Abstract
Background
The COVID‐19 pandemic has focused medical attention on treating affected patients and protecting others from infection. However, concerns have been raised regarding the pandemic´s impact and associated containment measures (eg curfew, lockdown) on non–coronavirus disease 2019 (COVID‐19)–related acute medical diseases.
Objectives
To investigate changes in the incidence of pulmonary embolism (PE) during the COVID‐19 pandemic compared to the period before the pandemic and reference periods in previous years.
Methods
In this single‐center study, we explored all diagnostic imaging tests performed for suspected PE between weeks 1 and 17 of the years 2018, 2019, and 2020. Incidence of PE (ie, primary outcome) was analyzed. Secondary outcomes included number of imaging tests for suspected PE.
Results
Compared to weeks 1 to 11, 2020, an abrupt decline in PE diagnosis (mean weekly rate, 5.2; 95% confidence interval [CI], 3.8‐6.6 vs 1.8; 95% CI, 0.0‐3.6) and imaging tests (mean weekly rate, 32.5; 95% CI, 27.5‐37.6 vs. 17.3; 95% CI, 11.6‐23.1) was observed from week 12, with beginning of the containment measures and public lockdown in Austria. Compared to weeks 12 to 17 of 2018 and 2019, PE incidence and imaging tests were similarly decreased from 5.3 (95% CI, 3.6‐7.1) to 1.8 (95% CI, 0.0‐3.6) and 31.5 (95% CI, 27.1‐35.9) to 17.3 (95% CI, 11.6‐23.1), respectively. The median simplified pulmonary embolism severity index (sPESI) score of PE patients during the pandemic was higher than in all other PE patients (3; interquartile range, 1‐3 vs 1; interquartile range, 0‐2; P = .002).
Conclusion
Our study demonstrates that the COVID‐19 pandemic has an impact on non–COVID‐19–related acute diseases as shown by the decline in incidence of PE and imaging procedures for diagnostic workup. Further studies from other hospitals are needed to confirm our findings.
Keywords: COVID‐19, diagnostic imaging, incidence, pulmonary embolism, severe acute respiratory syndrome coronavirus 2, thromboembolism
Essentials.
Concerns arise on the impact of the coronavirus disease 2019 (COVID‐19) pandemic on non–COVID‐19–related medical conditions.
We conducted a single‐center study on number of imaging tests and pulmonary embolism (PE) compared to prior reference periods.
A decline in PE diagnosis and imaging tests during the pandemic was observed.
Strategies for providing acute medical care for non–COVID‐19–related medical conditions are needed.
1. INTRODUCTION
On March 11, 2020, in recognition of the widespread global transmission of the corona virus disease 2019 (COVID‐19), a novel respiratory disease induced by the severe acute respiratory syndrome coronavirus 2, the World Health Organization (WHO) has formally declared the current outbreak of COVID‐19 a pandemic. As of April 26, 2020, the WHO reported over 2.8 million confirmed cases globally with a steady increase in daily new cases. 1 To prevent and control infections, stringent containment measures have been undertaken in many countries, such as public lockdown, advice to stay at home, social distancing, isolation, and quarantines. One central goal has been to avoid overburdening of the health care capacity with cases of COVID‐19. However, as the coronavirus pandemic has focused medical attention on treating affected patients and protecting others from infection, questions arise about the impact of the pandemic on the general medical care for people with non–COVID‐19–related diseases. 2
Concerns have been raised regarding missing diagnoses of acute medical conditions such as acute coronary syndrome, stroke, or other acute cardiovascular conditions such as pulmonary embolism (PE). A reduction in diagnostic procedures, number of new cases, and delay in treatments of patients with acute coronary syndrome has already been reported. 3 , 4 , 5 , 6
In context of the COVID‐19 pandemic, the impact on patients with suspected PE may be of particular interest because of potentially overlapping symptoms with COVID‐19, which include chest pain, shortness of breath, hemoptysis, and even fever. 7 , 8 While the population was generally instructed to stay at home and refrain from seeking medical attention in non–life‐threatening situations, persons experiencing the symptoms above, may have sought medical attention for PE late. PE is a potentially life‐threatening disease, 9 where early diagnosis and initiation of anticoagulation are crucial. 10 , 11 , 12
Therefore, we aimed at investigating how the COVID‐19 crisis has influenced the routine diagnostic workup and incidence of PE patients in a single‐center cohort study in a large tertiary care hospital in Vienna, Austria.
2. METHODS
2.1. Study design
This single‐center cohort study was conducted at the Vienna General Hospital of the Medical University of Vienna, a tertiary care center with a capacity of 1773 beds, including 137 intermediate and 130 intensive care beds. The national pandemic emergency plan intended specific hospitals to treat COVID‐19 patients, and in our hospital, it was anticipated to provide acute medical care for the urban population of Vienna not affected by COVID‐19. The study was approved by the ethics committee of the Medical University of Vienna, Vienna, Austria (EK‐Nr: 1305/2020).
2.2. Data sources
We obtained all diagnostic imaging reports for patients undergoing computed tomography pulmonary angiography (CTPA) or ventilation/perfusion (V/P) lung scanning in the calendar weeks 1 to 17 of the years 2018, 2019, and 2020. All imaging reports were screened, and examinations that were not performed for the diagnostic workup of a suspected PE including diagnostic for chronic thromboembolic pulmonary hypertension were excluded. Further, medical records and electronic patient charts of each patient were reviewed to confirm the diagnosis of PE and to obtain further information on patient demographics and clinical history and to assess the severity of PE. The data cutoff for the study was April 26, 2020, 6 weeks after the initiation of the public measures (ie, nationwide curfew and lockdown; advise to stay at home, social distancing and isolation, and quarantine in some parts of the country not including the city of Vienna) to prevent and control the COVID‐19 pandemic in Austria.
2.3. Study objectives and periods
The primary objective of this study was the incidence rate of PE per week. The secondary objectives were rate of imaging tests performed per week for the diagnostic workup of PE and the severity of the diagnosed PE events. PE severity was assessed according to the guidelines of the European Society of Cardiology. 13 Patients were categorized into low, intermediate‐low, intermediate‐high, and high‐risk PE using hemodynamic parameters, simplified pulmonary embolism severity index (sPESI), imaging of the right ventricle, and high‐sensitivity cardiac troponin T (hs‐cTnT).
To perform comparative analyses, study periods were defined as follows: The period of interest was calendar week 12 to 17, 2020, further referred to as the pandemic period. At the beginning of week 12 (March 16, 2020), the containment measures (nationwide curfew and lockdown) to control the widespread transmission of COVID‐19 started in Austria. After 30 days, on April 14, 2020, stepwise lifting of the nationwide containment measures started. The corresponding periods in years 2018 and 2019 were used as the reference period.
2.4. Statistical analysis
First, we analyzed the outcomes from week 1 to week 17 in 2020 (ie, intrayear analysis). Second, we compared the outcomes occurring between week 12 and week 17 in 2020 with the aggregated data of the reference period in 2018 and 2019 (ie, interyear analysis). Third, we compared severity of PE during the pandemic period (weeks 12‐17, 2020) with all other PE events occurring during the study period (weeks 1‐17 in years 2018 and 2019 and weeks 1‐11 in 2020).
Incidence rate for the primary outcome (ie, PE) and rate of imaging tests were calculated per week. Incidence‐rate ratio (IRR) of PE and rate ratio (RR) of imaging tests for inter‐ and intrayear analysis were calculated using the Poisson regression to model the number of cases per week. IRR and RR are presented with the corresponding 95% confidence interval (CI). Severity of PE was analyzed using Mann‐Whitney U test. The results were given as the mean (with 95% CI), median (interquartile range [IQR]) or number (percentage), as appropriate. A P value of <.05 was considered statistically significant. Data were analyzed using R (Version 3.6.2; R Core Team, 2019).
3. RESULTS
3.1. Diagnostic procedures and number of imaging tests
Overall, 1911 imaging tests (CTPA and V/P lung scans) performed during the observation period (week 1 to week 17 in the years 2018, 2019, and 2020) were identified, and 354 tests were excluded because they were not performed for the diagnostic workup of suspected acute PE. This resulted in 1557 (81.5%) imaging tests, consisting of 1468 CTPAs and 89 V/P lung scans, which were performed for the diagnostic workup of PE. Table 1 describes the number of diagnostic procedures per year and during the period of the pandemic compared to the reference period in the years 2018 and 2019.
TABLE 1.
Number of imaging tests and pulmonary embolism diagnoses in weeks 1 to 17 of the respective calendar year
| 2020 | 2019 | 2018 | |
|---|---|---|---|
| Total number of imaging tests | 462 | 586 | 509 |
| CTPA | 441 | 560 | 467 |
| V/P lung scanning | 21 | 26 | 42 |
| Tests in calendar weeks 1‐11 | 358 | 371 | 346 |
| CTPA | 339 | 355 | 314 |
| V/P lung scanning | 19 | 16 | 32 |
| Tests in calendar weeks 12‐17 | 104 | 215 | 163 |
| CTPA | 102 | 205 | 153 |
| V/P lung scanning | 2 | 10 | 10 |
| Intrayear analysis RR (95% CI) | 0.53 (0.42‐0.66) | 1.06 (0.85‐1.32) | 0.86 (0.69‐1.08) |
|
Interyear analysis RR (95% CI) |
0.55 (0.44‐0.68) | ||
| Total number of PEs | 68 | 93 | 88 |
| PEs in calendar weeks 1‐11 | 57 | 55 | 62 |
| PEs in calendar weeks 12‐17 | 11 | 38 | 26 |
| Intrayear analysis IRR (95% CI) | 0.35 (0.18‐0.67) | 1.27 (0.66‐2.42) | 0.77 (0.40‐1.47) |
| Interyear analysis IRR (95% CI) | 0.34 (0.18‐0.65) |
Abbreviations: CTPA, computed tomography pulmonary angiography; IRR, incidence‐rate ratio; PE, pulmonary embolism; RR, rate ratio; V/P, ventilation/perfusion
Intrayear analysis: comparison of weeks 1‐11 with 12‐17 in 2018, 2019, and 2020; interyear analysis: comparison of weeks 12‐17, 2020, with weeks 12‐17, 2018 and 2019.
The intrayear analysis, comparing weeks 1 to 11, 2020, with weeks 12 to 17, 2020 (pandemic period), showed a significant decrease in the weekly number of imaging tests with the start of the nationwide containment measures (mean weekly number of tests, 32.5; 95% CI, 27.5‐37.6 vs. 17.3; 95% CI, 11.6‐23.1, corresponding to a RR of 0.53; 95% CI, 0.42‐0.66). In week 17, number of imaging tests showed a trend toward weekly rates before the pandemic (Figure 1A).
FIGURE 1.
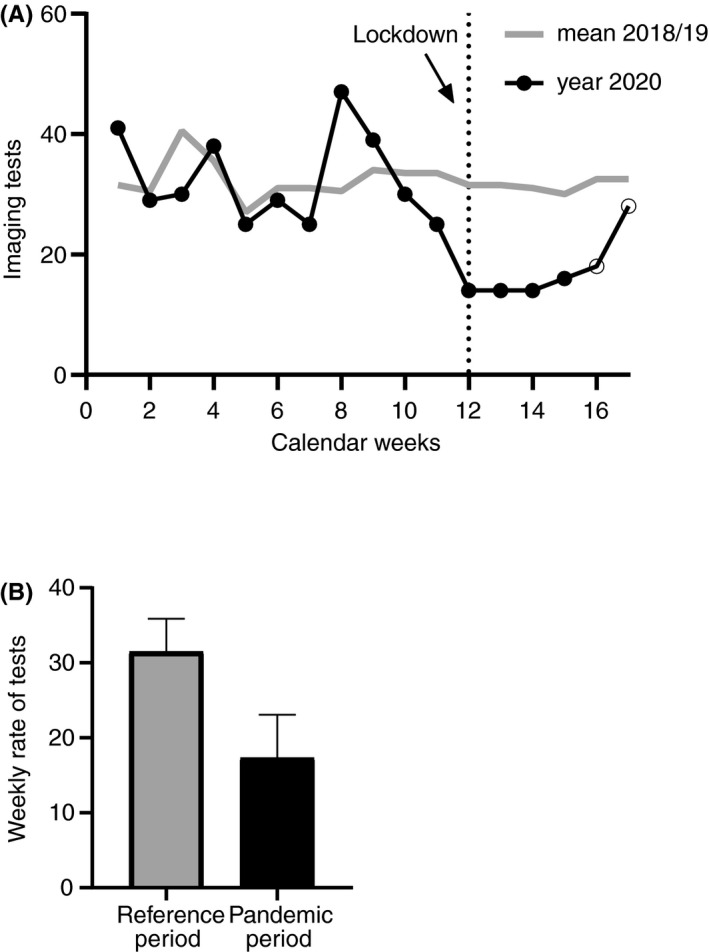
Reduction of imaging tests at the Vienna General Hospital carried out to diagnose pulmonary embolism. (A) Dotted vertical line shows start of the public health measures in Austria. (B) Interyear analysis between the pandemic period in year 2020 (weeks 12‐17) and the reference period in 2018 and 2019 displayed as mean (with 95% confidence interval [CI]) weekly rate of tests: Rate ratio of 0.55 (95% CI, 0.44‐0.68). Bars represent the mean, and whiskers represent the upper limit of the 95% CI.
The interyear analysis compared the pandemic period with the reference period of the years 2018 and 2019. Again, the mean number of imaging tests in the pandemic period (17.3; 95% CI, 11.6‐23.1) was significantly lower than the reference period (31.5; 95% CI, 27.1‐35.9, corresponding to a RR of 0.55; 95% CI, 0.44‐0.68) (Figure 1B).
In 17 (16%) CTPAs performed during the pandemic period, suspicion of COVID‐19 was part of the indication. In 2 of them, COVID‐19 was confirmed.
3.2. Incidence of pulmonary embolism
PE was diagnosed in 16.0% (249) of all imaging tests that were performed during the observation period of the years 2018, 2019, and 2020. The detailed numbers per year and for the pandemic period as compared to the reference period in years 2018 and 2019 are shown in Table 1.
PE rates per calendar week are shown in Figure 2A. Similar to the trends of imaging procedures, we observed a reduction of PE diagnoses from week 12 in year 2020. The intrayear analysis, comparing the mean weekly incidence rates between the 2 time periods in 2020 (5.2; 95% CI, 3.8‐6.6 vs 1.8; 95% CI, 0.0‐3.6) showed a significantly lower rate during the pandemic period (IRR, 0.35; 95% CI, 0.18‐0.67). The incidence of PE has risen with beginning of calendar week 17 and has reached similar rates as the period before the pandemic with the associated containment measures.
FIGURE 2.
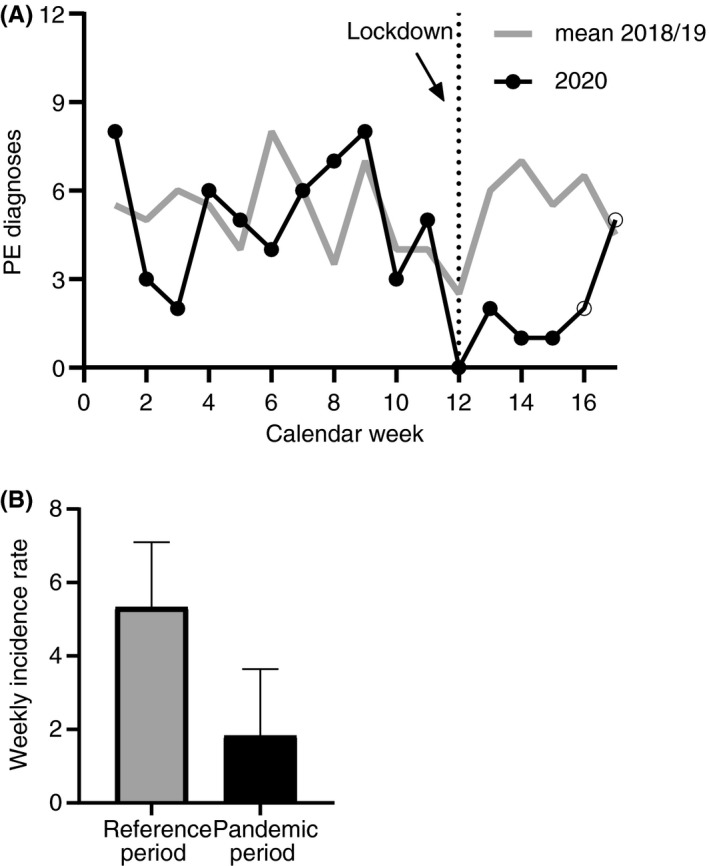
Decline in pulmonary embolus (PE) cases treated at the Vienna General Hospital associated with the beginning of COVID‐19 containment measures in Austria. (A) Dotted vertical line shows start of the public health measures in Austria. (B) Interyear analysis between the pandemic period in year 2020 (weeks 12‐17) and the reference period in 2018 and 2019 displayed as mean (with 95% confidence interval [CI]) weekly incidence rates: Incidence‐rate ratio of 0.34 (95% CI, 0.18‐0.65). Bars represent the mean, and whiskers represent the upper limit of the 95% CI.
Interyear analysis with the reference periods of 2018 and 2019 (mean incidence rate per week, 5.3; 95% CI, 3.6‐7.1) confirmed the significant drop of PE incidence rate during the pandemic period (1.8; 95% CI, 0.0‐3.6; IRR, 0.34; 95% CI, 0.18‐0.65) (Figure 2B).
3.3. Patient demographics and severity of pulmonary embolism
Patient demographics, clinical history, and severity of PE of all 11 patients suffering from acute PE during the pandemic period are presented in Table 2. For comparison, all other patients with PE in this study are displayed as a reference group. Patients with PE during the pandemic were in median 64 (IQR, 49‐83) years old, and 5 (45%) were female. Nine (82%) presented as outpatients and all were admitted to the hospital after confirmed diagnosis of PE. Four (36%) patients had active cancer, 2 (18%) had a history of cancer, 2 (18%) suffered from chronic lung disease, and none was previously diagnosed with congestive heart failure. One (9%) and 4 (36%) patients fulfilled the criteria for high‐risk and intermediate‐high‐risk PE, respectively. Six (55%) showed signs of right ventricular dysfunction on echocardiography or CTPA and 5 (45%) had also elevated hs‐cTnT and N‐terminal pro‐B‐type natriuretic peptide. None of the patients with PE was diagnosed with COVID‐19. Severity of PE (categorized into low, intermediate‐low, intermediate‐high, and high‐risk PE according to the definition of the European Society of Cardiology 13 ) of patients diagnosed during the pandemic period compared to all PE events in the comparative years did not significantly differ (P = .068). However, the median sPESI was significantly higher for patients diagnosed during the pandemic (3; IQR, 1‐3 vs 1; IQR, 0‐2; P = .002). The median hs‐cTnT and N‐terminal pro‐B‐type natriuretic peptide levels were 40 pg/mL (IQR, 19‐91) and 1609 ng/L (IQR, 406‐3908) for the patients in the pandemic period and 18 pg/mL (IQR, 9‐59) and 385 ng/L (IQR, 98‐2276) for the reference cohort, respectively. Furthermore, the proportion of patients with PE and active cancer was higher in the pandemic period (36%) than in the reference period (21%).
TABLE 2.
Patient demographics and severity of pulmonary embolism
| Variable | PE patients in pandemic period (n = 11) | All other PE patients a (n = 238) |
|---|---|---|
| Demographics baseline | ||
| Age, median (IQR) | 64 (49‐83) | 63 (47‐76) |
| Sex, female, n (%) | 5 (45) | 125 (53) |
| Inpatient, n (%) | 2 (18) | 57 (24) |
| Outpatient, n (%) | 9 (82) | 181 (76) |
| Admitted to hospital, n | 9 | 132 |
| Medical history, n (%) | ||
| Active cancer | 4 (36) | 51 (21) |
| History of cancer | 2 (18) | 8 (3) |
| Congestive heart failure | 0 (0) | 17 (7) |
| Chronic pulmonary disease | 2 (18) | 27 (11) |
| Severity of PE, b n (%) | ||
| High‐risk PE | 1 (9) | 11 (5) |
| Intermediate‐high risk PE | 4 (36) | 46 (19) |
| Intermediate‐low‐risk PE | 5 (45) | 113 (47) |
| Low‐risk PE | 1 (9) | 68 (29) |
| Right ventricular dysfunction c | 6 (55) | 67 (28) |
| sPESI, median (IQR) | 3 (1‐3) | 1 (0‐2) |
| Laboratory parameters, median (IQR) | ||
| NT‐proBNP, ng/L d | 1609 (406‐3908) | 385 (98‐2276) |
| hs‐cTnT, pg/mL e | 40 (19‐91) | 18 (9‐59) |
Abbreviations: hs‐cTnT, high‐sensitivity cardiac Troponin T; IQR, interquartile range; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PE, pulmonary embolism; sPESI, simplified pulmonary embolism severity index.
Severity of PE by risk categories did not differ significantly between the 2 groups, assessed by Mann‐Whitney U test (P = .068). However, scores for sPESI were statistically different (P = .002).
This included all patients diagnosed before the pandemic (weeks 1‐11, 2020, and weeks 1‐17 of 2018 and 2019). Two patients experienced a second PE event during the reference observation period, which were counted as a separate event.
Patients of the intermediate‐risk group, who had either no assessment of the right ventricular function or hs‐cTnT was not ordered, were assigned to the intermediate‐low‐risk group. This was done for 3 patients in the pandemic period and 65 patients of the reference group.
Right ventricular dysfunction was assessed by echocardiography and/or computed tomography images.
NT‐proBNP was assessed in 8 patients in the pandemic period and 77 patients of the reference group.
hs‐cTnT was assessed in 7 patients in the pandemic period and 85 patients of the reference group.
4. DISCUSSION
In this study, we investigated the effect of the COVID‐19 pandemic and the associated containment measures (curfew, lockdown, etc) on the general medical care as exemplified by the incidence of PE and the changes in diagnostic workup for suspected PE at a large single‐center hospital in Vienna, Austria. We have observed a dramatic reduction in cases of patients with diagnosed PE and the number of diagnostic procedures, associated with the start of the lockdown on March 16, 2020, in Austria. When comparing the pandemic period (weeks 12‐17) to the corresponding periods in the years 2018 and 2019, we have observed a 66% reduction in PE diagnoses and a 45% decrease in imaging tests for suspected PE.
The pandemic and lockdown measures resulted in a decline in PE diagnoses, but the reasons are elusive. Our worst suspicion is therefore that the strict measures during the lockdown and the call to not seek medical attention at hospitals in case of COVID‐19–associated symptoms could have prevented people with PE from going to the hospital. In Austria, a diagnosis of PE is generally performed in the hospital setting after general practitioners refer patients with suspected PE to a hospital or patients choose to directly go to a hospital. Early diagnosis and initiation of appropriate treatment are essential to improve clinical outcomes and reduce risk of mortality. Consequently, delays in diagnosis of PE may have adverse clinical consequences. Our data set is confined to diagnostic workup within the hospital, but centrally reported Austrian mortality rates have risen abruptly since the 12th calendar week, and these are not explained solely by the deaths associated with confirmed COVID‐19 cases. 14 Interestingly, observations worldwide show a similar gap between registered COVID‐19 deaths and the statistically increased mortality rate. 15 The trend toward normalization of PE incidence and test rates in week 17 in our study could be explained by the gradual lifting of lockdown measures, which was initiated on April 14, 2020, in Austria.
The Vienna General Hospital is one of the largest hospitals in Europe and the national pandemic emergency plan intended for the hospital to maintain non–COVID‐19–related acute medical care for the urban population of Vienna. A dramatic reduction in non–COVID‐19–related PE diagnoses and imaging may, therefore, indicate a true reduction in the incidence of PE. On the contrary, a recent consensus document suggested that risk of PE in people without COVID‐19 during the pandemic could be even increased. 16 Decreased daily activities and sedentary lifestyles could contribute to a higher risk during the pandemic. However, we must consider that reduced activities leading to trauma and the associated immobility with risk for thromboembolism as well as fewer surgeries, initiation or postponement of oncologic and new hormonal therapies, and reduction of long‐haul travel during the shutdown of routine medical care, could have led to a true decline in PE. Nevertheless, given that approximately a third of all PEs are provoked by trauma, surgery, or hospitalization, and given that these cases did not occur, the observed reduction of 66% cannot fully be explained by decline of provoked cases.
A major limitation of this study is that for COVID‐19 patients, who seem to be at high risk for PE, 17 , 18 , 19 effort was taken to treat them at specific hospitals and reserve our hospital, the Vienna General Hospital, for the acute treatment of non–COVID‐19 patients. Because symptoms for COVID‐19 and PE overlap, this might partly explain the massive decrease in PE diagnosis, as patients with respiratory symptoms might have been transferred to hospitals dedicated to taking care of patients with COVID‐19. However, our data are in agreement with recent results from a nationwide analysis of hospitalizations for acute coronary syndrome that similarly showed a decline in acute coronary syndrome–related hospitalization and support our findings. 3 Another potential limitation is that the findings of this study are based on data from a single center. Nevertheless, our hospital is one of the largest in Europe, and the homogeneous procedures for diagnostic workup might even be of advantage. Clinical severity of the PE cases diagnosed during the pandemic assessed by sPESI showed higher scores. However, the interpretation of this result is limited due to the unexpectedly low patient number and the retrospective nature of the study. Still, the design is considered a strength with regard to inclusion of consecutive patients and enabled us to compare results with the reference periods from the previous years.
In summary, our study demonstrates that the COVID‐19 pandemic has an impact on the management of non–COVID‐19–related diseases as shown with the decline in PE incidence and reduction of PE imaging for diagnostic workup. Therefore, we would like to call for awareness to establish strategies to tackle challenges during the COVID‐19 pandemic that may have an adverse impact on acute care of people who develop acute and life‐threatening medical conditions such as PE. However, further studies from other hospitals and with expanded analysis are needed to confirm our findings.
RELATIONSHIP DISCLOSURE
The authors report nothing to disclose.
AUTHOR CONTRIBUTIONS
SN and CA designed the study and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. IP, OK, HP, and KJ‐S contributed substantially to the interpretation of the data and critical writing and revision of the manuscript. IS developed the algorithm to screen for the imaging tests.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
The authors thank Florian Moik for statistical advice.
Nopp S, Janata‐Schwatczek K, Prosch H, et al. Pulmonary embolism during the COVID‐19 pandemic: Decline in diagnostic procedures and incidence at a university hospital. Res Pract Thromb Haemost. 2020;4:835–841. 10.1002/rth2.12391
Handling Editor: Dr Suzanne Cannegieter
Funding information
The study was supported by the Medical Scientific Fund of the Mayor of the City of Vienna (ID: CoVid015).
REFERENCES
- 1. World Health Organization . Coronavirus disease 2019 (COVID‐19): situation report, 97. Geneva: World Health Organization; 2020. [Google Scholar]
- 2. Rosenbaum L. The untold toll — the pandemic’s effects on patients without Covid‐19. N Engl J Med. 2020. 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 3. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID‐19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41 (19):1852–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodríguez‐Leor O, Cid‐Álvarez B, Ojeda S, Martín‐Moreiras J, Ramón Rumoroso J, López‐Palop R, et al. Impacto de la pandemia de COVID‐19 sobre la actividad asistencial en cardiología intervencionista en España. REC Interv Cardiol. 2020;2:82–9. [Google Scholar]
- 5. Tam C‐CF, Cheung K‐S, Lam S, Wong A, Yung A, Sze M, et al. Impact of Coronavirus Disease 2019 (COVID‐19) Outbreak on ST‐Segment‐Elevation Myocardial Infarction Care in Hong Kong. China. Circulation: Cardiovascular Quality and Outcomes. 2020;13(4):e006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Filippo O, D’Ascenzo F, Angelini F, Bocchino PP, Conrotto F, Saglietto A, et al. Reduced rate of hospital admissions for ACS during Covid‐19 outbreak in Northern Italy. N Engl J Med. 2020. 10.1056/NEJMc2009166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu BO, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan. China. JAMA. 2020;323(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stein PD, Beemath A, Matta F, Weg JG, Yusen RD, Hales CA, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. The American Journal of Medicine. 2007;120(10):871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keller K, Hobohm L, Ebner M, Kresoja K‐P, Münzel T, Konstantinides SV, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J. 2019;41(4):522–9. [DOI] [PubMed] [Google Scholar]
- 10. Kline JA, Hernandez‐Nino J, Jones AE, Rose GA, Norton HJ, Camargo CA. Prospective study of the clinical features and outcomes of emergency department patients with delayed diagnosis of pulmonary embolism. Acad Emerg Med. 2007;14(7):592–8. [DOI] [PubMed] [Google Scholar]
- 11. Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6):1382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soh S, Kim JM, Park JH, Koh SO, Na S. Delayed anticoagulation is associated with poor outcomes in high‐risk acute pulmonary embolism. J Crit Care. 2016;32:21–5. [DOI] [PubMed] [Google Scholar]
- 13. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G‐J, Harjola V‐P, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. 2019;41(4):543–603. [DOI] [PubMed] [Google Scholar]
- 14. Statistik Austria . Sterbefälle im Jahr 2020 und im Durchschnitt der Jahre 2016 bis 2019 nach Kalenderwoche und Alter. 2020, April 20; Available from: https://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/bevoelkerung/gestorbene/122934.html
- 15. Wu J, McCann A, Katz J, Peltier E 40,000 Missing Deaths: Tracking the True Toll of the Coronavirus Outbreak. 2020, April 27; Available from: https://www.nytimes.com/interactive/2020/04/21/world/coronavirus‐missing‐deaths.html
- 16. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up. J Am Coll Cardiol. 2020:27284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020. 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020. 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Llitjos JF, Leclerc M, Chochois C, Monsallier J‐M, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020. 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


