Abstract
Early reports of coronavirus disease 2019 (COVID‐19) clinical features describe a hypercoagulable state, and recent guidelines recommend prophylactic anticoagulation for patients with COVID‐19 with low‐molecular‐weight heparin, but this would be contraindicated in the presence of heparin‐induced thrombocytopenia (HIT). We address the key clinical question whether HIT is also present during COVID‐19. We report 3 cases of thrombocytopenia with antiplatelet factor 4 antibodies among 16 intubated patients with COVID‐19 with adult respiratory distress syndrome, a higher‐than‐expected incidence of 19%. Each patient had evidence of thrombosis (pulmonary embolism, upper extremity venous thromboses, and skin necrosis, respectively). The serotonin release assay confirmed HIT in 1 case, and 2 cases were negative. We believe this is the first reported case of HIT during the COVID‐19 pandemic. Recognition that the thrombocytopenia represented HIT in the confirmed case was delayed. We recommend clinicians monitor platelet counts closely during heparin therapy, with a low threshold to evaluate for HIT.
Keywords: coronavirus, heparin/ adverse effects, respiratory distress syndrome, adult, thrombocytopenia/chemically induced, thrombosis
Essentials.
A hypercoagulable state is recognized during COVID‐19, associated with severe or lethal disease.
Identifying thrombocytopenia from heparin‐induced thrombocytopenia is critical to properly treat.
We present the first reported case of heparin‐induced thrombocytopenia during COVID‐19.
Delayed recognition may have contributed to this poor outcome. Clinicians must monitor closely.
1. INTRODUCTION
During the coronavirus disease 2019 (COVID‐19) pandemic, early reports have identified clinical features of the disease suggesting an associated prothrombotic coagulopathy, with recommendations for use of anticoagulation in all patients with COVID‐19. Among 1099 patients with laboratory‐confirmed COVID‐19 in China in December 2019 and January 2020, a D‐dimer ≥0.5 mg/L was noted in 46.4% of patients tested, and was associated with more severe disease. 1 In February 2020, Han 2 compared 94 patients with severe acute respiratory sybdrome coronavirus 2 (SARS‐CoV‐2) to 40 negative controls; the COVID‐19 patients had lower antithrombin values and prothrombin activity, and higher concentrations of D‐dimer, fibrin degradation products (FDPs), and fibrinogen levels. Patients with severe COVID‐19 had higher FDP and D‐dimer levels. 2 Among 183 consecutive patients with COVID‐19 admitted to Huazhong University in Wuhan, disseminated intravascular coagulation (DIC) affected only 0.6% of survivors but 71.4% of nonsurvivors, who also had significantly higher D‐dimers, FDP levels, and prothrombin times. 3
Yin et al 4 compared 449 patients with COVID‐19 to 104 historical controls with nonCOVID pneumonia, all with severe symptoms. Among patients with COVID‐19 with elevated D‐dimers, those treated with heparin had a lower mortality (32.8% vs 52.4%; P = .017). 4 Consistent with these suggestions that patients with COVID‐19 may be hypercoagulable, Cui et al 5 reported venous thromboembolism (VTE) in 20 of 81 (25%) patients not treated with prophylactic anticoagulation, including 8 (40%) patients who died. The patients with VTE were older and had lower lymphocyte counts, longer activated partial thromboplastin times, and higher D‐dimer levels. 5
We add to these reports of COVID‐associated hypercoagulability 3 cases of thrombocytopenia with positive anti–platelet factor 4 (PF4)/heparin antibodies, addressing the key clinical question whether heparin‐induced thrombocytopenia (HIT) is also present during COVID‐19 (Table 1). One patient was proven by serotonin release assay (SRA) to have heparin‐induced thrombocytopenia (HIT) and confirmed pulmonary emboli, representing heparin‐induced thrombocytopenia with thrombosis (HITT) (Table 2). A PubMed search on May 7, 2020, for “COVID” AND “Heparin‐induced thrombocytopenia” revealed no matches, and a recent review article summarizing the data related to thrombotic disease in patients with COVID‐19 made no mention of HIT 6 ; we believe this to be the first report of HIT during the COVID‐19 pandemic. Clinicians must be aware of the possibility of HIT, especially with more liberal or aggressive use of unfractionated heparin or low‐molecular‐weight heparin for patients with COVID‐19.
TABLE 1.
Demographic, clinical, and laboratory findings
| Age, y | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| 70 | 74 | 53 | |
| Medical history | Hypertension, hyperlipidemia, benign prostatic hypertrophy | Coronary artery disease, chronic obstructive pulmonary disease, hypertension, alcoholic cirrhosis (remission × 2 y), pernicious anemia | Atrial fibrillation, depression, irritable bowel syndrome, obstructive sleep apnea |
| Symptoms at onset | Weakness, cough, dyspnea | Dyspnea, cough, hoarseness | Cough, fever, diaphoresis, diarrhea |
| Chest imaging features | Bilateral, patchy alveolar consolidation and interstitial coarsening | Bilateral airspace opacities predominantly affecting the mid and lower lungs | patchy opacity in mid‐left lung, right lung was clear |
| PaO2/FiO2 admit/lowest | 114/94 | 94/92 | 188/122 |
| Antiviral treatment (hospital day [HD]) | Hydroxychloroquine (HD 1‐5), tocilizumab (HD 5), remdesivir expanded access (HD 9‐17) | None | Hydroxychloroquine (HD 2‐6) |
| ARDS treatment | High PEEP, ARDSnet, prone osition, cisatracurium | High PEEP, ARDSnet, prone position | High PEEP, ARDSnet, prone position, cisatracurium |
| Admission laboratory findings (normal range) | |||
|
White blood cells (4.2‐9.9 per mm3) |
18 600 | 6300 | 17 600 |
|
Total neutrophils (2.4‐7.6 per mm3) |
17 100 | 4100 | 15 600 |
|
Total lymphocytes (1.0‐3.3 per mm3) |
370 | 1030 | 720 |
|
Hemoglobin (13.0‐17.4 g/dL) |
12.6 | 6.5 | 17.6 |
|
Creatinine (0.5‐1.3 mg/dL) |
1.7 | 0.7 | 1.2 |
|
Urea nitrogen (6‐19 mg/dL) |
52 | 13 | 17 |
|
eGFR (>60 mL/min/1.73 m2) |
40 | >60 | >60 |
|
Albumin (3.2‐5.2 g/dL) |
3.2 | 2.3 | 4.0 |
|
AST (0‐37 µ/L) |
109 | 151 | 48 |
|
ALT (0‐40 µ/L) |
194 | 48 | 43 |
| Lactate dehydrogenase (94‐250 µ/L) | 706 | 579 | 378 |
|
Creatine kinase (39‐308 µ/L) |
305 | NA | NA |
| Coagulation laboratory findings | |||
|
Platelet count (140 000‐440 000 per mm3) |
438 000 | 143 000 | 207 000 |
|
D‐dimer units (<230 ng/mL) |
1028 | 1639 | 203 |
|
aPTT (27‐37 s) |
28 | 47 | 32 |
|
INR (0.9‐1.2) |
1.2 | 1.5 | 1.4 |
|
Fibrinogen (160‐450 mg/dL) |
NA | NA | NA |
|
Troponin T (0‐0.02 ng/mL) |
<0.01 | <0.01 | <0.01 |
|
Ferritin (30‐400 ng/mL) |
1216 | 252 | 779 |
|
C‐reactive protein (0‐8.0 mg/L) |
256 | 84 | 123 |
ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; ARDS, adult respiratory distress syndrome; ARDSnet, acute respiratory distress syndrome network; AST, aspartate aminotransferase; FiO2, fraction of inhaled oxygen; INR, International Normalized Ratio; PaO2, arterial partial pressure for oxygen; PEEP, positive end‐expiratory pressure.
TABLE 2.
HIT‐related data
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Risk factors | |||
| Sex | Male | Male | Male |
| BMI | 28.1 kg/m2 | 45.8 kg/m2 | 42.5 kg/m2 |
| Recent heparin | No | No | No |
| Recent surgery | None | None | None |
| 4T score | 6 | 4 | 6 |
| Anti‐PF4 optical density/Hospital day | 2.0/20 | 1.3/9 | 0.48/11 |
|
Serotonin release assay (% release at low ‐ high heparin concentrations) |
Positive (48%‐0%) | Negative (0%‐0%) | Negative (0%‐0%) |
| Confirmed thrombosis | Pulmonary embolism | Upper‐extremity venous thromboses | Skin necrosis |
|
Peak D‐dimer/Hospital day (<230 ng/mL) |
9461/10 | 2108/10 | 939/13 |
|
Nadir platelets/Hospital day (140 000‐440 000 per mm3) |
90 000/20 | 68 000/12 | 22 000/11 |
Abbreviations: BMI, body mass index; HIT, heparin‐induced thrombocytopenia; PF4, platelet factor 4.
2. CASES
2.1. Case 1
A 70‐year‐old man experienced flulike symptoms and tested positive for COVID‐19 by reverse transcriptase polymerase chain reaction testing of a nasopharyngeal swab as an outpatient on March 16, 2020. Over the next 4 days, generalized weakness, cough, and dyspnea worsened, dramatically over the fourth night. In the emergency department, oxygen saturation by pulse oximetry (SpO2) was 71% on a nonrebreather mask. He was intubated and treated with ceftriaxone and azithromycin and received subcutaneous unfractionated heparin prophylaxis for deep venous thrombosis (5000 units twice daily). Clinical trials for COVID‐19 were still being processed for our institution, and he received open‐label hydroxychloroquine for 5 days. On hospital day (HD) 5, he had worsening oxygenation and ventilation and responded to prone positioning and a cisatracurium infusion. Tocilizumab was administered, and remdesivir was obtained via an expanded access protocol. By HD 7, oxygenation was improving, and platelets remained stable through HD 12 at ~ 310 000/mm3 (Figure 1).
FIGURE 1.
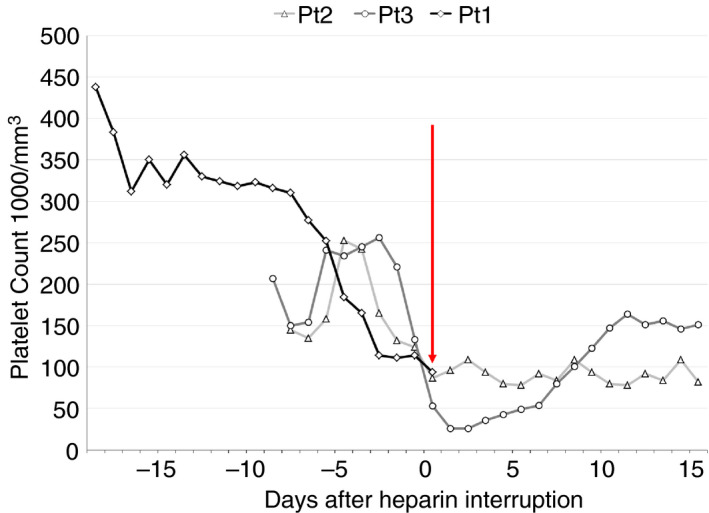
Serial platelet counts for all 3 cases, anchored on the day the anti–platelet factor 4/heparin antibody was sent and heparin interrupted (vertical red line). All 3 patients started on unfractionated heparin or low‐molecular‐weight heparin at admission, which was the first platelet count listed for them (19 days before red line for Patient 1, 8 days for Patient 2, and 9 days for Patient 3). Patient 1 expired before follow‐up platelet measurements
A percutaneous tracheostomy was placed on HD 17. The next day, his SpO2 abruptly declined, associated with tachycardia. A chest radiograph showed no pneumothorax and improved airspace infiltrates, and despite heparin prophylaxis, a computed tomography (CT) angiogram of the chest revealed segmental and subsegmental pulmonary emboli in the right upper and middle lobe, left lower lobe, and improving airspace disease consistent with COVID. He was started on an unfractionated heparin infusion at 18 units/kg/h. Later that day, a gradual decrease in his platelets beginning on HD 13 was recognized (see Figure 1), and HIT was suspected when his 4T score was calculated to be 6. A test for anti‐PF4/heparin antibody was sent, and heparin was transitioned to bivalirudin starting at 0.15 mg/kg/h. The next day, the anti‐PF4/heparin antibody returned positive (optical density 2.0), now meeting criteria for HITT, and a confirmatory SRA was ordered. Over the next 16 hours, his oxygen and ventilator requirements worsened with progressive hypotension requiring initiation of norepinephrine and vasopressin. Extracorporeal membrane oxygenation was requested but declined, a trial of inhaled nitric oxide was unsuccessful, and systemic tenecteplase was administered. After further clinical deterioration, his wife requested transition to comfort‐oriented care, and he expired. An autopsy was declined. The SRA from HD 18 returned positive (48% release low‐dose heparin, 0% release high‐dose) several days later.
2.2. Case 2
A 74‐year‐old man developed progressive cough, dyspnea, fatigue, and hoarseness over 2 weeks, and presented to the emergency department on March 31/2020. His SpO2 was 96% on nasal cannula at 6 L/min with a respiratory rate of 24, temperature 37.5°C. Physical examination was significant for obesity and 2+ lower‐extremity edema. A nasopharyngeal swab for COVID‐19 was sent, and he was admitted to an intermediate care unit with airborne precautions. He was started on vancomycin, piperacillin/tazobactam, azithromycin, and enoxaparin 40 mg every 12 hours for deep vein thrombosis prophylaxis. Overnight, his COVID swab returned positive, azithromycin was changed to doxycycline due to a prolonged QTc interval, and his oxygen requirements increased, prompting transfer to the intensive care unit. Despite diuresis, high‐flow nasal cannula, and antibiotics, his respiratory rate climbed into the 40s on HD 3, and he was intubated.
On HD 3, he developed hypotension requiring norepinephrine and vasopressin, and was started on stress‐dose hydrocortisone. Pulmonary embolism was suspected, but CT of the chest was deferred due to COVID isolation. Bilateral lower‐extremity Doppler ultrasound revealed no evidence of thrombosis, and he was started empirically on an unfractionated heparin infusion at 18 units/kg/h, with a platelet count of 158 000/mm3. A guaiac‐positive nonbloody stool prompted a reevaluation of the heparin infusion. A CT angiogram of the chest revealed no thromboembolic disease, but ground glass opacities were noted in both lungs. The heparin infusion was stopped, and he was started on subcutaneous weight‐adjusted unfractionated heparin (7500 units twice daily). With increasing oxygen needs, a trial of prone positioning for 20 hours on HD 5 was effective. With a prolonged QTc, a positive quantiferon test for latent tuberculosis infection, and pressor requirements, he was not a candidate for antiviral therapy.
On HD 9, we recognized that his platelet count had fallen to 87 000/mm3 from 165 000/mm3 on HD 6 and 124 000/mm3 on HD 8. His 4T score was 4, heparin was stopped, and anti‐PF4/heparin antibody and ultrasound examination of four extremities were ordered. Pending these results, and to minimize blood draws and contact with the patient, he received fondaparinux at 10 mg daily. On HD 10, the anti‐PF4/heparin antibody returned positive, with an optical density of 1.3. Ultrasound examination of the extremities revealed right cephalic, left basilic, and left cephalic thromboses, meeting criteria for HITT, and fondaparinux was changed to bivalirudin. The SRA drawn on this day returned negative (0% release low‐dose heparin, 0% release high‐dose) on HD 14; bivalirudin was discontinued, and gastrointestinal bleeding precluded anticoagulation. Over the next 3 weeks, he developed worsening hepatic and renal dysfunction, support was limited, and he died on HD 34.
2.3. Case 3
A 53‐year‐old man developed a dry cough, fever, diaphoresis, and diarrhea that worsened over 1 week. He stopped working after 7 days, and 5 days later (March 25, 2020) presented to the emergency department with chest pain, palpitations, and productive cough. Physical examination revealed tachypnea, diaphoresis, 2+ peripheral edema, and an irregular heartbeat at 151 beats per minute (atrial fibrillation by electrocardiogram). A chest radiograph revealed a left, midlung opacification. His heart rate improved with metoprolol, and he was started on an unfractionated heparin infusion (12 units/kg/h) for atrial fibrillation and received 2 g of ceftriaxone, 500 mg of azithromycin, and furosemide. Influenza A and B testing was negative, a nasopharyngeal swab for COVID testing was sent, and he was admitted to a telemetry isolation unit. His COVID swab returned positive overnight, and he was treated with hydroxychloroquine. His oxygen requirements worsened over the next 2 days, with a chest radiograph showing bilateral alveolar infiltrates, and on HD 3, he was intubated and treated with the acute respiratory distresas syndrome (ARDS) network high positive end‐expiratory pressure strategy and continued on the heparin infusion. On HD 11, a significant decrease in his platelets from 221 000/mm3 to 53 000/mm3 over the prior 2 days was recognized, and skin blisters with black eschars suggestive of skin necrosis were noted. His 4T score was 6, and his heparin was stopped. Argatroban was initiated at 2 µg/kg/min, and anti‐PF4/heparin antibodies were sent, which returned positive (optical density 0.48). An SRA was ordered, and over the next 5 days, his platelet count rebounded, and he improved from a respiratory standpoint. His SRA returned negative on HD 17 (0% release low‐dose heparin, 0% release high‐dose). On HD 19, he was extubated and received a percutaneous endoscopic gastrostomy tube on HD 22. His anticoagulation was converted to apixaban 5 mg twice daily, and after 2 negative COVID swabs, he was transferred to rehabilitation on HD 37.
3. DISCUSSION
A hypercoagulable state has been described in the early clinical reports of COVID‐19, with elevated D‐dimer concentrations (worse with more severe disease), increased fibrin degradation products, lower antithrombin levels, and a potential survival benefit from prophylactic low‐dose unfractionated heparin or low‐molecular‐weight heparin when D‐dimer concentrations were significantly increased. 1 , 2 , 4 More recently, 3 cases of antiphospholipid antibodies during COVID‐19 critical illness were associated with cerebral infarction. 7 These observations prompted recommendations from the ISTH for prophylactic anticoagulation including prophylactic low‐molecular‐weight heparin administration for all patients with COVID‐19. 8 Heparin‐induced thrombocytopenia is uncommon in critically ill patients, with an incidence estimated at 0.2%–0.45%. 9 , 10 Even among high‐risk populations such as patients undergoing cardiac surgery, the incidence remains low, reported at 1%–3%. 10 In this series of patients, we describe 3 patients with COVID‐19 with suspicion for HITT and positive anti‐PF4/heparin antibodies, with SRA confirmation in 1 of 3 tests. We believe this is the first case of HIT or HITT reported during the COVID‐19 pandemic.
The SRA assay was performed by the Blood Center of Wisconsin, using 14C‐serotonin, with a low heparin concentration of 0.1 U/mL, a high concentration of 100 U/mL, and a threshold of 20% serotonin release. The enzyme immunoassay used by our laboratory to detect anti‐PF4/heparin antibodies is the polyspecific Stago assay, with a cutoff optical density of 0.40. Our patients had few risk factors for HIT (no female patients; no recent cardiac, vascular, orthopedic, or trauma surgery; no heparin exposure in the past 90 days); although 2 patients had body mass index (BMI) values >40 kg/m2, the BMI for the confirmed HIT‐positive patient was <30 kg/m2. 9 All 3 patients received heparin at either prophylactic or therapeutic doses from the time of admission. We did not recognize or initially consider HIT with dropping platelets in a timely fashion for Patient 1, likely related to the continued (but dropping) platelet count >100 000/mm3, the well‐described presence of DIC associated with COVID‐19, and the elimination of face‐to‐face rounding with clinical pharmacists (who often bring these abnormalities to our attention) in our institution during the pandemic. We present these cases to bring the presence of HIT and HITT during the COVID‐19 pandemic to the attention of clinicians.
Wang et al 11 reported 3 cases of COVID‐19 associated ARDS treated with tissue‐type plasminogen activator (t‐PA) for suspected in‐situ thrombosis. All 3 patients improved dramatically after t‐PA but worsened again during subsequent heparin therapy, including 1 patient who died. No mention of HIT or anti‐PF4/heparin antibody testing was made in the report, and it is possible that the postthrombolytic heparin therapy contributed to a prothrombotic state if HIT was present. 11 A recent meta‐analysis of thrombocytopenia during the COVID‐19 pandemic identified that lower platelet counts are associated with increased risk of severe disease and mortality in patients with COVID‐19 but makes no mention of heparin or heparin‐induced thrombocytopenia. 12 Continued use of heparin during unrecognized HIT may contribute to mortality, and it is critical that clinicians consider HIT to allow early recognition, discontinuation of heparin, and initiation of an appropriate anticoagulant. Our 3 patients were treated with fondaparinux, argatroban, and bivalirudin after HIT was suspected or confirmed, as is recommended. 10 , 13 , 14
Two of our patients had anti‐PF4/heparin antibodies but negative SRA testing. After stopping heparin in both, the platelets of patient 2 (with advanced cirrhosis and acute kidney injury) did not rebound as expected, but the platelets of Patient 3 recovered, and he survived and was discharged. The limitations of these functional assays for HIT have been well described, 15 and this could have been a case of SRA‐negative HIT. 16 The prothrombotic and hyperinflammatory environment associated with COVID‐19 may contribute to this phenomenon. We did not pursue advanced testing to evaluate this, but if clinicians have a strong clinical suspicion for HIT with confirmed anti‐PF4/heparin antibodies but a negative conventional SRA test, they should consider investigating for SRA‐negative HIT. 17 , 18
4. CONCLUSION
Anticoagulation, including prophylactic or treatment doses of unfractionated heparin and low‐molecular‐weight heparin, is recommended for patients with COVID‐19. We report 3 patients with clinically suspected heparin‐induced thrombocytopenia with thrombosis, which was confirmed in 1 patient. Delayed recognition of HIT may have contributed to a poor outcome for this patient. We recommend that clinicians pay close attention to platelet counts during heparin therapy, with continued vigilance for HIT.
RELATIONSHIP DISCLOSURE
The authors declare nothing to report.
AUTHOR CONTRIBUTIONS
RR and DS: concept and design, analysis and interpretation of data, critical writing, final approval; TM, DG, and MB: analysis and interpretation of data, final approval; GF, WZ: analysis and interpretation of data, critical writing, final approval.
Riker RR, May TL, Fraser GL, et al. Heparin‐induced thrombocytopenia with thrombosis in COVID‐19 adult respiratory distress syndrome. Res Pract Thromb Haemost. 2020;4:936–941. 10.1002/rth2.12390
Handling Editor: Dr Pantep Angchaisuksiri
Contributor Information
Richard R. Riker, Email: rriker@cmamaine.com.
Teresa L. May, @TLMay22.
Gilles L. Fraser, @gilfraser.
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS‐CoV2 and non‐SARS‐CoV2. J Thromb Thrombolysis 2020; Apr 3. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020; Apr 9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up. J Am Coll Cardiol 2020; Apr 15. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med 2020; Apr 8. [Epub ahead of print].382(17):e38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ThachilJ TN, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thrombosis Haemostasis. 2020;18(5):1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. East JM, Cserti‐Gazdewich CM, Granton JT. Heparin‐induced thrombocytopenia in the critically ill patient. Chest. 2018;154:678–90. [DOI] [PubMed] [Google Scholar]
- 10. Greinacher A. Clinical practice. Heparin‐induced thrombocytopenia. N Engl J Med. 2015;373:252–61. [DOI] [PubMed] [Google Scholar]
- 11. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID‐19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020. Apr 8. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: A meta‐analysis. Clin Chim Acta 2020;506:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelton JG, Arnold DM, Bates SM. Nonheparin anticoagulants for heparin‐induced thrombocytopenia. N Engl J Med. 2013;368:737–44. [DOI] [PubMed] [Google Scholar]
- 14. Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin‐induced thrombocytopenia. Blood Adv. 2018;2:3360–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tardy B, Lecompte T, Mullier F, Vayne C, Pouplard C. Detection of platelet‐activating antibodies associated with heparin‐induced thrombocytopenia. J Clin Med. 2020;9:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warkentin TE, Nazy I, Sheppard JI, Smith JW, Kelton JG, Arnold DM. Serotonin‐release assay‐negative heparin‐induced thrombocytopenia. Am J Hematol. 2020;95:38–47. [DOI] [PubMed] [Google Scholar]
- 17. Vayne C, Guery EA, Kizlik‐Masson C, Rollin J, Bauters A, Gruel Y, et al. Beneficial effect of exogenous platelet factor 4 for detecting pathogenic heparin‐induced thrombocytopenia antibodies. Br J Haematol. 2017;179:811–9. [DOI] [PubMed] [Google Scholar]
- 18. Warkentin TE, Sheppard JI, Smith JW, Arnold DM, Nazy I. Timeline of heparin‐induced thrombocytopenia seroconversion in serial plasma samples tested using an automated latex immunoturbidimetric assay. Int J Lab Hematol. 2019;41:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]


