Since the first cases in Wuhan, China, during the month of December 2019, coronavirus disease (COVID‐19) evolved into a worldwide emergency, becoming one of the deadliest pandemics of modern history. As we write, the outbreak has led to 3 595 662 confirmed cases and 247 652 deaths worldwide. 1 Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), the virus responsible for the disease, is a single‐stranded RNA virus which can cause a wide clinical spectrum of symptoms. According to the available knowledge, a large but still unknown percentage of the infected patients remain asymptomatic, representing an important and insidious vehicle of transmission. 2 According to the largest case series to date, most of the symptomatic patients complain of a mild respiratory syndrome (81%), while approximately 14% develop a severe disease requiring hospitalization and a further 5% need intensive care. 3
At the Humanitas Cancer Center, we have been operating at the epicenter of the European outbreak since the end of February, when the first Italian case was registered. Shortly after, our government imposed a general lockdown in order to limit the spread of the infection across the population.
Italy has paid the highest price in Europe in terms of deaths, currently ranking third in the somber list of countries for number of victims, after the United States and the United Kingdom.
As we write this article, 14 611 victims have been recorded in our region of Lombardy to date, the most affected Italian region accounting for approximately 50% of all Italian cases. 4
We work at one of the major Italian cancer centres, which is at the same time also an academic centre and a general hospital with its own emergency room. Our team is mainly focused on liver cancer patients, including hepatocellular carcinoma (HCC) and biliary tract cancer (BTC), treated according to clinical practice or within research protocols. During the past 2 years, we enrolled almost two hundred liver cancer patients in 18 clinical trials from phase 1 to phase 3. At the beginning of the Italian outbreak, on the 21st of February, we were following 11 active protocols, with dozens of scheduled accesses in the following weeks. The protocols involved HCC and BTC patients, receiving oral, subcutaneous and intravenous drugs, including chemotherapy, immune checkpoint inhibitors and targeted agents.
According to early data, 5 , 6 , 7 cancer patients appear to be at higher risk of mortality, especially those who underwent surgery or received a systemic treatment in the previous month. In particular, liver cancer patients constitute an even more vulnerable category. These patients are often older, have multiple comorbidities and in most cases suffer from an underlying liver disease, which is responsible for a baseline dysfunction of both innate and adaptive immune responses. 8 Cirrhosis has already been linked to higher mortality in A/H1/N1/09 influenza 9 and liver impairment has been described in SARS‐CoV cases. 10 The initial data regarding liver involvement during the course of COVID‐19 are not univocal, 11 but for the above‐mentioned reasons it is fair to expect an increased risk of death or severe events for liver cancer patients infected by SARS‐CoV‐2. As a result, the issue of treatment procrastination for patients with active liver cancer was of great concern to us. 12 At the same time, patients who were responding to the treatment could feel abandoned with effectively nowhere else to turn, thus increasing the risk of dropouts from effective therapies. 13
Coronavirus disease outbreak opened dismal prospects for cancer clinical trials. In many cases, especially for industry‐sponsored trials, the enrolment of new patients, across all phases and types of diseases, was suspended by the sponsors; unnecessary procedures (eg biopsies) were withdrawn and previously planned screenings were cancelled. 14 This indiscriminate discontinuation was inevitable in order to ensure the safety of the patients, to spare them unnecessary exposures to the healthcare environment, and to respect travel restrictions. Moreover, many resources had to be diverted to the management of the emergency, thus not guaranteeing the minimum standards required by a clinical trial. In addition, the ongoing pandemic can further represent a potential bias on research outcomes, altering the clinical course and biochemical and imaging assessments of enrolled patients. Considered together, these factors could easily have led to a dead end, not only for scientific research but also for hopes and expectations of the patients.
Was it possible to reinvent our daily practice, ensuring satisfactory care to patients while simultaneously respecting the social distancing measures and minimizing the risks of infection for such a frail population?
For our liver cancer clinical trials, we tried to set a countertrend. In full coordination with the sponsors, we were able to guarantee the activities of 10 of 11 trials, with the only exception of a phase 1 trial. Since the 21st of February, we handled multiple accesses to our facility for 25 patients within research protocols. We evaluated new patients for recruitment and succeeded in not having to suspend biopsy or molecular analyses. Even though we work at an Institute whose emergency room admits SARS‐Cov‐2‐positive subjects on a daily basis (more than 820 patients so far), we created a safe region for our patients by building a rigid separation between the designated areas for infected and suspected individuals and the rest of the hospital. According to the guidelines of the main scientific societies, 15 , 16 we implemented a telemedicine routine, consisting in a phone call the day before a scheduled appointment to assess the health status and the presence of any suspected symptoms (fever, cough, rhinorrhea, myalgias or anosmia). We minimized unnecessary accesses. We agreed with the sponsors on the possibility of performing routine assessments, such as blood tests and imaging exams, in the closest accredited facility. Moreover, we were able to provide home delivery for oral drugs following a phone assessment of clinical conditions and blood tests. For those patients for whom we could not avoid a hospital visit, we implemented a double check system (Figure 1). In addition to the checkpoints at the entrance of our hospital, where everyone undergoes a screening of body temperature and hand sanitization, as well as being provided with a face mask, we established an extra triage before access to our Day Care facility, in order to once again sanitize patient's hands and verify the correct use of their face mask. Entrance to anyone except for the patients was denied. For patients who underwent an experimental injective therapy, we made sure that the distance between treatment chairs and beds complied with the safety range of at least 1 meter and a thorough sanitization was performed at the end of the day. In cases where patients complained of respiratory symptoms during the visit which had not arisen in the previous day phone call or in the double checkpoint screening, we alerted the COVID‐surveillance team, created an immediate isolation and conducted a nasopharyngeal swab within the hospital's COVID track. With the aim of minimizing the risk for our administrative staff, we ensured remote working facilities for our statisticians and data managers. We maintained our weekly multidisciplinary liver tumour board, strictly reducing participation to one member for each specialty and imposing the social distancing measures in our meeting room. With the collaboration of our hepatologists, we established a “liver safe track”, a preferential agenda to safeguard the regular follow up of patients with underlying liver diseases and the continuity of antiviral therapies. These patients are often at risk of decompensation, especially if infected, so they require constant monitoring of their liver function. For a cirrhotic patient enrolled in a clinical trial, the stakes are even higher: a worsening liver function could not only mean a worse prognosis but also the need to interrupt the current experimental treatment. Most clinical trials require a Child‐Pugh score A as an inclusion criterion, so it is mandatory to provide these patients with an adequate hepatic follow‐up. Furthermore, these patients do not always have a valid therapeutic standard‐of‐care alternative, so for us it was of utmost importance to ensure the continuum of care for these patients even during the pandemic.
FIGURE 1.
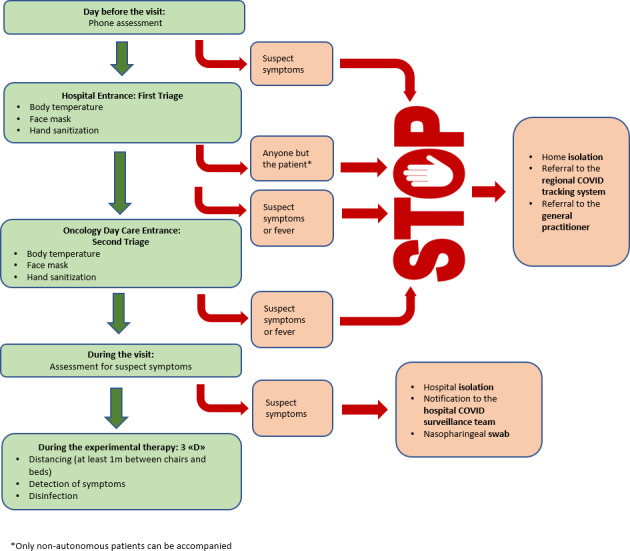
Double check system for Oncology Day Care admission
Moreover, we had to manage this complex situation while facing an additional challenge: half of our team independently contracted the virus, some of us were forced to stay home from 20 to more than 40 days, and our residents were recruited in COVID‐19‐positive wards. We had to further reorganize our work, training all the members on all the active research protocols while isolated members provided telematic support once they had clinically recovered. Thanks to these measures, we succeeded in providing all the scheduled treatments as planned, with zero cases of confirmed SARS‐CoV‐2 infections occurring in our liver cancer patients treated within clinical trials.
As the European Society of Medical Oncology's elected president Professor Solange Peters wrote to all the oncology community, 17 we must always be mindful of our additional responsibility of maintaining the pace of research, in particular for those patients who do not have alternatives and who are benefitting from experimental treatments. With the experience of our centre, we demonstrated the feasibility of conducting clinical trials during an emergency situation in a particularly frail population, ensuring safety to the patients without compromising our research quality standards.
CONFLICT OF INTEREST
NP reports receiving consulting fees from Amgen, ArQule, Merck Serono, Servier; lectures fees from AbbVie, Gilead, Lilly; and travel fees from Amgen, ArQule. AL has no conflict of interests relevant to this article; she has consulted for Takeda, Intercept Pharma; she has received lecture fees from AbbVie, Gilead, Intercept Pharma, Alfa‐Sigma and MSD. AS reports receiving advisory board fees from BMS (Bristol‐Myers‐Squibb), Servier, Gilead, Pfizer, Eisai, Bayer, MSD; consultancy fees from ArQule, Sanofi; and lecture fees from Takeda, BMS, Roche, AbbVie, Amgen, Celgene, Servier, Gilead, AstraZeneca, Pfizer, ArQule, Lilly, Sandoz, Eisai, Novartis, Bayer, MSD. LR reports receiving consulting fees from Amgen, ArQule, Basilea, Bayer, Celgene, Eisai, Exelixis, Hengrui, Incyte, Ipsen, Lilly, MSD, Roche, Sanofi; lectures fees from AbbVie, Amgen, Eisai, Gilead, Ipsen, Lilly, Roche, Sanofi; travel fees from Ipsen; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Incyte, Ipsen, Lilly and MSD. No other potential conflict of interests was reported.
AUTHORS' CONTRIBUTIONS
All of the authors performed the research, writing and review of all of the drafts of this paper and approved the final version.
Funding information
No financial support was provided for the writing of the manuscript.
REFERENCES
- 1. World Health Organization . Novel coronavirus (COVID‐19) situation. 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed May 6, 2020
- 2. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS‐CoV‐2). Science. 2020;368(6490):489‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 4. Istituto Superiore di Sanità . Epidemia COVID‐19. 2020. https://www.epicentro.iss.it/coronavirus/. Accessed May 6, 2020
- 5. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu J, Ouyang W, Chua MLK, Xie C. SARS‐CoV‐2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020. 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID‐19‐infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020. 10.1016/j.annonc.2020.03.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albillos A, Lario M, Álvarez‐Mon M. Cirrhosis‐associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61(6):1385‐1396. [DOI] [PubMed] [Google Scholar]
- 9. Marzano A, Marengo A, Ruggiero T, et al. Clinical impact of A/H1/N1/09 influenza in patients with cirrhosis: experience from a nosocomial cluster of infection. J Med Virol. 2013;85(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 10. Humar A, McGilvray I, Phillips MJ, Levy GA. Severe acute respiratory syndrome and the liver. Hepatology. 2004;39(2):291‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bangash M, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis MA. Between scylla and charybdis—oncologic decision making in the time of Covid‐19. N Engl J Med. 2020. 10.1056/NEJMp2006588 [DOI] [PubMed] [Google Scholar]
- 13. Rosenbaum L. The untold toll—The pandemic's effects on patients without Covid‐19. N Engl J Med. 2020. 10.1056/NEJMms2009984 [DOI] [PubMed] [Google Scholar]
- 14. EMA . Guidance on the management of clinical trials during the COVID‐19 (Coronavirus) pandemic, v2. 2020. https://ec.europa.eu/health/documents/eudralex/vol‐10_en. Accessed April 26, 2020.
- 15. Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Rep. 2020;2(3):100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer T, Chan S, Park JW. ILCA guidance for management of HCC during COVID‐19 pandemic. 2020. https://ilca‐online.org/management‐of‐hcc‐during‐covid‐19‐ilca‐guidance/. Accessed April 26, 2020.
- 17. Peters S. A word from your president. 2020. http://hubspot.esmo.org/maintaining‐the‐pace‐of‐research. Accessed April 26, 2020.


