The diagnosis and management of acute promyelocytic leukaemia (APML) in the context COVID‐19 poses a challenge for clinicians. We present a case illustrating this due to the masking of the typical laboratory pattern of APML coagulopathy and the potential heightened risks of thrombosis and differentiation syndrome (DS) when the two conditions are combined.
A 36‐year‐old man was admitted in April 2020 with fever, cough and sweats. Examination revealed a fever of 38·4°C, heart rate of 116 bpm, normal blood pressure, respiratory rate of 19, saturations of 95% on air and bilateral crepitations up to the mid‐zone. There was no bruising, petechiae, hepatosplenomegaly or lymphadenopathy.
The blood count showed haemoglobin 95 g/l, total white cell count 1·0 × 109/l, neutrophil count 0·5 × 109/l, lymphocyte count 0·4 × 109/l, platelet count 69 × 109/l. Blood film revealed teardrop poikilocytes, left‐shifted neutrophils with vacuolation and plasmacytoid lymphocytes. Prothrombin time (PT) was 18.5 s [normal range (NR) 9·1–12.5], activated partial thromboplastin time (APTT) 31 s (NR 26–40), D‐dimer 43 246 ng/ml (NR 0–230), Clauss fibrinogen >5·00 g/l (NR 1·8–3·6) and ferritin 4073 µg/l (NR 30–400). His creatinine was 193 µmol/l (NR 62–106), lactate dehydrogenase 452 iu/l (NR 135–225) and C‐reactive protein 382 mg/l (NR 0–5).
A nasopharyngeal swab detected SARS‐CoV‐2 RNA. His computed‐tomography pulmonary angiogram was negative for pulmonary embolus but showed extensive, predominantly peripheral, consolidative changes consistent with moderate/severe COVID‐19 (Fig 1). His clinical status including coagulopathy improved rapidly with antibiotics but neutrophils remained low, prompting an urgent bone marrow examination. Bone marrow aspirate was a dry tap but the trephine roll had bilobed, hypergranular mononuclear cells highly suggestive of APML. Peripheral blood fluorescence in situ hybridisation detected the presence of a low level PML‐RARA rearrangement. The bone marrow trephine biopsy became available two days later and showed 75% infiltration by promyelocytes (Fig 2).
Fig 1.
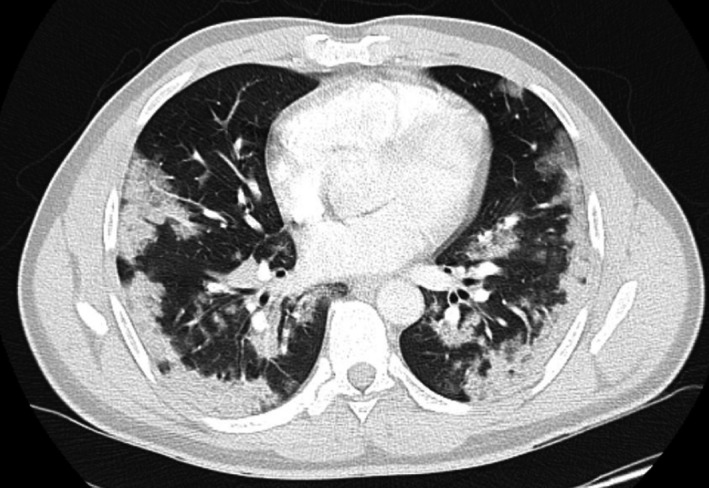
CT chest axial slice demonstrating extensive bilateral patchy, peripheral consolidative changes throughout the lungs.
Fig 2.
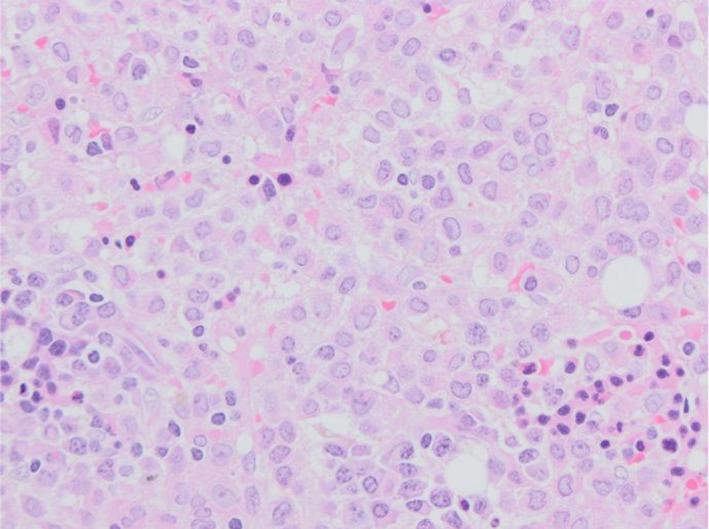
Bone marrow trephine biopsy: Bone marrow biopsy showing numerous blasts, many with bilobate/reniform nuclei and cytoplasmic granules. The background shows dysplastic erythropoiesis and scattered plasma cells. Haematoxylin and eosin staining, ×20.
He was commenced on all‐trans retinoic acid (ATRA) and arsenic trioxide (ATO) at 50% doses due to the risk of DS sequelae in the context of COVID‐19 lung disease. Intermediate dose enoxaparin was initiated to minimise thrombotic complications.
Our case remains clinically well following initial dose‐reduced ATRA/ATO treatment with slow titration to near full doses. He had sustained resolution of coagulopathy with no bleeding or thrombotic complications.
Coagulopathy is the leading cause of fatality in APML. Typically, due to a combination of disseminated intravascular coagulopathy, hyperfibrinolysis and thrombocytopenia, patients usually present with low platelet count, prolonged PT and APTT, elevated D‐dimers and low fibrinogen levels. 1 , 2 Survival rates have markedly improved with prompt initiation of ATRA/ATO and supportive measures addressing the coagulopathy.
COVID‐19 is also associated with coagulopathy, denoted the coagulopathy of Covid (CAC). 3 , 4 CAC is due to the inflammatory response to SARS‐CoV‐2 which results in thrombo‐inflammation and drives thrombosis. 3 Abnormal coagulation parameters in COVID‐19 include prolonged PT and APTT, raised D‐dimers (associated with increased mortality) and high fibrinogen, with thrombocytopenia uncommonly reported. 3 , 4 , 5
Although our case had features of APML, the concomitant diagnosis of COVID‐19 raised diagnostic and therapeutic challenges. Firstly he had no bleeding manifestations, reported in up to 76% of APML patients. 1 Furthermore, his fibrinogen was consistently raised and thrombocytopenia mild, discordant with the usual pattern in APML. His abnormal coagulation parameters largely corrected with supportive management for COVID‐19 prior to the initiation of ATRA, suggesting his coagulopathy was more consistent with CAC.
Whilst viral infection‐associated haemophagocytic lymphohistiocytosis can lead to pancytopenia, this is uncommon in COVID‐19. The main full blood count abnormality reported in COVID‐19 is lymphopenia, associated with worse prognosis. 6 The significant neutropenia in our case prompted us to conduct an urgent bone marrow examination. Although we were unable to attain an aspirate, the trephine roll was helpful and raised the suspicion of APML. Suspicion of APML would lead to prompt initiation of ATRA and help reduce mortality. DS, a complication of ATRA, which presents with fever, weight gain, dyspnoea, pulmonary infiltrates and pleuro‐pericardial effusions has a mortality rate of up to 30% due to hypoxic respiratory failure if untreated. 7 , 8 As our patient had respiratory compromise due to COVID‐19, we were reluctant to commence ATRA based on suspicion of APML diagnosis due to the risk of further respiratory compromise from DS. Furthermore, the atypical pattern of coagulation derangement and the improving trend of both platelet count and coagulation studies raised diagnostic uncertainty.
Once the diagnosis was confirmed with the presence of PML/RARA translocation in peripheral blood, the choice of appropriate treatment was the next dilemma. Given the low white cell count at presentation our case fell into the low–intermediate risk group and thus was a candidate for ATRA/ATO combination with associated survival rates of >90%. 9 Due to the perceived risk of DS on our patients’ COVID‐19‐compromised lung, we cautiously initiated treatment with ATRA and ATO at 50% doses. Whilst the benefit of prophylactic corticosteroids in prevention of DS is uncertain and mainly reserved for patients presenting with a white cell count >5 × 109/l, we used prophylactic low dose dexamethasone. 9
Although haemorrhagic complications of APML predominate and are reduced by ATRA, thrombosis is not uncommon; however, this risk is not reduced by ATRA. 1 , 2 The acute inflammatory state in COVID‐19, in addition to the known thrombotic risk of hospitalisation, results in a highly pro‐thrombotic state. This, when combined with APML thrombotic complications were felt to warrant intermediate dose enoxaparin prophylaxis in our case.
This case presented challenges due to atypical coagulation studies in the context of COVID‐19. Laboratory findings of APML can be disguised in the context of COVID‐19, thus stressing the need to suspect a potential acute leukaemia in COVID‐19 presenting with neutropenia. The complexities of balancing risk of DS on the background of already severely inflamed lungs and the risk/benefit of prophylaxis with steroids made it necessary to consider treatment alterations.
References
- 1. Breen KA, Grimwade D, Hunt BJ. The pathogenesis and management of the coagulopathy of acute promyelocytic leukaemia. Br J Haematol. 2012;156:24–36. [DOI] [PubMed] [Google Scholar]
- 2. David S, Mathews V. Mechanisms and management of coagulopathy in acute promyelocytic leukemia. Thromb Res. 2018;164:S82–S88. [DOI] [PubMed] [Google Scholar]
- 3. Connors J, States U, Levy J, States U. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020. 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rego EM, De Santis GC. Differentiation syndrome in promyelocytic leukemia: clinical presentation, pathogenesis and treatment. Mediterr J Hematol Infect Dis. 2011;3:e2011048. 10.4084/MJHID.2011.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sentero D, Hosenpud J. Retinoic acid syndrome in acute promyelocytic leukemia. Wisconsin Med J. 1997;96:35–8. [PubMed] [Google Scholar]
- 9. Sanz MA, Fenaux P, Tallman MS, Estey EH, Löwenberg B, Naoe T, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133:1630–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


