Abstract
Purpose
The role of stereotactic radiosurgery (SRS) alone for patients with ≥5 brain metastases is not fully understood. The objective of the study was to compare SRS-alone treatment results for 2 to 4 versus 5 to 15 tumors.
Methods and Materials
This was an institutional review board–approved, retrospective cohort study using our prospectively accumulated database including 1150 patients with 2 to 4 tumors and 939 with 5 to 15 tumors who underwent Gamma Knife SRS during a 20-year period (1998-2018). The Kaplan-Meier method was used to determine post-SRS survival times, and competing risk analyses were applied to estimate cumulative incidences of the secondary endpoints.
Results
The post-SRS median survival time was slightly longer in the group with 2 to 4 tumors (8.1 months) than in that with 5 to 15 tumors (7.2 months, P = .0010). Median survival time differences were statistically significant for non-small cell lung cancer, gastrointestinal tract cancer, and others but not for small cell lung cancer, breast cancer, and kidney cancer. Multivariable analysis demonstrated female sex, better Karnofsky Performance Status score, non-small cell lung cancer (vs gastrointestinal tract cancer), younger age, controlled primary cancer, and no extracerebral metastases to be significant predictors of a longer survival period in both tumor number groups. Crude and cumulative incidences of salvage whole brain radiation therapy were significantly higher in the group with 5 to 15 tumors than in that with 2 to 4 tumors, although those of other secondary endpoints were similar to or lower in the 5 to 15 tumor number group than those in the group with 2 to 4 tumors.
Conclusions
We conclude that carefully selected patients with ≥5 to 15 tumors are not unfavorable candidates for SRS alone.
Introduction
Before 2010, the standard treatment for patients with 3 to 4, or more, brain metastases (BMs) was whole brain radiation therapy (WBRT), although stereotactic radiosurgery (SRS) was applied only to patients with 1 to 4 BMs because several randomized clinical trials had demonstrated such patients to have fewer neurocognitive sequelae and improved quality of life with SRS compared with WBRT.1,2 However, our JLGK0901 study demonstrated that SRS treatment results for patients with 5 to 10 BMs were not inferior to those for patients with 2 to 4 BMs. Notably, we found that there was no significant difference in long-term neurocognitive function maintenance between the 2 tumor number groups.3,4 Moreover, recently performed randomized clinical trials demonstrated that decreased neurocognitive function was more common in patients undergoing WBRT than in those receiving SRS alone.5,6 Therefore, the tumor number criterion was recently eliminated from the major treatment guidelines (ie, the National Comprehensive Cancer Network Clinical Practice Guideline in Oncology, Central Nervous System Cancers, version 2.2018, and the guideline of the Congress of Neurologic Surgery).7,8
Very recently, Hughes et al9 reported that median survival times (MSTs) were 8.0, 6.3, and 4.7 months for patients with 1, 2 to 4, and 5 to 15 BMs, respectively (P = .14) and that salvage SRS and WBRT rates did not differ among the 3 tumor number groups. According to their study, however, 1-year distant brain failure occurred in 27%, 44%, and 40% of cases, respectively (P = .01).9 Now that the JLGK0901 study has been published,3,4 the next step is to test whether SRS alone for patients with tumor numbers exceeding 10 is inferior to results obtained in patients with fewer BMs. Thus, the Hughes et al study9 is very timely, although their results are essentially limited to hypothesis generation. In fact, a major weakness of their study was the relatively small patient numbers (ie, 190 and 68 patients having 2 to 4 and 5 to 15 BMs, respectively), such that statistical power was not sufficient. Even more recently, Hughes et al reported, based on an 8-institution study, that patients treated with initial SRS for 5 to 15 BMs experienced survival similar to that in patients with 2 to 4 BMs. However, the number of patients with 5 to 15 BMs was not large, only 212.10 Therefore, we conducted this retrospective cohort study, based on our SRS-treated cohort of more than 2000 patients with BM, including 939 with 5 to 15 tumors, to reappraise whether treatment results differed for tumor numbers of 5 to 15, compared with 2 to 4, and to identify factors relating to overall survival.
Methods and Materials
Patient population
This retrospective cohort study was conducted using our prospectively accumulated database composed of 3558 consecutive patients who underwent Gamma Knife (GK) SRS alone, without WBRT, for BMs during the 20-year period from July 1, 1998 through June 30, 2018. The Institutional Review Board of the Tokyo Women's Medical University gave approval for the present study (no. 1981). Four patients lost to follow-up were excluded, along with 1041 patients with a single BM and 424 with 16 or more BMs, such that we studied 2089 patients (839 females, 1250 males; median age, 66 [range, 19-96] years) in total. Pre-SRS clinical characteristics are presented in Table 1.
Table 1.
Summary of clinical characteristics of 2089 patients with 2 to 15 brain metastases
| Characteristics | Total | 2 to 4 (group A) | 5 to 15 (group B) | P values∗ |
|---|---|---|---|---|
| No. of patients | 2089 | 1150 | 939 | |
| No. of tumors | ||||
| Median (IQR) | 4 (2-7) | 3 (2-3) | 8 (6-11) | |
| Age (y) | ||||
| Median (IQR) | 66 (58-73) | 67 (58-73) | 65 (58-72) | .082 |
| Sex | ||||
| Female | 839 (40.2%) | 438 (38.1%) | 401 (42.7%) | .035 |
| Male | 1250 (59.8%) | 712 (61.9%) | 538 (57.3%) | |
| Primary cancer sites | ||||
| Lung (NSCLC) | 1157 (55.4%) | 634 (55.0%) | 525 (55.9%) | <.0001† |
| Lung (SCLC) | 233 (11.2%) | 112 (9.7%) | 121 (12.9%) | |
| Breast | 235 (11.3%) | 107 (9.3%) | 128 (13.6%) | |
| GI tract | 224 (10.7%) | 144 (12.5%) | 80 (8.5%) | |
| Kidney | 75 (3.4%) | 51 (4.4%) | 24 (2.6%) | |
| Others‡ | 165 (7.9%) | 104 (9.0%) | 61 (6.5%) | |
| Primary cancer status | ||||
| Controlled | 685 (32.8%) | 401 (34.9%) | 284 (30.2%) | .028 |
| Not controlled | 1404 (67.2%) | 749 (65.1%) | 655 (69.8%) | |
| Extra-cerebral METs | ||||
| No | 1021 (48.9%) | 570 (49.6%) | 451 (48.0%) | .51 |
| Yes | 1068 (51.1%) | 580 (50.4%) | 488 (52.0%) | |
| KPS | ||||
| ≥80% | 1597 (76.5%) | 888 (77.2%) | 709 (75.5%) | .38 |
| ≤70% | 492 (23.6%) | 262 (22.8%) | 230 (24.5%) | |
| Modified-RPA class | ||||
| 1 + 2a | 262 (12.5%) | 166 (14.4%) | 96 (10.2%) | .015 |
| 2b | 642 (30.7%) | 348 (30.3%) | 294 (31.3%) | |
| 2c + 3 | 1185 (56.7%) | 636 (55.3%) | 549 (58.5%) | |
| DS-GPA | ||||
| 3.5-4.0 | 91 (4.7%) | 58 (5.5%) | 33 (3.8%) | <.0001† |
| 3.0 | 203 (10.5%) | 125 (11.9%) | 78 (8.9%) | |
| 1.5-2.5 | 1051 (54.6%) | 618 (59.0%) | 433 (49.4%) | |
| 0-1.0 | 580 (30.1%) | 247 (23.6%) | 333 (38.0%) | |
| Neurologic symptoms | ||||
| No | 1001 (47.9%) | 520 (45.2%) | 481 (51.2%) | .0064 |
| Yes | 1088 (52.1%) | 630 (54.8%) | 458 (48.8%) | |
| Prior surgery | ||||
| No | 1707 (81.7%) | 910 (79.3%) | 797 (84.9%) | .0008 |
| Yes | 382 (18.3%) | 240 (20.9%) | 142 (15.1%) | |
| Prior WBRT | ||||
| No | 1977 (94.6%) | 1097 (95.4%) | 880 (93.7%) | .097 |
| Yes | 83 (4.0%) | 53 (4.3%) | 59 (6.3%) | |
| Tumor volume (mL) | ||||
| Cumulative | ||||
| Median (IQR) | 5.51 (1.84-13.38) | 5.04 (1.56-12.55) | 6.18 (2.17-14.84) | .016 |
| Largest tumor | ||||
| Median (IQR) | 3.65 (1.10-9.00) | 3.98 (1.20-9.80) | 3.40 (0.94-8.20) | .0029 |
| Peripheral dose (Gy) | ||||
| Median (IQR) | 24.00 (18.00-24.00) | 24.00 (20.00-24.00) | 22.00 (20.00-24.00) | .0009 |
| Single or 3 stage | ||||
| Single | 2006 (96.0%) | 1100 (95.7%) | 906 (96.5%) | .37 |
| 3-Stage | 112 (5.4%) | 50 (4.4%) | 33 (3.5%) | .45 |
| Treatment periods | ||||
| July 1998-June 2003 | 481 (23.0%) | 287 (25.0%) | 194 (20.7%) | .017† |
| July 2003-June 2008 | 685 (32.8%) | 352 (30.6%) | 333 (35.5%) | |
| July 2008-June 2013 | 501 (24.0%) | 255 (23.1%) | 235 (25.0%) | |
| July 2013-June 2018 | 422 (20.2%) | 245 (21.3%) | 177 (18.9%) | |
Abbreviations: DS-GPA = diagnostic-specific Graded Prognostic Assessment11; GI = gastrointestinal; IQR = interquartile ratio; KPS = Karnofsky Performance Status; MET = metastases; Modified-RPA = Modified-Recursive Partitioning Analysis10,11; WBRT = whole brain radiation therapy.
Student t test was used for continuous variables and Fisher exact test for pairs of categorical variables.
Pearson P value.
Twelve patients with melanoma were included.
Radiosurgical techniques
Our radiosurgical techniques were detailed in prior reports12, 13, 14 and thus are not repeated herein. In brief, we performed standard, single-session GK SRS with frame placement in all cases. Selected doses for the tumor periphery ranged from 10.0 Gy to 25.0 Gy (median, 24.0 Gy; interquartile range [IQR], 20.00-24.00 Gy). However, in 83 patients, a 3-stage treatment protocol was applied because there was only 1 or a few relatively large tumors or because, even in the event of a tumor being small, it was located at or near very critical anatomic structures (eg, the optic chiasma, hypothalamus, internal auditory canal).15,16 In these 83 patients, peripheral doses of 9 to 10 Gy were delivered at a 2-week interval. Multiple SRS procedures were required in 664 (31.8%) of our 2089 patients, 2, 3 and 4 or more times in 450, 130 and 84, respectively (IQR, 1-2; maximum, 8).
Statistical analysis
The primary outcome was overall survival. The secondary outcomes were neurologic death (defined as death caused by any form of intracranial diseases [ie, tumor recurrence, meningeal dissemination, and progression of other untreated intracranial tumors]), neurologic deterioration (deterioration; defined as Karnofsky Performance Status [KPS] score decrease ≥20% from baseline due to neurologic worsening), SRS-related complications, local recurrence, and the need for salvage SRS or WBRT. Major complications included Radiation Therapy Oncology Group neurotoxicity grades of 2 or worse. The Kaplan-Meier method was applied to assess overall survival, and competing risk analysis was used for the time-to-event outcome analyses of all secondary endpoints.17,18 In these time-to-event outcome analyses, interval (months) was calculated from the day of SRS (the day of the first procedure in the 83 patients who underwent 3-stage treatment). The Cox proportional hazard model was used for the multivariable analyses assessing longer survival. All statistical analyses were carried out by a statistician (Y.S.) using SAS software version 9.4 (SAS Institute, Cary, NC). Before the statistical analyses, the entire database was cleaned by one of the coauthors (Y.H.). Neither author was involved in the SRS treatments or any aspects of patient follow-up.
Results
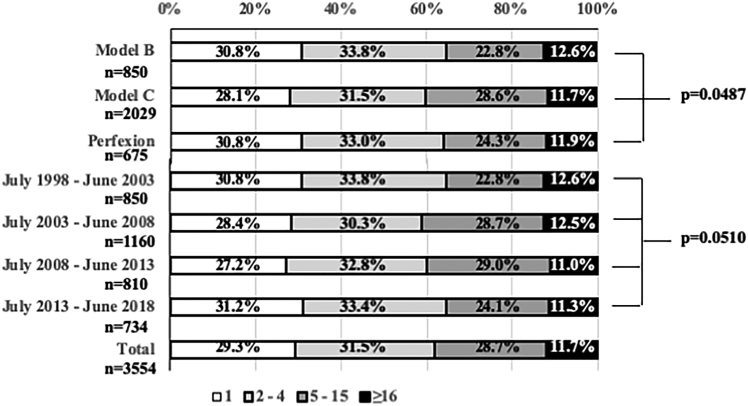
At the Mito GammaHouse, proportions of patients with 1, 2 to 4, 5 to 15, or ≥16 BMs remained nearly constant during the 20-year period of this study (Fig 1). Furthermore, proportions of patients with 1, 2 to 4, 5 to 15, or ≥16 BMs did not differ among 3 periods in which different gamma units were used (ie, the original Leksell Gamma Unit Model B [from July 1, 1998 through June 30, 2003], the subsequent Model C [from July 1, 2003 through December 31, 2013], and the currently used Perfexion [from January 1, 2014 through June 30, 2018]; Elekta AB, Stockholm, Sweden).
Figure 1.
Proportions of patients with 1, 2 to 4, 5 to 15 or ≥16 brain metastases: 3554 patients undergoing radiosurgery according to the 3 Gamma Knife models used or 5-year fractions (July 1, 1998 to June 30, 2018).
The median post-SRS follow-up duration for 295 censored observations was 7.8 (IQR, 1.3-22.8) months, with 1794 patients (92.3%) having died as of the end of June 2019. The overall MST after SRS was 7.7 (95% confidence interval [CI], 7.3-8.1) months. The respective actuarial post-SRS survival proportions were 34.9%, 16.8%, 9.3%, 6.9%, and 4.7% at the 12th, 24th, 36th, 48th, and 60th post-SRS months. Among the 1794 patients who died, the causes of death could not be determined in 69 patients but were confirmed in the remaining 1725: nonbrain diseases in 1537 (89.1%) and brain diseases (local and/or remote BM recurrence, tumor bleeding, SRS-related complications, and any other brain pathology, such as hemorrhage or infarct) in 188 patients (10.9%). Among the total 2089 patients, 664 (31.8%) underwent salvage SRS, generally for new lesions (586 patients, 88.3%) and less commonly for recurrence of a treated lesion (78 patients, 11.7%). Fifty-two patients (2.5%) required salvage WBRT and 17 (0.8%) surgical removal.
Overall survival difference between groups with 2 to 4 and 5 to 15 tumors
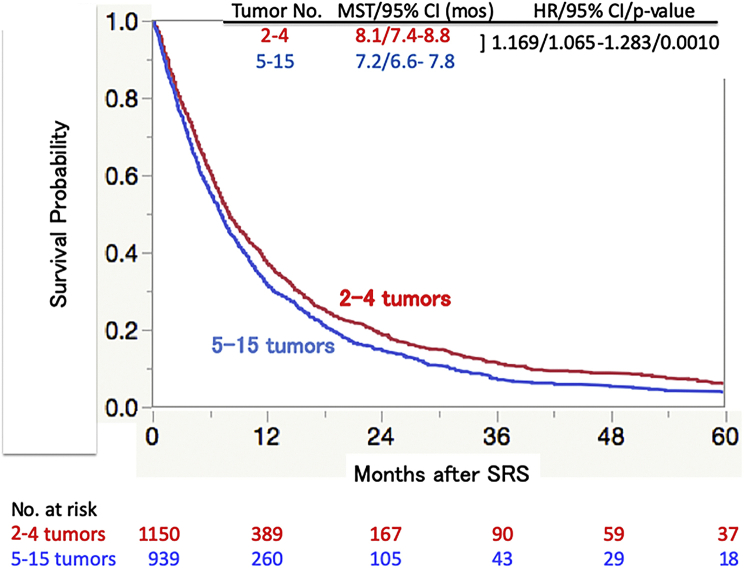
The post-SRS MST was slightly longer in the group with 2 to 4 (8.1 [95% CI, 7.4-8.8] months) tumors than in that with 5 to 15 tumors (7.2 [95% CI, 6.6-7.8] months), as shown in Figure 2. Although this MST difference reached statistical significance (hazard ratio [HR], 1.169; 95% CI, 1.065-1.283; P = .0010), the actual MST difference was only 0.9 months.
Figure 2.
Overall survival according to tumor numbers 2 to 4 and 5 to 15 estimated using the Kaplan-Meier method. Abbreviations: CI = confidence interval; HR = hazard ratio; mos = months; MST = median survival time; SRS = stereotactic radiosurgery.
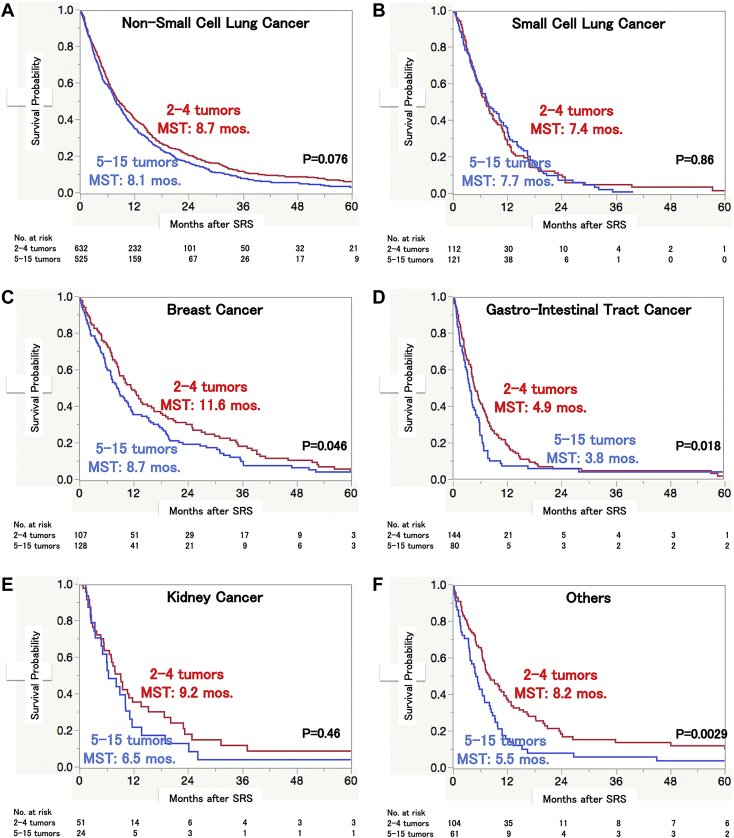
Kaplan-Meier plots of the 2 tumor number groups are presented according to the primary cancer categories: non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), breast cancer, gastrointestinal (GI) tract cancer, kidney cancer, and others (Fig 3). The MST did not differ significantly between patients with SCLC (HR, 0.992; 95% CI, 0.752-1.309; P = .96) and those with kidney cancer (HR, 1.228; 95% CI, 0.730-2.066; P = .44). Although the actual post-SRS MST difference was only 0.6 months in NSCLC patients, this difference was statistically significant (HR, 1.169; 95% CI, 1.030-1.326; P = .015). In contrast, the difference of 1.9 months in patients with breast cancer did not reach the level of statistical significance (HR, 1.281; 95% CI, 0.974-1.685; P = .077). There were significant post-SRS MST differences between the 2 tumor number groups in patients with GI tract cancer (HR, 1.361; 95% CI, 1.016-1.825; P = .039) versus other malignancies (HR, 1.667; 95% CI, 1.182-1.350; P = .0036).
Figure 3.
Overall survival according to tumor numbers of 2 to 4 and 5 to 15 estimated using the Kaplan-Meier method in (A) non-small cell lung cancer, (B) small cell lung cancer, (C) breast cancer, (D) gastrointestinal tract cancer, (E) kidney cancer, and (F) others. Abbreviations: mos = months; MST = median survival time.
Factors affecting longer survival period
As shown in Table 2, among various pre-SRS clinical factors, multivariable analysis demonstrated female sex, better KPS score, NSCLC diagnosis (vs a diagnosis of GI tract cancer), controlled primary cancer, and absence of extracerebral metastases to be significant predictors of longer survival for both tumor number groups. In the patient group with 2 to 4 tumors, being asymptomatic and not having undergone surgery before SRS were significantly favorable predictors of longer survival. Regarding the treatment period, we found statistically significant MST differences between July 1998 to June 2003 versus July 2003 to June 2008 and between July 2008 to June 2013 versus July 2013 to June 2018 in the group of patients with 2 to 4 tumors. However, in that with 5 to 15 tumors, there were no significant MST differences between any of the 2 subsequent 5-year periods.
Table 2.
Multivariable analyses of survival after SRS
| Variables | 2-4 tumors |
5-15 tumors |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | ||||
| Male vs female | 1.372 (1.197-1.572) | <.0001 | 1.246 (1.071-1.450) | .0043 |
| Age (y) | ||||
| ≥65 vs <65 | 1.121 (0.991-1.270) | .5630 | 1.158 (1.006-1.333) | .0412 |
| KPS (%) | ||||
| ≤70 vs ≥80 | 1.784 (1.531-2.080) | <.0001 | 1.875 (1.583-2.220) | <.0001 |
| Neurologic symptoms | ||||
| Yes vs no | 1.171 (1.025-1.337) | .0201 | 1.111 (0.958-1.289) | .1649 |
| Tumor volume (mL) | ||||
| Cumulative ≥10.0 vs <10.0 | 1.044 (0.823-1.324) | .7247 | 1.189 (0.986-1.434) | .0698 |
| Largest tumor ≥10.0 vs <10.0 | 1.155 (0.894-1.491) | .2706 | 1.055 (0.846-1.316) | .6320 |
| Dose (Gy) | ||||
| Minimum <20 vs ≥20 | 1.075 (0.879-1.316) | .4815 | 1.022 (0.830-1.258) | .8402 |
| Maximum <36 vs ≥36 | 0.993 (0.878-1.122) | .9062 | 1.005 (0.877-1.151) | .9429 |
| Primary cancer | ||||
| SCLC vs NSCLC | 1.171 (0.952-1.442) | .1350 | 1.041 (0.848-1.278) | .7011 |
| Breast vs NSCLC | 1.131 (0.886-1.444) | .3233 | 1.099 (0.854-1.415) | .4634 |
| GI tract vs NSCLC | 1.316 (1.083-1.598) | .0057 | 1.513 (1.180-1.941) | .0011 |
| Kidney vs NSCLC | 0.979 (0.730-1.312) | .8851 | 1.098 (0.722-1.669) | .6614 |
| Others vs NSCLC | 0.965 (0.774-1.204) | .7517 | 1.174 (0.884-1.561) | .2669 |
| Primary cancer status | ||||
| No vs good | 1.884 (1.644-2.160) | <.0001 | 1.886 (1.897-2.226) | <.0001 |
| Extracranial METs | ||||
| Not vs controlled | 1.325 (1.174-1.518) | <.0001 | 1.478 (1.281-1.706) | <.0001 |
| Pre-SRS WBRT | ||||
| Yes vs no | 0.850 (0.639-1.230) | .2625 | 1.096 (0.828-1.450) | .5217 |
| Pre-SRS surgery | ||||
| Yes vs no | 0.779 (0.668-0.908) | .0015 | 0.851 (0.703-1.019) | .0956 |
| Treatment period | ||||
| July 1998-June 2003 vs July 2003-June 2008 | 1.235 (1.048-1.455) | .0116 | 1.055 (0.876-1.270) | .5743 |
| July 2003-June 2008 vs July 2008-June 2013 | 0.882 (0.770-1.037) | .1279 | 1.166 (0.981-1.386) | .0807 |
| July 2008-June 2013 vs July 2013-June 2018 | 0.783 (0.654-0.936) | .0071 | 0.874 (0.714-1.069) | .1892 |
Abbreviations: CI = confidence interval; GI = gastrointestinal; HR = hazard ratio; KPS = Karnofsky Performance Status; METs = metastases; NSCLC = non-small cell lung cancer; SCSL = small cell lung cancer; SRS = stereotactic radiosurgery; WBRT = whole brain radiation therapy.
Prognostic indexes
Kaplan-Meier plots of the 2 tumor number groups (ie, 2-4 and 5-15 tumors) are presented in Figure 4 according to the Diagnostic Specific-Graded Prognostic Assessment (DS-GPA) system.11 A higher DS-GPA score is clearly associated with a longer MST, and the survival difference based on 4-group stratification was statistically significant in both the 2 to 4 BM group (P < .0001) and the 5 to 15 BM group (P = .0031). However, the MST differences between the 2 subgroups, one with a DS-GPA score of 3.0 and the other with scores of 3.5 to 4.0 (2-4 tumor group: P = .082; 5-15 tumor group: P = .97), as well as between the 2 DS-GPA subgroups with scores of 1.5 to 2.5 versus 3.0 (2-4 tumor group: P = .21; 5-15 tumor group: P = .010), did not reach statistical significance. In fact, only the survival difference between those with DS-GPA scores of 0 to 1.0 and 1.5 to 2.5 reached statistical significance (2-4 tumor group: P < .0001; 5-15 tumor group: P = .0075).
Figure 4.
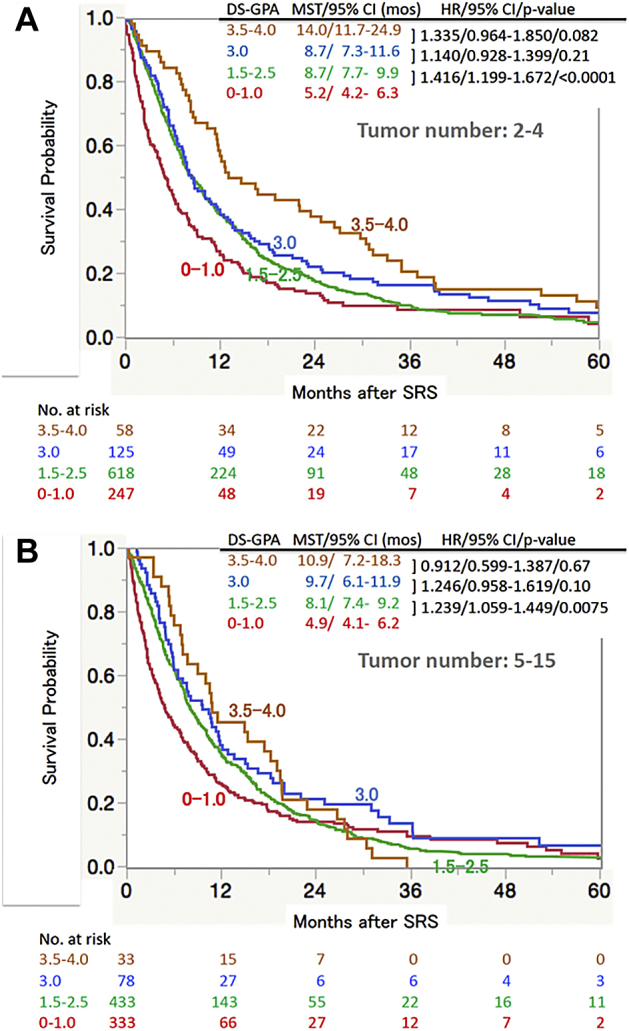
Overall survival according to subclasses of the diagnostic-specific Graded Prognostic Assessment (DS-GPA)18 estimated using the Kaplan-Meier method, tumor numbers of (A) 2 to 4 and (B) 5 to 15. Because the DS-GPA is not applicable to patients with other original cancers, 165 (104/2-4 tumors and 61/5-15 tumors) patients with other original cancers were excluded. Abbreviations: CI = confidence interval; HR = hazard ratio; mos = months; MST = median survival time; SRS = stereotactic radiosurgery.
Figure 5 is the Kaplan-Meier plots of the 2 tumor number patient groups (ie, 2-4 and 5-15 tumors), according to the Modified-Recursive Partitioning Analysis (M-RPA) system.12,19 In the group with 2 to 4 tumors, the respective MSTs of the 1 + 2a, 2b, and 2c + 3 subclasses were 22.3, 10.8, and 5.7 months (P < .0001). In those with 5 to 15 tumors, the corresponding values were 17.7, 11.2, and 4.6 months (P < .001). It is noteworthy that, in both tumor number groups, the MSTs are significantly different with no overlap of the 95% CI between the 2 subclasses (ie, 1 + 2a vs 2b and 2b vs 2c + 3).
Figure 5.
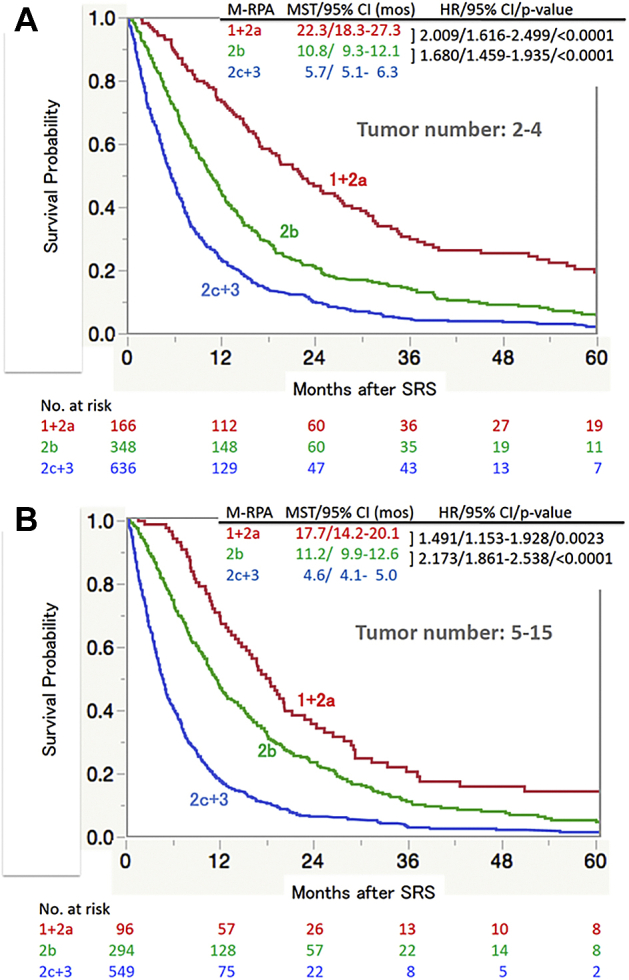
Overall survival according to subclasses of the Modified Recursive Partitioning Analyses (M-RPA)10,11 estimated using the Kaplan-Meier method, tumor numbers of (A) 2 to 4 and (B) 5 to 15. Abbreviations: CI = confidence interval; HR = hazard ratio; mos = months; MST = median survival time; SRS = stereotactic radiosurgery.
Secondary outcomes
The crude and cumulative incidences of neurologic death, repeat SRS, and SRS-related complications did not differ significantly between the 2 tumor number groups (Table 3). Crude and cumulative incidences of neurologic deterioration and local recurrence were both significantly lower in the group with 5 to 15 tumors than in that with the 2 to 4 tumors, although the numbers requiring salvage WBRT were significantly higher in the group with 5 to 15 tumors than in that with 2 to 4 tumors.
Table 3.
Posttreatment crude and cumulative incidences of the secondary endpoints determined using competing risk analysis
| Tumor no. group | Crude incidences (%) | P value | Cumulative incidences (post-SRS mo), % |
Adjusted HR (95% CI)/P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 12 | 24 | 36 | 48 | 60 | |||||
| Neurologic death∗ | 2-4 | 105 (11.2) | .59 | 5.7 | 9.2 | 10.00 | 10.2 | 10.5 | 0.952 (0.713-1.271)/.73 |
| 5-15 | 81 (10.3) | 6.3 | 8.2 | 9.2 | 9.9 | 10.3 | |||
| Neurologic deterioration† | 2-4 | 145 (12.6) | .021 | 8.2 | 11.8 | 13.0 | 13.4 | 13.8 | 0.741 (0.569-0.966)/.026 |
| 5-15 | 88 (9.4) | 6.9 | 8.8 | 9.8 | 10.1 | 10.7 | |||
| Local recurrence‡ | 2-4 | 60 (7.3) | .037 | 4.2 | 6.5 | 10.0 | 7.2 | 8.3 | 0.626 (0.405-0.970)/.035 |
| 5-15 | 30 (4.6) | 2.3 | 4.4 | 5.2 | 5.4 | 5.4 | |||
| Repeat SRS | 2-4 | 379 (33.0) | .22 | 27.0 | 33.5 | 35.0 | 35.6 | 35.6 | 0.914 (0.784-1.066)/.25 |
| 5-15 | 285 (30.4) | 26.4 | 31.6 | 32.7 | 33.1 | 33.1 | |||
| Salvage WBRT | 2-4 | 19 (1.7) | .0072 | 1.0 | 1.6 | 1.7 | 1.8 | 1.8 | 2.165 (1.233-3.803)/.0072 |
| 5-15 | 33 (3.5) | 3.0 | 3.6 | 3.9 | 3.9 | 3.9 | |||
| SRS-related complications | 2-4 | 11 (1.0) | .47 | 1.3 | 1.9 | 2.2 | 2.5 | 2.8 | 0.783 (0.453-1.352)/.38 |
| 5-15 | 6 (0.8) | 1.2 | 1.7 | 2.0 | 2.2 | 2.6 | |||
Abbreviations: MMSE = mini-mental status examination (decreased; defined as score decrease ≥3 from baseline); SRS = stereotactic radiosurgery, WBRT = whole brain radiation therapy.
Based on 1794 deceased patients (979 [85.1%] in 2-4 tumor group and 815 [86.8%] in 5-15 tumor group).
Deteriorated; defined as score decrease ≥20% from baseline.
Based on 1483 (827 [71.9%] in 2-4 tumor group and 656 [69.9%] in 5-15 tumor group) patients in whom neuroimaging results were available.
Discussion
Regarding SRS for patients with ≥5 BMs, Hughes et al recently stated that “due to technical advancements allowing for the treatment of a greater number of metastases, patients with multiple BMs are more frequently treated with SRS alone.”9 However, this statement does not appear to adequately reflect the history of SRS. In several GK facilities throughout Japan, SRS for patients with 4 to 5, or as many as 10 or more, BMs was already being performed in the years around 1995.20, 21, 22 In other words, SRS techniques for 10 or more BMs had already been established more than 20 years ago. The reluctance of many physicians in Western countries to undertake SRS for multiple BMs was not related to a lack of technical development. Rather, there is a long-established dogma that WBRT should be performed for patients with multiple BMs, despite a lack of scientific evidence showing the superiority of WBRT to SRS alone for such patients.
Kim et al reported 26 patients undergoing GK SRS for 10 or more BMs, with a post-SRS MST of 34 (range, 8-199) weeks.23 After 2010, several studies on SRS for multiple BMs were reported in the Journal of Neurosurgery or American Journal of Clinical Oncology: Chang et al (58 patients/6-10 BMs, 17/11-15 BMs),24 Grandhi et al (61/≥10 BMs),25 Raldow et al (103 patients/≥5 BMs),26 and Rava et al (53 patients/≥10 BMs).27 However, patient numbers were relatively small in these 4 studies. The authors performed a study based on 548 case-matched patients in 2 groups with 1 to 4 and ≥5 BMs.28 Although the post-SRS MST difference of 0.9 months between the 2 groups was statistically significant, this difference was not clinically meaningful. Furthermore, the 2 groups did not differ significantly in cumulative incidences, as determined using competing risk analyses of neurologic deterioration, neurologic death, local recurrence, re-SRS for new lesions, and SRS-related complications.
In the present study, although the post-SRS MST difference of 0.9 months between the 2 tumor number groups was statistically significant, this difference had no clinical effect. Crude and cumulative incidences of salvage WBRT were significantly higher in the patient group with 5 to 15 BMs (3.5%) than in that with 2 to 4 BMs (1.7%). However, the incidences were very low, and the difference in the absolute percentage, 1.8%, is not particularly meaningful clinically. Furthermore, the 2 groups did not differ significantly in cumulative incidences of neurologic death, re-SRS, or SRS-related complications. Rather, the cumulative incidences of neurologic deterioration or local recurrence were significantly lower in the group with 5 to 15 BMs than in that with 2 to 4 BMs.
As Hughes et al pointed out, this study, using the same data set as our previous studies, was conducted in Japan, where patients with lung cancer with prolonged post-SRS survival are more prevalent.9 Unlike Asian populations, in which up to 30% of patients with lung cancer harbor an EGFR gene mutation, the prevalence of mutations relevant to treatment (EGFR, ALK, and/or ROS-1) in patients with lung cancer is relatively low in Western countries. Furthermore, melanoma is very uncommon in Japan. In the data set used for this study, there were only 11 patients with melanoma (0.5% of 2,059 patients). This discrepancy may have contributed to the trend toward poorer survival demonstrated in the 5 to 15 BM group. In fact, as shown in Figure 3, the MST difference between the 2 tumor number groups was very small in patients with NSCLC and absent in those with SCLC, although MSTs in patients with the other 4 original cancers (ie, breast, GI, kidney, and other cancers) were, regardless of whether they were statistically significant, shorter in the 5 to 15 BM than in the other group. Also, as Hughes et al pointed out, their analysis did not take into account the need for salvage therapies. In the United States, given that SRS is several-fold more costly than WBRT, the need for multiple or early salvage regimens must be considered when deciding whether to administer upfront treatment of BM. In contrast, the cost of SRS in Japan is relatively low, set by the Japanese National Health Insurance system at JPY500,000. Therefore, salvage SRS can easily be repeated in Japan.
In a Rando phantom experiment, the first author (M.Y.) analyzed cumulative whole brain irradiation doses based on the treatment protocol for a patient with 48 lesions.20 The estimated cumulative irradiation doses were 2.60 Gy to 6.69 Gy at sites located some distance from the targets. These earlier results are highly consistent with those described in the present study. Yang et al, based on their dose-volume histogram analysis using a model with placement of 25 targets within the whole brain followed by irradiation with a maximum dose of 40 Gy, reported that the 50% whole brain dose was no more than 5 Gy.29 Furthermore, Boone et al recently reported that, based on their management of 10 patients with 6 to 15 BMs treated with a linear accelerator system, the largest calculated cumulative dose to the entire brain was approximately 5.0 Gy.30 In 2002, the first author (M.Y.) and colleagues reported that, based on a series of 80 patients with ≥10 BMs (median, 17; maximum, 43) undergoing SRS, the estimated absorbed doses to the whole brain ranged from 2.16 to 8.51 (median, 4.71) Gy.22 It was thus assumed that these doses had not exceeded the threshold level of radiation-induced injury to the whole brain. We also reported, based on 2996 patients, multivariable analyses that demonstrated solitary tumor, controlled primary cancer, no extracerebral metastases, KPS 80%, and largest tumor volume ≥3.3 cm3 to be independently significant predictors of a higher incidence of complications. Pre-SRS WBRT, cumulative tumor volume, 12 Gy brain volume, and total cumulative energy delivered to the whole skull had no effect on complication rates.14
Regarding patient selection being favorable for SRS treatment, the widely used DS-GPA system is not considered to be sufficient because, as shown in Figure 4, MST differences between DS-GPA scores of 3.5 to 4.0 versus 3.0 and between a DS-GPA score of 3.0 versus 1.5 to 2.5 did not reach the level of statistical significance. However, patients with DS-GPA scores over 1.5 are considered to be good candidates for SRS treatment. In contrast, our M-RPA system is apparently ideal for patient selection because, as shown in Figure 5, MST differences between M-RPA 1 + 2a versus 2b and between modified-RPA 2b versus 2c + 3 were statistically significant. Thus, patients with M-RPA 1 + 2a or 2b are good candidates for SRS treatment.
Limitations
The major weakness of this study might be its retrospective design, based largely on one neurosurgeon’s experiences in a single institute. Given the long study period, 20 years, we must interpret our treatment results in light of progression in the management of patients with cancer (ie, one factor being the development of imaging technology and the other advances in systemic anticancer agent treatments). Regarding the first issue, our imaging technique for dose planning has remained unchanged for the past 20 years (ie, T1-weighted axial image with a 2 mm slice thickness with no gap and administration of a single gadolinium dose). Our magnetic resonance (MR) unit was updated from a 1.0 Tesla MR unit to a 1.5 Tesla MR unit at the end of August 2002. Only the first 19% of our patients were examined using a 1.0 Tesla MR unit; therefore, the MR unit update was considered to have minimally affected our treatment results. Regarding the impacts of medical treatment advancements, there was a large difference in treatment results between 2 periods (ie, the earlier and the most recent 10-year period). In fact, in our cohort study (3554 patients), there was a statistically significant difference in overall survival between the 2 study periods: 7.3 versus 10.8 months (HR, 1.464; 95% CI, 1.356-1.580; P < .0001). However, in the present study, comparisons were made within the same periods. Therefore, as shown in Table 4, medical treatment advances were considered to have minimally affected our comparative results in this study.
Table 4.
Median survival times (mo) and cumulative survival incidences in 2 tumor number groups (2-4 vs 5-15 tumors, according to 5-year periods)
| Tumor no. | n | MST/95% CI | Cumulative incidence (no. at risk) |
HR/95% CI | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12 mo | 24 mo | 36 mo | 48 mo | 60 mo | ||||||
| July 1998-June 2003 | 2-4 | 287 | 7.4/6.1-8.1 | 0.289 (84) | 0.100 (29) | 0.054 (16) | 0.046 (13) | 0.038 (10) | 1.202/1.000-1.446 | .0499 |
| 5-15 | 194 | 5.4/4.5-6.5 | 0.242 (48) | 0.080 (16) | 0.021 (5) | 0.021 (5) | 0.011 (3) | |||
| July 2003-June 2008 | 2-4 | 352 | 7.6/6.7-9.6 | 0.352 (126) | 0.168 (60) | 0.091 (33) | 0.068 (25) | 0.034 (13) | 1.237/1.062-1.469 | .0061 |
| 5-15 | 333 | 6.9/5.7-7.7 | 0.258 (88) | 0.091 (31) | 0.045 (16) | 0.030 (11) | 0.015 (6) | |||
| July 2008-June 2013 | 2-4 | 266 | 7.4/6.6-8.7 | 0.343 (91) | 0.154 (41) | 0.090 (24) | 0.057 (14) | 0.048 (12) | 1.124/0.939-1.347 | .2033 |
| 5-15 | 235 | 7.0/5.7-7.8 | 0.300 (71) | 0.139 (33) | 0.062 (14) | 0.033 (8) | 0.033 (7) | |||
| July 2013-June 2018 | 2-4 | 245 | 16.4/11.6-22.8 | 0.566 (92) | 0.409 (43) | 0.322 (19) | 0.277 (9) | 0.202 (2) | 0.971/0.728-1.295 | .8400 |
| 5-15 | 177 | 17.6/10.4-26.3 | 0.547 (54) | 0.422 (28) | 0.273 (9) | 0.234 (5) | 0.176 (2) | |||
Abbreviations: CI = confidence interval; HR = hazard ratio.
Another weakness might have been the inclusion in our database of neither original cancer phenotypes nor information on systemic anticancer agent treatment, both of which are regarded as correlating with survival. However, in our view, the present study supports the hypothesis generated by Hughes et al9; SRS for 5 to 15 BMs is well tolerated, and there is no evidence of associated increases in toxicity, treatment failure, and salvage therapy. Although before 2010 there was a clear reluctance in the West to apply SRS to patients with a large number of BMs, clinical trials including patients with numerous BMs have recently been initiated. Ongoing prospective randomized studies (eg, NCT01731704, NCT03075072, NCT03550391, NCT03297788, NCT02953717) are anticipated to provide more robust support for the hypothesis that relatively large numbers of BMs can be treated with SRS in appropriately selected patients.
Conclusions
We conclude that carefully selected patients (ie, DS-GPA 1.5 or better or M-RPA 1 + 2a and 2b) with 5 to 15 tumors are not unfavorable candidates for SRS alone. However, a randomized controlled trial should be conducted in the near future to clarify the optimal role of SRS alone in patients with 5 or more BMs.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: The authors have no conflicts of interest to declare.
References
- 1.Andrews D.W., Scott C.B., Sperduto P.W. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet North Am Ed. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 2.Aoyama H., Shirato H., Tago M. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M, Serizawa T, Shuto T. Results of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective study. Lancet Oncology. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M., Serizawa T., Higuchi Y. A Multi-Institutional Prospective Observational Study of Stereotactic Radiosurgery for Patients with Multiple Brain Metastases (JLGK0901 Study Update): Irradiation-Related Complications and Long-Term Maintenance of Mini-Mental State Examination Scores. Int J Radiat Oncol Biol Phys. 2017;99:31–40. doi: 10.1016/j.ijrobp.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 5.Chang E.L., Wefel J.S., Hess K.R. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomized controlled trail. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 6.Brown P.D., Jaeckle K., Ballman K.V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The National Comprehensive Cancer Network Clinical Practice Guideline in Oncology, Central Nervous System Cancers, version 2. 2018. 2018. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
- 8.Graber J.J., Cobbs C.S., Olson J.J. Congress of Neurological Surgeons systematic review and evidence-based guidelines on the use of stereotactic radiosurgery in the treatment of adults with metastatic brain tumors. Neurosurgery. 2019;84:E168–E170. doi: 10.1093/neuros/nyy543. [DOI] [PubMed] [Google Scholar]
- 9.Hughes R.T., McTyre E.R., LeCompte M. Clinical outcomes of upfront stereotactic radiosurgery alone for patients with 5 to 15 brain metastases. Neurosurgery. 2019;85:257–263. doi: 10.1093/neuros/nyy276. [DOI] [PubMed] [Google Scholar]
- 10.Hughes R.T., Masters A.H., McTyre E.R. Initial SRS for patients with 5-15 brain metastases: Results of a multi-institutional experience. Int J Radiat Oncol Biol Phys. 2018;104:1091–1098. doi: 10.1016/j.ijrobp.2019.03.052. [DOI] [PubMed] [Google Scholar]
- 11.Sperduto P.W., Chao S.T., Sneed P.K. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4259 patients. Int J Radiat Oncol Biol Phys. 2010:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M., Sato Y., Serizawa T. Subclassification of recursive partitioning analysis Class II patients with brain metastases treated radiosurgically. Int J Radiat Oncol Biol Phys. 2012;83:1399–1405. doi: 10.1016/j.ijrobp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M., Kawabe T., Sato Y. Stereotactic radiosurgery for patients with multiple brain metastases: a case-matched study comparing treatment results for 2-9 vs ≥10 tumors. J Neurosurg. 2014;21(Suppl 2):16–25. doi: 10.3171/2014.8.GKS141421. [DOI] [PubMed] [Google Scholar]
- 14.Aiyama H., Yamamoto M., Kawabe T. Complications after stereotactic radiosurgery for brain metastases: Incidences, correlating factors, treatments and outcomes. Radiother Oncol. 2018;129:364–369. doi: 10.1016/j.radonc.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi Y., Serizawa T., Nagano O. Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys. 2009;74:1543–1548. doi: 10.1016/j.ijrobp.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M., Higuchi Y., Serizawa T. Three-stage Gamma Knife treatment for metastatic brain tumors larger than 10 cm3: a 2-institute study including re-analyses of earlier results using competing risk analysis. J Neurosurg. 2018;129(Suppl):77–85. doi: 10.3171/2018.7.GKS181392. [DOI] [PubMed] [Google Scholar]
- 17.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Gooley T.A., Leisenring W., Crowley J., Storer B.E. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M., Serizawa T., Sato Y. Validity of two recently-proposed prognostic grading indices for lung, gastro-intestinal, breast and renal cell cancer patients with radiosurgically-treated brain metastases. J Neurooncol. 2013;111:327–335. doi: 10.1007/s11060-012-1019-9. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M., Ide M., Jimbo M. In: Kondziolka D, editor. Basel: Karger; 1998. pp. 94–109. 2. [Google Scholar]
- 21.Suzuki S., Omagari J., Nishio S., Nishiye E., Fukui M. Gamma Knife radiosurgery for simultaneous multiple metastatic tumors. J Neurosurg. 2000;93(Suppl 3):30–31. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M., Ide M., Nishio S., UrakawaGamma Y. Knife radiosurgery for numerous brain metastases: Is this a safe treatment? Int J Radiat Oncol Biol Phys. 2002;53:1279–1283. doi: 10.1016/s0360-3016(02)02855-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim C.H., Im Y.S., Nam D.H., Park K., Kim J.H., Lee J.I. Gamma knife radiosurgery for ten or more brain metastases. J Korean Neurosurg Soc. 2008;44:358–363. doi: 10.3340/jkns.2008.44.6.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang W.S., Kim H.Y., Chang J.W., Park Y.G., Chang J.H. Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: Is stereotactic radiosurgery effective for multiple brain metastases? J Neurosurg. 2010;113(Suppl):73–78. doi: 10.3171/2010.8.GKS10994. [DOI] [PubMed] [Google Scholar]
- 25.Grandhi R., Kondziolka D., Panczykowski Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg. 2012;117:237–245. doi: 10.3171/2012.4.JNS11870. [DOI] [PubMed] [Google Scholar]
- 26.Raldow A.C., Chiang V.L., Knisely J.P., Yu J.B. Survival and intracranial control of patients with 5 or more brain metastases treated with gamma knife stereotactic radiosurgery. Am J Clin Oncol. 2013;36:486–490. doi: 10.1097/COC.0b013e31825494ef. [DOI] [PubMed] [Google Scholar]
- 27.Rava P., Leonard K., Sioshansi S. Survival among patients with 10 or more brain metastases treated with stereotactic radiosurgery. J Neurosurg. 2013;119:457–462. doi: 10.3171/2013.4.JNS121751. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto M., Kawabe T., Sato Y. A case-matched study of stereotactic radiosurgery for patients with multiple brain metastases: comparing treatment results for 1-4 vs ≥ 5 tumors: clinical article. J Neurosurg. 2013;118:1258–1268. doi: 10.3171/2013.3.JNS121900. [DOI] [PubMed] [Google Scholar]
- 29.Yang C.C., Ting J., Wu X. Dose volume histogram analysis of the Gamma Knife radiosurgery treating twenty-five metastatic intracranial tumors. Stereotact Funct Neurosurg. 1998;70(Suppl 1):41–49. doi: 10.1159/000056405. [DOI] [PubMed] [Google Scholar]
- 30.Boone R.A., Solberg T.D., Selch M.T. Cumulative dose to targets, critical structures, and the whole brain from the treatment of multiple intracranial metastases. In: Alexander E. 3rd, Kondziolka D., Lindquist C., editors. Radiosurgery 1999. Karger; Basel: 2000. pp. 247–256. [Google Scholar]