Short abstract
Linked articles: COVID‐19 SPECIAL FORUM. J Eur Acad Dermatol Venereol 2020; 34: e346–e380.
On 31 December 2019 from the Chinese city of Wuhan, Hubei, comes the first case of ‘atypical ARDS/interstitial pneumonia’. On 11 February 2020, the WHO officially announced the name of the emergent disease associated with new coronavirus identified as SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2): COVID‐19 (Coronavirus Disease 2019). 1 On 13 February, 72 000 cases have been officially recorded in China, among which 15 000 were registered in Hubei province only, with 242 deaths in a single day. After that, Chinese republic has ordered drastic containment measures for fighting SARS‐CoV‐2 virus spreading. 1 On 11 March 2020, the World Health Organization (WHO) stated that the international outbreak of SARS‐CoV‐2 new coronavirus infection had to be considered a pandemic. 2 To date, 14 April 2020, 213 countries are affected worldwide with 1 812 734 confirmed cases and 113 675 deaths worldwide since the epidemic outbreak. 3
In this scenario, probably the most important strategic goal for dermatologists is to assess what their active involvement in this global pandemic might be. For this reason, our goal is to provide the ‘state of the art’ on this topic. Our personal experience, and a full extensive review of literature, led us to focus our attention on several topics in the management of dermatological patients during the SARS‐CoV‐2 pandemic, and four main topics have been identified. They are: (ⅰ) cutaneous manifestations related to COVID‐19, (ⅰⅰ) the implications of SARS‐CoV‐2 infection for use of biological drugs and other immunosuppressive and immunomodulatory therapies in dermatological patients, (ⅰⅰⅰ) occupational skin diseases related to hygienic washing and use of personal protective equipment (PPE) to avoid SARS‐CoV‐2 infection among patients and clinicians, and (ⅳ) strategic implications in planning dermatological clinical assistance to population during SARS‐CoV‐2 pandemic.
Skin manifestations due to SARS‐CoV‐2 infection are different and often underestimated, since the general conditions of patients are critical, as in several cases of hospitalized COVID‐19. However, the most common symptoms of COVID‐19 disease are reported in Table 1.
Table 1.
Clinical features of COVID‐19 disease in a selected cohort of 1099 Chinese patients with laboratory‐confirmed disease during the first 2 months of the pandemic 4
| Symptoms | N (%) |
|---|---|
| Fever | 473 (43.8) |
| Conjunctival congestion | 9 (0.8) |
| Nasal congestion | 53 (4.8) |
| Headache | 150 (13.6) |
| Cough | 745 (67.8) |
| Sore throat | 153 (13.9) |
| Sputum production | 370 (33.7) |
| Fatigue | 419 (38.1) |
| Haemoptysis | 10 (0.9) |
| Shortness of breath | 205 (18.7) |
| Nausea or vomiting | 55 (5.0) |
| Diarrhoea | 42 (3.8) |
| Myalgia or arthralgia | 164 (14.9) |
| Chills | 126 (11.5) |
Validated studies encompassing a sufficiently large number of patients to fully describe the most frequent ongoing skin manifestations of COVID‐19 are not yet available. However, many clinical reports have been published through the direct observation of infected patients, thus literature report several skin manifestations related to COVID‐19 disease, whose real pathogenic link with SARS‐CoV‐2 has yet to be proved.
Joob et al. 5 described a single case of skin petechial rash associated with decrease in platelet count in a patient SARS‐CoV‐2 positive, initially diagnosed as Dengue disease, which is an infection commonly reported in Thailand.
The skin manifestation most frequently reported by several authors is urticarial rash (Fig. 1).
Figure 1.
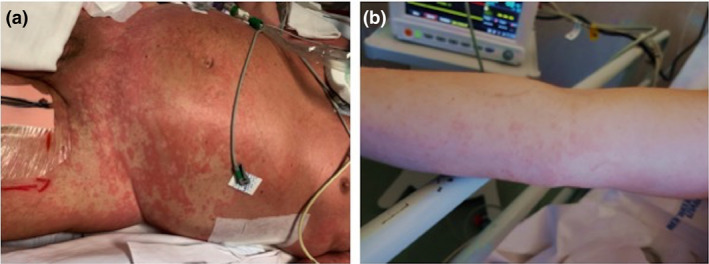
Urticarial rash in (a) patient with severe respiratory distress syndrome related to COVID‐19; (b) patient on the way to recovery after bilateral interstitial pneumonia from COVID‐19.
Lu et al., describing a familiar cluster of three COVID‐19 asymptomatic patients, stated that that the only skin evident clinical feature was an urticarial rash in one patient with no previous or ongoing clinical history of food allergy, drug allergy or chronic urticarial. 6 Recalcati, in Italy, studying cutaneous manifestations in 88 patients with COVID‐19, described three patients with widespread urticaria. 7 In addition to urticaria, other dermatological manifestations in patients suffering from COVID‐19 reported by Recalcati are erythematous rash (14 patients) and chickenpox‐like vesicles (1 patient). 7 Finally, Zhang analysing 140 patients with COVID‐19 stated that urticaria were self‐reported by 1.4% of patients. 8 Urticarial skin manifestations did not appear to be correlated with severity of COVID‐19 disease, and it resembled to many other urticaria rashes, commonly reported both during and after other well‐known viral infections. 7
Other frequently described skin manifestations are related to acro‐ischaemia. With a retrospective study of 7 critical COVID‐19 pneumonia patients, Zhang et al. reported acro‐ischaemia findings related to a hypercoagulation status. Patients showed acro‐ischaemia presentations including finger/toe cyanosis (Fig. 2), skin bullae and dry gangrene. D‐dimer, fibrinogen and fibrinogen degradation product (FDP) were significantly increased in most of the patients. Within the reported case series, 4 patients were diagnosed with disseminated intravascular coagulation (DIC), and 5 out from 7 patients finally died. 9 For these reasons, authors concluded that acro‐ischaemia associated with hypercoagulation status could have a negative prognostic significance in COVID‐19 evolution. Although it represents a non‐specific clinical sign of disease, it could be interpreted as a telltale sign of COVID‐19 progression towards fatal outcome. The median time from the appearance of acro‐ischaemia to death in Zhang’s study was 12 days. 9
Figure 2.
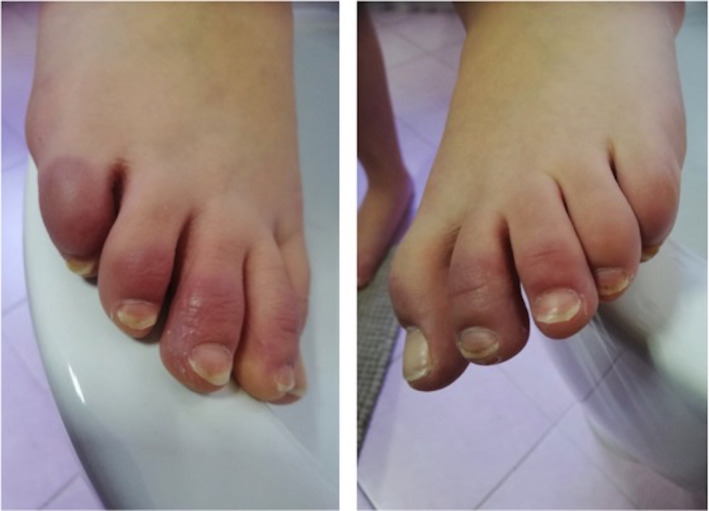
Acro‐ischaemia presentation as finger toe cyanosis in a SARS‐CoV‐2 positive adolescent patient.
Again, Manalo et al. described a transient livedo reticularis as possible skin related manifestation of COVID‐19. 10 Despite poor data from literature, other dermatological manifestations identified in the course of SARS‐CoV‐2 infection come from social networks, which are teeming with clinical pictures of skin involvement, since dermatologists coming from different countries share in real time their clinical cases during COVID‐19 pandemic, in order to fill their diagnostic doubts.
Therefore, analysis of all sources shows that even if skin is not the main target of SARS‐CoV‐2, dermatological manifestations can appear during the ongoing viral infection.
SARS‐CoV‐2 implication in the inflammatory process leading to occlusion of small skin vessels could be traced into skin vasculitis, which could also be responsible for several other symptoms such as rashes, exanthemas, frostbite‐like painful chilblains, urticarias, livedo, petechias or itching.
Moreover, SARS‐CoV‐2 is able to reduce the haemoglobin oxygen saturation, thus causing hypoxia that reflects in a skin pallor or a bluish appearance of the extremities, for example fingertips, toes lips and tongue.
Several contingent criticisms can be signalled in the attempt to estimate skin involvement in COVID‐19. Skin manifestations due to SARS‐CoV‐2 infection are often underestimated; since general conditions in most cases of hospitalized COVID‐19 are critical, and dermatologic evaluation, skin biopsies or photographic documentation of skin lesions are lacking in the most part of the patients.
These documenting limits make difficult to directly attribute the described skin manifestations to SARS‐CoV‐2 infection. Further extensive dermatological evaluations should be recommended both on symptomatic and asymptomatic patients, in order to elucidate the still open questions: Which is the pathogenetic link underlying skin involvement during SARS‐CoV‐2 infection? Could skin involvement be disease phase related (e.g. more prominent in the early rather than in the late stage of disease)? Could it have a prognostic value on COVID‐19 clinical course?
As regards biological drugs and other immunosuppressive and immunomodulatory therapies (IMT), pandemic of SARS‐CoV‐2 is making dermatologists wonder whether their immunosuppressed patients are at higher risk of being infected and/or developing a more severe disease and whether they need to discontinue the treatment preemptively. There are not precise guidelines and we still do not know if these drugs could make the patient more susceptible to SARS‐CoV‐2 infection, and/or aggravate the clinical course of COVID‐19.
A small Italian survey carried out in Lombardy during COVID‐19 outbreak in rheumatologic patients, showed that infected patients with chronic arthritis treated with biologics or DMARDs do not seem to be at increased risk of respiratory or life‐threatening complications from SARS‐CoV‐2 compared with the general population. 11 Beyond the immunomodulation, these drugs exert many other pharmacodynamics effects. For example, cyclosporine A (CsA) is able to block the replication of diverse coronaviruses by affecting the function of many members of the cyclophilin family. 12 Moreover, we have no specific clinical data on COVID‐19 evolution in patients in treatment with biologics for dermatological diseases. The only way to rationally deal with the topic is to try to extrapolate data of susceptibility, by considering the risk of respiratory tract infections for psoriasis patients in treatment with IMT, and knowing that upper respiratory tract infections represent the most common adverse events leading to biological therapy withdrawal in psoriasis. 13
Interleukin (IL)‐23 blockers and secukinumab (anti‐IL 17A monoclonal antibody – mAb) showed slightly increase of upper respiratory infection in some trials. The others IL‐17 inhibitors, ixekizumab and brodalumab, mainly seem to predispose to candida infections but not to respiratory infections. 14 Even under TNF inhibitors (TNFi) therapy, which carries the major warnings among biologics (it showed to increase the overall risk of infections, especially upper respiratory infections, by up to 7% compared to placebo during clinical trials), serious and life‐threatening infections (e.g. pneumonia, sepsis, osteomyelitis and invasive fungal) are uncommon. These severe infections are even more uncommon with etanercept (that is not a mAb but a soluble form of TNF alpha receptor 2), 15 which administration in a previous animal model study on coronavirus infection did not worsen the disease course nor ameliorate it. 16 , 17 Moreover, there are evidences that in some patients COVID‐19 morbidity could be attributed to a ‘cytokine storm’ rather than a viral direct cytopathic effect. 18 This is a massive release of pro‐inflammatory cytokines as TNF alpha, IL‐6, interferon (IFN)‐alpha, IFN‐gamma and other mediators contributing to the development of the acute respiratory distress syndrome (ARDS). In fact, many trials are experimenting mAb as tocilizumab (anti‐IL‐6) and adalimumab (TNFi) or other molecules with anti‐inflammatory properties (e.g. hydroxychloroquine) in the attempt to limit this exaggerated immune response in critical ill patients. 19 , 20
Owing to insufficient evidences to determine whether dermatological patients on IMT are at increased risk of developing COVID‐19 infection or more likely to have severe disease, or paradoxically potentially protected from the evolution towards severe interstitial pneumonia, we think that dermatologists need to assess the benefit‐to‐risk ratio on a case‐by‐case basis. Some patient factors may indicate a higher risk of severe COVID‐19 evolution, like age over 60, uncontrolled or multiple chronic comorbidities (including but not limited to cardiovascular or chronic pulmonary disease), chronic kidney disease, diabetes, hypertension and some malignancies, high doses or multiple immunomodulators, and history of severe or recurrent respiratory tract infections. Dose reductions or drug temporary cessation might be considered in psoriasis patients at ‘high risk’ for severe COVID‐19 evolution, especially if satisfying control of psoriasis has been already achieved. An integrative therapeutic approach, based on the association with topical treatments, phototherapy and/or drugs with a lower impact on the immune system (e.g. retinoids), could be taken into consideration for this subset of patients. For the majority of the patients, who are at ‘COVID‐19 low‐risk’ and in most children, we think, in accordance with SIDeMaST guidelines, 21 that IMT could be continued, recommending the patients to strictly follow opportune hygienic measures (hand washing hygiene, PPE adoption and social distancing). A close monitoring is necessary: patients should promptly report suspicious symptoms, also by encouraging a strict follow‐up via telemedicine. 22 Moreover, in agreement with the Australia/New Zealand consensus statement on immunomodulatory and biologic agents use during COVID‐19 pandemic, 23 we assert that in patients with suspected or confirmed COVID‐19 disease, all immunomodulators used for skin diseases should be immediately withheld, with the possible exception of systemic corticosteroid therapy, which needs to be weaned. Doses of predniso(lo)ne >20 mg/day are considered immunosuppressive, but sudden stopping, or significant reduction of dose, in patients on long‐term systemic corticosteroids, is unwise, particularly if they have suddenly become physiologically stressed. Note that systemic corticosteroids are part of many ARDS protocols, but are not currently recommended for SARS‐CoV‐2 infection, as there is weak evidence of harm when used in Influenza‐associated ARDS. 24 Therefore, long‐term use of high dosages during the pandemic should be avoided and, if reduction of corticosteroid therapy is indicated to mitigate infection risk during the pandemic, a graduated reduction is advised, aiming for a dose of ≤10 mg of predniso(lo)ne or equivalent. 22 We also recommend to temporary stop IMT in case of confirmed exposure to COVID‐19, as it had been suggested for other previous pandemics. 25 Otherwise, in patients who develop symptoms or signs of upper respiratory tract infection, but COVID‐19 is not yet confirmed, consider dose reduction or temporarily cessation for 1–2 weeks. As reported by Price et al., non‐biologic medications including small molecule inhibitors and immunosuppressants are typically easier to stop and re‐start within days to weeks due to shorter half‐life. 26 , 27 On the contrary, biologics generally have a longer half‐life and include a risk of anti‐drug antibody formation with treatment cessation and subsequent continuation. However, biologics also tend to be more targeted and less involved in the previously mentioned components of the viral immune response. Therefore, in general, biologics can be continued if viral symptoms are mild, but consider stopping if viral symptoms worsen or high fever develops. For what concerns conventional immunosuppressants and rituximab, consider prompt interruption whether viral symptoms present especially with known or potential exposure. On the other hand, it seems that hydroxychloroquine, mostly used by dermatologists in cutaneous lupus treatment, can be continued, as its in vitro activity against COVID‐19 was reported and several in vivo clinical trials are underway. 28 , 29
For the patients who are being considered for the initiation of IMT, we recommend to weigh the risk versus benefits on a case‐by‐case basis, pondering the severity of the disease, patient’s age and comorbidities that may put at higher risk for serious illness from COVID‐19. 30 If starting an IMT is appropriate, we suggest to consider drugs with the shortest half‐life (e.g. etanercept if a TNFi is indicated) and viral screening for SARS‐CoV‐2 before initiation of IMT, even if patients are asymptomatic or do not have history of COVID‐19 exposure. 31
Concerning atopic dermatitis, we agree with the European Task Force on Atopic Dermatitis (ETFAD) statement on SARS‐CoV‐2 infection and atopic dermatitis (AD) 32 to continue all immune‐modulating treatments, including immunosuppressive therapy, since exacerbations of underlying diseases can have a large negative impact on patients’ immunity. It is also recommended to carefully observe hygienic procedures using hand wash and topical disinfectants, favouring the use of non‐irritant soap substitutes, and applying moisturizers afterwards.
As regards occupational skin diseases related to hygienic washing and use of personal protective equipment (PPE) to avoid SARS‐CoV‐2 infection, the adequate protection of health personnel plays a primary role in order to guarantee safety in the workplace and ensure continuity of care. Adequate respiratory hygiene, careful hand washing and the use of PPE such as gloves, face masks and glasses are the main precautionary measures. However, their use for a long time can lead to the onset of various skin reactions of dermatological interest, such as mechanic irritation, acute or chronic allergic contact dermatitis, worsening of previous skin diseases and increased risk of secondary infection. 33
Major skin problems PPE‐related can be mainly linked to humid skin environment induced by prolonged use of PPE, repeated friction, fissurations of the skin barrier and late secondary skin reactions, which can worsening any skin diseases already present. The most affected areas were the nasal bridge (83.1%), hands (74.5%), cheeks (78.5%) and forehead (57.2%). Commonly related symptoms were xerosis, itching, burning and pain, and frequently observed skin lesions ranged from desquamation and erythema, to maceration with fissures, until rare cases of ulcerations and vesicles. 34 The lesions most frequently reported on the face by the Health Care Workers (HCWs) were pressure damage, delayed urticaria, contact dermatitis and skin xerosis. In addition, the worsening of existing diseases such as acne, seborrhoeic dermatitis, rosacea and folliculitis, favoured by rupture of blackheads and associated with a humid environment with anomalies of the microcirculation by prolonged pressure, should be noted. Both the correct use of PPE and its proper positioning on the face are crucial to ensure their effective use. Moreover, the correct use of PPE is mandatory in order to avoid a well‐known vicious circle: incorrect use followed by skin complication may lead to skin damage which cause in turn wrong positioning of PPE, trying to shift points of pressure and abrasion. 35
Hand washing is a basic important hygienic measure to reduce the risk of spreading viral disease. 36 Several studies 33 , 34 , 37 , 38 report that excessively frequent hand washing can alter the superficial hydro‐lipid layer of the skin and favour the development of irritative phenomena up to full‐blown dermatitis. The clinical features most frequently recorded for excessive hand washing are dryness/tightness (70.3%), tenderness 299 (56.8%), itching (52%) and burning/pain (38%), and about 3/4 of the HCWs reported hand desquamation, erythema, maceration, fissure and more rarely erosion/ulcers, vesicles and wheal. 34 For this reason, various recommendations are proposed to limit skin damage, such as the frequent use of a moisturizing and barrier cream, especially after hand washing, the use of detergents with emollients in hygiene outside work, avoid using excessively hot water. 33 Although the application of barrier creams is among the most effective measures only 22% of the HCWs use them. 34
As regards strategic implications in planning dermatological clinical assistance to population during SARS‐CoV‐2 pandemic, the spread of COVID‐19 worldwide has overburden the health systems of the whole world, obliging urgent reorganizations of the routine and planning of outpatient activities. Dermatology has found itself fully engaged in this process of reviewing activities, since it is mainly an outpatient discipline. All the dermatology services in the world have had to adapt and transform quickly, with the aim of solving two apparently antithetical aspects: to remain close to the needs of the patients, and to prevent the diffusion of SARS‐CoV‐2 infection among patients who normally crowd the waiting rooms of dermatological clinics.
As widely demonstrated, dermatological visits can be a source of contagion for various infectious diseases, including COVID‐19, both for the close proximity to which patients and operators are exposed, and the turnout in waiting rooms, reason why, as described by Kwatra et al., rapid and drastic measures should be taken to minimize risk of contagion. 39 Some of these measures include the booking suspension of non‐urgent visits, rescheduling of previous booked and of further clinical visits in telemedicine modality, the systematic adoption of PPE both for clinicians, and patients and interpersonal distancing. Furthermore, HCWs with risk factors (such as age >60 years, pregnancy and immunosuppression), as well as trainees and students, should not have direct contact with patients, in order to preserve the pool of workers. 39
A valid model was offered by the Wuhan group of Tao et al., who decided to let the access only to those patients who had underwent a first triage for COVID‐19 at the hospital entrance and, if negative, a second evaluation before any dermatological visit. Suspected cases, even if only by one of the two controls, were then referred to the fever clinic, in order to exclude the infection in progress. Following this preliminary path, only patients deemed not at risk had received authorization to access dermatological clinic. 40
Another management model has been provided by Radi et al. 41 in relation to a medium‐sized dermatological centre in Italy, the first European country most affected by COVID‐19. In order to avoid the gathering of patients, the deferred visits had been rescheduled, allowing the temporary suspension of activities in the period over high risk of transmissibility. Only urgent dermatological cases were regularly provided, such as severe acute skin diseases and surgery of skin neoplasms, not otherwise treatable. In‐hospital consultations were regularly realized. All patients had to undergo a preliminary triage to access dermatological services. To reduce the risk of infection among HCWs, shifts have been reorganized, reducing the number of operating staff to the minimum necessary and ensuring ample shifting. In addition, all HCWs were recommended to strictly follow individual protection protocols through the correct use of PPEs.
Telemedicine represents an excellent aid for the disposal of waiting lists, already widely exploited during the past epidemics of SARS‐CoV, MERS‐CoV, Ebola and Zika. 42 This healthcare procedure can guarantee continuity of care, meanwhile reducing the risk of COVID‐19 transmission, ensuring at the same time an adequate and safe triage.
Various authors suggest some tips to optimize the use of telemedicine, such as exploiting widespread and free telecommunication systems (WhatsApp, Skype or Zoom) which, however, cannot guarantee respect for privacy. Other paid information technology (IT) solutions integrated with the healthcare system are available, although their diffusion is limited, according to the technology level of specific country. 43
In the COVID‐19 pandemic scenario, it seems mostly important to guarantee telemedicine access, primarily for urgent and high‐risk patients of COVID‐19, identifying specific pathways for their taking charge, as well as postponing as much as possible the deferred visits, so as not to overload the network. 44
From the first experiences since 2015, the Ohannessian et al. 45 group proposed a new framework model potentially applicable on a large scale. In the case of asymptomatic patients for COVID‐19, the use of telemedicine should be systematic for routine care. If suspected asymptomatic patients or having close contact with confirmed cases, telephone or online triage could be used: in case of severe clinical symptoms, patient should be sent to the emergency medical centre. In case of stable clinical conditions, in the absence of worsening risk factors, patients should be referred first to the teleconsultation with General Practitioner and, if necessary, with the specialist.
The limits of this method could be related to inadequacy of Internet connection and the lack of structural integration with the various health systems. For example, the reimbursability of these services has only recently been provided in France and Italy. 45
As described by Jakhar et al. 46 in the field of dermatology, teledermatology, which is the telematic comparison between inter‐ or intradepartmental specialists, can be a valuable support to avoid overcrowding by clinic operators.
In conclusion, during the COVID‐19 pandemic, although the skin is not the main target of the infection, dermatologists result strongly involved, both directly and indirectly, in many aspects of the disease management. 47 , 48 Although it can be believed that the role of dermatologists is marginal in the management of the pandemic, an in‐depth analysis of the situation leads to understand that dermatologists can be strategic both in the containment of the infection, and in the treatment of patients and clinicians in deal with SARS‐CoV‐2 infection.
Conflicts of Interest
None declared.
Funding sources
None.
Acknowledgment
The patients in this manuscript have given written informed consent to publication of their case details.
References
- 1. Fan J, Liu X, Pan W, Douglas MW, Bao S. Epidemiology of 2019 novel coronavirus disease‐19 in Gansu Province, China, 2020. Emerg Infect Dis 2020; 26 6:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahase E. Covid‐19: WHO declares pandemic because of "alarming levels" of spread, severity, and inaction. BMJ 2020; 12: m1036. [DOI] [PubMed] [Google Scholar]
- 3. Coronavirus disease (COVID‐19) outbreak situation . Available at https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019.
- 4. Wei‐jie G, Zheng‐yi N, Yu H et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for Dengue. J Am Acad Dermatol 2020; 82: e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu S, Lin J, Zhang Z et al. Alert for non‐respiratory symptoms of Coronavirus Disease 2019 (COVID‐19) patients in epidemic period: A case report of familial cluster with three asymptomatic COVID‐19 patients. J Med Virol 2020. 10.1002/jmv.25776. [published online ahead of print, 2020 Mar 19] [DOI] [PubMed] [Google Scholar]
- 7. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34: e212–e213. [DOI] [PubMed] [Google Scholar]
- 8. Zhang JJ, Dong X, Cao YY et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy 2020; 10.1111/all.14238. [published online ahead of print, 2020 Feb 19] [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Cao W, Xiao M et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi 2020; 41: E006. [DOI] [PubMed] [Google Scholar]
- 10. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID‐19: Transient livedo reticularis. J Am Acad Dermatol 2020. 10.1016/j.jaad.2020.04.018. [published online ahead of print, 2020 Apr 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monti S, Balduzzi S, Delvino P. et al. Clinical course of COVID‐19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Annals Rheumatic Dis 2020; 79: 667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka Y, Sato Y Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses 2013; 5: 1250–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim WB, Marinas JE, Qiang J, Shahbaz A, Greaves S, Yeung J. Adverse events resulting in withdrawal of biologic therapy for psoriasis in real‐world clinical practice: a Canadian multicenter retrospective study. J Am Acad Dermatol 2015; 73: 237–241. [DOI] [PubMed] [Google Scholar]
- 14. Lebwohl M, Rivera‐Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID‐19? J Am Acad Dermatol 2020; 82: 1217–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campanati A, Paolinelli M, Diotallevi F, Martina E, Molinelli E, Offidani A. Pharmacodynamics OF TNF α inhibitors for the treatment of psoriasis. Expert Opin Drug Metab Toxicol 2019; 15: 913–925. [DOI] [PubMed] [Google Scholar]
- 16. Atanasova K, Van Gucht S Van Reeth K. Anti‐TNF‐alpha therapy does not ameliorate disease in a model of acute virus‐endotoxin mediated respiratory disease in pigs. Vet Immunol Immunopathol. 2010; 137: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Campanati A, Diotallevi F, Martina E, Paolinelli M, Radi G Offidani A. Safety update of etanercept treatment for moderate to severe plaque psoriasis. Expert Opin Drug Saf 2020; 19: 439–448. [DOI] [PubMed] [Google Scholar]
- 18. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu T, Zhang J, Yang Y et al. The potential role of IL‐6 in monitoring coronavirus disease 2019. SSRN Electronic J 2020; 10.2139/ssrn.3548761. [DOI] [Google Scholar]
- 20. A randomized, open‐label, controlled trial for the efficacy and safety of Adalimumab Injection in the treatment of patients with severe novel coronavirus pneumonia (COVID‐19) Shanghai, China: Chinese Clinical Trial Registry: ChiCTR2000030089; 2020. [cited 2020 Feb 22].
- 21. SIDeMaST (Società Italiana di Dermatologia e Malattie Sessualmente Trasmesse) coronavirus vademecum. Available at https://www.sidemast.org/blog/coronavirus/ Published online 25 Feb 2020.
- 22. Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease . 2019. (COVID‐19) or persons under investigation for COVID‐19 in healthcare settings: centers for disease control and prevention; 2020. [cited 2020 Feb 21].
- 23. Wang C, Rademaker M, Baker C Foley P. COVID‐19 and the use of immunomodulatory and biologic agents for severe cutaneous disease: An Australia/New Zealand consensus statement. Australas J Dermatol 2020. 10.1111/ajd.13313. [published online ahead of print, 2020 Apr 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rademaker M, Baker C, Foley P, Sullivan J Wang C. Advice regarding COVID‐19 and use of immunomodulators, in patients with severe dermatological diseases. Australas J Dermatol 2020; 61: 158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swine flu press release . British Society for Rheumatology (2009) http://www.rheumatology.org.uk/media/pressreleases/swineflu (accessed 31 July 2009).
- 26. Price KN, Frew JW, Hsiao JL, Shi VY. COVID‐19 and immunomodulator/immunosuppressant use in dermatology. J Am Acad Dermatol 2020; 82: e173–e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radi G, Campanati A, Diotallevi F, Molinelli E, Offidani A. Skin involvement in patients with psoriatic arthritis: preliminary results of treatment with apremilast in real world setting. G Ital Dermatol Venereol 2019; 154: 166–169. [DOI] [PubMed] [Google Scholar]
- 28. Liu J, Cao R, Xu M et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov 2020; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gbinigie K, Frie K. Should chloroquine and hydroxychloroquine be used to treat COVID‐19? A rapid review. BJGP Open 2020. bjgpopen20X101069 10.3399/bjgpopen20X101069. [published online ahead of print, 2020 Apr 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Academy of Dermatology (AAD) , Guidance on the use of biologic agents during COVID‐19 outbreak, 18 March, 2020.
- 31. Zingone F Savarino EV. Viral screening before initiation of biologics in patients with inflammatory bowel disease during the COVID‐19 outbreak. Lancet. Gastroenterol Hepatol 2020; 5: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wollenberg A, Flohr C, Simon D et al. European Task Force on Atopic Dermatitis (ETFAD) statement on severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2)‐infection and atopic dermatitis. J Eur Acad Dermatol Venereol 2020; 34: e241‐e242. [DOI] [PubMed] [Google Scholar]
- 33. Yan Y, Chen H, Chen L et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for healthcare workers fighting against coronavirus disease 2019. Dermatol Ther 2020; 13: e13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lan J, Song Z, Miao X et al. Skin damage among healthcare workers managing coronavirus disease‐2019. J Am Acad Dermatol 2020; 82: 1215–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kantor J. Behavioral considerations and impact on personal protective equipment (PPE) use: Early lessons from the coronavirus (COVID‐19) outbreak. J Am Acad Dermatol 2020; 82: 1087–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta‐analysis. Am J Public Health. 2008; 98: 8:1372–1381. 10.2105/ajph.2007.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID‐19) epidemic. J Am Acad Dermatol. 2020; 82: 5:1085–1086. 10.1016/j.jaad.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Darlenski R Tsankov N. Covid‐19 pandemic and the skin ‐ What should dermatologists know? Clin Dermatol 2020. 10.1016/j.clindermatol.2020.03.012. [published online ahead of print, 2020 Mar 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID‐19 transmission: A call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020; 82: 5:e179–e180. 10.1016/j.jaad.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tao J, Song Z, Yang L, Huang C, Feng A, Man X. Emergency management for preventing and controlling nosocomial infection of the 2019 novel coronavirus: implications for the dermatology department. Br J Dermatol. 2020; 10.1111/bjd.19011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Radi G, Diotallevi F, Campanati A, Offidani A. Global coronavirus pandemic (2019‐nCOV): implication for an Italian medium size dermatological clinic of a II level hospital. J Eur Acad Dermatol Venereol. 2020; 34: e213–e214. [DOI] [PubMed] [Google Scholar]
- 42. Ohannessian R. Telemedicine: Potential applications in epidemic situations. Eur Res Telemedicine/La Recherche Européenne en Télémédecine. 2015; 4: 3:95–98. 10.1016/j.eurtel.2015.08.002. [DOI] [Google Scholar]
- 43. Hollander JE, Carr BG. Virtually Perfect? Telemedicine for Covid‐19. N Engl J Med. 2020; 382: 18 18:1679–1681. 10.1056/nejmp2003539. [DOI] [PubMed] [Google Scholar]
- 44. Lee I, Kovarik C, Tejasvi T, Pizarro M, Lipoff JB. Telehealth: Helping your patients and practice survive and thrive during the COVID‐19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020; 82: 5:1213–1214. 10.1016/j.jaad.2020.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohannessian R, Duong TA, Odone A. Global Telemedicine Implementation and Integration Within Health Systems to Fight the COVID‐19 Pandemic: A Call to Action. JMIR Public Health and Surveillance. 2020;6: 2:e18810. 10.2196/18810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jakhar D, Kaur I, Kaul S. Art of performing dermoscopy during the times of coronavirus disease (COVID‐19): simple change in approach can save the day!. J Eur Acad Dermatol Venereo. 2020; 34: e242–e244. [DOI] [PubMed] [Google Scholar]
- 47. Diotallevi F, Campanati A, Bianchelli T et al. Skin Involvement in SARS‐CoV‐2 Infection: Case Series. J Med Virol. 2020; https://doi:10.1002/jmv.26012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Radi G, Simonetti O, Diotallevi F. How Can I Take Care Of You? The Dermatologist Meets Patients’ Needs During The Covid19 Pandemic. Dermatol Ther. 2020; 10.1111/dth.13740. [published online ahead of print, 2020 June 1] [DOI] [PMC free article] [PubMed] [Google Scholar]


