Thrombocytopenia is a risk factor for increased morbidity and mortality in patients infected with the new severe acute respiratory syndrome coronavirus 2, SARS‐CoV‐2 (COVID‐19 infection). 1 Thrombocytopenia in COVID‐19 patients may be caused by disseminated intravascular coagulation (DIC), sepsis or be drug‐induced. Recently a single case report suggested immune thrombocytopenia (ITP) may be associated with COVID‐19 infection. 2 ITP is a rare autoimmune disease characterised by a platelet count <100 × 109/l, leading to an increased risk of bleeding. 3 Several risk factors have been described for ITP including environmental (e.g. infection, malignancy and drugs) and genetic predisposition. 4 We report here the first case series of three patients with ITP associated with COVID‐19 infection.
Patient 1 is a 59‐year‐old man, known for 10 years with a stage IV neuroendocrine tumour (NET) of the small bowel, who presented with oral mucosal petechiae and spontaneous skin haematomas. He also experienced symptoms of coughing and fever 10 days before presentation and his partner had a documented COVID‐19 infection. Full blood counts showed an isolated thrombocytopenia (<3 × 109/l) without signs of dysplasia in the peripheral blood film and he tested positive for COVID‐19 by polymerase chain reaction (PCR) on a nasopharyngeal swab. Additional diagnostic procedures showed no signs of NET progression or other infections (Table I; Figure [Link], [Link]A, B). After excluding other causes of thrombocytopenia, including DIC, bacterial sepsis and medication, he was diagnosed with COVID‐19‐associated ITP. He was treated with platelet transfusion, without increment, followed by intravenous immunoglobulins (IVIG) 1 g/kg for two days. Platelet autoantibodies were tested positive. After an increase to 47 × 109/l, platelet count dropped to 19 × 109/l when dexamethasone was started leading to a platelet count of 51 × 109/l on day 27 (Fig 1A).
Table I.
Patient characteristics of the three patients with COVID‐19‐associated immune thrombocytopenia.
| Patient 1 | Patient 2 | Patient 3 | Reference range | |
|---|---|---|---|---|
| Age | 59 | 66 | 67 | |
| Sex | Male | Female | Male | |
| Haemoglobin level (mmol/l) | 8·3 | 8·0 | 9·3 | 8·6–10·5 |
| Platelet count (×109/l) | <3 | 2 | 338 | 150–370 |
| Leucocyte count (×109/l) | 3·9 | 5·8 | 11·2 | 3·5–10 |
| Lymphocyte count (×109/l) | 0·4 | 0·7 | 0·86 | 0·5–5 |
| PT (s) | 12·5 | 10·4 | n.d. | 10·9–13·3 |
| APTT (s) | 22·0 | 22·8 | n.d. | 22·0–32·0 |
| D‐dimer (μg/l) | n.d. | 1411 | >35 200 | 0–500 |
| Autoantibodies | ||||
| GPIb | Positive | Negative | n.d. | |
| GPIIBIIa | Positive | Negative | n.d. | |
| GPV | Positive | Negative | n.d. | |
| Anti‐cardiolipin antibodies | n.d. | Negative | n.d. | |
| Anti‐beta‐2‐glycoprotein I | n.d. | Negative | n.d. | |
| Thrombopoietin (Units/ml) | 56 | 100 | n.d. | 4–32 |
| Virus serology | ||||
| HIV | Negative | Negative | n.d. | |
| Hepatitis B and C | Negative | Negative | n.d. | |
| EBV | Negative | Negative | n.d. | |
| Parvo B19 virus | Negative | n.d. | n.d. | |
| CMV virus | Negative | n.d. | 288/P | |
| Helicobacter pylori | Negative | Negative | n.d. | |
| Interleukin‐6 (ng/l) | 19 | 3 | 270 | <10 |
GP, glycoprotein; HIV, human immunodeficiency virus; EBV, Epstein–Barr virus; CMV, cytomegalovirus; n.d., not done.
Fig 1.
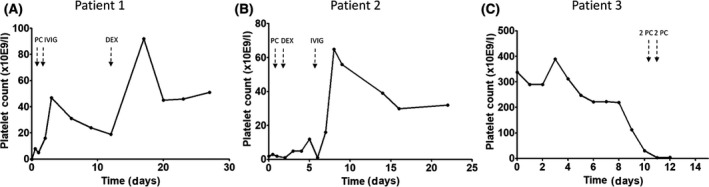
The course of platelet counts of three patients (A‐C) with COVID‐19‐associated immune thrombocytopenia. Patient 3 (C) died at day 13 because of an intracerebral bleeding. IVIG, intravenous immunoglobulins (1 g/kg on two consecutive days); PC, platelet concentrate transfusion; DEX, 4 days 40 mg/day treatment with dexamethasone.
Patient 2, a 66‐year‐old woman with hypertension, presented with petechiae, spontaneous epistaxis and increased blood loss from haemorrhoids since three weeks (Figure S2). Four weeks before admission she experienced fever, dyspnoea and coughing during a week, followed by diarrhoea and vomiting for several days. On admission, oropharyngeal swab PCR confirmed COVID‐19 infection. Platelet counts at admission were 2 × 109/l (Table I). Additional diagnostics showed no signs of other infections, recent medication changes or antiphospholipid antibodies. Autoantibodies to platelets tested negative. One unit of platelets was administered without increment. She was diagnosed with COVID‐19‐associated ITP and treated with dexamethasone 40 mg daily for four days. Without any response on day 6 patient received IVIG resulting in a platelet count of 32 × 109/l on day 22 (Fig 1B).
Patient 3, a 67‐year‐old man with a history of hypertension and diabetes mellitus, presented with fever, coughing and dyspnoea since nine days. Blood counts were normal at admission and his computed‐tomography (CT) scan showed bilateral infiltrates. He was diagnosed with COVID‐19 by PCR on oropharyngeal swab. Because of respiratory failure he was transferred to the ICU at day 2 and intubated on day 3. On day 10 a CT scan was repeated due to lack of respiratory improvement, which showed segmental pulmonary embolism for which he was treated with unfractionated heparin. Platelet counts dropped from 112 × 109/l on day 10 to 3 × 109/l on day 12 (Fig 1C). His clinical condition as well as other coagulation parameters remained stable and heparin‐induced thrombocytopenia was excluded (Table I). Laboratory examination excluded pseudothrombocytopenia and thrombotic thrombocytopenic purpura. Anticoagulant therapy was continued with a target platelet count of >40 × 109/l. Unfortunately, he did not have increments on platelet transfusions and died of an intracerebral bleeding within 24 h. Based on the course of platelet counts and exclusion of other causes we consider the thrombocytopenia as COVID‐19‐associated ITP.
Viral infections are associated with ITP. 4 Here we describe the first case series of three patients with COVID‐19‐associated ITP. The ITP occurred not only during active COVID‐19 infection, but also up to 10 days after the clinical COVID‐19 symptoms subsided. Diagnosis of COVID‐19‐associated ITP may be difficult because of several other potential causes, for instance the coagulation activation by COVID‐19 infection leading to DIC and subsequent thrombocytopenia. Also, treatments for COVID‐19, including heparin, azithromycin and hydroxychloroquine, may lead to thrombocytopenia. 1
The goal of ITP treatment is preventing severe bleeding by providing a safe platelet count. 3 Treatment for COVID‐19‐associated ITP may pose several issues. Commonly used treatments include IVIG, glucocorticoids or thrombopoietin receptor agonists (TPO‐RAs). 3 IVIG are generally reserved for ITP patients who require a rapid increase in platelet count. A disadvantage of IVIG is that they are not curative and often poorly tolerated. 3 As IVIG inhibit the phagocytic capabilities of macrophages, 4 treatment with IVIG in an early stage of COVID‐19 may be successful. 5 Additionally, some COVID‐19 patients who suffered deterioration of clinical symptoms have been salvaged by IVIG treatment. 6 IVIG was chosen in patient 1 because of active bleeding and the relative contraindication of glucocorticoid treatment, which may interfere with the somatostatin analogue therapy for the NET, and in patient 2 because of failure of dexamethasone treatment. Glucocorticoids comprise the primary treatment of ITP. 3 However, glucocorticoids are considered harmful for patients with COVID‐19 infection as they inhibit immune responses and clearance of the SARS‐COV‐2 virus. 7 Patient 2 received treatment with dexamethasone because she had no symptoms of the COVID‐19 infection for over a week. She received a short course of high‐dose dexamethasone rather than a longer period of prednisone which is associated with significant toxicities and a longer time to response. 8 COVID‐19 infection may be accompanied by thrombocytopenia as a result of DIC, which is associated with a strongly increased risk of thromboembolism reported in 30% of the patients. 9 As treatment with TPO‐RA has shown in selected cases to increase the risk of venous thromboembolism, 10 it should be used with caution in COVID‐19 infection.
In conclusion, this is the first case series of three patients with a COVID‐19‐associated ITP. It is important to be aware of this severe complication of a COVID‐19 infection and should be diagnosed and treated immediately. Failure to recognise it on time may ultimately lead to fatal complications, as shown in one of our patients. Choice of treatment of ITP should be based on balancing the risk of bleeding due to ITP versus potential deterioration of COVID‐19 infection due to immunosuppressive therapy.
Author Contributions
FNC and AJGJ designed the project. GB, FNC, FWGL and AJGJ analysed the data and wrote the manuscript. GB, PGNJM, FWGL, PAWB and JH interpreted the data, commented, provided essential clinical input and reviewed the manuscript. All authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest in carrying out this study.
Supporting information
Fig S1. (A) Bone marrow aspirate with May–Grünwald Giemsa staining and at 613× magnification. Normal cellularity and an increase in ther amount of megakaryocytes were found.
Fig S1. (B) Bone marrow trephine biopsy (PAS staining, original object lens magnification 20×) with a cluster of three megakaryocytes (Meg). The number is increased; however, they appear morphologically normal. Note the erythropoiesis organised in regularly spaced erythrons (E) and the mestastasis (M) of the neuroendocrine tumour. Although minimal localisation of NET cells was found in the bone marrow, no signs of dysplasia were found. Additionally, with a normal haemoglobin value and leucocyte count we assumed localisation of the NET cells in the bone marrow could not explain the low platelet count, making the diagnosis of ITP more likely.
Fig S2. Petechiae on the left leg of patient 2.
References
- 1. Liu Y, Sun W, Guo Y, Chen L, Zhang L, Zhao S, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;209:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zulfiqar AA, Lorenzo‐Villalba N, Hassler P, Andres E. Immune thrombocytopenic purpura in a patient with COVID‐19. N Engl J Med. 2020;382(18):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381:945–55. [DOI] [PubMed] [Google Scholar]
- 4. Swinkels M, Rijkers M, Voorberg J, Vidarsson G, Leebeek FWG, Jansen AJG. Emerging concepts in immune thrombocytopenia. Front Immunol. 2018;9:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao W, Liu X, Bai T, Fan H, Hong K, Song H, et al. High‐dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7:ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie Y, Cao S, Dong H, Li Q, Chen E, Zhang W, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID‐19. J Infect. 2020. 10.1016/j.jinf.2020.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395:473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang L, Xu L, Hao H, Jansen AJG, Liu G, Li H, et al. First line treatment of adult patients with primary immune thrombocytopenia: a real‐world study. Platelets. 2020;31:55–61. [DOI] [PubMed] [Google Scholar]
- 9. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020. pii: S0049–3848(20)30120–1. 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jansen AJG, Swart RM, te Boekhorst PAW. Thrombopoietin‐receptor agonists for immune thrombocytopenia. N Engl J Med. 2011;365:2240–1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. (A) Bone marrow aspirate with May–Grünwald Giemsa staining and at 613× magnification. Normal cellularity and an increase in ther amount of megakaryocytes were found.
Fig S1. (B) Bone marrow trephine biopsy (PAS staining, original object lens magnification 20×) with a cluster of three megakaryocytes (Meg). The number is increased; however, they appear morphologically normal. Note the erythropoiesis organised in regularly spaced erythrons (E) and the mestastasis (M) of the neuroendocrine tumour. Although minimal localisation of NET cells was found in the bone marrow, no signs of dysplasia were found. Additionally, with a normal haemoglobin value and leucocyte count we assumed localisation of the NET cells in the bone marrow could not explain the low platelet count, making the diagnosis of ITP more likely.
Fig S2. Petechiae on the left leg of patient 2.


