Abstract
Corona virus disease 2019 (COVID‐19) outbreak has attracted worldwide attention. The COVID‐19 outbreak is unique in its rapid transmission and results in heavy stress for the front‐line health care workers (HCWs). The current study aimed to exam posttraumatic stress symptoms (PTSSs) of HCWs fighting for the COVID‐19 and to evaluate their sleep quality after 1‐month stressful suffering. Three hundred seventy‐seven HCWs working in different provinces of China participated in the survey between February 1 and 5. The demographic information was collected first. Posttraumatic Stress Disorder Checklist for DSM‐5 (PCL‐5) and the Pittsburgh Sleep Quality Index (PSQI) were selected to measure PTSSs and sleep quality. Results showed that 1 month after the outbreak, the prevalence of PTSSs was 3.8% in HCWs. Female HCWs were more vulnerable to PTSSs with hazard ratio of 2.136 (95% CI = 1.388–3.286). HCWs with higher exposure level also significantly rated more hyperarousal symptoms (hazard ratio = 4.026, 95% CI = 1.233–13.140). There was a significant difference of sleep quality between participants with and without PTSSs (z value = 6.014, p < .001) and among different groups with various contact frequencies (chi‐square = 7.307, p = .026). Path analysis showed that there was a significant indirect effect from exposure level to PTSSs through sleep quality (coefficient = 1.750, 95% CI of Boostroop test = 0.543–2.998). In summary, targeted interventions on sleep contribute to the mental recovery during the outbreak of COVID‐19. Understanding the mental health response after a public health emergency might help HCWs and communities prepare for a population's response to disaster.
Keywords: COVID‐19, health care workers (HCWs), psychological guidance, PTSD, sleep quality
Abbreviations
- CI
confidence interval
- COVID‐19
corona virus disease 2019
- HCWs
health care workers
- PTSSs
posttraumatic stress symptoms
- SARSs
severe acute respiratory syndromes
- WHO
World Health Organization
1. INTRODUCTION
On February 11, 2020, this novel coronavirus was officially named as the corona virus disease 2019 (COVID‐19) by the World Health Organization (WHO). It is more likely to affect older males with comorbidities and can result in severe and even fatal respiratory diseases such as acute respiratory distress syndrome (Chen, Zhou, & Dong, 2020). About 1 month after the first confirmed case, the number of confirmed cases soared up to more than 20,000 in the mainland China. By April 14, 2020, the COVID‐19 has hit 211 countries and regions in the world, and confirmed cases reach to 1,883,183. Therefore, due to its rapid transmission, the COVID‐19 outbreak not only raises public health concerns but also causes tremendous psychological distress (Xiang et al., 2020), particularly among health care workers (HCWs) who are the very core of any public health response and form the crucible base, which must not melt in the heat of crisis.
It is alarmed that HCWs are vulnerable to the sustained psychological impacts of the epidemic (Kang, Li, Hu, Chen, & Yang, 2020). HCWs who are at the first line of battle under the influence of high fatality and intense media coverage will undoubtedly show the emotion of fear, anxiety, anger and frustration (Lu, Shu, Chang, & Lung, 2004;Maunder, Hunter, Vincent, Bennett, & Mazzulli, 2003). A survey of psychological impacts of 2003 SARS outbreak on HCWs in Taiwai showed that social functioning and immediate mental health were among the worst subscales after caring for patients with SARS (Chen et al., 2007). These results highlighted the emotional and occupational vulnerability of HCWs. Worsely, the outbreak of COVID‐19 was at the corner of the Spring festival, one of the biggest festivals in a year for Chinese people to enjoy the happiness of family reunion and relieve from a year's hardworking. However, COVID‐19 terminated the holiday, and HCWs have to work continually. Undoubtedly, at the harsh moment, HCWs will weather not only physical but also mental sufferings. According to previous studies about the Public Health Emergency of International Concerns (PHEIC), angers, fears and anxiety might be at peak of the outbreak (Robertson, Hershenfield, Grace, & Stewart, 2004), yet they would decrease along with the stability of the spreading of the virus (Wu, Chan, & Ma, 2005a). Nevertheless, most of survivors and aid personnel experiencing a stress reaction in the disaster had the poor performance on the mood, cognition, physical health and interpersonal relationships for several days or weeks (Grimm, Hulse, Preiss, & Schmidt, 2012; Kaya, 2001). As reported in recent investigations of COVID‐19 in China, most people were preoccupied with the thoughts of COVID‐19 and had paranoia about infecting virus, among who 18.2% reported sleep difficulties and enhanced the perception of mental health care need (Huang & Zhao, 2020). Worringly, a study of the immediate psychological impact of COVID‐19 on HCWs showed that 29.8%, 13.5% and 24.1% HCWs reported stress, depression and anxiety symptoms (Lua, Wang, Linc, & Li, 2020). Hereby, there are reasons to believe that HCWs will experience more troubles confronting with such tough situation, and it is urgent to focus on the mental health of HCWs during the COVID‐19 outbreak.
Posttraumatic stress disorder (PTSD) is a state of psychological unbalance following an exposure to traumatic events, with which people often re‐experience traumatic events, demonstrate avoidance behaviour and become irritable (Blake, Weathers, & Nagy, 1995). The reported occurrence rate of PTSD features for SARS survivors was in the middle of the range, which was investigated in previous samples of other medical diseases (Hawryluck, Gold, Robinson, Pogorski, & Styra, 2004; Wu et al., 2005a). Nevertheless, subsequent empirical researches consistently demonstrate that substantial rates of subclinical posttraumatic stress symptoms (PTSSs) were more discerning. PTSSs follow traumatic occurrences outside the range of common human experience such as violent physical assaults, torture, accidents, rape or natural disasters and are characterized by a typical symptom pattern of intrusions, persistence of trauma, relevant stimuli avoidance, emotional numbing and physiological hyperarousal (Deja, Denke, Weber‐Carstens, & Schröder, 2006). Zhang et al. (2006) investigated that the occurrences of PTSS in three groups of people (patients, HCWs and the public) related to SARS showing the incidence of PTSS, respectively, were 55.1%, 25.8% and 31.18% (Zhang et al., 2006). Also, those respondents who had been isolated, worked in high‐risk workplaces such as SARS wards or had friends or close relatives who contacted SARS were two to three times more likely to develop high levels of PTSS than those who were not exposed to the virus (Wu, Chan, & Ma, 2005b). An investigation in a regional general hospital reported that a total of 177 out of 661 participants (27%) had psychiatric symptoms (35% of physicians and 25% of nurses) and approximately 20% of the participants were diagnosed with PTSD after 2 months of the epidemic outbreak (Chan & Yiong, 2004). More notably, HCWs, after a long period of the horrified situation, are subjective to the early PTSS (Brondolo, Eftekharzadeh, Clifton, Schwartz, & Delahanty, 2017; Lazarus, 2014). At the first period of the epidemic, HCWs were exposed to high level of unfamiliarity and uncontrollability about the virus, which, literally documented, could in turn affect their likelihoods for PTSS and developing PTSD (Marshall, Bryant, Amsel, Suh, & Neria, 2007; Schlenger et al., 2002). However, a paucity of studies caught the detection of PTSS of HCWs at the fastigium of epidemic outbreak. These explorations could avail to the future retrospective studies. Therefore, we focussed on the PTSS in HCWs immediately after the outbreak of COVID‐19 and investigated the potential related factors to PTSD in the present study.
Importantly, evidence suggests that sleep is not simply a secondary symptom of PTSD but rather is a risk factor for worsening symptoms of the disorder (Schlenger et al., 2002; Wright et al., 2011). As PTSD endures for many years, factors such as expectations of disturbed sleep, conditioning to environmental stimuli and psychiatric and medical comorbidity likely confound the relationship, yet investigations during the early aftermath of trauma reduce it to some extent (Mellman, Pigeon, Nowell, & Nolan, 2007). To the best of our knowledge, sleep disorders and exhaustion tolled on the HCWs at the early stage of the SARS outbreak (approximately 1 month later). It was estimated that 10.67% HCWs had the problem to fall asleep, while 40.31% of them were extremely overwhelmed and even fainted (Yang, 2004). Being burn out and allotting all time to care the patients, HCWs are also under the condition of sleep deprivation and miss their opportunity for restorative break (Geiger‐Brown et al., 2012). Notably, research suggests that poor sleep quality is a risk factor for PTSD (Spoormaker & Montgomery, 2008). For example, insomnia 1 month after exposure to a traumatic event predicts the development of PTSD 6 weeks (Mellman, David, Kulick‐Bell, Hebding, & Nolan, 1995), 6 months (Harvey & Bryant, 1998) and 1 year after exposure (Koren, Arnon, Lavie, & Klein, 2002). A limited number of laboratory studies during periods closer to the time of trauma exposure have also generated findings that an index of REM sleep continuity, the average duration of uninterrupted segments of REM sleep (REM segment duration), was significantly reduced in the group who subsequently showed PTSD (Mellman, Bustamante, David, & Fins, 2002; Mellman, Bustamante, Fins, Pigeon, & Nolan, 2002). However, even though the association between sleep quality and PTSD is clearly established, the bulk of related researches only focusses on the epidemiology and disease process or organizational issues. Relatively little work has sought to investigate mechanisms in sleep and PTSS during the early aftermath of trauma. Moreover, previous studies have not directed the mechanism at first‐line HCWs under the pressure from the epidemic.
1.1. Current study
Hence, this cross‐sectional study was to document and describe the PTSS of the HCWs combating for the COVID‐19, to evaluate the staff's sleep quality after 1‐month stressful suffering and to explore mechanisms in sleep and PTSS during the early aftermath of trauma. The findings of this study will offer better psychological guidance to HCWs inside or outside China dealing with COVID‐19 or other similar diseases/disasters, as well as be important in planning for future outbreaks of emerging diseases.
2. METHODS
2.1. Participants
A cross‐sectional online survey was conducted using snowball sampling through e‐mail, WeChat, shares and online website to the contacts of the investigators in different provinces of China between February 1 and 5, which was the 1‐month time point of the comprehensive epidemic defensive work and the fastigium of the COVID‐19 outbreak. All participants voluntarily completed the scale. The participants were also explained that the survey results would be used exclusively for research purposes and that the information they would provide would not be leaked. The survey began with an informed consent procedure that included information on research purposes and confidentiality. The questionnaire can be completed anonymously. In this study, the inclusion criteria were as follows: 1—health care workers, 2—among 18–60 years old (the age range for incumbent HCWs), 3—understanding the question literally and 4—no diagnosed mental disorders like depression, anxiety disorder and PTSD. Finally, 377 HCW participants met all the criteria, and the average time for their responding was 257.7 ± 150 s. With six HCWs whose response time below 100 s excluded, thereby, the valid rate was 98.41%. Figure 1 shows each step of the enrolment. This project was approved by the Ethics Committee of the Navy Medical University.
FIGURE 1.
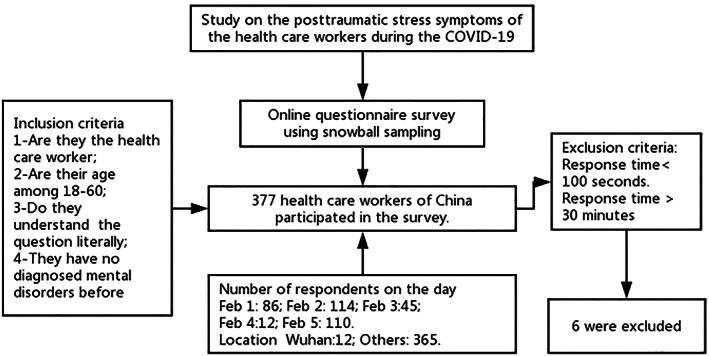
Sampling frame
3. MEASURES
3.1. Demographic characteristics
The demographic information was collected by asking for their personal information (current location, occupation, gender, education background, age and occupations), exposure history (whether they have contacted suspected or confirmed patients of COVID‐19 and their contact frequencies) and quarantined status (whether being quarantined in home or other safety places). According to the exposure history, we categorized the traumatic exposure into three levels: HCWs without contact with potentially infected people were thought to be experiencing low exposure level, having contacted the potential patients but not in a persistent exposure to virus rated as under middle level and HCWs working in Wuhan, the centre of the epidemic, were deemed to be highly frequently contacted with the virus and were at high‐exposure‐level risk.
3.2. The PTSD Checklist for DSM‐5
The PTSD Checklist for DSM‐5 (PCL‐5) was developed by an American PTSD research centre following the Diagnostic and Statistical Manual of Mental Disorders‐V (DSM‐5). According to Weathers (2013), exposure to an epidemic was a traumatic event. Thus, in present study, participants of epidemic‐hit cities were selected and were instructed to rate how much they were bothered by the outbreak of COVID‐19 in the last month upon answering PCL‐5. This scale includes 20 items for evaluating four clusters of PTSS, including intrusive symptoms (Criterion B: Questions 1–5), avoidance symptoms (Criterion C: Questions 6 and 7), negative alteration in cognition and mood (Criterion D: Questions 8–14) and hyperarousal symptoms (Criterion E: Questions 15–20). Respondents were asked to rate how bothered they have been by each of 20 items in the past month on a 5‐point Likert scale ranging from 0 to 4. Items are summed to provide a total severity score (range = 0–80). The total PCL, Criterion B, Criterion C, Criterion D and Criterion E had Cronbach's α coefficients of 0.906, 0.798, 0.791, 0.750 and 0.829, respectively, thereby indicating high internal consistency. Each item rated as 2 = moderately or higher was treated as a symptom endorsed. The PCL‐5 can determine that a provisional prediction according to summing all 20 items and using cut‐point score of 33 for PTSS appears to be a reasonable based upon current psychometric work, considering the goals of the assessment and the population being assessed and giving a probable differentiation (Blevins, Weathers, Davis, Witte, & Domino, 2015).
3.3. Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) is a self‐administrated questionnaire, which assesses sleep quality and disturbances over a 1‐month time interval (Buysse, Iii, Monk, Berman, & Kupfer, 1989). Chinese vision of PSQI was also proved to be valid (Liu & Tang, 1996). It is indicated that the use of ‘component’ scores in the PSQI is also empirical and clinical (Buysse, Sonia, Edinger, Lichstein, & Morin, 2006; Harvey, Kathleen, Whitaker, Damian, & Harvinder, 2008). Therefore, we selected four questions from PSQI in the present study to save time for HCWs, including subjective sleep quality by asking ‘how good is your sleep quality’, sleep disturbances by asking ‘do you have easy waking during sleep and early waking in the morning’, sleep latency by asking ‘do you have difficulty in starting sleep’ and sleep duration with asking ‘what's your actual sleep time recently’. Each question weighted equally on a 0–3 scale, respectively, presents different choices. In items for subjective sleep quality, ‘0–3’ was equal to ‘very good to very bad’; in terms of sleep disturbance, it was equal to ‘no more than 3 times a week’; in sleep latency, it was equal to ‘no more than 3 times a week’, and then, in sleep duration, it was equal to ‘more than 7 h to less than 5’. The scores of four questions were summed to yield a global PSQI score; higher scores indicate worse sleep quality. Cronbach's α coefficient of the global score was 0.793.
3.4. Statistical analysis
Statistical analysis was performed using SPSS 19.0, Release Version 19.0 (2010, Armonk, NY) for Windows. The differences of PTSS in characteristic of HCWs were compared using chi‐square tests for dichotomous variables, which were characterized using frequency statistics to examine differences in key variables as a function of sociodemographic characteristics (i.e., age and sex). Sleep quality summed by the four components of PSQI was coded as a continuous variable whose mean values and SDs were calculated. Nonparametric test was performed in comparing the group differences of sleep quality, which was not normally distributed, to describe its interactions with exposure level or the severity of PTSS. Furthermore, the total score of PCL‐5, which was normally distributed and represented the severity of PTSS, was evaluated by hierarchical multiple regression models to examine the relationships among these variables and potential predictors of PTSS. Furthermore, mediation analyses were conducted to determine the potential mediating effect on the relationship between PTSS and epidemic exposures based on the regression results from hierarchical multiple regression. Specifically, path analyses via a structural equation modelling framework and bootstrap estimation were performed with SPSS Amos, Release Version 20.0 (2010, Armonk, NY), and statistical tests were two sided with α = 0.05. We hypothesized that sleep quality could be a potential mediator. Hence, first, a model planned to exam bidirectional influences of PTSS and exposure level was regressed on their respective baseline levels as well as each other at baseline. Second, sleep quality at baseline was indicated by a global PSQI score and was predicted by baseline PTSS and exposure level. Given significant correlations between PTSS and gender, analyses were adjusted for gender; sleep quality then predicted change in exposure level and PTSS adjusting for their respective baseline levels.
4. RESULTS
4.1. Description of sample
The demographics information was showed in Table 1. A total of 371 HCWs were recruited in this survey, among which 143 (38.5%) were males and 228 (61.5%) were females. The average age was 35.30 ± 9.48, and the average level of education was the bachelor, indicating that the participants might be mainly from the middle‐age group with rich work experiences. Most of these participants (96.8%) were not distributed in Wuhan, where the epidemic situation was not as serious as that of Wu Han, and in the high alert and tight sense. About 18.1% of the HCWs were doctors, 71.2% were nurses, and 10.2% were other HCW jobs like the medical technicians. Two hundred forty five (66%) of our cohort were quarantined at home and safe at this moment (according to the national mandate, all people should strictly maintain the social distance at home and stop regular outsider activities), whereas 126 (34%) were still working at the first line and undertaking the risk of infection. Frequency of contact with COVID‐19 patients in the daily working environment represented the exposure level. Two hundred eighty eight (77.6%), 71 (19.1%) and 12 (3.2%) HCWs belong to low, middle and high exposure groups, respectively.
TABLE 1.
Demographic characteristic of participated health care workers (HCWs)
| Demographics | Samples | Percentage | |
|---|---|---|---|
| Gender | |||
| Male | 143 | 38.5 | |
| Female | 228 | 61.5 | |
| Education | |||
| Senior high school or below | 10 | 2.7 | |
| Academy or bachelor | 271 | 73.0 | |
| Master or above | 90 | 24.3 | |
| Ages | |||
| 20–30 years | 142 | 38.3 | |
| 31–40 years | 116 | 31.3 | |
| Above 40 years | 113 | 30.5 | |
| Occupation | |||
| Doctors | 67 | 18.1 | |
| Nurse | 264 | 71.2 | |
| Others | 40 | 10.2 | |
| Location | |||
| Wu Han | 12 | 3.2 | |
| Other cities | 359 | 96.8 | |
| Quarantined | |||
| Not quarantined | 126 | 34.0 | |
| Quarantined | 245 | 66.0 | |
| Exposure level | |||
| Low | 288 | 77.6 | |
| Middle | 71 | 19.1 | |
| High | 12 | 3.2 | |
4.2. Prevalence of PTSS in HCWs
The outcome data from the PCL‐5 scale were showed in Table 2. A 3.8% (14 in 371) HCWs' total score of PCL‐5 was over 33, which indicated the diagnosis of PTSS according to the criterion. The intrusive symptoms assessed by Criterion B were more common 1 month after the epidemic (with the positive rate of 44.5%) compared with other PTSS (12% HCWs met Criterion C, 16.4% met Criterion D, and 16.2 met Criterion E). Female HCWs were more vulnerable to PTSS and significantly showed more intrusive symptoms than males with hazard ratio of 2.136 (95% confidence interval = 1.388–3.286; p = .010). Moreover, HCWs with higher exposure level also significantly rated more hyperarousal symptoms of the Criterion E. HCWs working in Wuhan had an over fourfold greater risk of showing hyperarousal symptoms (hazard ratio = 4.026, 95% confidence interval = 1.233–13.140; p = .021). However, there was no significant difference of PTSS in the groups of different education background or occupations.
TABLE 2.
Prevalence of posttraumatic stress symptom (PTSS) in health care workers (HCWs)
| Criterion | B | C | D | E | Total | |
|---|---|---|---|---|---|---|
| Total | Positive rate (%) | 44.5 | 12.7 | 16.4 | 16.2 | 3.8 |
| Gender | ||||||
| Male (%) | 32.7 | 8.8 | 15.0 | 14.3 | 2.0 | |
| Female (%) | 50.9 | 14.8 | 17.0 | 17.0 | 4.8 | |
| Chi‐square | 12.092 ** | 2.899 | 0.262 | 0.478 | 1.885 | |
| Education | ||||||
| Senior high school or below (%) | 20.0 | 20.0 | 10.0 | 40.0 | 0.0 | |
| Academy or bachelor (%) | 44.7 | 10.9 | 15.6 | 14.5 | 4.0 | |
| Master or above (%) | 43.5 | 16.3 | 18.5 | 17.4 | 3.3 | |
| Chi‐square | 2.401 | 2.373 | 0.700 | 4.870 | 0.502 | |
| Ages | ||||||
| 20–30 years (%) | 46.3 | 12.2 | 19.0 | 20.4 | 3.4 | |
| 31–40 years (%) | 40.2 | 13.7 | 16.2 | 12.8 | 4.3 | |
| Above 40 years (%) | 44.2 | 11.5 | 12.4 | 13.3 | 3.5 | |
| Chi‐square | 0.996 | 0.259 | 2.089 | 3.644 | 0.152 | |
| Occupation | ||||||
| Doctor | 37.5 | 7.5 | 12.5 | 17.5 | 0 | |
| Nurse | 44.7 | 12.9 | 17.8 | 15.5 | 4.2 | |
| Other | 47.8 | 14.9 | 13.4 | 17.9 | 7.5 | |
| Chi‐square | 1.086 | 1.285 | 1.250 | 0.282 | 3.427 | |
| Exposure level | ||||||
| Low (%) | 43.0 | 11.0 | 16.2 | 13.4 | 2.7 | |
| Middle (%) | 47.3 | 16.2 | 14.9 | 21.6 | 6.8 | |
| High (%) | 41.7 | 25.0 | 25.0 | 41.7 | 8.3 | |
| Chi‐square | 0.474 | 3.257 | 0.783 | 9.121 ** | 3.390 | |
p value less than 0.01.
4.3. Sleep quality of HCWs
Sleep quality was assessed by four questions including sleep satisfaction, sleep disturbance, sleep latency and sleep duration. As shown in Table 3, 2.7% HCWs scored ‘very bad’ for sleep satisfaction; 11.3% reported frequent sleep disturbance; 6.7% complained having difficulty falling asleep; and 6.2% had less than 6‐h sleeping time. Hence, sleep disturbance was more common than other sleep problems in HCWs. HCWs with high exposure level significantly had more sleep disturbance and longer sleep latency, indicating the poorer sleep quality than those with less exposure. Notably, HCWs categorized by the total score of PCL‐5 into two groups showed significant between‐group differences in all the components of sleep quality. On the basis of the overall sleep quality, the sum of the four questions, there was a significant difference in the two groups—one without PTSS and the other with PTSS (z value = 6.014, p < .001)—and also among different groups with frequency of contact (chi‐square = 7.307, p = .026). As illustrated in Figure 2, HCWs with PTSS had poorer overall sleep quality. In the group with PTSS, there were no significant differences in sleep quality of HCWs with different exposure level (chi‐square = 0.391, p = .823). Nonetheless, in the group without PTSS, the higher exposure level seemed to relate to poorer sleep quality, yet the difference was neither significant (chi‐square = 5.519, p = .063). Hence, there was no significant interaction effect between exposure level and sleep quality on the potential diagnosis of PTSS.
TABLE 3.
Group differences on the four components of Pittsburgh Sleep Quality Index (PSQI)
| Sleep satisfaction | Sleep disturbance | Sleep latency | Sleep duration | ||
|---|---|---|---|---|---|
| Total | |||||
| Mean ± SD | 0.91 ± 0.782 | 0.61 ± 0.948 | 0.82 ± 1.068 | 1.03 ± 0.915 | |
| Percentage | |||||
| 0 | 31.5 | 55.5 | 64.7 | 33.4 | |
| 1 | 46.9 | 16.7 | 15.4 | 34.8 | |
| 2 | 18.9 | 16.4 | 13.2 | 25.6 | |
| 3 | 2.7 | 11.3 | 6.7 | 6.2 | |
| Exposure level | |||||
| Lower | 0.87 ± 0.748 | 0.54 ± 0.879 | 0.76 ± 1.047 | 1 ± 0.915 | |
| Middle | 1 ± 0.876 | 0.74 ± 1.061 | 0.93 ± 1.064 | 1.15 ± 0.902 | |
| High | 1.33 ± 0.888 | 1.42 ± 1.379 | 1.75 ± 1.215 | 1.25 ± 0.965 | |
| Chi‐square | 5.289 | 7.343 * | 11.375 ** | 2.567 | |
| PTSS | |||||
| Without PTSS | 0.85 ± 0.727 | 0.54 ± 0.877 | 0.75 ± 1.013 | 0.98 ± 0.879 | |
| With PTSS | 2.43 ± 0.646 | 2.43 ± 0.938 | 2.64 ± 0.842 | 2.5 ± 0.519 | |
| z value | 5.826 *** | 5.923 *** | 5.485 *** | 5.407 *** | |
Note. Each item of the four components of PSQI weighted equally on a 0–3 scale; higher scores indicate a worse degree.
Abbreviation: PTSS, posttraumatic stress symptom.
p value less than 0.1.
p value less than 0.01.
p value less than 0.001.
FIGURE 2.
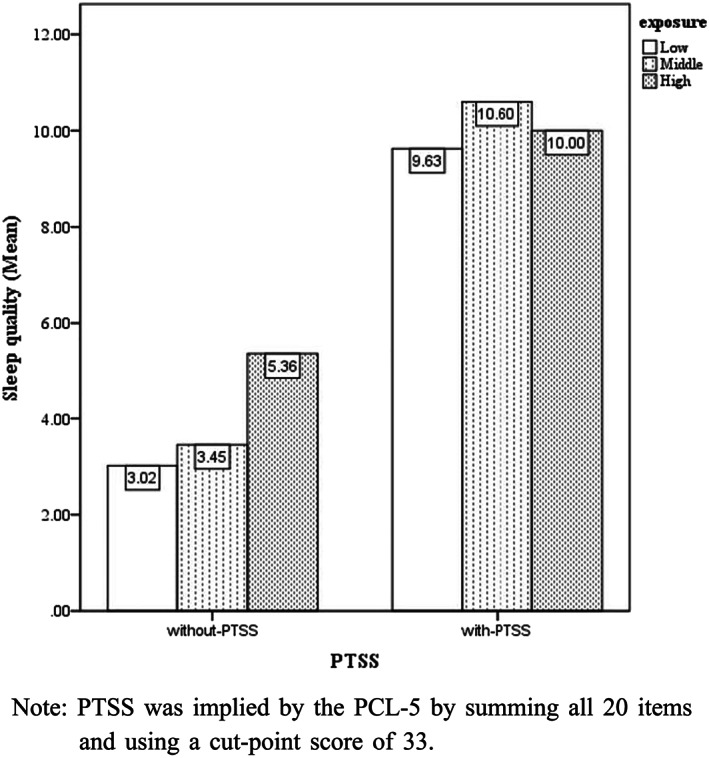
Group differences on the summed scores of Pittsburgh Sleep Quality Index (PSQI). Note: Posttraumatic stress symptom (PTSS) was implied by the Posttraumatic Stress Disorder Checklist for DSM‐5 (PCL‐5) by summing all 20 items and using a cut‐point score of 33
4.4. Association among exposure, sleep quality and PTSS
Correlation analysis results were demonstrated in Table 4, suggesting the significantly close interrelation in sleep quality components and clusters of PTSS (all the p values were less than .01). Exposure level was significant positive associated with sleep satisfaction (p = .018), sleep disturbance (p = .002) and sleep latency (p = .001). However, it was only significantly correlated to hyperarousal symptom clusters of PTSS (p = .003). On the basis of the above analysis, further exploration was conducted in explaining the role of different variables in the genesis of PTSS. As shown in Table 5, PTSS assessed by the total score of PCL‐5 was regressed on the demographic variables in Model 1, and only gender was a significant predictor indicating that female HCWs were reported more PTSS. In Model 2, exposure level was also a significant related factor to PTSS, and gender remained associated with PTSS. However, after the entering of sleep quality, only sleep quality turned significant related to PTSS. Hence, sleep quality was more strongly associated with PTSS, which was also proved by the results of mediation analysis shown in Figure 3. The total score of PCL‐5 was entered into the mediation model as a dependent variable, with sleep quality as a possible mediator, exposure level as a predictor and gender as a covariate. There were significant direct effects whereby higher exposure level was associated with worse sleep quality (coefficient = 1.03, p = .006) and sleep quality predicted PTSS (coefficient = 1.700, p < .001). There was a significant indirect effect from exposure level to PTSS through sleep quality (coefficient = 1.750, 95% CI of Boostroop test = 0.543–2.998). Coefficient of the total effect was 2.399 with p value at .0143. However, the direct effect from exposure level to PTSS was not significant, indicating that sleep quality completely mediated the relationship between exposure level and PTSS in this model (Figure 3). Moreover, it was not possible to infer causality from the aforementioned analysis given the concurrent measurement of sleep and PTSS. And we reran the model in the reverse direction to substantiate our conclusions from the mediation analysis. In this analysis, PTSS was entered as a predictor. This mediation model did not fit.
TABLE 4.
Correlations among exposure level, sleep quality components and posttraumatic stress symptom (PTSS)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Exposure | 1.000 | ||||||||
| 2. Sleep satisfaction | .123 * | 1.000 | |||||||
| 3. Sleep disturbance | .158 ** | .529 ** | 1.000 | ||||||
| 4. Sleep latency | .169 ** | .618 ** | .595 ** | 1.000 | |||||
| 5. Sleep duration | 0.080 | .527 ** | .357 ** | .358 ** | 1.000 | ||||
| 6. Criterion B | 0.030 | .313 ** | .229 ** | .198 ** | .157 ** | 1.000 | |||
| 7. Criterion C | 0.096 | .202 ** | .218 ** | .186 ** | .123* | .328 ** | 1.000 | ||
| 8. Criterion D | 0.020 | .275 ** | .197 ** | .216 ** | .145 ** | .232 ** | .334 ** | 1.000 | |
| 9. Criterion E | .154 ** | .361 ** | .375 ** | .399 ** | .170 ** | .285 ** | .339 ** | .477 ** | 1.000 |
p < 0.05.
p < 0.01.
TABLE 5.
Regression analysis with the total score of Posttraumatic Stress Disorder Checklist for DSM‐5 (PCL‐5) for health care workers (HCWs) as the outcome variable
| Model | B | SE | β | t | p value | R 2 | |
|---|---|---|---|---|---|---|---|
| 1 | Constant | 8.697 | 3.048 | 2.853 | .005 | 0.021 | |
| Gender | 2.752 | 1.017 | 0.140 | 2.707 | .007 | ||
| Age | −0.033 | 0.052 | −0.033 | −0.629 | .530 | ||
| Education | 0.727 | 1.048 | 0.036 | 0.694 | .488 | ||
| 2 | Constant | 8.387 | 3.030 | 2.768 | .006 | 0.037 | |
| Gender | 2.802 | 1.010 | 0.143 | 2.774 | .006 | ||
| Age | −0.046 | 0.052 | −0.045 | −0.881 | .379 | ||
| Education | 0.785 | 1.041 | 0.039 | 0.753 | .452 | ||
| Exposure | 2.409 | 0.974 | 0.128 | 2.473 | .014 | ||
| 3 | Constant | 3.035 | 2.631 | 1.154 | .249 | 0.298 | |
| Gender | 1.754 | 0.868 | 0.089 | 2.02 | .044 | ||
| Age | −0.028 | 0.045 | −0.028 | −0.636 | .525 | ||
| Education | 0.779 | 0.890 | 0.039 | 0.875 | .382 | ||
| Exposure | 0.686 | 0.846 | 0.036 | 0.811 | .418 | ||
| Sleep quality | 1.700 | 0.146 | 0.522 | 11.649 | .000 |
Note. Dependent variable was the total score of PCL‐5.
FIGURE 3.
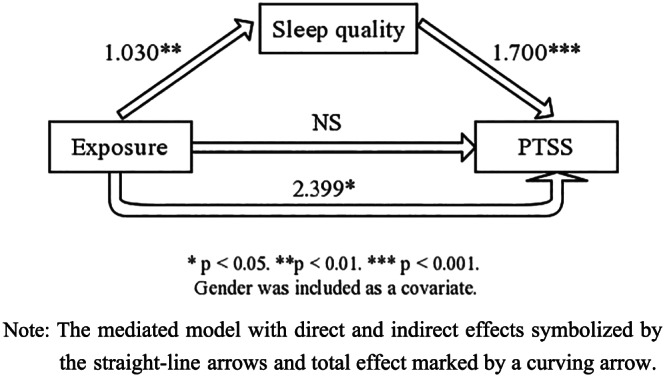
Sleep quality mediates the relationship between exposure and posttraumatic stress symptom (PTSS). Note: The mediated model with direct and indirect effects symbolized by the straight‐line arrows and total effect marked by a curving arrow
5. DISCUSSION
The COVID‐19 was declared to be a PHEIC by the WHO worldwide, as millions of people stay at home to minimize transmission of the virus. On the contrary, HCWs leave for clinics and hospitals, putting themselves at high risk of contracting COVID‐19, which is worthy respect but at the meantime also aggravates the mental risk. Therefore, the present study first investigated the prevalence of PTSS or PTSS 1 month after traumatic exposure and sleep quality of HCWs, aiming at understanding the mental health of HCWs on the backdrop of such a PHEIC and helping medical workers and communities with targeted psychological interventions.
The total prevalence of PTSS was 3.8% in the current 371 participants along with different prevalence in single cluster of PTSS including intrusive symptoms (44.5%), avoidance symptoms (12.7%), negative alteration in cognition and mood symptoms (16.4%) and hyperarousal symptoms (16.2%). HCWs experiencing high‐level exposure were more vulnerable to having PTSS (with the prevalence reaching 8.3%), who also significantly presented more hyperarousal symptoms. Referred to a study conducted in 2003 about the 3‐month follow‐up investigation of PTSS in 476 SARS survivors (with 131 responded at the both l and 3 months), 13 health workers (six at 1 month and seven at 3 months) had the diagnosis of PTSD, and HCWs showed higher scores at the subscale of hyperarousal symptoms than other survivors (Wu et al., 2005a). Although using a different measure assessing PTSS, our result was consistent with the study in 2003, suggesting that HCWs were vulnerable to have PTSS, especially the hyperarousal symptoms. Moreover, our recent published research reported the prevalence of PTSS after COVID epidemic for hardest‐hit areas in China reached 7% less than that of the HCWs in the same areas (with the prevalence reaching 8.3%) (Liu et al., 2020). As speculated, the prevalence of 3.8% in the overall HCWs might be lower than the previous studies, given the time point of our study and the backdrop against the early stage of COVID epidemic. Hence, the follow‐up studies are needed and should focus on deterring the development of PTSS. In fact, figures from China's National Health Commission show that more than 3,300 HCWs have been infected as of the early March and, by the end of February, at least 22 died according to local media. In Italy, 20% of responding HCWs were infected, and some have died. Reports from medical staff describe physical and mental exhaustion, the torment of difficult triage decisions and the pain of losing patient and colleagues, all in addition to the infection risk (Lancet, 2020), given that HCWs at higher risk of being infected were in the more nervous and alerted status than usual.
Meanwhile, HCWs might report more difficulty in falling asleep (longer sleep latent) and short sleep duration compared with the public in hardest‐hit areas (according to our previous study, 8.4% of the public were unable to feel asleep frequently, and 4.2% of them have less than 5‐h sleep time). Besides, combined with the results of sleep quality, more sleep disturbance and longer sleep latency in HCWs of high exposure responded to the hyperarousal symptoms, which is a consequence of having heightened anxiety and arousal responses and in the long term may result in difficulty falling or staying asleep or even a bad sleep pattern (Pace‐Schott, Germain, & Milad, 2015). To date, medical workers all over the world have been facing enormous pressure, including a high risk of infection and inadequate protection from contamination, overwork, frustration, discrimination, isolation, patients with negative emotions, a lack of contact with their families and exhaustion (Kang et al., 2020; Zhou et al., 2020). These distresses have proved to exert an obvious impact on their sleep quality and elicit a lasting effect on their mental health such as long‐term PTSD (Lancee, 2008; Liu, Zhou, & Shi, 2008). Therefore, protecting the mental health of these medical workers by reducing exposure risk would be important.
It is important to note that the sleep quality statistically mediated the association between exposure level and PTSS. Consistent with previous researches, poor sleep quality has been linked to both the onset and maintenance of PTSD (Babson & Feldner, 2010; Harvey, Jones, & Schmidt, 2003; Koren et al., 2002). Current theory suggests that the development of PTSD is in a dynamic process. That is, poor sleep quality may result in a context in which individuals are more prone to avoid trauma reminders. Accordingly, when reminders cannot be avoided, individuals with elevated levels of anxiety and alertness lead to the genesis of PTSS. However, to the best of our knowledge, less information is available about the role of sleep quality playing in the relationship between exposure experience and PTSS, especially in the public emergency involving a various and unavoidable exposure. Therefore, this study further explored the obtained preliminary results, showing that the mediated role of sleep quality played in the association between exposure level and PTSS. With gender as the covariate—considering gender was the only significant demographic predictor for PTSS in the regression, sleep quality was a complete mediator, which signalled that the poor sleep quality might be a major reason for different exposure level developing PTSS in HCWs on the backdrop of the epidemic. Notably, this kind of exposure, specified by the contact frequency of the confirmed or suspected patients, related to the genesis of the PTSS, mainly depended on its relation to sleep quality. This finding raises the possibility that HCWs with poor sleep quality, who were most frequently contacted with patients infected by COVID‐19, suffered from more PTSS. Moreover, such findings provided preliminary evidences that the association of higher exposure level with reduced sleep quality could comprise a significant predictor and effective intervention point for PTSS.
5.1. Limitations
Despite several important results of this study, there are some limitations that should be warranted. First is the small sample size of HCWs, especially those working at the first line. This is due to most HCWs in Wuhan, who have abided to the strict quarantine standard and devoted to the overwhelming works. It was difficult for them to participate in or finish the questionnaires. There was no definite number of the large population of HCWs from the frontline, but we assured that the actual statue of PTSS in HCWs could be more serious than our results as the limited number of HCWs in the hardest‐hit area sent their reports. Second, other potentially significant associated factors for PTSS were not fully discussed in the current study such as gender, married status, infection of family members/friends/colleagues, life stress, which could be intertwined with the exposure level on the effect of trauma on PTSS, and coping styles and personality, many of which have potential confounding effects on sleep quality and PTSS. Besides, considering the design was a cross‐sectional study, results gleaning from the mediating analysis on the role of sleep quality played in the traumatic experiences and PTSS should be interpreted cautiously. Third, the measures of stress symptoms and sleep quality used in this study may be vulnerable to inherent bias because of their online self‐reports. Attentionally, this research did not include a structured diagnostic interview and thus was not able to evaluate comparability of the PCL‐5 scores with those based on clinicians' ratings or evaluate diagnostic utility of the PCL‐5 against a clinical PTSD diagnosis. Moreover, referred to the researches, the cut‐off at 33 of PCL‐5 total score could imply for PTSS. Nevertheless, the cut‐off score was not reified and still required appropriate concerns about the populations and purposes for validity. A dynamic measure and obviation could be more objective and helpful for diagnosis of PTSD in the future researches.
5.2. Implications
These results of current studies have important implications. First, our findings demonstrated a significant psychosocial impact of COVID‐19 outbreak on HCWs combating at the front line, when knowledge of the diseases was limited and the number of confirmed and suspected patients were rapidly increasing. The diagnosis and prevalence of PTSS in our participants, especially those working in the epic centre of the epidemic, highlight the need for greater supports to HCWs during such crises with psychological interventions to address psychosocial distress and concerns. Second, low sleep quality resulted from stress and overload of work has crippling effect on HCWs including woeful psychological consequences like PTSS. Hence, as recommended, more shifts and breaktime should be guaranteed for HCWs by redeploying the hospital personal arrangements, despatching more health workers to the epic centre where the patients are gregarious and so on. Through the immediate intervention of the sleep quality, traumas with high exposure level could remain less psychological distress, and PTSD could be never developed into chronic. As seen in China, most general hospitals have established a shift system to allow front‐line medical workers to rest and to take turns in high‐pressured roles; online platforms with medical advice have been provided to share information on how to decrease the risk of transmission between the patients in medical setting; psychological invention teams have been set up; and assistance hotline teams composed of volunteers provided telephone guidance. Hundreds of medical workers are receiving these interventions, with good response, and their provision is expanding to more people and hospitals. As a whole, understanding the mental health response after a public health emergency might help HCWs and communities prepare for a population's response to disaster.
6. CONCLUSION
In sum, HCWs showed PTSS after 1 month of the COVID‐19 outbreak, and 3.8% of them could be at high risk of PTSS. Gender, exposure level (contact frequency of COVID‐19 patients) and sleep quality were all significantly associated with PTSS, with sleep quality playing an important role in the development of PTSS after epidemic. Importantly, the influencing factors identified in this study may be extremely effective in defining the high‐risk HCWs group in the similar influenza epidemics and offer hints on how HCWs can cope with future epidemics. HCWs deserve more attention and multifaceted psychological guidance, which are urgently needed after the COVID‐19 outbreak.
AUTHOR CONTRIBUTIONS
Q. Y. and Z. S. contributed to the writing of this article and part of statistical analysis. W. L. leaded the whole study, including putting forward this study and carrying out the study, and was the corresponding author. T. L. and X. N. contributed to revise this article and part of statistical analysis. X. D., Y. J. and Z. S. contributed to perform the investigation and collection of all data. Y. Z. helped to collect the data.
We are all accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interests.
DATA AVAILABILITY STATEMENT
Data obtained for the study will not be made available to others.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the HCWs who participated in the study and appreciate all the endeavour of the workers contributing to the fight for the COVID‐19. We also pay tribute to the cooperation of affiliated hospital of Navy Medical University and involved hospital.
Yin Q, Sun Z, Liu T, et al. Posttraumatic stress symptoms of health care workers during the corona virus disease 2019. Clin Psychol Psychother. 2020;27:384–395. 10.1002/cpp.2477
Qianlan Yin, Zhuoer Sun, Tuanjie Liu and Xiong Ni contributed equally to this work.
REFERENCES
- Babson, K. A. , & Feldner, M. T. (2010). Temporal relations between sleep problems and both traumatic event exposure and PTSD: A critical review of the empirical literature. Journal of Anxiety Disorders, 24(1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondolo, E. , Eftekharzadeh, P. , Clifton, C. , Schwartz, J. E. , & Delahanty, D. (2017). Work‐related trauma, alienation, and posttraumatic and depressive symptoms in medical examiner employees. Psychological Trauma, 10(6), 689. [DOI] [PubMed] [Google Scholar]
- Blake, D. D. , Weathers, F. W. , & Nagy, L. M. (1995). The development of a Clinician‐Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- Blevins, C. A. , Weathers, F. W. , Davis, M. T. , Witte, T. K. , & Domino, J. L. (2015). The Posttraumatic Stress Disorder Checklist for DSM‐5 (PCL‐5): Development and Initial Psychometric Evaluation. Journal of Traumatic Stress, 28, (6), 489–498. 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- Buysse, D. J. , Iii, C. F. R. , Monk, T. H. , Berman, S. R. , & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Buysse, D. J. , Sonia, A. I. , Edinger, J. D. , Lichstein, K. L. , & Morin, C. M. (2006). Recommendations for a standard research assessment of insomnia. Sleep, 29(9), 1155–1173. [DOI] [PubMed] [Google Scholar]
- Chan, A. O. M. , & Yiong, H. C. (2004). Psychological impact of the 2003 severe acute respiratory syndrome outbreak on health care workers in a medium size regional general hospital in Singapore. Occupational Medicine, 53(3), 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. H. , Wang, P. C. , Hsieh, M. J. , Huang, C. C. , Kao, K. C. , Chen, Y. H. , … Tsai, Y. H. (2007). Impact of severe acute respiratory syndrome care on the general health status of healthcare workers in Taiwan. Infection Control and Hospital Epidemiology, 28(1), 75–79. 10.1086/508824 [DOI] [PubMed] [Google Scholar]
- Chen, N. S. , Zhou, M. D. , & Dong, Q. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet, 395(10222), 391–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deja, M. , Denke, C. , Weber‐Carstens, S. , & Schröder, J. (2006). Social support during intensive care unit stay might improve mental impairment and consequently health‐related quality of life in survivors of severe acute respiratory distress syndrome. Critical Care, 10(5), R147. 10.1186/cc5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger‐Brown, J. , Rogers, V. E. , Trinkoff, A. M. , Kane, R. L. , Bausell, R. B. , … Scharf, S. M. (2012). Sleep, sleepiness, fatigue, and performance of 12‐hour‐shift nurses. Chronobiology International, 29(2), 211–219. 10.3109/07420528.2011.645752 [DOI] [PubMed] [Google Scholar]
- Grimm, A. , Hulse, L. , Preiss, M. , & Schmidt, S. (2012). Post‐ and peritraumatic stress in disaster survivors: An explorative study about the influence of individual and event characteristics across different types of disasters. European Journal of Psychotraumatology, 3(1), 7382. 10.3402/ejpt.v3i0.7382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, A. , & Bryant, R. (1998). The relationship between acute stress disorder and posttraumatic stress disorder: A prospective evaluation of motor vehicle accident survivors. Journal of Consulting and Clinical Psychology, 66, 507–512. 10.1037/0022-006X.66.3.507 [DOI] [PubMed] [Google Scholar]
- Harvey, A. G. , Jones, C. , & Schmidt, D. A. (2003). Sleep and posttraumatic stress disorder: A review. Clinical Psychology Review, 23(3), 377–407. 10.1016/S0272-7358(03)00032-1 [DOI] [PubMed] [Google Scholar]
- Harvey, A. G. , Kathleen, S. , Whitaker, K. L. , Damian, M. , & Harvinder, V. (2008). The subjective meaning of sleep quality: A comparison of individuals with and without insomnia. Sleep, 31(3), 383–393. 10.1093/sleep/31.3.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluck, L. , Gold, W. L. , Robinson, S. , Pogorski, S. , & Styra, R. (2004). SARS control and psychological effects of quarantine, Toronto, Canada. Emerging Infectious Diseases, 10(7), 1206–1212. 10.3201/eid1007.030703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , & Zhao, N. (2020). Generalized anxiety disorder, depressive symptoms and sleep quality during COVID‐19 epidemic in China: A web‐based cross‐sectional survey. MedRxiv. 10.1101/2020.02.19.20025395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, L. , Li, Y. , Hu, S. , Chen, M. , & Yang, B. (2020). The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry, 7(3), e14. 10.1016/S22150366(20)30047-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya, M. S. B. (2001). The onset and longitudinal course of a man‐made post‐traumatic morbidity: Survivors of the Sivas disaster. International Journal of Psychiatry in Clinical Practice, 5(3), 195–202. 10.1080/136515001317021662 [DOI] [PubMed] [Google Scholar]
- Koren, D. , Arnon, I. , Lavie, P. , & Klein, E. (2002). Sleep complaints as early predictors of posttraumatic stress disorder: A 1‐year prospective study of injured survivors of motor vehicle accidents. American Journal of Psychiatry, 159(5), 855–857. 10.1176/appi.ajp.159.5.855 [DOI] [PubMed] [Google Scholar]
- Lancee, W. J. M. R. (2008). Prevalence of psychiatric disorders among Toronto hospital workers one to two years after the SARS outbreak. Psychiatric Services, 1(59), 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus, A. (2014). Traumatized by practice: PTSD in physicians. The Journal of Medical Practice Management, 30(2), 131–134. [PubMed] [Google Scholar]
- Liu, X. C. , & Tang, M. Q. (1996). A study on the reliability and validity of Pittsburgh Sleep Quality Index. Chinese Journal of Psychiatry, 2(29), 103–107. [Google Scholar]
- Liu, H. , Zhou, J. , & Shi, Q. (2008). A survey of PTSD in health‐care workers one and a half years after the Wenchuan earthquake. Nerve Damage and Functional Reconstruction, 005(4), 277–281. 300 [Google Scholar]
- Liu, N. , Zhang, F. , Wei, C. , Wu, L. , Sun, Z. , Zhou, Y. , … Liu, W. (2020). Prevalence and predictors of PTSS during COVID‐19 outbreak in China hardest‐hit areas: Gender differences matter. Psychiatry, 287, 112921. 10.1016/j.psychres.2020.112921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. C. , Shu, B. C. , Chang, Y. Y. , & Lung, F. W. (2004). The mental health of hospital workers dealing with severe acute respiratory syndrome. Psychotherapy and Psychosomatics, 75(6), 370–375. [DOI] [PubMed] [Google Scholar]
- Lua, W. , Wang, H. , Linc, Y. , & Li, L. (2020). Psychological status of medical workforce during the COVID‐19 pandemic: A cross‐sectional study. Psychiatry Research, 288, 112936. 10.1016/j.psychres.2020.112936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, R. D. , Bryant, R. A. , Amsel, L. , Suh, E. J. , & Neria, Y. (2007). The psychology of ongoing threat—Relative risk appraisal, the September 11 attacks, and terrorism‐related fears. American Psychologist, 62(4), 304–316. 10.1037/0003-066X.62.4.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunder, R. , Hunter, J. , Vincent, L. , Bennett, J. , & Mazzulli, T. (2003). The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. Canadian Medical Association Journal, 168(10), 1245–1251. [PMC free article] [PubMed] [Google Scholar]
- Mellman, T. A. , David, D. , Kulick‐Bell, R. , Hebding, J. , & Nolan, B. (1995). Sleep disturbance and its relationship to psychiatric morbidity after Hurricane Andrew. American Journal of Psychiatry, 152(11), 1659–1663. 10.1176/ajp.152.11.1659 [DOI] [PubMed] [Google Scholar]
- Mellman, T. A. , Bustamante, V. , David, D. , & Fins, A. I. (2002). Hypnotic medication in the aftermath of trauma. Journal of Clinical Psychiatry, 63(12), 1183–1184. [DOI] [PubMed] [Google Scholar]
- Mellman, T. A. , Bustamante, V. , Fins, A. I. , Pigeon, W. R. , & Nolan, B. (2002). REM sleep and the early development of posttraumatic stress disorder. The American Journal of Psychiatry, 159(10), 1696–1701. [DOI] [PubMed] [Google Scholar]
- Mellman, T. A. , Pigeon, W. R. , Nowell, P. D. , & Nolan, B. (2007). Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. Journal of Traumatic Stress, 20(5), 893–901. 10.1002/jts.20246 [DOI] [PubMed] [Google Scholar]
- Pace‐Schott, E. F. , Germain, A. , & Milad, M. R. (2015). Sleep and REM sleep disturbance in the pathophysiology of PTSD: The role of extinction memory. Biology of Mood & Anxiety Disorders, 5(1), 3. 10.1186/s13587-015-0018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, E. , Hershenfield, K. , Grace, S. L. , & Stewart, D. E. (2004). The psychosocial effects of being quarantined following exposure to SARS: A qualitative study of Toronto health care workers. The Canadian Journal of Psychiatry, 49(6), 403–407. [DOI] [PubMed] [Google Scholar]
- Schlenger, W. E. , Caddell, J. M. , Ebert, L. , Jordan, B. K. , Rourke, K. M. , Wilson, D. , … Kulka, R. A. (2002). Psychological reactions to terrorist attacks: Findings from the National Study of Americans' Reactions to September 11. JAMA, 288(5), 581–588. 10.1001/jama.288.5.581 [DOI] [PubMed] [Google Scholar]
- Spoormaker, V. I. , & Montgomery, P. (2008). Disturbed sleep in post‐traumatic stress disorder: Secondary symptom or core feature? Sleep Medicine Reviews, 12(3), 184. [DOI] [PubMed] [Google Scholar]
- The Lancet . (2020). COVID‐19: Protecting health‐care workers April 9, 2020, from https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30644-9/fulltext [DOI] [PMC free article] [PubMed]
- Wright, K. , Britt, T. , Bliese, P. D. , Adler, A. B. , Picchioni, D. , & Moore, D. (2011). Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. Journal of Clinical Psychology, 67(12), 1240–1258. 10.1002/jclp.20845 [DOI] [PubMed] [Google Scholar]
- Wu, K. K. , Chan, S. K. , & Ma, T. M. (2005a). Posttraumatic stress after SARS. Emerging Infectious Diseases, 11(8), 1297–1300. 10.3201/eid1108.041083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, K. K. , Chan, S. K. , & Ma, T. M. (2005b). Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS). Journal of Traumatic Stress: Official Publication of the International Society for Traumatic Stress Studies, 18(1), 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Y. T. , Yang, Y. , Li, W. , Zhang, L. , Zhang, Q. , Cheung, T. , … Ng, C. H. (2020). Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry, 3(7), 228–229. 10.1016/S2215-0366(20)30046-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. J. , & X. L. Z. L . (2004). Mental status of medical working in atyoical pneumonia wards or outpatient department in Beijing. Health Education in China, 20(5), 29–30. [Google Scholar]
- Zhang, K. R. , Xu, Y. , Yang, H. , Liu, Z. G. , Chen, Z. Q. , … Wang, Y. Q. (2006). Investigation by comparison on the posttraumatic stress response among SARS patients, hospital staffs and the public exposed to SARS. Chinese Journal of Behavioral Medical Science, 15(4), 358–360. [Google Scholar]
- Zhou, Z. , Xu, S. , Wang, H. , Liu, Z. , Wu, J. , Li, G. , … Wang, W. (2020). COVID‐19 in Wuhan: Immediate psychological impact on 5062 health workers. MedRxiv. 10.1101/2020.02.20.20025338 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data obtained for the study will not be made available to others.


