Abstract
Purpose
There are limited clinical data on scanning-beam proton therapy (SPT) in treating locally advanced lung cancer, as most published studies have used passive-scatter technology. There is increasing interest in whether the dosimetric advantages of SPT compared with photon therapy can translate into superior clinical outcomes. We present our experience of SPT and photon intensity modulated radiation therapy (IMRT) with clinical dosimetry and outcomes in patients with stage III lung cancer.
Methods and Materials
Patients with stage III lung cancer treated at our center between 2013 and May 2018 were identified in compliance with our institutional review board (64 patients = 34 SPT + 30 IMRT). Most proton patients were treated with pencil beam scanning (28 of 34), and 6 of 34 were treated with uniform scanning. Fisher exact test, χ2 test, and Mann-Whitney test were used to compare groups. All tests were 2-sided.
Results
Patient characteristics were similar between the IMRT and SPT patients, except for worse lung function in the IMRT group. Mean dose to lung, heart, and esophagus was lower in the SPT group, with most benefit in the low-dose region (lungs, 9.7 Gy vs 15.7 Gy for SPT vs IMRT, respectively [P = .004]; heart, 7 Gy vs 14 Gy [P = .001]; esophagus, 28.2 Gy vs 30.9 Gy [P = .023]). Esophagitis and dermatitis grades were not different between the 2 groups. Grade 2+ pneumonitis was 21% in the SPT group and 40% in the IMRT group (P = .107). Changes in blood counts were not different between the 2 groups. Overall survival and progression-free survival were not different between SPT and IMRT (median overall survival, 41.6 vs 30.7 months, respectively [P = .52]; median progression-free survival, 19.5 vs 14.6 months [P = .50]).
Conclusions
We report our experience with SPT and IMRT in stage III lung cancer. Our cohort of patients treated with SPT had lower doses to normal organs (lungs, heart, esophagus) than our IMRT cohort. There was no statistically significant difference in toxicity rates or survival, although there may have been a trend toward lower rates of pneumonitis.
Introduction
Lung cancer remains the leading cause of cancer death in the United States, and survival for locally advanced nonsmall cell lung cancer (NSCLC) remains limited at around 30% at 5 years.1,2 Efforts to improve outcomes by intensifying radiation treatment for unresectable NSCLC have encountered challenges, as Radiation Therapy Oncology Group 0617 showed that dose escalation to 74 Gy was inferior to 60 Gy.3 Radiation dose to normal organs remains a predictor of morbidity and mortality, with heart dose and esophagitis grade shown to be associated with survival, and lung dose associated with radiation pneumonitis.3,4 Although photon intensity modulated radiation therapy (IMRT) has dosimetric advantages over conformal photon radiation, dose to normal tissues continues to cause significant treatment toxicity.5,6 Proton beam therapy has been increasing in prevalence in the United States, and possesses different physical characteristics from photon radiation owing to the Bragg peak.7 Dosimetry studies have shown that proton beam therapy can improve dose to normal tissues while maintaining tumor dose coverage.8,9
Multiple single-arm studies have been published, with promising results, using proton beam therapy and chemotherapy in locally advanced NSCLC.10,11 A National Cancer Database analysis of proton versus photon radiation therapy for NSCLC saw better survival with proton therapy in a retrospective analysis, although survival was not significantly different between proton and IMRT, which comprised 9% of the photon cohort.12 The enthusiasm for proton therapy led to the conduct of a phase II randomized trial comparing photon IMRT versus conformal passive scattering proton therapy for unresectable NSCLC.13 The trial found that for patients who had IMRT and proton plans that could meet prespecified dosimetric constraints, passive scatter proton therapy did not improve mean radiation dose to the lungs and esophagus, and in fact increased lung volume, receiving at least 20 Gy to 80 Gy (V20-80 Gy), while reducing V5-10 Gy. There was also no improvement in the rate of pneumonitis with proton therapy, which may not be unexpected given the mean lung dose was similar between the 2 groups. However, the trial did note the rate of pneumonitis for the proton group declined over time in the trial, and authors theorize that this may be due to a learning curve in proton planning, as replans of earlier patients led to improved dosimetry. Compared with passive scattering proton therapy, newer proton centers typically have scanning beams, and intensity modulated proton therapy with scanning beam technology has dosimetric advantages over passive scattering proton therapy.9,14 The rate of pneumonitis in definitive chemoradiation for lung cancer is especially important in the era of immunotherapy, as adjuvant durvalumab is now standard of care, and immune-mediated pneumonitis is also a treatment-limiting toxicity for patients.15 Beyond pneumonitis, there has also been clinical data that proton therapy can decrease heart dose compared with IMRT, and decrease lymphopenia, both of which may be associated with improved outcomes.13,16
To address some of these open questions, we present here our institutional experience in patients with locally advanced lung cancer being treated with curative intent radiation therapy. We present real-life dosimetry and clinical outcomes with the newer generation scanning beam proton therapy and photon IMRT/volumetric modulated arc therapy (VMAT). In addition to real-life dosimetric comparisons between scanning-beam proton therapy (SPT) and IMRT, we also assess potential toxicity differences between the 2 patient groups.
Methods and Materials
Patients
Records from patients with stage III lung cancer treated in our department between 2013 and May 2018 were reviewed in an institutional review board–approved study. This period was chosen because our proton center opened in 2013, and therefore a contemporary group of proton and photon patients could be analyzed. Patients were excluded if they received <50 Gy or if they did not receive conventional fractionation (1.8-2.0 Gy fractions). A total of 64 patients were identified, 34 patients treated with SPT and 30 patients treated with IMRT. Of the 34 SPT patients, pencil beam scanning (PBS) was used in 28, and uniform scanning (US) was used in 6 patients. The decision to use protons versus IMRT was based on patient or physician choice and insurance coverage. All photon patients were treated with IMRT or VMAT. Clinical staging was based on the American Joint Committee on Cancer Staging, 8th edition. All patients underwent pretreatment workup including brain magnetic resonance imaging and positron emission tomography/computed tomography (CT).
Radiation treatment procedures
All patients were simulated supine with 4-dimensional CT at 2.5-mm thick slices. Gross target volume (GTV) was primary tumor and involved lymph nodes on CT, positron emission tomography/CT, or biopsy. Clinical target volume was the GTV plus a 0.5 to 1cm margin as appropriate to account for subclinical tumor extension. Internal target volume was generated on 4-dimensional CT to account for motion, which was limited to <1 cm. Planned target volume (PTV) was a 5-mm expansion from clinical target volume in all directions. Beam-specific PTVs were used for proton plans. Our lung contours excluded the GTV.
Proton therapy was delivered using the Proteus Plus system (Ion Beam Applications, Louvain-la-Neuve, Belgium). Patients were treated with US from 2013 to March 2015 and PBS afterward because our center switched from US to PBS at that time. Treatment planning and delivery have been described previously.17 Briefly, in US beam delivery, patient-specific brass apertures were created, with wax range compensators for range uncertainty of 2.5% + 2 mm added to the distal and proximal ranges, as well as 1- to 2-cm smearing margins, designed using Xio treatment planning software (Impac Medical Systems, Maryland Heights, MO). For PBS delivery, treatment plans were created using RayStation (RaySearch Laboratories AB, Stockholm, Sweden). Single-field uniform dose optimization was used. Pretreatment quality assurance used ion chambers in water and the MatrixxPT ion chamber array device (IBA Dosimetry GmbH, Schwarzenbruck, Germany). Standard for verification was a gamma pass rate of >90% using acceptance criteria of 3% or 3 mm. To mitigate organ motion with PBS beam delivery (interplay effects), volumetric rescanning was used as needed.18 This approach has produced satisfactory results in phantom patients used for protocol credentialing at the center (Imaging and Radiation Oncology Core lung phantom). All patients underwent slow CT scans (GE Optima CT580; GE Hangwei Medical Systems, Beijing, China) at a slice thickness of 2.5 mm, revolution time 4 s/slice, 120 kV, for quality assurance and replanning at 15 Gy, 30 Gy, and 45 Gy to ensure target coverage and normal tissue dose were still within tolerance levels. A constant relative biological effectiveness factor of 1.1 was used to convert physical dose to relative biological effectiveness-adjusted dose.
Beginning in 2017, photon-based treatment planning was performed using the Pinnacle (Philips Medical Systems, Madison, WI) treatment planning system and Raystation. Both IMRT and VMAT were used. All plans were calculated on a 2-mm isotropic dose voxel grid, optimized for delivery by a clinically commissioned 6 MV Infinity LINAC with Agility collimator (Elekta, Stockholm, Sweden). VMAT dose was calculated by collapsed cone convolution.19
Endpoints and statistical analysis
Patients were followed at least every 3 months for the first 2 years and at least every 6 months thereafter. Adverse events were evaluated according to Common Toxicity Criteria (version 4.0). All new respiratory symptoms posttreatment were classified as radiation pneumonitis, regardless of whether the source of the symptoms was felt to be related to radiation or immune mediated (for patients receiving immunotherapy) and whether fibrosis or effusions were possibly also contributing to the shortness of breath. There were no cases of respiratory symptoms posttreatment that were felt to be exclusively due to effusion or fibrosis without accompanying pneumonitis. Acute toxic effects were defined as occurring within 90 days after last treatment and late toxic effects thereafter. Statistical analysis was performed using Graphpad Prism software version 5.0 and Statistical Package for the Social Sciences 19.0 (SPSS Statistics for Windows; IBM Corp., Armonk, NY). Comparisons between groups were performed using Fisher’s exact test, Χ2 test, and Mann-Whitney test. Locoregional recurrence was defined as recurrence inside or adjacent to radiation fields at the site of first recurrence (which could present at the same time as distant recurrence). Survival times were calculated from the date of diagnosis and compared with Mantel-Cox. All tests were 2-sided and P values <.05 was considered to be statistically significant.
Results
Patient characteristics
From 2013 through May 2018, 64 patients with stage III lung cancer were treated with definitive dose (>50 Gy) radiation at our institution. Patient characteristics are listed in Table 1. Most baseline characteristics were comparable between the proton and IMRT groups, except the IMRT group had worse forced expiratory volume in 1 second at baseline (only 42 patients had data), and there was a trend toward more photon patients being treated with concurrent chemotherapy. Chemotherapy regimens typically consisted of a platinum doublet, with carboplatin-paclitaxel and cisplatin/etoposide being the most common regimens.
Table 1.
Patient characteristics
| Characteristic | All, no. (%) | Proton no. (%) | IMRT no. (%) | P |
|---|---|---|---|---|
| No. of patients | 64 | 34 | 30 | |
| Sex | ||||
| Female | 41 (64.1) | 21 (61.8) | 20 (66.7) | .796 |
| Male | 23 (35.9) | 13 (38.2) | 10 (33.3) | |
| Median age, years | 67 (25-90) | 67 (25-85) | 66 (35-90) | .463 |
| <65 | 28 (43.8) | 16 (47.1) | 12 (40.0) | .620 |
| ≥65 | 36 (56.3) | 18 (52.9) | 18 (60.0) | |
| Ethnicity | ||||
| White | 53 (82.8) | 28 (82.4) | 25 (83.3) | .989 |
| Black | 4 (6.2) | 2 (5.9) | 2 (6.7) | |
| Asian | 2 (3.1) | 1 (2.9) | 1 (3.3) | |
| Others | 5 (7.8) | 3 (8.8) | 2 (6.7) | |
| ECOG | ||||
| 0-1 | 61 (95.3) | 32 (94.1) | 29 (96.7) | 1.000 |
| 2 | 3 (4.7) | 2 (5.9) | 1 (3.3) | |
| >2 | 0 | 0 | 0 | |
| Smoking history | ||||
| Never | 7 (10.9) | 6 (17.6) | 1 (3.3) | .109 |
| Ever | 57 (89.1) | 28 (82.4) | 29 (96.7) | |
| Median FEV1, L (range)∗ | 2.2 (0.83-4.2) | 2.35 (0.83-4.2) | 1.78 (0.96-3.38) | .080 |
| Median FEV1, % predicted (range)∗ | 75.5 (39-149) | 84 (44-149) | 66 (39-113) | .020 |
| Median DLCO, % predicted (range)† | 63 (33-102) | 65 (33-102) | 62 (35-90) | .344 |
| T stage | ||||
| T1 | 10 (15.6) | 4 (11.8) | 6 (20) | .913 |
| T2 | 17 (26.6) | 9 (26.5) | 8 (26.7) | |
| T3 | 14 (21.9) | 8 (23.5) | 6 (20.0) | |
| T4 | 23 (35.9) | 13 (38.2) | 10 (33.3) | |
| N stage | ||||
| N0 | 5 (7.8) | 3 (8.8) | 2 (6.7) | .370 |
| N1 | 2 (3.1) | 0 (0.0) | 2 (6.7) | |
| N2 | 42 (65.6) | 24 (70.6) | 18 (60.0) | |
| N3 | 15 (23.4) | 7 (20.6) | 8 (26.7) | |
| Stage | ||||
| IIIA | 23 (35.9) | 12 (35.3) | 11 (36.7) | .824 |
| IIIB | 37 (57.8) | 19 (55.9) | 18 (60.0) | |
| IIIC | 4 (6.3) | 3 (8.8) | 1 (3.3) | |
| Tumor histology | ||||
| Adenocarcinoma | 36 (56.3) | 19 (55.9) | 17 (56.7) | .740 |
| SCC | 21 (32.8) | 11 (32.4) | 10 (33.3) | |
| NSCLC unspecified | 1 (1.6) | 0 (0.0) | 1 (3.3) | |
| Small cell | 6 (9.4) | 4 (11.8) | 2 (6.7) | |
| Induction chemotherapy | ||||
| Yes | 15 (23.4) | 9 (26.5) | 6 (20.0) | .571 |
| No | 49 (76.6) | 25 (73.5) | 24 (80.0) | |
| Concurrent chemotherapy | ||||
| Yes | 43 (67.2) | 19 (55.9) | 24 (80.0) | .061 |
| No | 21 (32.8) | 15 (44.1) | 6 (20.0) | |
| Adjuvant chemotherapy | ||||
| Yes | 23 (35.9) | 13 (38.2) | 10 (33.3) | .796 |
| No | 41 (64.1) | 21 (61.8) | 20 (66.7) | |
| Any chemotherapy | ||||
| Yes | 60 (93.8) | 31 (91.2) | 29 (96.7) | .820 |
| No | 4 (6.3) | 3 (8.8) | 1 (3.3) | |
| Receiving adjuvant radiation therapy (50-54 Gy) | ||||
| Yes | 11 (17.2) | 7 (20.6) | 4 (13.3) | .443 |
| No | 53 (82.8) | 27 (79.4) | 26 (86.7) | |
| Surgery | ||||
| Yes | 14 (21.9) | 9 (26.5) | 5 (16.7) | .381 |
| No | 50 (78.1) | 25 (73.5) | 25 (83.3) | |
| Immunotherapy | ||||
| Yes | 23 (35.9) | 15 (44.1) | 8 (26.7) | .197 |
| No | 41 (64.1) | 19 (55.9) | 22 (73.3) | |
Abbreviations: DLCO = diffusing capacity of the lung for carbon monoxide; ECOG = Eastern Cooperative Oncology Group; FEV1 = forced expiratory volume in 1 second; IMRT = intensity modulated radiation therapy; NSCLC = nonsmall cell lung cancer; SCC = squamous cell carcinoma.
Data available for 42 patients (20 SPT and 22 IMRT).
Data available for 37 patients (17 SPT and 20 IMRT).
Dosimetry comparison
Dosimetric comparisons between proton versus IMRT patients are summarized in Table 2. All treatment plans were approved for clinical treatment and delivered. Tumor volumes and prescription doses were similar between proton and IMRT patients. Mean lung dose was lower with proton therapy, with most of the advantage in the low-dose region (V5-20 Gy). For lung-PTV, mean dose was 9.70 Gy with proton therapy and 15.77 Gy with IMRT (P < .001), with V20 Gy at 18.81% for proton therapy and 27.98% for IMRT (P < .001). Esophageal mean dose was also lower with protons, with V5-30 Gy lower with proton therapy (P < .05). There was a large reduction in heart dose with proton radiation, with a mean dose of 6.95 Gy with proton therapy and 14.04 Gy with IMRT (P = .001). Heart V5-35% were all significantly lower with proton therapy (P < .05).
Table 2.
Dosimetric comparison between SPT and IMRT
| Characteristic | All | Proton | IMRT | P |
|---|---|---|---|---|
| Prescription dose (Gy/CGE) | 61.2 (50.4-74.0) | 61.2 (50.4-74.0) | 61.5 (50.4-66.6) | .820 |
| Median target volumes, cm3 (range) | ||||
| PTV | 599.1 (94.10-1639) | 607.9 (94.10-1243) | 587.6 (135.30-1639) | .845 |
| GTV | 156.1 (1.39-647.8) | 173.5 (1.39-486.3) | 131.5 (28.16-647.8) | .445 |
| CTV | 370 (37.49-1202) | 382.5 (37.49-729.3) | 334.2 (45.81-1202) | .755 |
| Dosimetric comparison between proton and IMRT | ||||
| Lung | ||||
| Mean dose in Gy (CGE) | 15.78 (1.4-24.35) | 13.38 (5.11-24.35) | 17.89 (1.40-22.69) | .004 |
| V5 (%) | 43 (5.21-73.83) | 34.19 (18.90-73.83) | 58.45 (5.21-72.42) | <.001 |
| V10 (%) | 37.28 (3.61-55.77) | 29.26 (15.66-51.83) | 44.44 (3.61-55.77) | <.001 |
| V20 (%) | 28.84 (1.86-42.27) | 24.29 (9.99-41.1) | 33.39 (1.86-42.27) | <.001 |
| V30 (%) | 21.74 (1.30-33.55) | 19.99 (4.73-33.55) | 24.21 (1.30-32.61) | .127 |
| V40 (%) | 17.27 (0-27.96) | 17.05 (2.70-27.96) | 18.88 (0.98-26.83) | .957 |
| V50 (%) | 12.05 (0-23.84) | 12.99 (0.80-23.84) | 11.43 (0.70-22.19) | .264 |
| V60 (%) | 5.84 (0-19.77) | 7.30 (0-19.77) | 4.52 (0-14.38) | .184 |
| Lung-PTV | ||||
| Mean dose in Gy (CGE) | 13.09 (1.28-19.77) | 9.70 (4.87-17.53) | 15.77 (1.28-19.77) | <.001 |
| V5 (%) | 39.14 (5.01-70.67) | 29.02 (15.55-70.67) | 57.53 (5.01-69.34) | <.001 |
| V10 (%) | 34.42 (3.41-55.19) | 23.58 (13.86-47.19) | 41.66 (3.41-55.19) | <.001 |
| V20 (%) | 24.69 (1.66-35.55) | 18.81 (9.51-35.44) | 27.98 (1.66-35.55) | <.001 |
| V30 (%) | 18.1 (1.10-27.17) | 14.27 (4.22-27.17) | 19.69 (1.10-26.82) | .015 |
| V40 (%) | 11.8 (0.78-21.72) | 10.55 (2.18-21.72) | 12.96 (0.78-21.06) | .341 |
| V50 (%) | 6.74 (0.09-17.25) | 6.98 (0.29-17.25) | 6.13 (0.09-13.71) | .400 |
| V60 (%) | 1.25 (0-12.48) | 1.85 (0-12.48) | 1.1 (0-5.96) | .245 |
| Esophagus | ||||
| Mean dose in Gy (CGE) | 29.76 (10.78-60.43) | 28.19 (10.78-54.14) | 30.91 (17.67-60.43) | .023 |
| V10 (%) | 58.28 (30.39-98.50) | 56.1 (30.39-97.06) | 64.53 (37.99-98.50) | .007 |
| V20 (%) | 52.76 (16.46-95.18) | 51.5 (16.46-95.18) | 59.54 (34.89-94.10) | .028 |
| V30 (%) | 48.80 (13.57-93.49) | 45.5 (13.57-93.49) | 53.1 (18.5-90.88) | .038 |
| V40 (%) | 42.8 (6.48-91.70) | 41.92 (6.48-91.70) | 45.67 (8.610-88.57) | .223 |
| V50 (%) | 34.19 (0-89.30) | 32.35 (0-89.30) | 36.63 (0.84-85.6) | .423 |
| V55 (%) | 27.17 (0-83.44) | 26.36 (0-83.39) | 30.56 (0-83.44) | .134 |
| V60 (%) | 15.26 (0-79.16) | 16.52 (0-78.81) | 14.6 (0-79.16) | .715 |
| Heart | ||||
| Mean dose in Gy (CGE) | 11.65 (0-39.51) | 6.95 (0-39.51) | 14.04 (0-35.43) | .001 |
| V5 (%) | 32.16 (0-100.0) | 22.12 (0-100) | 55.44 (0-98.39) | <.001 |
| V10 (%) | 28.32 (0-99.80) | 18.87 (0-99.80) | 41.9 (0-85.60) | <.001 |
| V20 (%) | 19.98 (0-94.63) | 14.49 (0-94.63) | 26.68 (0-71.33) | .006 |
| V30 (%) | 13.2 (0-83.45) | 10.86 (0-83.45) | 18.06 (0-58.18) | .020 |
| V35 (%) | 11.5 (0-71.30) | 9.36 (0-71.30) | 15.18 (0-51.27) | .043 |
| V40 (%) | 9.94 (0-44.89) | 7.95 (0-44.89) | 13.06 (0-43.39) | .079 |
| V50 (%) | 6 (0-36.57) | 5.83 (0-26.09) | 6.155 (0-36.57) | .312 |
| V60 (%) | 1.49 (0-20.91) | 1.17 (0-20.36) | 1.535 (0-20.91) | .838 |
| Spinal cord | ||||
| D0.03cc Gy (CGE) | 43.78 (0.63-53.35) | 37.54 (0.63-49.96) | 45.83 (31.4-53.35) | <.001 |
Abbreviations: CGE = cobalt Gy equivalent; CTV = clinical target volume; GTV = gross target volume; IMRT = intensity modulated radiation therapy; PTV = planned target volume; RBE = relative biologic effectiveness; SPT = scanning-beam proton therapy.
Treatment toxicities
All patients completed the planned course of radiation therapy. Nonhematological toxicities are summarized in Table 3. No significant differences were seen in the rates of esophagitis, dermatitis, weight loss, or pneumonitis, except a trend toward lower pneumonitis with proton therapy (40.0% grade 2+ with IMRT vs 20.6% with protons, P = .107). Because adjuvant/postop patients received a lower dose of radiation than the definitive patients (50-54 Gy vs ≥60 Gy, respectively), we analyzed the grade 2+ pneumonitis rate in the definitive cohort and saw similar results (42.3% grade 2+ with IMRT vs 18.5% with protons, P = .077; Table E1, available online at https://doi.org/10.1016/j.adro.2020.03.001).
Table 3.
Nonhematological toxicities comparison between SPT and IMRT
| All | Proton | IMRT | P | |
|---|---|---|---|---|
| Esophagitis | ||||
| Grade 0 | 7 (10.9) | 4 (11.8) | 3 (10.0) | .600 |
| Grade 1 | 19 (29.7) | 8 (23.5) | 11 (36.7) | |
| Grade 2 | 29 (45.3) | 16 (47.1) | 13 (43.3) | |
| Grade 3 | 9 (14.1) | 6 (17.6) | 3 (10) | |
| Grade 4 | 0 | 0 | 0 | |
| Grade 5 | 0 | 0 | 0 | |
| Grade 0-1 | 26 (40.6) | 12 (35.3) | 14 (46.7) | .842 |
| Grade ≥ 2 | 38 (59.4) | 22 (64.7) | 16 (53.3) | |
| Pneumonitis | ||||
| Grade 0 | 4 (6.3) | 3 (8.8) | 1 (3.3) | .198 |
| Grade 1 | 41 (64.1) | 24 (70.6) | 17 (56.7) | |
| Grade 2 | 11 (17.2) | 3 (8.8) | 8 (26.7) | |
| Grade 3 | 4 (6.3) | 2 (5.9) | 2 (6.7) | |
| Grade 4 | 2 (3.1) | 0/0 | 2 (6.7) | |
| Grade 5 | 2 (3.1) | 2 (5.9) | 0 | |
| Grade 0-1 | 45 (70.3) | 27 (79.4) | 18 (60.0) | .107 |
| Grade ≥ 2 | 19 (29.7) | 7 (20.6) | 12 (40.0) | |
| Acute dermatitis | ||||
| Grade 0 | 4 (6.3) | 1 (2.9) | 3 (10.0) | .379 |
| Grade 1 | 38 (59.4) | 19 (55.9) | 19 (63.3) | |
| Grade 2 | 19 (29.7) | 13 (38.2) | 6 (20.0) | |
| Grade 3 | 3 (4.7) | 1 (2.9) | 2 (6.7) | |
| Grade 4 | 0 | 0 | 0 | |
| Grade 5 | 0 | 0 | 0 | |
| Grade 0-1 | 42 (65.6) | 20 (58.8) | 22 (73.3) | .294 |
| Grade ≥2 | 22 (34.4) | 14 (41.2) | 8 (26.7) | |
| Acute weight loss (lbs) | ||||
| Grade 0 | 41 (64.1) | 19 (55.9) | 22 (73.3) | .139 |
| Grade 1 | 17 (26.6) | 11 (32.4) | 6 (20.0) | |
| Grade 2 | 5 (7.8) | 4 (11.8) | 1 (3.3) | |
| Grade 3 | 1 (1.6) | 0 | 1 (3.3) | |
| Grade 4 | 0 | 0 | 0 | |
| Grade 5 | 0 | 0 | 0 | |
| Grade 0-1 | 58 (90.6) | 30 (88.2) | 28 (93.3) | .345 |
| Grade ≥ 2 | 6 (9.4) | 4 (11.8) | 2 (6.7) |
Abbreviations: IMRT = intensity modulated radiation therapy; SPT = scanning-beam proton therapy.
Changes in blood counts were tracked pre- and postradiation. Median hemoglobin (Hb), white-blood-cell count (WBC), and neutrophil counts were all within normal limits at baseline pretreatment. Median Hb counts in SPT and IMRT groups were 12.0 and 12.45 g/L, respectively. Median WBC counts in SPT and IMRT groups were 6.97 and 7.63 x103/μL, respectively. Median neutrophil counts in SPT and IMRT groups were 4.62 and 5.61 x103/μL, respectively. Changes in weekly Hb, WBC, and neutrophil counts after starting radiation showed no statistically significant differences between the SPT versus IMRT groups (Fig 1, P > .2 for comparison of nadirs between SPT and IMRT).
Figure 1.
Hematological toxicity comparison of scanning-beam proton therapy (SPT) and intensity modulated radiation therapy (IMRT) treatment using percent changes in blood counts from baseline after the start of radiation therapy. (A) White blood cell count (WBC); (B) neutrophil; (C) hemoglobin. Error bars represent standard error of the mean.
Locoregional control and survival analysis
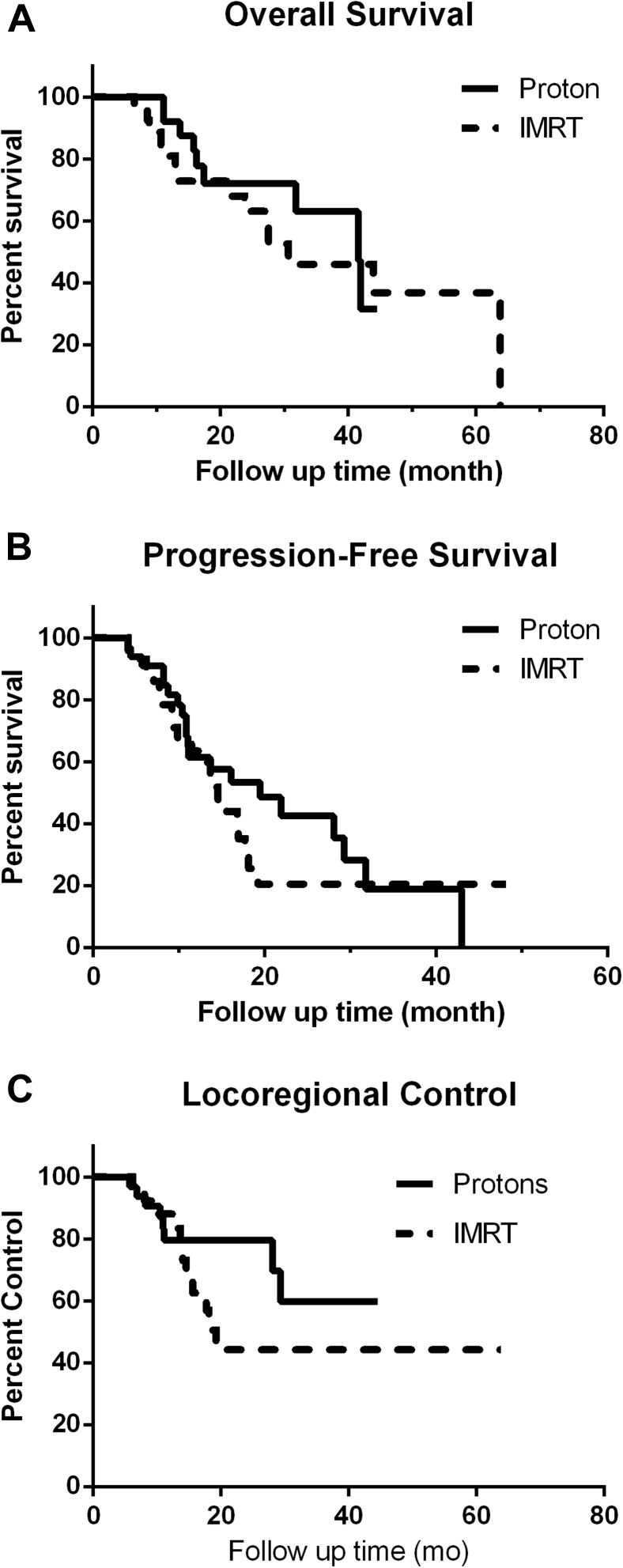
Median follow-up was 16.8 months (range, 3.1-63.8 months; 20.2 for IMRT group and 16.1 for proton group). There was no statistically significant difference in overall survival (OS) and progression-free survival between proton and IMRT patients (Fig 2A,B; median OS, 41.6 months for protons and 30.7 months for IMRT, P = .52; median progression-free survival, 19.5 months for protons vs 14.6 months for IMRT, P = .50). Locoregional control was also not statistically significantly different between the 2 groups, with locoregional control of 59.7% for the proton group and 44.2% for the IMRT group (P = .26; Fig 2C).
Figure 2.
Overall survival (A), progression free survival (B), and locoregional control (C) comparison between scanning-beam proton therapy (SPT) and intensity modulated radiation therapy (IMRT) treatment.
Discussion
We present here our institution’s experience in advanced SPT for locally advanced lung cancer, with real-life treated dosimetry (as opposed to dosimetric planning studies) and clinical outcomes. We also provide a retrospective comparison with our cohort of contemporary patients treated with photon IMRT/VMAT. For comparable tumor volumes in these 2 patient cohorts, patients treated with SPT had significantly lower mean dose to the heart and lungs, with most of the dosimetric advantage in the low-dose region. Esophageal mean dose was also lower with proton therapy but the magnitude of the difference was smaller than for heart and lungs. Toxicity rates seemed comparable between our proton therapy and IMRT cohorts, although there was a suggestion of lower pneumonitis with proton therapy. Survival and locoregional control were similar between the 2 patient groups.
Our results appear similar to other published proton patient series (see Table 4), such as 2 MD Anderson series with passive scattering proton therapy showing median OS of 26.5 to 30.4 months for patients with stage III NSCLC and another series with intensity modulated proton therapy showing median OS 33.9 months.10,20,21 Our grade 2+ pneumonitis rate of 20.6% in our proton cohort appears similar to the rates of 14%, 23.5%, and 28% published in these 3 series. Our grade 3 esophagitis rate was 17.6% in our proton cohort, compared with 4%, 6%, and 12% in these 3 series. One reason for variable toxicity rates from different series may be related to intervention strategies, as our institution intervenes with intravenous hydration and oral medications for early signs of esophagitis. A comparison of results from select published proton patient series is included in Table 4.22, 23, 24
Table 4.
Outcomes comparison between select published proton series on locally advanced NSCLC
| Patient no. | Proton technology | Overall survival | Locoregional control | Pneumonitis | Esophagitis | |
|---|---|---|---|---|---|---|
| Present study | 34 | Scanning beam | Median, 41.6 mo | 59.7% | Grade 2+, 20.6%; Grade 3+, 11.8% |
Grade 2+, 64.7%; Grade 3+, 17.6% |
| Oshiro et al22 | 57 | Passive scatter | Median, 21.3 mo | 2-y, 64.1% | Acute grade 2+, 12.3%; Acute grade 3+, 5.3% |
Grade 2+, 1.8%; Grade 3+, 0% |
| Hatayama et al23 | 27 | Passive scatter | 2-y, 51.5% | 1-y local control, 68.1%; 2-y local control, 36.4% |
Grade 2+, 29.6%; Grade 3+, 7.4% |
Grade 2+, 22.2%; Grade 3+, 3.7% |
| Chang et al20 | 64 | Passive scatter | Median, 26.5 mo | 72% | Grade 2+, 28%; Grade 3+, 12% |
Acute grade 2+, 36%; Acute grade 3+, 8%; Late grade 2+, 9%; Late grade 3+, 4% |
| Liao et al13 | 57 | Passive scatter | Median, 26.1 mo | 1-y local control, 89.5%; 5-y, ~65% (estimated from figure) |
1-y grade 3+, 10.5%; | |
| Elhammali et al21 | 51 | Intensity modulated proton therapy | Median, 33.9 mo | 64.5% | Grade 2+, 15%; Grade 3+, 0% |
Grade 2+, 49%; Grade 3+, 6% |
| Yu et al24 | 33 | Intensity modulated proton therapy | 1-y, 68% | 1-y, 86% | Grade 3, 6.1% | Grade 3, 6.1% |
Abbreviation: NSCLC = nonsmall cell lung cancer.
Heart dose has been found to be correlated with survival in some clinical series of locally advanced NSCLC, and more stringent dose constraints have been recommended for the heart in recent years.3,6 Our cohort of patients treated with proton therapy had significantly reduced mean heart dose compared with our cohort of patients treated with IMRT or VMAT. However, we did not see a significant difference in survival between proton therapy and IMRT in our patient groups, although the absolute numbers were better in the proton group. We are limited by our small sample size.
Perhaps related to heart dose, there is also increasing clinical data on the negative effect of lymphopenia on survival, with the suggestion that proton therapy may be correlated to less severe lymphopenia compared with photon therapy.16,25,26 We do not have lymphocyte data available for our patients, but changes in total WBC count, neutrophils, and hemoglobin were not different between proton therapy and IMRT in our series. Based on modeling series, even a low dose of radiation to a small portion of the circulation per fraction results in the entire circulation being radiated after 30 fractions.27 Therefore, although proton therapy drastically reduces the heart dose, it may not have an effect on lymphopenia in conventionally fractionated radiation treatment.
Beyond retrospective comparisons such as our series presented here, there are ongoing efforts to generate prospective randomized evidence to evaluate the benefit of proton therapy, such as “Radiation Therapy Oncology Group 1308 (NCT01993810): Comparing Photon Therapy to Proton Therapy to Treat Patients With Lung Cancer.”28 Given the heterogeneity of patients with locally advanced NSCLC, it is unclear whether testing across an unselected treatment population will ever prove the value of a new technology.7 Given the clear dosimetric advantage of proton therapy in some clinical scenarios, other methods have been proposed in value-based care, such as using normal tissue complication probability models (NTCP) to select patients most likely to benefit from proton therapy.29,30 Typically, a threshold is set for improvement in toxicity based on NTCP modeling, and if a proton treatment plan meets that threshold, patients are recommended to receive proton therapy instead of photon therapy. However, it is not clear which endpoint or combination of endpoints should be used in modeling, and to date, NTCP models are based on photon-treated patients, and it is not clear whether the models will be the same for proton patients.
Implementing any new technology comes with a learning curve, including proton therapy.13 There are guidelines for implementing PBS for thoracic tumors, which is an especially challenging region from the technical perspective owing to a mix of factors including motion (both tumor and normal tissues) and tissue heterogeneity.31 At our center, we follow the best practice recommendations and limit motion to <10 mm with compression or breath hold as needed.31
In conclusion, we showed that for 2 contemporary cohorts of patients with locally advanced lung cancer, the cohort treated with SPT had lower normal tissue doses compared with the IMRT cohort, with most of the dosimetric differences in the low-dose region. There was no statistically significant difference in toxicity rates or survival, although there may have been a trend toward lower rates of pneumonitis. Large patient numbers will be needed to demonstrate whether this dosimetric difference translates into better clinical outcomes.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Rengan has had travel/food funding from IBA, and consulting fees/food from AstraZeneca.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.03.001.
Supplementary data
References
- 1.Goldstraw P., Chansky K., Crowley J. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA: Can J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palma D.A., Senan S., Tsujino K. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun S.G., Hu C., Choy H. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speirs C.K., DeWees T.A., Rehman S. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12:293–301. doi: 10.1016/j.jtho.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 7.Rengan R., Redman M., Zeng J. Challenge of proving the value of proton therapy in an unselected patient population in the era of precision oncology: The fallacy of a one-size-fits-all strategy in radiotherapy for lung cancer. J Clin Oncol. 2018;36:2003–2004. doi: 10.1200/JCO.2018.78.3803. [DOI] [PubMed] [Google Scholar]
- 8.Berman A.T., Teo B.K., Dolney D. An in-silico comparison of proton beam and IMRT for postoperative radiotherapy in completely resected stage IIIA non-small cell lung cancer. Radiat Oncol. 2013;8:144. doi: 10.1186/1748-717X-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X., Li Y., Pan X. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: A virtual clinical study. Int J Radiat Oncol Biol Phys. 2010;77:357–366. doi: 10.1016/j.ijrobp.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen Q.N., Ly N.B., Komaki R. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol. 2015;115:367–372. doi: 10.1016/j.radonc.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoppe B.S., Henderson R., Pham D. A phase 2 trial of concurrent chemotherapy and proton therapy for stage iii non-small cell lung cancer: Results and reflections following early closure of a single-institution study. Int J Radiat Oncol Biol Phys. 2016;95:517–522. doi: 10.1016/j.ijrobp.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Higgins K.A., O'Connell K., Liu Y. National Cancer Database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97:128–137. doi: 10.1016/j.ijrobp.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Liao Z., Lee J.J., Komaki R. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small-cell lung cancer. J Clin Oncol. 2018 doi: 10.1200/JCO.2017.74.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kase Y., Yamashita H., Fuji H. A treatment planning comparison of passive-scattering and intensity-modulated proton therapy for typical tumor sites. J Radiat Res. 2012;53:272–280. doi: 10.1269/jrr.11136. [DOI] [PubMed] [Google Scholar]
- 15.Antonia S.J., Villegas A., Daniel D. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. New Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 16.Shiraishi Y., Fang P., Xu C. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018;128:154–160. doi: 10.1016/j.radonc.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng Y.C., Vyas S., Dang Q. Proton therapy posterior beam approach with pencil beam scanning for esophageal cancer: Clinical outcome, dosimetry, and feasibility. Strahlenther Onkol. 2016;192:913–921. doi: 10.1007/s00066-016-1034-4. [DOI] [PubMed] [Google Scholar]
- 18.Grassberger C., Dowdell S., Sharp G., Paganetti H. Motion mitigation for lung cancer patients treated with active scanning proton therapy. Med Phys. 2015;42:2462–2469. doi: 10.1118/1.4916662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahnesjo A., Aspradakis M.M. Dose calculations for external photon beams in radiotherapy. Phys Med Biol. 1999;44:R99–R155. doi: 10.1088/0031-9155/44/11/201. [DOI] [PubMed] [Google Scholar]
- 20.Chang J.Y., Verma V., Li M. Proton beam radiotherapy and concurrent chemotherapy for unresectable stage III non-small cell lung cancer: Final results of a phase 2 study. JAMA Oncol. 2017;3 doi: 10.1001/jamaoncol.2017.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elhammali A., Blanchard P., Yoder A. Clinical outcomes after intensity-modulated proton therapy with concurrent chemotherapy for inoperable non-small cell lung cancer. Radiother Oncol. 2019;136:136–142. doi: 10.1016/j.radonc.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Oshiro Y., Mizumoto M., Okumura T. Results of proton beam therapy without concurrent chemotherapy for patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2012;7:370–375. doi: 10.1097/JTO.0b013e31823c485f. [DOI] [PubMed] [Google Scholar]
- 23.Hatayama Y., Nakamura T., Suzuki M. Preliminary results of proton-beam therapy for stage III non-small-cell lung cancer. Cur Oncol. 2015;22:e370–e375. doi: 10.3747/co.22.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu N, DeWees TA, Liu C, et al. Proton therapy versus intensity-modulated radiation therapy: The Mayo Clinic Experienc. Adv Radiat Oncol In press. 10.1016/j.adro.2019.08.001. [DOI] [PMC free article] [PubMed]
- 25.Campian J.L., Ye X., Brock M., Grossman S.A. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013;31:183–188. doi: 10.3109/07357907.2013.767342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yovino S., Grossman S.A. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol. 2012;1:149–154. doi: 10.2217/cns.12.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yovino S., Kleinberg L., Grossman S.A., Narayanan M., Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comparing Photon Therapy to Proton Therapy to Treat Patients with Lung Cancer, 2018. ClinicalTrials.gov identifier: NCT01993810. Available at: https://clinicaltrials.gov/ct2/show/NCT01993810. Accessed April 9, 2020.
- 29.Widder J., van der Schaaf A., Lambin P. The quest for evidence for proton therapy: Model-based approach and precision medicine. Int J Radiat Oncol Biol Phys. 2016;95:30–36. doi: 10.1016/j.ijrobp.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Brodin N.P., Kabarriti R., Pankuch M. A quantitative clinical decision-support strategy identifying which patients with oropharyngeal head and neck cancer may benefit the most from proton radiation therapy. Int J Radiat Oncol Biol Phys. 2019;104:540–552. doi: 10.1016/j.ijrobp.2018.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang J.Y., Zhang X., Knopf A. Consensus guidelines for implementing pencil-beam scanning proton therapy for thoracic malignancies on behalf of the PTCOG Thoracic and Lymphoma Subcommittee. Int J Radiat Oncol Biol Phys. 2017;99:41–50. doi: 10.1016/j.ijrobp.2017.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.