. For example, in bacteria, osmotic changes result in a significant motility response, possibly through changes in IMF causing flagellar rotation [42]. Such a coupling between flagellar rotation and IMF has been characterized [43] and recently been used to monitor changes in MP and cell metabolism through changes in flagellar rotation speeds [36].
There is a large family of voltage-gated ion channels and non-specific porins that can be influenced by MP and, in turn, can influence MP and IMF by their actions [44]. It is now increasingly clear that, in prokaryotic and eukaryotic cells, some ion channels can also respond to local mechanical forces within the membrane [45], thereby providing a direct link between MP, cell physiology and mechanical forces. The mechanical forces can come from the environment, including neighbouring cells within tissues or biofilms, or can be caused within the cell itself. Within the tissue context, for example, electrical and mechanical forces can act synergistically to re-model the extracellular matrix and further enable contractions of engineered muscle tissue [46]. Within cells, (de)stabilization of microtubules is implicated to alter the mitochondrial MP in cancer cells [47], while, in bacteria, the alteration of MP can change the membrane distribution of structural proteins involved in cell division [48,49]. It has also been suggested that action potentials in neurons are accompanied by a mechanical wave (soliton wave) across the membrane [50]. These examples highlight the possibility that the link between MP and mechanical forces can provide a key integrator of information from external and internal sources. Interestingly, one such process that potentially requires the integration of internal and external clues—the initiation of bacterial sporulation—was linked to bioelectrical changes in a recent study [51]. Whether such bioelectrical changes during sporulation are caused by mechanical forces and/or involve other contributing factors remains an open research question to pursue.
In plants, the best characterized electrical signalling responses are to mechanical stimuli caused by wounding and herbivory [9]. A landmark study demonstrated that electrical potentials were rapidly initiated upon wounding, which propagated to systemic leaves, initiating the classical jasmonate-based wound response [52]. Such wound-induced ‘electrical’ signals in plants are known as variation potentials (VPs) and show slower dynamics than action potentials seen in neurons. VPs appear to facilitate information transfer on a large scale, alerting all the intervening tissue of distal stimuli [53], and arise from the activation of ligand-dependent or mechano-sensitive calcium channels, facilitating Ca2+ influx. These wound VPs were shown to be dependent upon glutamate-like receptors (GLRs), which facilitate the generation of waves of Ca2+. Consistent with this finding, glutamate triggers long-distance, Ca2+-mediated plant defences against herbivory via GLRs [54]. Notably, the application of a synthetic electrical stimulus of similar magnitude and duration to wound VPs activated transcriptional re-programming remarkably similar to that activated systemically following wounding. This study thus provides tantalizing evidence for using bioelectrical stimuli to engineer beneficial responses in plants.
4. Bioelectrical engineering of cell biology: potential and challenges
The above-highlighted couplings between cell physiology and bioelectricity can only be pursued experimentally through the development of integrated quantitative measurement techniques. To this end, electrochemistry offers a range of quantitative techniques that are capable of measuring the concentration of ions (e.g. H+ (i.e. pH), Ca2+ or Na+) or specific redox-active compounds (e.g. neurotransmitters, glucose, flavins, hydrogen peroxide and oxygen). The use and combination of such techniques have already resulted in the development of specific and powerful bio-electrochemical tools. Multi-electrode arrays allow the measurement of electrochemical events occurring at time scales of the order of microseconds [55] and at the tissue level [56], while scanning ion conductance (SICM) and scanning electrochemical microscopy (SECM) allow mapping of ionic conductivity and redox reactions, respectively, at nano-scale to single-cell levels [57].
Electrochemical approaches can also be used to modify the chemical composition of the cell microenvironment in a selective and controlled manner by producing or delivering reactant species that will trigger specific processes [58,59] or by exposing cells to electrical fields and pulses [60–62]. This opens up the possibility of ‘dialling in’ on cellular behaviour at the single-cell and tissue levels. It has been shown for example that electric fields can influence or stop mammalian cell division [61,63] or trigger specific cellular responses [60,64] and can be used to distinguish between metabolically active and dormant bacterial cells [35]. Techniques such as SECM can directly deliver redox-active compounds to individual cells and measure their responses; for example, to interrogate the metabolism of cancerous versus normal cells [65].
Regardless of the specifics of a given technique, using electrochemical approaches to study and engineer cell behaviour poses challenges. By its nature, an electrochemical measurement is complex, involving processes at the interface of an electrode and solution. In the case of cellular methods, there would even be multiple interfaces among cells, solution and electrodes. Thus, the characteristics of such interfaces must be considered to correctly evaluate the measurements. At single- and sub-cell scales, untangling the interface effects can be complicated and requires the application of modelling approaches, such as finite-element modelling to solve coupled mass transport–reactivity problems [57,66]. The application of measurement and modelling in tandem provides a roadmap for elucidating cell/solution interface properties, as has been shown for understanding surface charge heterogeneities at the sub-cellular level [66]. Another challenge for the application of electrochemical techniques is signal acquisition and processing. Recent efforts have focused on improving signal sensitivity (both signal/noise ratio and the specificity of signal) and temporal resolution. For example, it is possible to minimize electrode impedance [67] by optimizing electrode size, morphology and materials. The use of such low-noise measuring systems revealed minute, yet regular, membrane capacitive current oscillations across large populations of mammalian cells, which have previously been considered electrically quiescent, such as C6 glioma cells [67,68].
A number of technical challenges need to be considered when combining electrode measurements with cells and tissues, including sample preparation, interfaces of electrodes for in vitro or in vivo studies and maintenance of the integrity of the sensing/stimulating electrodes and the living system. For example, for in vitro spatially resolved electrochemical measurements, cells must be immobilized, which may not reflect their natural state. Irrespective of the application (sensing or modifying cell behaviour), the electrode should not be cytotoxic to cells. It must be ensured that the physiological medium used neither is prohibitive for specific electrochemical techniques nor interferes with the measurement. By-products of a measurement (e.g. in the case of redox-based measurements) should not drastically disturb the cell microenvironment. This can be of particular concern, as by-products and coupled reactions, such as solvent breakdown, could impact and obscure experimental conclusions.
These challenges are far outweighed by the potential of developing a bioelectrical basis of cell behaviour. As we argued above, this will not only open up completely new ways of understanding cells, but also allow the development of novel bioelectrical means to control and engineer cell behaviour. The latter is happening already with emerging applications of so-called electroceuticals to treat biofilms and cancer tumours [61,69–71]. On the former premise, the ability to use nano- and micro-scale electrodes allows the elucidation of single-cell properties and responses in ways previously not possible. For example, recent applications of SICM have revealed cellular charge heterogeneities [66], which cannot be detected with bulk methods such as zeta-potential measurements of cells [72]. SECM combined with SICM opens up new methods for stimulating cells with ions or redox-active compounds and following their bioelectrical responses in real time and space [73]. The combination of such single-cell electrochemical measurements with other existing methods, such as fluorescence microscopy of ion-binding or MP-responsive dyes [6,35,74,75], and the tandem use of these measurements with modelling will provide a significantly improved theoretical basis for understanding the cell–ionic environment interface. The continued developments taking place in electrode manufacturing, electrochemical techniques and signal processing will enable bioelectrical control of cells and their behaviour at single-cell, tissue and perhaps even organ levels, using also implantable devices. These exciting prospects will require a true integration of cell biology, electrochemistry, physics and engineering in the years to come.
5. Concluding remarks
A bioelectrical view on cell behaviour, as advocated here, can help to establish a unifying framework that allows us to see cell behaviour arising from interfaces with its microenvironment through the fluxes of ions and redox-active compounds. This framework offers a new synthesis that can bridge molecular studies with a bioelectrical basis of cellular physiology. The resulting science can have a transformative effect on our understanding of cellular behaviour and pave the way to its direct control through predictive bioelectrical engineering, a process that is already advancing in the context of neuronal systems [76].
Acknowledgements
We thank all delegates attending the 'Electrical Cell Biology' workshop and Mary Coates for her support in the event organization.
Data accessibility
This paper has no additional data.
Authors' contributions
All authors participated in the 'Electrical Cell Biology' workshop organized at the University of Warwick, Warwick, UK, in March 2019, where the core idea of the manuscript was discussed. All authors contributed to the writing of the manuscript. P.T. contributed additionally with figure design.
Competing interests
We declare we have no competing interests
Funding
We recognize funding for the aforementioned workshop from the University of Warwick, and the UK's Biological and Biotechnological Sciences (grant no. BB/S506783/1) and Engineering and Physical Sciences Research Councils.
References
- 1.Hoff HE. 1936. Galvani and the pre-Galvanian electrophysiologists. Ann. Sci. 1, 157–172. ( 10.1080/00033793600200131) [DOI] [Google Scholar]
- 2.Harold FM. 1977. Ion currents and physiological functions in microorganisms. Annu. Rev. Microbiol. 31, 181–203. ( 10.1146/annurev.mi.31.100177.001145) [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P. 1961. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144–148. ( 10.1038/191144a0) [DOI] [PubMed] [Google Scholar]
- 4.Nicholls DG, Ferguson SJ. 2013. Bioenergetics, 4th edn London, UK: Elsevier. [Google Scholar]
- 5.Liu J, Prindle A, Humphries J, Gabalda-Sagarra M, Asally M, Lee D, Ly S, Garcia-Ojalvo J, Süel GM. 2015. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 523, 550–554. ( 10.1038/nature14660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Süel GM. 2015. Ion channels enable electrical communication in bacterial communities. Nature 527, 59–63. ( 10.1038/nature15709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin M. 2007. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 17, 261–270. ( 10.1016/j.tcb.2007.04.007) [DOI] [PubMed] [Google Scholar]
- 8.Chang F, Minc N. 2014. Electrochemical control of cell and tissue polarity. Annu. Rev. Cell Dev. Biol. 30, 317–336. ( 10.1146/annurev-cellbio-100913-013357) [DOI] [PubMed] [Google Scholar]
- 9.Choi W-G, Hilleary R, Swanson SJ, Kim S-H, Gilroy S. 2016. Rapid, long-distance electrical and calcium signaling in plants. Annu. Rev. Plant Biol. 67, 287–307. ( 10.1146/annurev-arplant-043015-112130) [DOI] [PubMed] [Google Scholar]
- 10.Kral N, Hanna Ougolnikova A, Sena G. 2016. Externally imposed electric field enhances plant root tip regeneration. Regeneration 3, 156–167. ( 10.1002/reg2.59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucerova R, Walczysko P, Reid B, Ou J, Leiper LJ, Rajnicek AM, McCaig CD, Zhao M, Collinson JM. 2011. The role of electrical signals in murine corneal wound re-epithelialization. J. Cell. Physiol. 226, 1544–1553. ( 10.1002/jcp.22488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheikh AQ, Taghian T, Hemingway B, Cho H, Kogan AB, Narmoneva DA. 2013. Regulation of endothelial MAPK/ERK signalling and capillary morphogenesis by low-amplitude electric field. J. R. Soc. Interface 10, 20120548 ( 10.1098/rsif.2012.0548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathews J, Levin M. 2018. The body electric 2.0: recent advances in developmental bioelectricity for regenerative and synthetic bioengineering. Curr. Opin. Biotechnol. 52, 134–144. ( 10.1016/j.copbio.2018.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mclaughlin KA, Levin M. 2018. Bioelectric signaling in regeneration: mechanisms of ionic controls of growth and form. Dev. Biol. 433, 177–189. ( 10.1016/j.ydbio.2017.08.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin M, Martyniuk CJ. 2018. The bioelectric code: an ancient computational medium for dynamic control of growth and form. Biosystems 164, 76–93. ( 10.1016/j.biosystems.2017.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benarroch JM, Asally M. 2020. The microbiologist's guide to membrane potential dynamics. Trends Microbiol. 28, 304–314. ( 10.1016/j.tim.2019.12.008) [DOI] [PubMed] [Google Scholar]
- 17.Kay AR. 2017. How cells can control their size by pumping ions. Front. Cell Dev. Biol. 5, 41 ( 10.3389/fcell.2017.00041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zerfaß C, Asally M, Soyer OS, Zerfass C, Asally M, Soyer OS. 2018. Interrogating metabolism as an electron flow system. Curr. Opin. Syst. Biol. 13, 59–67. ( 10.1016/j.coisb.2018.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottschalk G. 1986. Bacterial metabolism. New York, NY: Springer. [Google Scholar]
- 20.Dingledine R, Borges K, Bowie D, Traynelis SF. 1999. The glutamate receptor ion channels. Pharmacol. Rev. 51, 7–61. [PubMed] [Google Scholar]
- 21.Zong Y, Zheng T, Martins P, Lanceros-Mendez S, Yue Z, Higgins MJ. 2017. Cellulose-based magnetoelectric composites. Nat. Commun. 8, 1–7. ( 10.1038/s41467-017-00034-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz-ruiz R, Uribe-carvajal S, Devin A, Rigoulet M. 2009. Tumor cell energy metabolism and its common features with yeast metabolism. BBA — Rev. Cancer 1796, 252–265. ( 10.1016/j.bbcan.2009.07.003) [DOI] [PubMed] [Google Scholar]
- 23.Basan M, Hui S, Okano H, Zhang Z, Shen Y, Williamson JR, Hwa T. 2015. Overflow metabolism in Escherichia coli results from efficient proteome allocation. Nature 528, 99–104. ( 10.1038/nature15765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzl-Raz E, Kafri M, Yaakov G, Soifer I, Gurvich Y, Barkai N. 2017. Principles of cellular resource allocation revealed by condition-dependent proteome profiling. eLife 6, 1–21. ( 10.7554/eLife.28034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang M, Brackenbury WJ. 2013. Membrane potential and cancer progression. Front. Physiol. 4, 185 ( 10.3389/fphys.2013.00185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vemuri GN, Altman E, Sangurdekar DP, Khodursky AB, Eiteman MA. 2006. Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Appl. Env. Microbiol. 72, 3653–3661. ( 10.1128/AEM.72.5.3653-3661.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vemuri GN, Eiteman MA, McEwen JE, Olsson L, Nielsen J. 2007. Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 104, 2402–2407. ( 10.1073/pnas.0607469104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry MN. 1981. An electrochemical interpretation of metabolism. FEBS Lett. 134, 133–138. ( 10.1016/0014-5793(81)80585-6) [DOI] [PubMed] [Google Scholar]
- 29.Lovley DR. 2012. Electromicrobiology. Annu. Rev. Microbiol. 66, 391–409. ( 10.1146/annurev-micro-092611-150104) [DOI] [PubMed] [Google Scholar]
- 30.Kato S. 2015. Biotechnological aspects of microbial extracellular electron transfer. Microbes Environ. 30, 133–139. ( 10.1264/jsme2.ME15028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinowitz JD, Vacchino JF, Beeson C, McConnell HM. 1998. Potentiometric measurement of intracellular redox activity. J. Am. Chem. Soc. 120, 2464–2473. ( 10.1021/ja973560f) [DOI] [Google Scholar]
- 32.Rawson FJ, Downard AJ, Baronian KH. 2014. Electrochemical detection of intracellular and cell membrane redox systems in Saccharomyces cerevisiae. Sci. Rep. 4, 1–9. ( 10.1038/srep05216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y, et al. 2014. Regulation of the cyanobacterial circadian clock by electrochemically controlled extracellular electron transfer. Angew. Chem. Int. Ed. 53, 2208–2211. ( 10.1002/anie.201309560) [DOI] [PubMed] [Google Scholar]
- 34.Tschirhart T, et al. 2017. Electronic control of gene expression and cell behaviour in Escherichia coli through redox signalling. Nat. Commun. 8, 1–11. ( 10.1038/ncomms14030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stratford JP, Edwards CLA, Ghanshyam MJ, Malyshev D, Delise MA, Hayashi Y, Asally M. 2019. Electrically induced bacterial membrane-potential dynamics correspond to cellular proliferation capacity. Proc. Natl Acad. Sci. USA 116, 9552–9557. ( 10.1073/pnas.1901788116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krasnopeeva E, Lo CJ, Pilizota T. 2019. Single-cell bacterial electrophysiology reveals mechanisms of stress-induced damage. Biophys. J. 116, 2390–2399. ( 10.1016/j.bpj.2019.04.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pu Y, et al. 2019. ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance. Mol. Cell 73, 143–156.e4. ( 10.1016/j.molcel.2018.10.022) [DOI] [PubMed] [Google Scholar]
- 38.Sukumar M, et al. 2016. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 23, 63–76. ( 10.1016/j.cmet.2015.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pchelintseva E, Djamgoz MBA. 2018. Mesenchymal stem cell differentiation: control by calcium-activated potassium channels. J. Cell. Physiol. 233, 3755–3768. ( 10.1002/jcp.26120) [DOI] [PubMed] [Google Scholar]
- 40.Sundelacruz S, Levin M, Kaplan DL. 2013. Depolarization alters phenotype, maintains plasticity of predifferentiated mesenchymal stem cells. Tissue Eng. Part A 19, 1889–1908. ( 10.1089/ten.tea.2012.0425.rev) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Menorval M-A, Andre FM, Silve A, Dalmay C, Français O, Le Pioufle B, Mir LM. 2016. Electric pulses: a flexible tool to manipulate cytosolic calcium concentrations and generate spontaneous-like calcium oscillations in mesenchymal stem cells. Sci. Rep. 6, 32331 ( 10.1038/srep32331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosko J, Martinez VA, Poon WCK, Pilizota T. 2017. Osmotaxis in Escherichia coli through changes in motor speed. Proc. Natl Acad. Sci. USA 114, E7969–E7976. ( 10.1073/pnas.1620945114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo CJ, Leake MC, Pilizota T, Berry RM. 2007. Nonequivalence of membrane voltage and ion-gradient as driving forces for the bacterial flagellar motor at low load. Biophys. J. 93, 294–302. ( 10.1529/biophysj.106.095265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delcour AH. 2013. Electrophysiology of bacteria. Annu. Rev. Microbiol. 67, 179–197. ( 10.1146/annurev-micro-092412-155637) [DOI] [PubMed] [Google Scholar]
- 45.Anishkin A, Loukin SH, Teng J, Kung C. 2014. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl Acad. Sci. USA 111, 7898–7905. ( 10.1073/pnas.1313364111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H, Kim M-C, Asada HH. 2019. Extracellular matrix remodelling induced by alternating electrical and mechanical stimulations increases the contraction of engineered skeletal muscle tissues. Sci. Rep. 9, 2732 ( 10.1038/s41598-019-39522-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maldonado EN, Patnaik J, Mullins MR, Lemasters JJ. 2010. Free tubulin modulates mitochondrial membrane potential in cancer cells. Cancer Res. 70, 10 192–10 201. ( 10.1158/0008-5472.CAN-10-2429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strahl H, Hamoen LW. 2010. Membrane potential is important for bacterial cell division. Proc. Natl Acad. Sci. USA 107, 12 281–12 286. ( 10.1073/pnas.1005485107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chimerel C, Field CM, Piñero-Fernandez S, Keyser UF, Summers DK. 2012. Indole prevents Escherichia coli cell division by modulating membrane potential. Biochim. Biophys. Acta Biomembr. 1818, 1590–1594. ( 10.1016/j.bbamem.2012.02.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson AS, Winlow W. 2018. The soliton and the action potential—primary elements underlying sentience. Front. Physiol. 9, 1–10. ( 10.3389/fphys.2018.00779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sirec T, Benarroch JM, Buffard P, Garcia-Ojalvo J, Asally M. 2019. Electrical polarization enables integrative quality control during bacterial differentiation into spores. iScience 16, 378–389. ( 10.1016/j.isci.2019.05.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. 2013. Glutamate receptor-like genes mediate leaf-to-leaf wound signalling. Nature 500, 422–426. ( 10.1038/nature12478) [DOI] [PubMed] [Google Scholar]
- 53.Davies E. 2004. New functions for electrical signals in plants. New Phytol. 161, 607–610. ( 10.1111/j.1469-8137.2003.01018.x) [DOI] [PubMed] [Google Scholar]
- 54.Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S. 2018. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361, 1112–1115. ( 10.1126/science.aat7744) [DOI] [PubMed] [Google Scholar]
- 55.Edwards MA, Robinson DA, Ren H, Cheyne CG, Tan CS, White HS. 2018. Nanoscale electrochemical kinetics & dynamics: the challenges and opportunities of single-entity measurements. Faraday Discuss. 210, 9–28. ( 10.1039/C8FD00134K) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon DT, Gabrielsson EO, Tybrandt K, Berggren M. 2016. Organic bioelectronics: bridging the signaling gap between biology and technology. Chem. Rev. 116, 13 009–13 041. ( 10.1021/acs.chemrev.6b00146) [DOI] [PubMed] [Google Scholar]
- 57.Page A, Perry D, Unwin PR. 2017. Multifunctional scanning ion conductance microscopy. Proc. R. Soc. A 473, 20160889 ( 10.1098/rspa.2016.0889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun P, Laforge FO, Abeyweera TP, Rotenberg SA, Carpino J, Mirkin MV. 2008. Nanoelectrochemistry of mammalian cells. Proc. Natl Acad. Sci. USA 105, 443–448. ( 10.1073/pnas.0711075105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen B, Perry D, Page A, Kang M, Unwin PR. 2019. Scanning ion conductance microscopy: quantitative nanopipette delivery–substrate electrode collection measurements and mapping. Anal. Chem. 91, 2516–2524. ( 10.1021/acs.analchem.8b05449) [DOI] [PubMed] [Google Scholar]
- 60.Taghian T, Narmoneva DA, Kogan AB. 2015. Modulation of cell function by electric field: a high-resolution analysis. J. R. Soc. Interface 12, 20150153 ( 10.1098/rsif.2015.0153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirson ED, et al. 2007. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl Acad. Sci. USA 104, 10 152–10 157. ( 10.1073/pnas.0702916104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grosse C, Schwan HP. 1992. Cellular membrane potentials induced by alternating fields. Biophys. J. 63, 1632–1642. ( 10.1016/S0006-3495(92)81740-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao M, Forrester JV, McCaig CD. 1999. A small, physiological electric field orients cell division. Proc. Natl Acad. Sci. USA 96, 4942–4946. ( 10.1073/pnas.96.9.4942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mycielska ME, Djamgoz MBA. 2004. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J. Cell Sci. 117, 1631–1639. ( 10.1242/jcs.01125) [DOI] [PubMed] [Google Scholar]
- 65.Liu B, Rotenberg SA, Mirkin MV. 2000. Scanning electrochemical microscopy of living cells: different redox activities of nonmetastatic and metastatic human breast cells. Proc. Natl Acad. Sci. USA 97, 9855–9860. ( 10.1073/pnas.97.18.9855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou L, Gong Y, Hou J, Baker LA. 2017. Quantitative visualization of nanoscale ion transport. Anal. Chem. 89, 13 603–13 609. ( 10.1021/acs.analchem.7b04139) [DOI] [PubMed] [Google Scholar]
- 67.Rocha PRF, Schlett P, Kintzel U, Mailänder V, Vandamme LKJ, Zeck G, Gomes HL, Biscarini F, De Leeuw DM. 2016. Electrochemical noise and impedance of Au electrode/electrolyte interfaces enabling extracellular detection of glioma cell populations. Sci. Rep. 6, 34843 ( 10.1038/srep34843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rocha PRF, et al. 2016. Extracellular electrical recording of pH-triggered bursts in C6 glioma cell populations. Sci. Adv. 2, e1600516 ( 10.1126/sciadv.1600516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dusane DH, et al. 2019. Electroceutical treatment of Pseudomonas aeruginosa biofilms. Sci. Rep. 9, 2008 ( 10.1038/s41598-018-37891-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giladi M, Porat Y, Blatt A, Wasserman Y, Kirson ED, Dekel E, Palti Y. 2008. Microbial growth inhibition by alternating electric fields. Antimicrob. Agents Chemother. 52, 3517–3522. ( 10.1128/AAC.00673-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banerjee J, et al. 2015. Silver-zinc redox-coupled electroceutical wound dressing disrupts bacterial biofilm. PLoS ONE 10, e0119531 ( 10.1371/journal.pone.0119531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson WW, Wade MM, Holman SC, Champlin FR. 2001. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 43, 153–164. ( 10.1016/S0167-7012(00)00224-4) [DOI] [PubMed] [Google Scholar]
- 73.Page A, Kang M, Armitstead A, Perry D, Unwin PR. 2017. Quantitative visualization of molecular delivery and uptake at living cells with self-referencing scanning ion conductance microscopy-scanning electrochemical microscopy. Anal. Chem. 89, 3021–3028. ( 10.1021/acs.analchem.6b04629) [DOI] [PubMed] [Google Scholar]
- 74.Farkas DL, Wei MD, Febbroriello P, Carson JH, Loew LM. 1989. Simultaneous imaging of cell and mitochondrial membrane potentials. Biophys. J. 56, 1053–1069. ( 10.1016/S0006-3495(89)82754-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loew LM, Tuft RA, Carrington W, Fay FS. 1993. Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys. J. 65, 2396–2407. ( 10.1016/S0006-3495(93)81318-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao RP. 2019. Towards neural co-processors for the brain: combining decoding and encoding in brain-computer interfaces. Curr. Opin. Neurobiol. 55, 142–151. ( 10.1016/j.conb.2019.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This paper has no additional data.