Abstract
Immunotherapy strategies targeting the programmed cell death ligand 1 (PD-L1)/programmed cell death 1 (PD-1) pathway in clinical treatments have achieved remarkable success in treating multiple types of cancer. However, owing to the heterogeneity of tumors and individual immune systems, PD-L1/PD-1 blockade still shows slow response rates in controlling malignancies in many patients. Accumulating evidence has shown that an effective response to anti-PD-L1/anti-PD-1 therapy requires establishing an integrated immune cycle. Damage in any step of the immune cycle is one of the most important causes of immunotherapy failure. Impairments in the immune cycle can be restored by epigenetic modification, including reprogramming the environment of tumor-associated immunity, eliciting an immune response by increasing the presentation of tumor antigens, and by regulating T cell trafficking and reactivation. Thus, a rational combination of PD-L1/PD-1 blockade and epigenetic agents may offer great potential to retrain the immune system and to improve clinical outcomes of checkpoint blockade therapy.
Key words: Epigenetic regulation, Immune cycle, PD-L1/PD-1 blockade, Cancer, Immunotherapy
Abbreviations: 5-AzaC, 5-azacitidine; ACE1, angiotensin converting enzyme; ACP1, human red cell acid phosphatase; APC, antigen-presenting cell; BETi, bromodomain and extra-terminal motif inhibitors; CCL22 (MDC), macrophage-derived chemokine; CLL, chronic lymphocytic leukemia; CTA, cancer testis antigen; CTLA-4, cytotoxic T lymphocyte antigen 4; CTLs, cytotoxic T lymphocytes; CX3CL1, C-X3-C motif chemokine ligand 1; CXCL, CXC chemokine ligand; DC, dendritic cell; DNMT1, DNA methyltransferase 1; DNMTi, DNA methyltransferase inhibitors; EZH2, enhancer of zeste homolog 2; FDA, U. S. Food and Drug Administration; FOXP3, forkhead box P3; H3K27me3, tri-methylation of lysine 27 on histone H3; HDACi, histone deacetylase inhibitor; IDO, indoleamine 2,3-dioxygenase; IFN-γ, interferon-gamma; LAG-3, lymphocyte activation gene-3; MDSCs, myeloid-derived suppressor cells; MHC, major histocompatibility complex; OS, overall survival; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; PRC2, polycomb repressive complex 2; TAA, tumor-associated antigen; TET2, ten-eleven translocation 2; TH-1, T helper type 1; TIL, tumor infiltrating lymphocytes; TIM-3, T cell immunoglobulin and mucin domain 3; Tregs, regulatory T cells; UHRF1, ubiquitin-like PHD and RING finger domain-containing 1
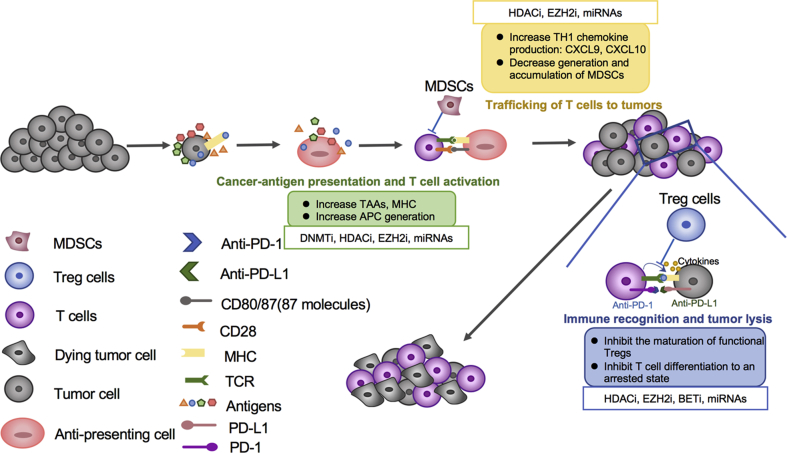
Graphical abstract
An effective response to anti-PD-L1/anti-PD-1 therapy requires the establishment of an integrated immune cycle. Impaired immune cycle can be restored by epigenetic modification, including reprogramming the tumor-associated immunity and eliciting an immune response. Epigenetic combination therapies may be optimally integrated to enhance the response rates of PD-L1/PD-1 blockade.
1. Introduction
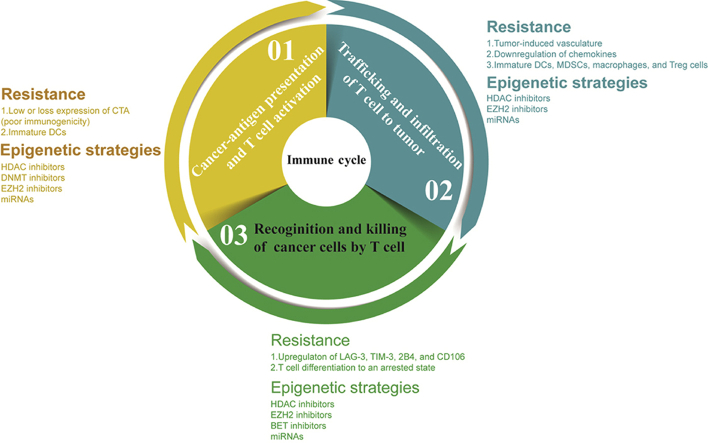
Due to a better understanding of fundamental principles of cancer biology and immunology, cancer immunotherapy has made significant achievements in the last several years, particularly in the immune checkpoint blockade field1. Cancer immunotherapy is based on the stimulation and engineering of the immune system, such as the restoration of T lymphocytes, to combat cancer2. Sequential steps (Fig. 1) are combined to create an integrated cancer immune cycle3. One of the most promising ways to activate the immune system is the blockade of the immune checkpoints. Immune checkpoints are inhibitory pathways that are excessively hardwired into the immune system and are critical for maintaining self-tolerance and regulating the duration and magnitude of physiological immune responses to minimize incidental tissue damage4. However, immune checkpoint molecules can mediate tumor immune escape, leading to malignant tumor progression. In the last decade, cancer immunotherapies targeting the immune checkpoint pathway, have achieved an unprecedented, long-lasting response rate in various cancers. This sustained response rate can be achieved primarily by blockade of the programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) pathway. Due to constant genetic changes in tumor cells, the varied conditions and complexity of the tumor immune microenvironment, most patients with advanced tumors have no response solely to immunotherapy5. An objective response rate of 20%–40% is observed in the majority of solid tumors6, 7. Hence, combined therapies are urgently needed to improve the antitumor activity of anti-PD-L1/anti-PD-1 strategies. Encouraging outcomes from combinations with PD-L1/PD-1 blockade have been reported in a set of preclinical and clinical trials. These include combinations with other immunotherapy treatments, such as anti-CTLA-4 (cytotoxic T lymphocyte antigen 4) blockade8, 9, and co-therapy with radiation strategy10, 11, standard-of-care chemotherapy12, and small-molecular inhibitors, especially epigenetic agents13.
Figure 1.
The cancer immune cycle must be initiated and completed successfully to elicit an effective therapeutic immune response, and this cycle involves efficient (1) cancer-antigen presentation, (2) trafficking and infiltration of T cells to tumors, and (3) recognition and elimination of tumor cells by T cells. Many immune evasion mechanisms present at each of these steps can contribute to primary or acquired resistance to PD-L1/PD-1 immunotherapy. Potential epigenetic strategies can be used at each step to overcoming immunotherapy resistance.
Prerequisites for eliciting an immune response include the pre-existing antitumor T cells that are restricted by a particular immune checkpoint and a complete immune cycle1. In most tumor patients, the antitumor response can maintain long-term disease control, but one-third of patients still have a recurrence. Nevertheless, there is evidence that changes in tumor antigen presentation and interferon-gamma (IFN-γ) signaling pathways play essential roles in primary and acquired resistance of PD-L1/PD-1 blockade, demonstrating a critical need for identifying combination therapies to overcome the resistance of immune checkpoint therapies. Recently, researchers have become increasingly aware of the role of the immune system in identifying tumors that have an effective clinical benefit in checkpoint blockade. Pre-existing immune responses can predict the response of PD-L1/PD-1 blockade by detecting gene expression of CTLA-4 and T helper type (TH) 1, as well as the absence of the C-X3-C motif chemokine ligand 1 (CX3CL1) in the baseline of tumor tissues14, 15. Therefore, an effective response to anti-PD-L1/anti-PD-1 therapies requires the establishing an intact immune cycle and identifying tumors by trafficking immune cells to the tumor microenvironment. First, cancer cells will synthesize and present tumor antigens, which are recognized by immune cells, and then the signals are presented to T lymphocytes. Then, the activated T cells infiltrate and traffic into the tumor tissues, targeting malignant cells. Finally, activated T cells eliminate these tumor cells by releasing factors such as IFN-γ and promoting the release of tumor antigens. We believe that every step of the immune cycle is critical to the function of immunotherapy, and every change in the immune cycle may improve the response rate of the PD-L1/PD-1 blockade.
Currently, with the advent of many small molecule inhibitors that target epigenetic regulatory enzymes, epigenetic reprogramming is becoming a viable and effective therapeutic route for chemotherapy and cancer chemoprevention16, 17. More importantly, each step of the immune cycle can be regulated by epigenetic therapies to improve antigen presentation, T cell trafficking and infiltration, and disruption of the immunosuppressive state. Epigenetic therapy, combined with immune checkpoint inhibitors, can restore immune recognition and tumor elimination, thus improving clinical response rates18. Given the importance of the pre-existing immune cycle in the adoption of checkpoint inhibitors and the profound impact of epigenetics on the immune system, this review will examine how epigenetic modifications affect various aspects of the immune cycle and discuss how epigenetic modification therapy regulates immune responses in cancer patients treated with PD-L1/PD-1 blockade therapies. We will focus on epigenetic strategies in combination therapy in the following text.
2. Current PD-L1 targeted immunotherapy
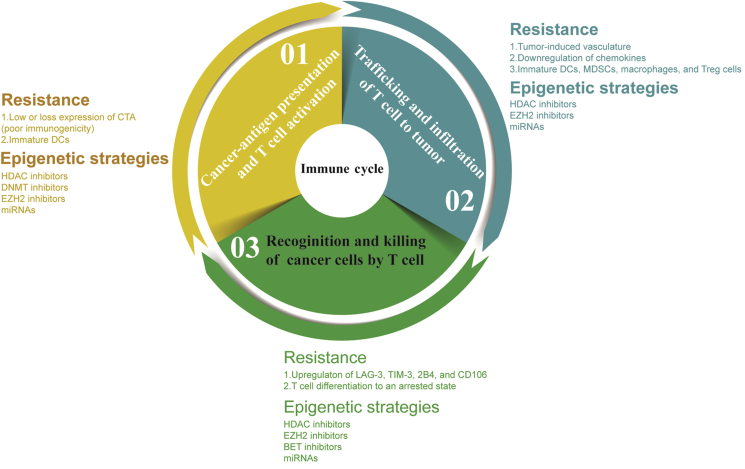
PD-L1 (B7-H1, CD274) can be detected on the cell surface of multiple tumor types, as well as several types of endothelial cells, epithelial cells, and several lymphocytes, thus playing a role in maintaining peripheral tolerance19. The binding of PD-L1 to its corresponding receptor PD-1 results in immune evasion via counteracting activation signals on T cells20 (Fig. 2). Therefore, PD-L1 is a shield for tumor cells which protects them from T cell-mediated elimination. Furthermore, CD80 (B7-1) binds to PD-L1 and transmits negative signals in both humans and mouse models21. A recent study also found that binding of CD80 to the PD-1 ligand PD-L1 in cis form on primary activated dendritic cells restricts PD-1 function during the activation of T lymphocytes22.
Figure 2.
Modeling of the interaction of PD-L1 and PD-1. The binding of PD-L1 to its corresponding receptor PD-1 triggers the apoptosis of T cells, leads to T cell exhaustion and results in immune evasion. During immunotherapy, PD-1/PD-L1 antibodies disrupt the interaction between PD-1 and PD-L1, enable T cell reactivation, proliferation, and target the tumor cell for destruction. PD-L1 is upregulated in response to some inflammatory signals (e.g., IFN-γ), which are produced by active T cells during anti-tumor immune responses.
The potential biological and long-lasting response rates in many types of cancer patients suggest that the PD-L1/PD-1 blockade therapy is one of the greatest advances in the history of cancer therapy. Several clinical trials on various solid tumor types with anti-PD-1 therapy showed significant extensions in overall survival (OS), such as non-small-cell lung cancer23, urothelial carcinoma24, and melanoma25. This remarkable outcome prompted the FDA (U. S. Food and Drug Administration) to approve six PD-L1/PD-1 blockade antibodies for various tumor indications: Keytruda (pembrolizumab), Opdivo (nivolumab), and most recently, Libtayo (cemiplimab) for PD-1 blockade; and Tecentrip (atezolizumab), Bavencio (avelumab) and Imfinzi (durvalumab) for anti-PD-L1 therapy. The PD-L1/PD-1 axis plays a crucial role in escaping the immune system in various advanced cancer types, the studies showing benefits of PD-L1/PD-1 blockade led to FDA approval of PD-L1/PD-1 inhibitors in more than 10 cancer indications26.
In particular, for therapeutic uses of anti-PD-L1/anti-PD-1 agents, clinical response to monotherapy has been shown in patients with various tumors, including melanoma, Hodgkin's lymphoma, non-small-cell lung cancer, and renal cancer27, 28, 29, 30. However, the main limitation of this therapy is the failure to elicit a response in most cancer patients. The response rate of this therapy varies across different tumor types, ranging from ∼19% in patients with head and neck cancer to ∼70% in patients with Hodgkin lymphoma, with an objective response rate of 20%–40% in majority of solid tumors6, 7. These results indicate that pre-existing immunity mediated by T cells exists in patients who respond to PD-L1/PD-1 blockade therapy, and patients who do not respond are likely to have damages in each step of the immune cycle. However, the PD-L1/PD-1 axis is not the sole mechanism to restrain immune responses31. In addition to negatively regulatory factors in the immune cycle, an individual's immune heterogeneity also reflects the contribution of a range of factors. For instance, epigenetic downregulation of cytokines results in lower T lymphocyte infiltration, and increased expression of endothelin receptors causes higher cancer survival and angiogenesis32. These immunosuppressive factors represent the obstacles that must be overcome for a cancer patient to be successfully treated with immunotherapy. Studies on the epigenetic regulation of the immune system reveal fundamental interactions between epigenetic regulation and immunoregulation. In this review, we will address the immune-cycle-related reasons for initial or acquired tolerance to PD-L1/PD-1 immunotherapy and suggest the possibility of epigenetics in elevated PD-L1/PD-1 immunotherapy.
3. Insights into resistance to anti-PD-L1 therapy
Many efforts have been made to obtain insights into the mechanisms of resistance to anti-PD-L1 or anti-PD-1 therapy. Primary resistance occurs in approximately 40%–65% of patients with melanoma treated with anti-PD-1-based therapy33, 34, 35, and 43% of responders develop acquired resistance by three years36. Before proceeding to the next steps, each step in the cancer immune cycle must be initiated and completed successfully to elicit an effective therapeutic immune response with immunotherapy. This cycle involves efficient (1) cancer-antigen presentation and T cell activation, (2) trafficking and infiltration of T cells to tumors, and (3) recognition and elimination of tumor cells by T cells31. In this regard, the reasons for failed anti-PD-L1/anti-PD-1-based therapies are primarily associated with disorders in T cell function within the tumor environment32. More specifically, the reasons that have been linked to primary and acquired resistance are as followed: (1) insufficient antigen presentation and recognition37, 38; (2) absence of T cells in the tumor microenvironment39, 40, 41, 42, 43; (3) upregulation of immunosuppressive markers [such as indoleamine 2,3-dioxygenase (IDO) and regulatory T cells (Tregs), T cell immunoglobulin and mucin domain 3 (TIM-3), lymphocyte activation gene-3 (LAG-3)]4,44, 45, 46, 47, 48, 49, 50, 51; (4) insufficient T-cell activation52, 53, 54; (5) decreased sensitivity to IFN-γ signaling.
Many advanced cancer patients do not respond to monotherapy of PD-L1 blockade5. This problem requires researchers to identify efficient combinatorial therapies urgently. Recent studies indicate that epigenetic modulations can trigger an immune response, enhance trafficking and infiltration of T cells, and improve the sensitivity of anti-PD-L1/anti-PD-1 therapy. Based on these research outcomes, we seek to find workable epigenetic strategies to cooperate with anti-PD-L1/anti-PD-1 immunotherapy.
4. Epigenetic regulation of the tumor microenvironment
Epigenetic modulations refer to a large-scale of, reversible, and heritable changes in gene expression without changing DNA sequences55. Recent studies have revealed that epigenetic modifications drive phenotypic changes in not only cancer cells, but also immune cells56. Epigenetic modifications, including changes in histone modifications, DNA methylation, and noncoding RNAs57, are often linked to cancer development, progression, and metastasis. Epigenetic dysregulation plays a vital role in the immunogenic deficiency of cancer cells and leads to the presence of more immunosuppressive immune and stromal cells58. The accumulation of changes in epigenetic modifications during tumorigenesis might contribute to proteomic transcriptional regulation and profound changes in genetic stability for the promotion of tumor immune escape58.
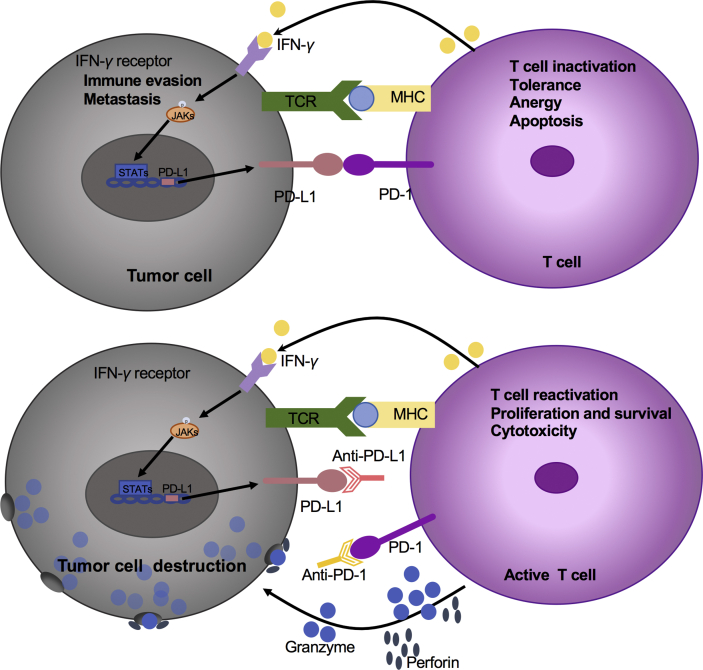
There is increasing evidence suggesting that epigenetic changes can alter the function and phenotype of immune cells, for cellular killing and functional adjustment. Epigenetic modification factors can activate many silent genes. Some of them are immune checkpoint regulators that trigger the immune response, while others turn them off, leading to immune evasion59. It is feasible for pharmacological agents to affect the epigenetic regulation of immune checkpoints, and the second generation of “episomal modifiers” is under development and has shown potential immunomodulatory properties60. Recent clinical trials have tested the combination of epigenetic agents and immunotherapy as a promising cancer treatment strategy35. Furthermore, several studies have indicated the importance of tumor epigenomic data such as a loss of the IFN-γ signaling pathway, which is closely related to the resistance of anti-CTLA-4 treatment. Therefore, these data reveal the critical role of patients who choose to receive immunological checkpoint treatments36 (see Fig. 3).
Figure 3.
The roles of epigenetic agents in multiple aspects of the immune cycle. Histone deacetylase inhibitors (HDACis) increase immune recognition by restoring the expression of various tumor-associated antigens (TAAs), major histocompatibility complex (MHC) molecules, and the generation of antigen-presenting cell (APC). Epigenetic agents may also enhance the migration of T cells to the tumor microenvironment by targeting the production of TH1 chemokine and generation of immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs). Targeting epigenetic regulators can provide promising and safe methods to restore T cell activation.
4.1. Epigenetic regulation of cycle 1: cancer-antigen presentation
The ideal tumor-associated antigen (TAA) is essential for cancer cell function, which is expressed only on cancer cells and elicits an immune response in the host. Finding a specific TAA as a specific target is one of the most important goals of immunotherapy61.
One of the most effective escape strategies adopted by cancer cells is damaging antigen presentation, which is an initial step in the immune cycle and plays an important role in tumor immunotherapy. Quantitative levels of costimulatory genes, as well as tumor antigens, and major histocompatibility complex (MHC) are essential for the determination of T cell priming in immune therapy. Available studies have indicated that antigen-specific T cell levels close to 1% CD8+ cells might be necessary to establish an effective antitumor response62. However, current studies indicate that the majority of cancer cells have lower levels of antigen presentation or even have none, which is lower than the recognized limits of the immune system, to prime an immune response63. Epigenetic regulation appears to represent the primary mechanism regulating TAAs expression in different tumor cells64, and treatments with DNMT inhibitors can increase tumor immunogenicity and immune recognition by re-expression of TAAs65, 66, 67, 68, 69.
Epigenetic events, especially DNA methylation, are thought to be the primary mechanism regulating the expression of cancer testis antigen (CTA). CTA represents a family of tumor-associated antigens expressed on tumors. Weber et al.70 obtained first-hand proof that DNA methylation plays a massive role in CTA expression. Moreover, the level of MHC class I is also downregulated by reversible methylation71. Upregulation of MHC class I and MHC class II by DNA methyltransferases inhibitors (DNMTi) has appeared in many cancers. Decitabine (5-aza-2-deoxycytidine, DAC) is a potent inhibitor of DNA methylation. In vitro treatment of a chronic lymphocytic leukemia (CLL) cell line with DAC upregulated the expression of MHC class I and MHC class II72. DAC treatment also upregulated the surface expression of MHC class I and exhibited an increase in IFN-γ release by tumor-specific cytotoxic T lymphocytes (CTLs)69. Treatment of RM-1 prostate cancer cells with another epigenetic modifier 5-azacitidine (5-AzaC) enhanced CTA expression. Moreover, 5-AzaC enhanced proinflammatory functions of dendritic cells (DC) by increasing MHC class I, MHC class II, CD80, CD86, CD205, and CD4073. Many studies have shown that exposure to demethylating agents can lead to the expression of several CTAs in multiple tumor cell lines, including melanoma, lung cancer, colon cancer, and malignant glioma74, 75, 76, 77, 78. The emerging study indicated that EZH2 (enhancer of zeste homolog 2) was activated or overexpressed by the mutation in partial melanoma and other tumors, resulting in the silencing of antigen presenting relevant genes and cancer suppressor genes79, 80.
The abovementioned studies indicate that epigenetic agents can effectively improve antigen presentation. On the one hand, these agents can enhance immunogenicity by increasing the expression of tumor-associated antigens; on the other hand, they can advance the proinflammatory functions of DCs to boost T-cell proliferation and effector T cell trafficking. The analysis of DNA methylation on a genome-wide scale showed that the maturation and differentiation of DC were related to hypomethylation at DNA enhancer regions, which represent binding domains for known transcription factors of DCs lineage specification, and along with dynamic changes in epigenetic modulator enzymes DNA methyltransferase 1 (DNMT1), DNMT3, and TET2 (ten-eleven translocation 2)81. Moreover, the expression of miRNAs, including miR-14a, miR-14b, and miR-22, inhibited the function of antigen presentation in DCs81, 82. Given that patients with primary resistance to PD-L1/PD-1 antibodies have a partial absence of activated immune cells, this inspires us to enhance the recognition and activation of immune cells by treatment with epigenetic agents, in order to further increase immune cells infiltration and the activity of PD-L1/PD-1 antibodies.
4.2. Epigenetic regulation of cycle 2: trafficking and infiltration of T cells
Tumors can be broadly classified into hot (T cell-infiltrated) and cold (non-T-cell- infiltrated) tumors76, 77, 78. Hot tumors are abundant in tumor infiltrating lymphocytes (TIL) and hold a primary immune response. However, cold tumors lack a liberal quantity of pre-existing TIL. Here, we discuss the epigenetic regulations that affect the migration of T cells to the tumor microenvironment. It is imperative to understand these epigenetic mechanisms in order to target them therapeutically. Through this summary, we hope to provide an effective epigenetic method to improve the response rate of PD-L1/PD-1 immunotherapy.
Previous studies have summarized the reasons for the inability of T cells to infiltrate tumors. These include vasculature induced by tumor cells, chemokines, and existing suppressive immune cells73. Other research suggests that epigenetic regulation is involved in all three aspects. It has been found that the polycomb repressive complex 2 (PRC2) component and the demethylase tri-methylation of lysine 27 on histone H3 (H3K27me3) inhibited the trafficking of effector T cells by downregulating the expression of CXC chemokine ligand (CXCL) 9 and CXCL10. Besides, PRC2-mediated epigenetic silencing was correlated with the suppression of effector T-cell trafficking and improved outcomes of anti-PD-L1 therapy in the mouse model83. In addition, DNA methylation associated with EZH2-mediated DNMT1 and H3K27me3 may suppress the expression of the TH1-type chemokines CXCL9 and CXCL10 and the subsequent transport of effector T cells to the tumor microenvironment84. Administration of epigenetic agents can increase the infiltration of effector T cells to the tumor and enhance the clinical efficacy of PD-L1 checkpoint blockade85, 86. Therefore, epigenetic silencing of TH1-type chemokines is an important mechanism of immune evasion. The formation of vascular trees is the result of complex interactions between genetic and epigenetic factors87.
The tumor microenvironment is often associated with immunosuppressive cells which have a negative impact on T cell activation, migration, and proliferation. These include MDSCs, immature DCs, macrophages, and Treg cells. Even the immune cells (granulocytes) are thought to participate73. The deletion of the epigenetic regulator ubiquitin-like PHD and RING finger domain-containing 1 (UHRF1) in CD4+ T cells demonstrated flawed proliferation and functional maturation of Treg cells and maintained gut immunological homeostasis88. Many Treg-specific epigenetic signature genes, such as CTLA4, IKZF4 (EOS), and TNFRSF18 (GITR), showed complete demethylation, which allowed forkhead box P3+ (FOXP3+) T cells to acquire Treg-specific gene expression, lineage stability, and specific immunosuppressive activity89. On the other hand, M2 macrophages can recruit Treg cells through the production of CCL22 (MDC, macrophage-derived chemokine)90. Epigenetic modifications of both human red cell acid phosphatase (ACP1) promoter and angiotensin converting enzyme (ACE1) distal regulatory elements appear to enhance specific gene expression programs in M2 macrophages, including metabolic pathway remodeling91. Moreover, EZH2 plays a vital role in the differentiation of peripheral Tregs, which may also contribute to the efficacy of PD-1 immunotherapy92. This finding suggests that the effect of epigenetic modification on macrophages may also trigger an effect on T cell infiltration. In another study, immunosuppressive MDSCs were reduced by the DNA-demethylating agent 5-aza-C in the tumor microenvironment, thereby facilitating an antitumor immune response93. Epigenetic reprogramming is significantly associated with T cell trafficking and infiltration, as well as patient survival rate. Therefore, this is another effective means that can promote tumors with high T lymphocyte cells infiltration to improve the efficacy of current immunotherapies.
4.3. Epigenetic regulation of cycle 3: T cell immunity
In a normally-functioning immune system, T cells spot cancer cells and fill the site with cytotoxic T lymphocytes (CTL), thereby infiltrating and killing cancer cells. However, tumor cells can upregulate immune suppression signals through different immunization steps to block T cell activation and induce cell death, resulting in immune evasion and eventual metastasis. A growing body of research and clinical trials has shown that immunotherapeutic methods involving the inhibition of immunological checkpoint modulators can restore T cell activation and promote the antitumor activity of the immune system. Emerging evidence indicates that targeting epigenetic regulators can provide promising and safe methods to restore T cell activation57.
Tumor-specific CD8+ T cell dysfunction in solid tumors causes tumor progression. Recent studies suggest that T cell exhaustion is associated with epigenetic alterations94. It directs T cell differentiation to an arrested state, causing T cell exhaustion95. Also, miRNAs are known to participate. These include miR-21496, miR-12697, and miR-56898, which can promote the development of Tregs and improve their function, thus downregulating CTL activity. Recent research illustrates that epigenetic control in Tregs occurs through the regulation of FOXP399,100. DNA hypomethylation is also necessary for Treg-specific gene expression and functional suppression101. The above studies suggest that epigenetic agents can facilitate the inhibition of the maturation of functional Tregs99.
Other new studies reveal that targeting epigenetic regulators can provide safe and effective methods to restore T-cell activation. T cells obtain a variety of inhibitory receptors such as LAG-3, PD-1, TIM-3, 2B4 (CD244), and CD10618, which can at least partially and synergistically mediate T cell exhaustion through nonredundant signaling pathways. Initial studies suggested that the PD-L1/PD-1 pathway mediates CD8+ T cell exhaustion, and PD-1 is considered as a marker of exhausted T cells102. Owing to the crucial influence of PD-1 and PD-L1 on CD8+ T cell dysfunction, we hope to identify transcriptional mechanisms involved in the regulation of gene expression encoding PD-1 and PD-L1.
It is worth noting that treatment with a demethylating agent, decitabine, results in dose-dependent upregulation of PD-1, its ligands (PD-L1 and PD-L2) and CTLA-4 mRNA expression103. Another study investigated the connection between epigenetic modifications and the upregulation of immune checkpoint genes. The results indicated that TIM-3, PD-1, LAG-3, and CTLA-4 were significantly hypomethylated and upregulated in tumors compared with normal tissues in breast cancer patients104. Two other preclinical studies have validated that HDAC inhibitors can upregulate the expression of PD-L1 and T cell chemokines71, 105. Bromodomain and extra-terminal motif inhibitors (BETi) can also increase the expression of PD-L1 through an MYC-dependent pathway16. Other research has identified a novel mechanism of PD-L1 upregulation in non-small-cell lung cancer through epigenetic regulation. These results demonstrated that PD-L1 is upregulated by P53 via miR-3417. Those results indicate a potential synergy between immune checkpoint inhibitors and epigenetic agents, as the former could upregulate the expression of immune checkpoints, which may improve the therapeutic effect and sensitivity of immune checkpoint inhibitors106.
5. Combination strategies between epigenetic agents and PD-1/PD-L1 blockade
Epigenetic reprogramming has been demonstrated to play a vital role in protecting tumor cells from immune surveillance107. All of these studies suggest that epigenetic reprogramming influences both cancer cells and immune cells, and suggest a potential combination of epigenetic agents and anti-PD-L1/anti-PD-1 immunotherapy. Of note, some clinical trials are designed to evaluate the effect of PD-L1/PD-1 antibodies combined with epigenetic agents among different cancer types, which are summarized in Table 1.
Table 1.
Current clinical trials.
| Clinicial trial indentifier | Status | Phase | Cancer type | Epigenetic drug (target) | PD-1/PD-L1 |
|---|---|---|---|---|---|
| NCT02936752 | Recruiting | I | Treating patients with myelodysplastic syndrome after deoxyribonucleic acid (DNA) methyltransferase inhibitor (DNMTi) therapy failure | Entinostat (HDAC1, HDAC2, HDAC3) | Pembrolizumab |
| NCT03250962 | Recruiting | I/II | Hodgkin lymphoma | Decitabine (DNMT1, DNMT3A, DNMT3B) | SHR-1210 |
| NCT02961101 | Recruiting | I/II | Relapsed or refractory malignancies, including non-Hodgkin's lymphoma, hepatocellular carcinoma, breast cancer, ovarian cancer or lung cancer or renal-cell cancer | Decitabine (DNMT1, DNMT3A, DNMT3B) | Anti-PD-1 antibody |
| NCT03346642 | Recruiting | I/II | Primary mediastinal large B-cell lymphoma | Decitabine (DNMT1, DNMT3A, DNMT3B) | SHR-1210 |
| NCT02892318 | Suspended | I | Acute myeloid leukemia | Guadecitabine (DNMT1) | Atezolizumab |
| NCT03308396 | Recruiting | I/II | Advanced kidney cancer, clear cell renal cell carcinoma | Guadecitabine (DNMT1) | Durvalumab |
| NCT02508870 | Suspended | I | Myelodysplastic syndromes | Azacitidine (DNMT1) | Atezolizumab |
| NCT03161223 | Recruiting | I/II | Lymphoma | 5-Azacitidine (DNMT1) | Durvalumab |
| NCT01928576 | Recruiting | II | Non-small-cell lung cancer | Azacitidine (DNMT1), Entinostat (HDAC1, HDAC2, HDAC3) |
Nivolumab |
| NCT02890069 | Recruiting | I | Colorectal cancer, non-small-cell lung carcinoma, triple negative breast cancer, renal cell carcinoma | Panobinostat (HDAC) | PDR001 |
| NCT02619253 | Recruiting | I | Renal cell carcinoma, urinary bladder neoplasms | Vorinostat (HDAC1, HDAC2, HDAC3, HDAC7, HDAC11) | Pembrolizumab |
| NCT02512172 | Active, not recruiting | I | Colorectal cancer | Romidepsin/CC-486 (HDAC1, HDAC2) | Pembrolizumab |
| NCT02453620 | Recruiting | I | Breast adenocarcinoma, HER2/Neu negative invasive breast carcinoma | Entinostat (HDAC1, HDAC2, HDAC3) | Ipilimumab/Nivolumab |
| NCT02959437 | Active, not recruiting | I/II | Solid tumors advanced malignancies metastatic cancer | INCB057643 (BET), Azacitidine (DNMT1) |
Pembrolizumab |
| NCT03854474 | Recruiting | I/II | Advanced urothelial carcinoma, locally advanced urothelial carcinoma, metastatic bladder urothelial carcinoma | Tazemetostat (EZH2) | Pembrolizumab |
| NCT02220842 | Active, not recruiting | I | Lymphoma | Tazemetostat (EZH2) | Atezolizumab |
| NCT03337698 | Recruiting | I/II | Non-small-cell lung cancer | Tazemetostat (EZH2) | Atezolizumab |
| NCT03019003 | Recruiting | I/II | Head and neck cancer | Azacitidine (DNMT1) | Durvalumab |
| NCT03390296 | Recruiting | II | Acute myeloid leukemia | Azacitidine (DNMT1) | Avelumab |
| NCT02811497 | Recruiting | II | Microsatellite stable colorectal carcinoma, platinum resistant epithelial ovarian cancer type II, estrogen receptor positive and HER2 negative breast cancer | Azacitidine (DNMT1) | Durvalumab |
| NCT02935361 | Recruiting | I/II | Chronic myelomonocytic leukemia, myelodysplastic syndrome, recurrent acute myeloid leukemia with myelodysplasia-related changes | Guadecitabine (DNMT1) | Atezolizumab |
| NCT03179943 | Recruiting | II | Urothelial carcinoma | Guadecitabine (DNMT1) | Atezolizumab |
| NCT03206047 | Suspended | I/II | Platinum-resistant ovarian carcinoma, recurrent fallopian tube carcinoma, recurrent ovarian carcinoma, recurrent primary peritoneal carcinoma | Guadecitabine (DNMT1) | Atezolizumab |
| NCT03590054 | Recruiting | I | Stage III cutaneous melanoma, stage IV cutaneous melanoma, locally advanced melanoma, locally advanced solid neoplasm | Abexinostat (pan-HDAC inhibitor) | Pembrolizumab |
| NCT03812796 | Recruiting | II | GI cancer | Domatinostat (HDAC1, HDAC2, HDAC3) | Avelumab |
| NCT03982134 | Not yet recruiting | I | Melanoma, non-small-cell lung cancer | Panobinostat (HDAC) | PDR001 |
6. Conclusions
The impact of recent cancer immunotherapies has demonstrated the importance and ability of the immune system to fight malignancies, leading to the successful use of PD-L1/PD-1 blocking antibodies to combat many tumors during this process. Even though PD-L1/PD-1 blockade therapies have proven to have significant benefits in clinical trials, numerous patients with advanced cancer do not respond to anti-PD-L1/anti-PD-1 monotherapy. In addition, the development of primary or acquired resistance is a limitation of using these agents, and ongoing studies are pursuing strategies to overcome resistance and improve the efficacy of checkpoint treatment. This phenomenon calls for an urgent need to identify efficient combinatorial treatments.
We have presented three aspects of preclinical evidence to demonstrate how epigenetic treatment can optimize the outcome of patients with checkpoint treatment through several parts of the immune system involving tumor cells and host immune cells. Epigenetic agents can induce T cell attraction and reactivation by synergistically upregulating tumor antigen presentation, as well as by downregulating immune suppression signals. Although there are promising ongoing research programs to evaluate the clinical outcomes of epigenetic agents in combination with immunological checkpoint inhibitors for different cancers, other epigenetic mechanisms (such as lncRNA or miRNA) cannot yet be clinically targeted.
Recent studies have shown that epigenetic agents improve antitumor outcomes to a great extent through combination with PD-L1/PD-1 blockade therapy and shed new light on cancer immunotherapies. These epigenetic combination therapies may be optimally integrated to enhance the response rates of PD-L1/PD-1 blocking antibodies, which could save the lives of patients with uncontrolled malignancies in the near future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China for Distinguished Young Scholar (No. 81625024 to Bo Yang) and the National Natural Science Foundation of China (No. 81773754 to Ling Ding).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Bo Yang, Email: yang924@zju.edu.cn.
Ling Ding, Email: ld362@zju.edu.cn.
Author contributions
Xi Chen designed and wrote the paper. Xiaohui Pan, Wenxin Zhang, Hongjie Guo, Shuyuan Cheng, and Qiaojun He revised the manuscript. Bo Yang and Ling Ding were responsible for the conception and design of the review.
Conflicts of interest
The authors claim that the researchers in this study have no conflict of interest.
References
- 1.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruella M., Kalos M. Adoptive immunotherapy for cancer. Immunol Rev. 2014;257:14–38. doi: 10.1111/imr.12136. [DOI] [PubMed] [Google Scholar]
- 3.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J., Hu L. Immunomodulators targeting the PD-1/PD-L1 protein–protein interaction: from antibodies to small molecules. Med Res Rev. 2019;39:265–301. doi: 10.1002/med.21530. [DOI] [PubMed] [Google Scholar]
- 7.Yarchoan M., Hopkins A., Jaffee E.M. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wainwright D.A., Chang A.L., Dey M., Balyasnikova I.V., Kim C.K., Tobias A. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20:5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lussier D.M., Johnson J.L., Hingorani P., Blattman J.N. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer. 2015;3:21. doi: 10.1186/s40425-015-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed K.A., Kim S., Arrington J., Naghavi A.O., Dilling T.J., Creelan B.C. Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J Neuro Oncol. 2017;133:331–338. doi: 10.1007/s11060-017-2437-5. [DOI] [PubMed] [Google Scholar]
- 11.Deng L., Liang H., Burnette B., Weicheslbaum R.R., Fu Y.X. Radiation and anti-PD-L1 antibody combinatorial therapy induces T cell-mediated depletion of myeloid-derived suppressor cells and tumor regression. OncoImmunology. 2014;3 doi: 10.4161/onci.28499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S.V., Powderly J.D., Camidge D.R., Ready N., Heist R.S., Hodi F.S. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with platinum-based doublet chemotherapy in patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2015;33:8030. [Google Scholar]
- 13.Hogg S.J., Vervoort S.J., Deswal S., Ott C.J., Li J., Cluse L.A. BET-bromodomain inhibitors engage the host immune system and regulate expression of the immune checkpoint ligand PD-L1. Cell Rep. 2017;18:2162–2174. doi: 10.1016/j.celrep.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchbinder E.I., Hodi F.S. Immune-checkpoint blockade—durable cancer control. Nat Rev Clin Oncol. 2016;13:77–78. doi: 10.1038/nrclinonc.2015.237. [DOI] [PubMed] [Google Scholar]
- 15.Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo C.B., Jones P.A. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 17.Baylin S.B., Jones P.A. Epigenetic determinants of cancer. Cold Spring Harb Perspect Biol. 2016;8:a019505. doi: 10.1101/cshperspect.a019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Héninger E., Krueger T.E., Lang J.M. Augmenting antitumor immune responses with epigenetic modifying agents. Front Immunol. 2015;6:29. doi: 10.3389/fimmu.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manjili M.H. A theoretical basis for the efficacy of cancer immunotherapy and immunogenic tumor dormancy: the adaptation model of immunity. Adv Cancer Res. 2018;137:17–36. doi: 10.1016/bs.acr.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Butte M.J., Peña-Cruz V., Kim M.J., Freeman G.J., Sharpe A.H. Interaction of human PD-L1 and B7-1. Mol Immunol. 2008;45:3567–3572. doi: 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugiura D., Maruhashi T., Okazaki I.M., Shimizu K., Maeda T.K., Takemoto T. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019;364:558–566. doi: 10.1126/science.aav7062. [DOI] [PubMed] [Google Scholar]
- 23.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E., Poddubskaya E. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellmunt J., De Wit R., Vaughn D.J., Fradet Y., Lee J.L., Fong L. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggermont A.M., Blank C.U., Mandala M., Long G.V., Atkinson V., Dalle S. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 26.Sanmamed M.F., Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2019;176:677. doi: 10.1016/j.cell.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powles T., Eder J.P., Fine G.D., Braiteh F.S., Loriot Y., Cruz C. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 29.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansell S.M., Lesokhin A.M., Borrello I., Halwani A., Scott E.C., Gutierrez M. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 32.Park Y.J., Kuen D.S., Chung Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med. 2018;50:109. doi: 10.1038/s12276-018-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 34.Larkin J., Hodi F.S., Wolchok J.D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 35.Gide T.N., Wilmott J.S., Scolyer R.A., Long G.V. Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res. 2018;24:1260–1270. doi: 10.1158/1078-0432.CCR-17-2267. [DOI] [PubMed] [Google Scholar]
- 36.Robert C., Ribas A., Hamid O., Daud A., Wolchok J.D., Joshua A.M. Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol. 2016;34:9503. [Google Scholar]
- 37.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGranahan N., Furness A.J., Rosenthal R., Ramskov S., Lyngaa R., Saini S.K. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong M., Puaux A.L., Huang C., Loumagne L., Tow C., Mackay C. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71:6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 40.Harlin H., Meng Y., Peterson A.C., Zha Y., Tretiakova M., Slingluff C. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue C., Shen S., Deng J., Priceman S.J., Li W., Huang A. STAT3 in CD8+ T cells inhibits their tumor accumulation by downregulating CXCR3/CXCL10 axis. Cancer Immunol Res. 2015;3:864–870. doi: 10.1158/2326-6066.CIR-15-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redondo P., Bandrés E., Solano T., Okroujnov I., García-Foncillas J. Vascular endothelial growth factor (VEGF) and melanoma. N-acetylcysteine downregulates VEGF production in vitro. Cytokine. 2000;12:374–378. doi: 10.1006/cyto.1999.0566. [DOI] [PubMed] [Google Scholar]
- 43.Rajabi P., Neshat A., Mokhtari M., Rajabi M.A., Eftekhari M., Tavakoli P. The role of VEGF in melanoma progression. J Res Med Sci. 2012;17:534–539. [PMC free article] [PubMed] [Google Scholar]
- 44.Dong Z.Y., Wu S.P., Liao R.Q., Huang S.M., Wu Y.L. Potential biomarker for checkpoint blockade immunotherapy and treatment strategy. Tumor Biol. 2016;37:4251–4261. doi: 10.1007/s13277-016-4812-9. [DOI] [PubMed] [Google Scholar]
- 45.Holmgaard R.B., Zamarin D., Li Y., Gasmi B., Munn D.H., Allison J.P. Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep. 2015;13:412–424. doi: 10.1016/j.celrep.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gajewski T.F. Identifying and overcoming immune resistance mechanisms in the melanoma tumor microenvironment. Clin Cancer Res. 2006;12 doi: 10.1158/1078-0432.CCR-05-2517. 2326s-30s. [DOI] [PubMed] [Google Scholar]
- 47.Jandus C., Bioley G., Speiser D.E., Romero P. Selective accumulation of differentiated FOXP3+ CD4+ T cells in metastatic tumor lesions from melanoma patients compared to peripheral blood. Cancer Immunol Immunother. 2008;57:1795–1805. doi: 10.1007/s00262-008-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viguier M., Lemaître F., Verola O., Cho M.S., Gorochov G., Dubertret L. Foxp3 expressing CD4+CD25high regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 49.Strauss L., Bergmann C., Szczepanski M., Gooding W., Johnson J.T., Whiteside T.L. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-β1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 50.Koyama S., Akbay E.A., Li Y.Y., Herter-Sprie G.S., Buczkowski K.A., Richards W.G. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taube J.M., Young G.D., McMiller T.L., Chen S., Salas J.T., Pritchard T.S. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21:3969–3976. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ladányi A., Kiss J., Somlai B., Gilde K., Fejős Z., Mohos A. Density of DC-LAMP+ mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56:1459–1469. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu W., Wang W., Wang Y., Li W., Yu G., Li Z. IL-37b suppresses T cell priming by modulating dendritic cell maturation and cytokine production via dampening ERK/NF-κB/S6K signalings. Acta Biochim Biophys Sin (Shanghai) 2015;47:597–603. doi: 10.1093/abbs/gmv058. [DOI] [PubMed] [Google Scholar]
- 54.Emeagi P.U., Maenhout S., Dang N., Heirman C., Thielemans K., Breckpot K. Downregulation of Stat3 in melanoma: reprogramming the immune microenvironment as an anticancer therapeutic strategy. Gene Ther. 2013;20:1085–1092. doi: 10.1038/gt.2013.35. [DOI] [PubMed] [Google Scholar]
- 55.Ramachandran S., Ient J., Gottgens E.L., Krieg A.J., Hammond E.M. Epigenetic therapy for solid tumors: highlighting the impact of tumor hypoxia. Genes (Basel) 2015;6:935–956. doi: 10.3390/genes6040935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wachowska M., Gabrysiak M., Golab J. Epigenetic remodeling combined with photodynamic therapy elicits anticancer immune responses. OncoImmunology. 2014;3 doi: 10.4161/onci.28837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dawson M.A., Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Liu M., Zhou J., Chen Z., Cheng A.S. Understanding the epigenetic regulation of tumours and their microenvironments: opportunities and problems for epigenetic therapy. J Pathol. 2017;241:10–24. doi: 10.1002/path.4832. [DOI] [PubMed] [Google Scholar]
- 59.Ali M.A., Matboli M., Tarek M., Reda M., Kamal K.M., Nouh M. Epigenetic regulation of immune checkpoints: another target for cancer immunotherapy?. Immunotherapy. 2017;9:99–108. doi: 10.2217/imt-2016-0111. [DOI] [PubMed] [Google Scholar]
- 60.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiriva-Internati M., Kim E., Figueroa A., Littlefield L., Saadeh C., Wade R. Cancer/testis antigens for immunotherapy and detection of multiple myeloma. Cancer Res. 2013;73:3535. [Google Scholar]
- 62.Tomasi T.B., Magner W.J., Khan A.N. Epigenetic regulation of immune escape genes in cancer. Cancer Immunol Immunother. 2006;55:1159–1184. doi: 10.1007/s00262-006-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ploegh H.L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 64.Roman-Gomez J., Jimenez-Velasco A., Agirre X., Castillejo J.A., Navarro G., Jose-Eneriz E.S. Epigenetic regulation of PRAME gene in chronic myeloid leukemia. Leuk Res. 2007;31:1521–1528. doi: 10.1016/j.leukres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Q.M., Shen N., Xie S., Bi S.Q., Luo B., Lin Y.D. MAGED4 expression in glioma and upregulation in glioma cell lines with 5-aza-2ʹ-deoxycytidine treatment. Asian Pac J Cancer Prev. 2014;15:3495–3501. doi: 10.7314/apjcp.2014.15.8.3495. [DOI] [PubMed] [Google Scholar]
- 66.Kim K.M., Song M.H., Kim M.J., Daudi S., Miliotto A., Old L. A novel cancer/testis antigen KP-OVA-52 identified by SEREX in human ovarian cancer is regulated by DNA methylation. Int J Oncol. 2012;41:1139–1147. doi: 10.3892/ijo.2012.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Filho P.A., López-Albaitero A., Xi L., Gooding W., Godfrey T., Ferris R.L. Quantitative expression and immunogenicity of MAGE-3 and -6 in upper aerodigestive tract cancer. Int J Cancer. 2009;125:1912–1920. doi: 10.1002/ijc.24590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou J., Li Y., Yao Y., Wang L., Gao L., Gao X. The cancer-testis antigen NXF2 is activated by the hypomethylating agent decitabine in acute leukemia cells in vitro and in vivo. Mol Med Rep. 2013;8:1549–1555. doi: 10.3892/mmr.2013.1659. [DOI] [PubMed] [Google Scholar]
- 69.Fonsatti E., Nicolay H.J., Sigalotti L., Calabrò L., Pezzani L., Colizzi F. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2ʹ-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clin Cancer Res. 2007;13:3333–3338. doi: 10.1158/1078-0432.CCR-06-3091. [DOI] [PubMed] [Google Scholar]
- 70.Weber J., Salgaller M., Samid D., Johnson B., Herlyn M., Lassam N. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2ʹ-deoxycytidine. Cancer Res. 1994;54:1766–1771. [PubMed] [Google Scholar]
- 71.Bubenik J. MHC class I down-regulation: tumour escape from immune surveillance? (Review) Int J Oncol. 2004;25:487–491. [PubMed] [Google Scholar]
- 72.Dubovsky J.A., McNeel D.G., Powers J.J., Gordon J., Sotomayor E.M., Pinilla-Ibarz J.A. Treatment of chronic lymphocytic leukemia with a hypomethylating agent induces expression of NXF2, an immunogenic cancer testis antigen. Clin Cancer Res. 2009;15:3406–3415. doi: 10.1158/1078-0432.CCR-08-2099. [DOI] [PubMed] [Google Scholar]
- 73.Van Der Woude L.L., Gorris M.A., Halilovic A., Figdor C.G., De Vries I.J. Migrating into the tumor: a roadmap for T cells. Trends Cancer. 2017;3:797–808. doi: 10.1016/j.trecan.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Gunda V., Frederick D.T., Bernasconi M.J., Wargo J.A., Parangi S. A potential role for immunotherapy in thyroid cancer by enhancing NY-ESO-1 cancer antigen expression. Thyroid. 2014;24:1241–1250. doi: 10.1089/thy.2013.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wachowska M., Gabrysiak M., Muchowicz A., Bednarek W., Barankiewicz J., Rygiel T. 5-Aza-2ʹ-deoxycytidine potentiates antitumour immune response induced by photodynamic therapy. Eur J Cancer. 2014;50:1370–1381. doi: 10.1016/j.ejca.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palucka A.K., Coussens L.M. The basis of oncoimmunology. Cell. 2016;164:1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28:383–391. doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gajewski T.F. The next hurdle in cancer immunotherapy: overcoming the non-T-cell-inflamed tumor microenvironment. Semin Oncol. 2015;42:663–671. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Comet I., Riising E.M., Leblanc B., Helin K. Maintaining cell identity: PRC2-mediated regulation of transcription and cancer. Nat Rev Cancer. 2016;16:803–810. doi: 10.1038/nrc.2016.83. [DOI] [PubMed] [Google Scholar]
- 80.Tiffen J., Gallagher S.J., Hersey P. EZH2: an emerging role in melanoma biology and strategies for targeted therapy. Pigment Cell Melanoma Res. 2015;28:21–30. doi: 10.1111/pcmr.12280. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X., Ulm A., Somineni H.K., Oh S., Weirauch M.T., Zhang H.X. DNA methylation dynamics during ex vivo differentiation and maturation of human dendritic cells. Epigenet Chromatin. 2014;7:21. doi: 10.1186/1756-8935-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Du J., Wang J., Tan G., Cai Z., Zhang L., Tang B. Aberrant elevated microRNA-146a in dendritic cells (DC) induced by human pancreatic cancer cell line BxPC-3-conditioned medium inhibits DC maturation and activation. Med Oncol. 2012;29:2814–2823. doi: 10.1007/s12032-012-0175-2. [DOI] [PubMed] [Google Scholar]
- 83.Nagarsheth N., Peng D., Kryczek I., Wu K., Li W., Zhao E. PRC2 epigenetically silences Th1-type chemokines to suppress effector T-cell trafficking in colon cancer. Cancer Res. 2016;76:275–282. doi: 10.1158/0008-5472.CAN-15-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng D., Kryczek I., Nagarsheth N., Zhao L., Wei S., Wang W. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Curiel T.J., Wei S., Dong H., Alvarez X., Cheng P., Mottram P. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 86.Zou W., Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 87.Jones E.A., Le Noble F., Eichmann A. What determines blood vessel structure? Genetic prespecification vs. hemodynamics. Physiology (Bethesda) 2006;21:388–395. doi: 10.1152/physiol.00020.2006. [DOI] [PubMed] [Google Scholar]
- 88.Zeng H., Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015;36:3–12. doi: 10.1016/j.it.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang B.H., Hagemann S., Mamareli P., Lauer U., Hoffmann U., Beckstette M. Foxp3+ T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 2016;9:444–457. doi: 10.1038/mi.2015.74. [DOI] [PubMed] [Google Scholar]
- 90.Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 91.Saeed S., Quintin J., Kerstens H.H., Rao N.A., Aghajanirefah A., Matarese F. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bolton H.A., Zhu E., Terry A.M., Guy T.V., Koh W.P., Tan S.Y. Selective Treg reconstitution during lymphopenia normalizes DC costimulation and prevents graft-versus-host disease. J Clin Investig. 2015;125:3627–3641. doi: 10.1172/JCI76031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Daurkin I., Eruslanov E., Vieweg J., Kusmartsev S. Generation of antigen-presenting cells from tumor-infiltrated CD11b myeloid cells with DNA demethylating agent 5-aza-2ʹ-deoxycytidine. Cancer Immunol Immunother. 2010;59:697–706. doi: 10.1007/s00262-009-0786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schietinger A., Greenberg P.D. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Philip M., Fairchild L., Sun L., Horste E.L., Camara S., Shakiba M. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545:452–456. doi: 10.1038/nature22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin Y., Cai X., Chen X., Liang H., Zhang Y., Li J. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res. 2014;24:1164–1180. doi: 10.1038/cr.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin A., Wen Z., Zhou Y., Li Y., Li Y., Luo J. MicroRNA-126 regulates the induction and function of CD4+ Foxp3+ regulatory T cells through PI3K/AKT pathway. J Cell Mol Med. 2013;17:252–264. doi: 10.1111/jcmm.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li W., Kong L.B., Li J.T., Guo Z.Y., Xue Q., Yang T. MiR-568 inhibits the activation and function of CD4+ T cells and Treg cells by targeting NFAT5. Int Immunol. 2014;26:269–281. doi: 10.1093/intimm/dxt065. [DOI] [PubMed] [Google Scholar]
- 99.Ohkura N., Kitagawa Y., Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 100.Waight J.D., Takai S., Marelli B., Qin G., Hance K.W., Zhang D. Cutting edge: epigenetic regulation of Foxp3 defines a stable population of CD4+ regulatory T cells in tumors from mice and humans. J Immunol. 2015;194:878–882. doi: 10.4049/jimmunol.1402725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohkura N., Hamaguchi M., Morikawa H., Sugimura K., Tanaka A., Ito Y. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 102.Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 103.Yang H., Bueso-Ramos C., DiNardo C., Estecio M.R., Davanlou M., Geng Q.R. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28:1280–1288. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sasidharan Nair V., El Salhat H., Taha R.Z., John A., Ali B.R., Elkord E. DNA methylation and repressive H3K9 and H3K27 trimethylation in the promoter regions of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, and PD-L1 genes in human primary breast cancer. Clin Epigenet. 2018;10:78. doi: 10.1186/s13148-018-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Woods D.M., Sodré A.L., Villagra A., Sarnaik A., Sotomayor E.M., Weber J. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol Res. 2015;3:1375–1385. doi: 10.1158/2326-6066.CIR-15-0077-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roulois D., Yau H.L., De Carvalho D.D. Pharmacological DNA demethylation: implications for cancer immunotherapy. OncoImmunology. 2016;5 doi: 10.1080/2162402X.2015.1090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maio M., Covre A., Fratta E., Di Giacomo A.M., Taverna P., Natali P.G. Molecular pathways: at the crossroads of cancer epigenetics and immunotherapy. Clin Cancer Res. 2015;21:4040–4047. doi: 10.1158/1078-0432.CCR-14-2914. [DOI] [PubMed] [Google Scholar]