Abstract
Background
Coronavirus disease (COVID‐19) is a growing concern worldwide. Approximately 5% of COVID‐19 cases require intensive care. However, the optimal treatment for respiratory failure in COVID‐19 patients is yet to be determined.
Case presentation
A 79‐year‐old man with severe acute respiratory distress syndrome due to COVID‐19 was admitted to our intensive care unit. Prone ventilation was effective in treating the patient’s hypoxemia. Furthermore, the patient received lung protective ventilation with a tidal volume of 6–8 mg/kg (predicted body weight). However, the patient’s respiratory failure did not improve and he died 16 days after admission because of multiple organ failure. Serial chest computed tomography revealed a change from ground‐glass opacity to consolidation pattern in both lungs.
Conclusions
We report a protracted case of COVID‐19 in a critically ill patient in Japan. Although prone ventilation could contribute to treating hypoxemia, its efficacy in preventing mortality from COVID‐19 is unknown.
Keywords: Acute respiratory distress syndrome, COVID‐19, critically ill, prone ventilation, SARS‐CoV‐2
COVID‐19 is a growing concern worldwide. In our seriously ill case with COVID‐19, despite the effectiveness of prone ventilation in ameliorating hypoxemia, enabling lung protective ventilation, and bypassing the need for extracorporeal membrane oxygenation, the patient died because of multiple organ failure accompanied by secondary bacterial infection.
Introduction
Coronavirus disease (COVID‐19) is a major growing concern worldwide. By 11 February 2020, 72,314 cases were reported in mainland China, 1 and among 44,672 confirmed cases, 1,023 deaths were reported (case‐fatality rate of 2.3%). In Japan, 1,160 confirmed cases, including 113 asymptomatic cases, were reported by the Ministry of Health, Labor and Welfare on 25 March 2020. 2 Among the 1,047 symptomatic patients, 43 died (case‐fatality rate of 4.1%).
The Chinese Center for Disease Control and Prevention reported that approximately 5% of COVID‐19 cases progress to a critical stage and require intensive care, including mechanical ventilation. 1 , 3 According to the Surviving Sepsis Campaign guideline for COVID‐19, low tidal volume ventilation and prone ventilation are recommended for treating severe hypoxemic respiratory failure. 4 However, the optimal treatment for respiratory failure in COVID‐19 patients is yet to be determined, likely because of the lack of specific information on the management and outcome of patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection.
Case Report
A 79‐year‐old man without relevant medical history presented to our hospital with progressive hypoxemia due to refractory pneumonia. Seven days before admission, he had presented to a different hospital with complaints of 5 days of fever, nausea, and loss of appetite. A chest computed tomography (CT) revealed small bilateral ground‐glass opacities (GGOs) (Fig. 1C, D) in both lungs. He was diagnosed with pneumonia and admitted to the aforementioned hospital; he was treated with ceftazidime, followed by meropenem. At the day of admission to our hospital, he developed hypoxemia and was referred to the emergency department of our tertiary care hospital.
Fig. 1.
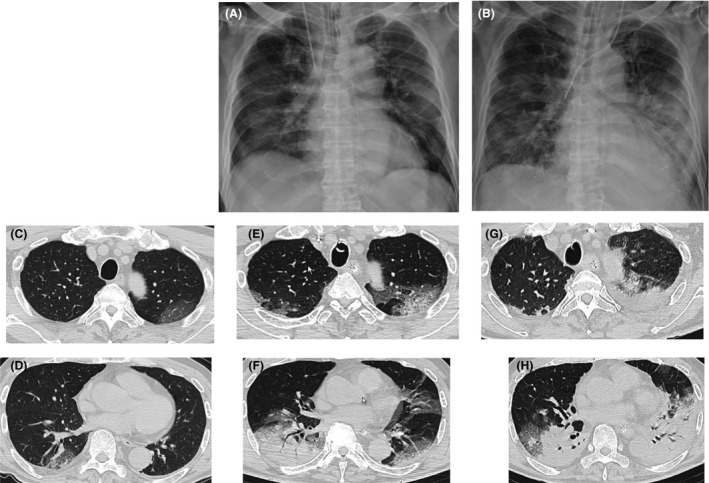
Radiological findings in a critically ill 79‐year‐old Japanese man with COVID‐19. A, Chest radiograph obtained on the day of admission showing bilateral ground‐glass opacity (GGO) without an air bronchogram. B, On the 13th hospital day, consolidation was visible in the left lower lung, in addition to bilateral GGO. C, D, Chest computed tomography scan obtained 7 days before admission to our hospital showing bilateral faint GGOs. E, F, On the day of admission to our hospital, the lesions had changed to a mixed pattern of GGOs and consolidation. G, H, On the 13th hospital day, the lesions progressed to a consolidation pattern.
On arrival at the emergency department, the patient’s blood pressure was 120/74 mmHg; heart rate, 107 b.p.m.; Glasgow Coma Scale score, E4V5M6; body temperature, 38.6°C; respiratory rate, 40 breaths/min; and oxygen saturation, 92% while receiving 10 L/min oxygen through a face mask with reservoir. His Sequential Organ Failure Assessment (SOFA) scores were 10. He was immediately intubated and placed on mechanical ventilation. Chest CT and radiography revealed worsening of lesions, illustrated by a mix of GGO and consolidation patterns (Fig. 1A, E, F). The patient had lymphopenia, with slightly elevated creatinine level accompanied by markedly elevated C‐reactive protein (Table 1). Even though the patient had no history of any epidemiological exposure to Wuhan or Hubei provinces, we undertook a nucleic acid amplification test (NAAT) of a throat swab sample for SARS‐CoV‐2 virus because of radiological findings and the refractory pneumonia despite treatment with antibiotics. Six hours after the admission, the NAAT was found to be positive, and the patient was diagnosed as having COVID‐19 pneumonia. Blood culture was negative, and sputum culture revealed only normal flora.
Table 1.
Laboratory results of a critically ill 79‐year‐old Japanese man with COVID‐19
| Variable | Seven days before admission † | At the emergency department | Day 13 of hospitalization |
|---|---|---|---|
| White‐cell count (per mm3) | 4,,400 | 8,670 | 14,520 |
| Lymphocyte count (per mm3) | 1,100 | 1,040 | 990 |
| Hemoglobin (g/dL) | 16.8 | 15.2 | 9.5 |
| Hematocrit (%) | 48.8 | 42.9 | 27.7 |
| Platelet count (per mm3) | 129,000 | 152,000 | 149,000 |
| Sodium (mmol/L) | 138 | 138 | 144 |
| Potassium (mmol/L) | 4.2 | 4.1 | 4.1 |
| Chloride (mmol/L) | 98 | 107 | 117 |
| Urea nitrogen (mg/dL) | 24.3 | 18.8 | 28.1 |
| Creatinine (mg/dL) | 1.1 | 1.1 | 1.7 |
| Aspartate aminotransferase (IU/L) | 36 | 118 | 60 |
| Alanine aminotransferase (IU/L) | 23 | 61 | 27 |
| Lactate dehydrogenase (IU/L) | 215 | 717 | 453 |
| Total bilirubin (mg/dL) | 0.9 | 1.1 | 6.3 |
| International normalized ratio | Not done | 1.14 | 1.50 |
| Fibrinogen (mg/dL) | Not done | 703 | 498 |
| C reactive protein (mg/dL) | 6.7 | 23.5 | 22.0 |
| Procalcitonin (ng/mL) | Not done | 0.43 | Not done |
| Arterial blood gas analysis | |||
| pH | Not done | 7.443 ‡ | 7.434 § |
| PaO2 (mmHg) | Not done | 75.5 ‡ | 89.7 § |
| PaCO2 (mmHg) | Not done | 34.0 ‡ | 29.1 § |
| (mmol/L) | Not done | 24.5 ‡ | 19.2 § |
| Lactate (mmol/L) | Not done | 1.2 ‡ | 1.2 § |
| Rapid antigen test | |||
| Rapid antigen test for influenzae virus | Negative | Negative | Not done |
| Rapid urinary antigen test for Pneumococcus | Not done | Negative | Not done |
| Rapid urinary antigen test for Legionella | Not done | Negative | Not done |
Laboratory tests carried out at the initial hospital.
Analysis undertaken while patient was receiving 10 L/min oxygen through a face mask with reservoir.
Analysis undertaken while patient was under mechanical ventilation using pressure support mode with positive end‐expiratory pressure 8 cmH2O and FiO2 0.5.
After admission to our intensive care unit, the patient was treated with piperacillin/tazobactam, azithromycin, and norepinephrine. Although we initially administered methylprednisolone for severe pneumonia, we discontinued it after the cause was found to be a SARS‐CoV‐2 infection. On the first day of admission, the PaO2/FiO2 ratio was 91 during a positive end‐expiratory pressure of 10 cmH2O. Low tidal volume pressure‐controlled ventilation of 6–8 mL/kg (predicted body weight) with prone ventilation was started for severe acute respiratory distress syndrome (ARDS). The PaO2/FiO2 ratio rapidly improved to 282, 2 h after introducing prone ventilation. However, after going from prone to supine position, the oxygenation deteriorated and prone ventilation had to be restarted. Overall, we carried out six rounds of prone ventilation with a median duration of 18.5 h (range, 12–43 h). The tidal volumes and oxygenation values are shown in Figure 2.
Fig. 2.
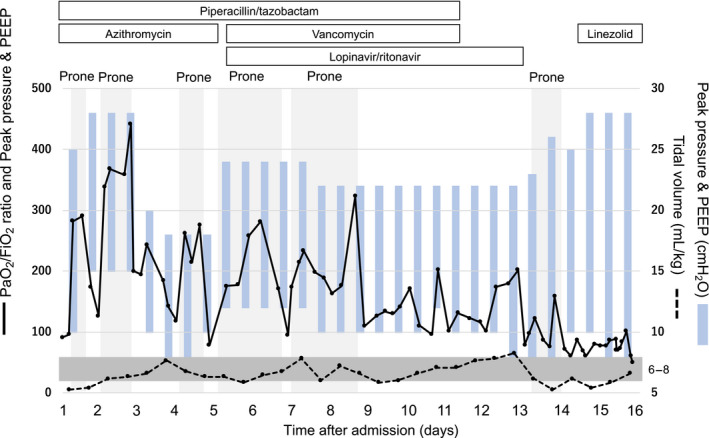
Course of ventilatory settings and PaO2/FiO2 ratio in a critically ill 79‐year‐old Japanese man with COVID‐19. Light gray shading indicates prone ventilation values over time. Dark gray shading indicates a tidal volume between 6 and 8 mL/kg. Although PaO2/FiO2 improved during prone ventilation, it deteriorated following return to the supine position. Tidal volume was maintained between 6 and 8 mL/kg (predicted body weight). PEEP, positive end‐expiratory pressure.
On the 5th hospital day, we started the patient on lopinavir/ritonavir (400/100 mg twice daily) and vancomycin for the unresolved pneumonia. On the 11th hospital day, we discontinued piperacillin/tazobactam and vancomycin because of worsening renal functions and generalized fixed drug eruptions, coupled with lack of evidence regarding the ongoing bacterial infection. On the 10th and 12th hospital days, two NAATs of sputum specimens were negative, and we discontinued lopinavir/ritonavir. The chest CT on the 13th day revealed worsening of the consolidation pattern (Fig. 1B, G, H). The laboratory findings revealed coagulopathy and elevated serum creatinine and bilirubin levels (Table 1). On the 14th hospital day, we initiated continuous renal replacement therapy for worsening kidney functions and hyperkalemia. Furthermore, we increased the dose of norepinephrine and undertook a blood culture because of worsening hypotension. Therefore, the SOFA score increased to 16, suggesting multiple organ failure. On the 15th hospital day, we started the patient on linezolid because of the presence of gram‐positive bacteria in the blood and sputum culture, which was later identified as methicillin‐resistant Staphylococcus aureus, indicating secondary bacterial pneumonia (Table S1). On the 16th hospital day, the patient died because of multiple organ failure.
Discussion
Here we reported a 79‐year‐old man with severe ARDS due to SARS‐CoV‐2 infection. According to our previous experience, we have applied prone ventilation for a longer duration than that stated in the abovementioned guideline, with a maximum duration of 43 h, which temporally cured his hypoxemia. 4 , 5 This allowed us to implement low tidal volume ventilation, bypassing the need for more invasive and resource‐intensive procedures, such as extracorporeal membrane oxygenation (ECMO). Although prone ventilation is recommended for severe ARDS, published reports on the outcomes of prone ventilation used in COVID‐19 patients are limited. 4 A recent case series study from Wuhan, which included critically ill patients with COVID‐19, reported that approximately 10% of patients were treated with prone ventilation. 6 Although prone ventilation might mitigate hypoxemia, it is unclear whether it contributes to a decrease in mortality of patients experiencing ARDS.
In our case, the chest CT showed GGO predominantly in the dorsal lung area, which could explain the effectiveness of prone ventilation. The GGO did not improve; instead, it progressed to a consolidation pattern 3 days before the patient died, which could explain the dependence on prone ventilation and refractory clinical course. A recent observational study of COVID‐19 patients showed that the prevalence of GGO decreases with time, and tends to be replaced by consolidation. 7 Our reported radiological findings are in line with this.
The use of venovenous (VV)‐ECMO for managing ARDS is controversial, and recent guidelines do not provide any specific recommendation regarding its use, due to the limited evidence on its efficacy. 8 Accordingly, a recent randomized controlled trial failed to determine a significant improvement in the mortality rate as a result of early VV‐ECMO in patients with severe ARDS. 9 In our hospital, we routinely use VV‐ECMO for refractory hypoxemia (PaO2/FiO2 ratio < 100) under low tidal volume ventilation with prone positioning and/or a neuromuscular blocking agent. In this case, because the PaO2/FiO2 ratio rose to close to 300 after prone positioning, we did not use VV‐ECMO or neuromuscular blocking agents, while successfully maintaining low tidal volume ventilation.
We introduced lopinavir/ritonavir and corticosteroids. Lopinavir/ritonavir was reported to be a promising antiviral agent for the treatment COVID‐19, even though its efficacy has not yet been fully proven. 10 By contrast, the use of corticosteroids has been discouraged because of the lack of evidence of their benefit in COVID‐19 patients. 11 Further studies will be required to fully understand the efficacy of these agents in the treatment of COVID‐19.
Conclusion
We reported a case of a critically ill COVID‐19 patient in Japan. Despite the effectiveness of prone ventilation in ameliorating hypoxemia, respiratory failure could not be improved and the patient finally died because of multiple organ failure accompanied by secondary bacterial infection. The lack of clinical or radiological improvement during hospitalization suggests refractory lung damage caused by severe ARDS from SARS‐CoV‐2 infections, which could require lung protective ventilation to be maintained for a long time, along with strict vigilance for secondary complications.
Disclosure
Approval of the research protocol: N/A.
Informed consent: Written informed consent was obtained from the patient’s family for publication. For privacy reasons, minimal epidemiological information is presented.
Registry and registration no. of the trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Supporting information
Table S1. In vitro susceptibility of Staphylococcus aureus detected using blood culture on day 15 of hospitalization
Acknowledgements
We thank Shuji Kawashima, Masaou Tanaka, and Naoaki Shibata for their help in managing the patient, Daisuke Norimura, Satomi Kotani, Mitsue Kojima, Ai Tsujita, and Machiyo Nakanishi for their efforts in implementing infection prevention and control measures in our hospital, and Editage for English language editing.
Funding information
No funding information provided.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239. [DOI] [PubMed] [Google Scholar]
- 2. Japanese Ministry of Health, Labour and Welfare . Current status about novel coronavirus infection in Japan and response of Japanese Ministry of Health, Labour and Welfare (25 March 2020 edition). 2020. [cited 28 Mar 2020]. https://www.mhlw.go.jp/stf/newpage_10442.html. [in Japanese].
- 3. Guan WJ, Ni ZY, Hu Y et al Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alhazzani W, Møller MH, Arabi Y et al Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID‐19). Intensive Care Med. 2020; 46: 854–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyamoto K, Kawazoe Y, Yasuda M et al Oxygenation improves during the first 8 h of extended‐duration prone positioning in patients with respiratory failure: a retrospective study. J. Intensive Care 2014; 2: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang X, Yu Y, Xu J et al Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir. Med. 2020; 8: 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi H, Han X, Jiang N et al Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020; 20: 425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan E, Del Sorbo L, Goligher EC et al An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017; 195: 1253–63. [DOI] [PubMed] [Google Scholar]
- 9. Combes A, Hajage D, Capellier G et al Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N. Engl. J. Med. 2018; 378: 1965–75. [DOI] [PubMed] [Google Scholar]
- 10. Cao B, Wang Y, Wen D et al A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N. Engl. J. Med. 2020; 382: 1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet 2020; 395: 473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. In vitro susceptibility of Staphylococcus aureus detected using blood culture on day 15 of hospitalization


