Abstract
Objective
Mortality from coronavirus disease 2019 (COVID‐19) is increased in patients with chronic obstructive pulmonary disease (COPD). Furthermore, higher BMI is related to severe disease. Severe acute respiratory syndrome coronavirus 2 utilizes angiotensin converting enzyme 2 (ACE2) to gain cellular entry.
Methods
Whether ACE2 bronchial epithelial expression is increased in COPD patients who have overweight compared with those who do not was investigated by RNA sequencing.
Results
Increased ACE2 expression was observed in patients with COPD with overweight (mean BMI, 29 kg/m2) compared with those without overweight (mean BMI, 21 kg/m2) (P = 0.004).
Conclusions
Increased ACE2 expression may cause increased severe acute respiratory syndrome coronavirus 2 infection of the respiratory tract. Overweight COPD patients may be at greater risk for developing severe COVID‐19.
Study Importance.
What is already known?
-
►
Severe coronavirus disease 2019 is associated with comorbidities including chronic obstructive pulmonary disease (COPD) and with higher BMI.
What does this study add?
-
►
We demonstrate increased expression of the severe acute respiratory syndrome coronavirus 2 receptor, angiotensin converting enzyme 2, in the epithelium of patients with COPD who are overweight.
How might these results change the direction of research or the focus of clinical practice?
-
►
Patients with COPD who are overweight may be at greater risk for developing severe COVID‐19.
Introduction
The rapid emergence and transmission of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has led to the global coronavirus disease 2019 (COVID‐19) pandemic. COVID‐19 presents with symptoms including fever, cough, and dyspnea (1). Lower respiratory tract infection is a characteristic feature of COVID‐19, with diffuse bilateral ground‐glass opacification and consolidation associated with respiratory failure in severe cases (2). The mortality from COVID‐19 is higher in patients with comorbidities, including chronic obstructive pulmonary disease (COPD), cardiovascular diseases, and diabetes (1, 3, 4). Furthermore, a higher BMI is related to increased hospital admission rates and a greater need for mechanical ventilation, independent of hypertension and diabetes (5, 6, 7). More severe COVID‐19 is associated with higher levels of proinflammatory cytokines and ferritin in the blood and lower lymphocyte and platelet counts, consistent with hyperinflammation or a cytokine storm (8, 9).
SARS‐CoV‐2 is a beta coronavirus that gains cellular entry via angiotensin converting enzyme 2 (ACE2) (10). ACE2 is an enzyme of the renin‐angiotensin system that degrades angiotensin II, thereby promoting vasodilation and increased water excretion. ACE2 is expressed in many human organs, including the lungs. Leung et al. (11) recently showed increased ACE2 expression in the bronchial epithelium of COPD patients compared with controls, a negative association between ACE2 expression and the degree of airflow obstruction, and increased expression in current smokers. These findings suggest that current smoking and/or the presence of COPD may facilitate enhanced SARS‐CoV‐2 cellular entry because of increased access to the virus receptor ACE2.
COPD patients often suffer from systemic comorbidities, with some patients showing muscle wasting and weight loss, whereas others have obesity (12). The expression of ACE2 is increased in the adipose tissue of people with obesity compared to those without (13). As higher BMI and COPD are both associated with more severe COVID‐19 (1, 3, 4, 6), we have investigated whether ACE2 bronchial epithelial expression in COPD patients is increased in those who are overweight.
Methods
A total of 37 COPD patients aged > 40 with a smoking history of > 10 pack‐years, a post‐bronchodilator forced expiratory volume in 1 second (FEV1) and forced vital capacity ratio < 0.7, and no history of asthma were recruited for bronchoscopy. The cohort was separated into two groups based on a BMI threshold of > 24.9, which defines overweight and includes individuals with obesity (at ≥ 30). There were 14 patients with COPD with BMI ≤ 24.9 (mean BMI, 21), including 1 individual who was underweight (BMI < 18.5), and 23 patients with BMI > 24.9, including 6 individuals with obesity (mean BMI, 29). Patients receiving oral corticosteroids or antibiotics within 6 weeks of the study were excluded. Sample collection was approved by South Manchester REC (06/Q1403/156). All participants provided written informed consent. The relationship between blood and lung eosinophil counts in these patients have been previously reported (14).
Bronchial brushings were collected in bronchial epithelial basal medium (Lonza, Slough, UK) and stored on ice before centrifugation (400 g for 10 minutes at 4°C). The cell pellet was resuspended in RLT buffer (Qiagen) with β‐mercaptoethanol added according to manufacturer’s instructions. Following removal of the supernatants during sputum processing, the remaining sputum cell pellet was resuspended in RLT buffer plus β‐mercaptoethanol. Total RNA was extracted from bronchial epithelial brushings and sputum cells by using ZR RNA MicroPrep kit (Zymo Research, Orange, California), and RNA‐sequencing libraries were prepared using TruSeq Stranded mRNA Prep kit (Illumina, San Diego, California) per manufacturer's protocols. To confirm RNA quality, RNA integrity number (RIN) scores were evaluated by using the 2100 Bioanalyzer system (Agilent, Santa Clara, California). Paired‐end sequencing (75 base pairs per end) with sequencing depth at 80 million reads was performed on the HiSeq2000 platform (Illumina) to generate FASTQ files. These were aligned to human genome (version HG19) using HiSAT2 (John Hopkin’s University, Baltimore, Maryland) and SAMtools (Genome Research Limited, Cambridge, UK). Normalized read counts were generated per transcript using DESeq2. Read counts were then transformed to log2 scale (after adding 1 to account for zero read counts).
Comparisons between groups were made by χ2 tests or unpaired t tests, as the data were normally distributed. Pearson correlations were performed to determine associations.
Results
Patient demographics are shown in Table 1. There were no differences in clinical characteristics, except for a higher modified Medical Research Council score indicating greater dyspnea in the BMI > 24.9 group (mean, 1.9 versus 1.0; P = 0.02). The bronchial expression of ACE2 was significantly higher in the BMI > 24.9 group compared with the BMI ≤ 24.9 group (means, 6.91 vs. 7.35, respectively; P = 0.004; Figure 1A). There was moderate positive correlation between BMI and ACE2 expression, which marginally failed to reach statistical significance (r = 0.3; P = 0.057; Figure 1B).
TABLE1 1.
Clinical characteristics of study population
| BMI ≤ 24.9 (n = 14) | BMI > 24.9 (n = 23) | P value | |
|---|---|---|---|
| Gender (female, %) | 36 | 30 | 0.7 |
| Age (y) | 61 (4) | 62 (6) | 0.4 |
| BMI (kg/m2) | 21 (2) | 29 (3) | < 0.0001 |
| Current smokers (%) | 71 | 40 | 0.06 |
| Pack years | 42 (15) | 39 (14) | 0.5 |
| FEV1 (L) | 2.0 (0.4) | 1.9 (0.4) | 0.3 |
| FEV1 (%) | 68 (11) | 63 (11) | 0.2 |
| FEV1/FVC ratio | 0.51 (0.06) | 0.52 (0.1) | 0.7 |
| ICS (%) | 64 | 70 | 0.7 |
| LABA (%) | 50 | 70 | 0.2 |
| LAMA (%) | 57 | 61 | 0.8 |
| Total SGRQ | 35 (21) | 42 (18) | 0.3 |
| CAT | 19 (9) | 17 (9) | 0.7 |
| mMRC | 1.0 (1.0) | 1.9 (1.1) | 0.02 |
| Exacerbation rate 12 months prior | 0.9 (1.2) | 0.7 (1.0) | 0.5 |
Data presented as % or mean ± SD.
CAT, COPD Assessment Test, FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroids; LABA, long‐acting beta 2 agonist; LAMA, long‐acting muscarinic antagonist; mMRC, modified Medical Research Council; SGRQ, St George’s Respiratory Questionnaire.
Figure 1.
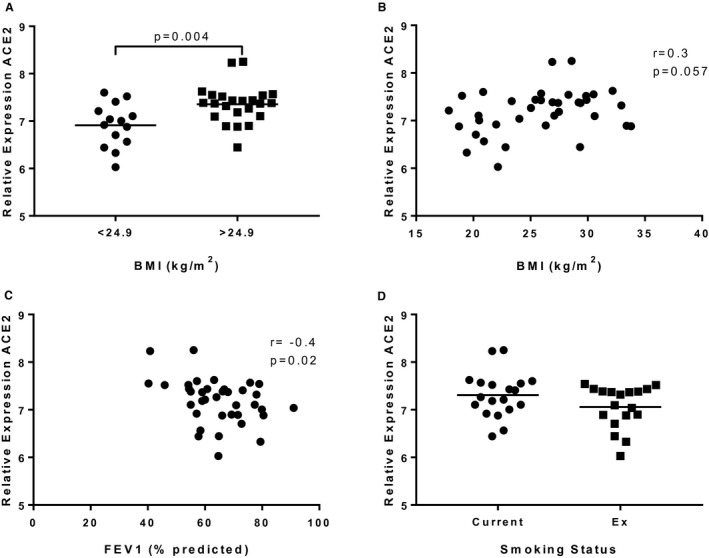
Bronchial epithelial ACE2 gene expression in patients with COPD. RNA was collected from bronchial brushings obtained during bronchoscopy, and ACE2 gene expression was examined by RNA sequencing. (A) COPD patients were separated into two groups: BMI < 24.9 (n = 14) and BMI > 24.9 (n = 23). Data presented as individual points with mean. (B) ACE2 gene expression was plotted against BMI, and a Pearson correlation coefficient was calculated. (C) ACE2 gene expression was plotted against FEV1% predicted, and a Pearson correlation coefficient was calculated. (D) ACE2 gene expression was examined in COPD current smokers (n = 19) and COPD ex‐smokers (n = 18). Data are presented as individual points with mean.
There was a trend for increased ACE2 expression in bronchial epithelial cells in COPD current versus COPD ex‐smokers, but this did not reach statistical significance (P = 0.1; Figure 1C). There was no correlation between pack‐year smoking history and ACE2 expression (data not shown). There was a negative association between FEV1% predicted and ACE2 expression (r = −0.4 P = 0.02; Figure 1D). There was no association between FEV1% predicted and BMI (r = −0.1; P = 0.5). There were no differences in ACE2 expression in individuals with hypertension (n = 10) and without hypertension (n = 27), nor in patients with hypertension receiving ACE inhibitors compared with those not (n = 5 each group; data not shown).
The expression of transmembrane protease serine 2 (TMPRSS2) in the bronchial epithelium of the BMI > 24.9 group compared with the BMI ≤ 24.9 group was similar, and we found no difference (P = 0.2; means, 11.36 vs. 11.55, respectively).
Discussion
Patients with COPD hospitalized with COVID‐19 have more severe disease and increased mortality (1, 3, 4). Leung et al. (11) showed increased ACE2 bronchial epithelial expression in patients with COPD compared with controls. As SARS‐CoV‐2 utilizes ACE2 for cellular entry (10), increased ACE2 expression in the bronchial epithelium may enhance opportunities for viral entry and survival. We now demonstrate increased bronchial epithelium ACE2 expression in patients with COPD who are overweight compared with those who are not overweight. Obesity is a risk factor for worse clinical outcomes in COVID‐19 (5, 6, 7). If increased ACE2 expression allows greater virus uptake, then our data suggest that the subgroup of overweight patients with COPD have a higher risk for developing severe COVID‐19.
Information regarding BMI has not been included in all cohort studies of patients hospitalized with COVID‐19. The relationship between BMI and outcomes may be masked by other coexisting systemic comorbidities (15). Our study highlights a subgroup of individuals with a comorbidity (COPD) who are overweight; this subgroup expresses higher levels of the receptor for SARS‐CoV‐2.
Similar to Leung et al. (11), we observed a negative association between FEV1% predicted and ACE2 expression. Additionally, recent studies have shown that current smoking increases ACE2 expression in the bronchial epithelium (16). Similarly, we observed a numerical increase in ACE2 expression in current smokers compared with ex‐smokers with COPD. The proportion of current smokers was numerically higher in the BMI ≤ 24.9 group and the BMI > 24.9 group, suggesting current smoking may have reduced the size of the differences we observed regarding weight.
SARS‐CoV‐2 cellular entry depends on priming of the spike glycoprotein to facilitate attachment to host cells. The serine protease TMPSSR2 is important for this process (17). We did not observe a difference in the expression of TMPRSS2 between the BMI ≤ 24.9 group and the BMI > 24.9 group.
A limitation of this study is that we studied gene expression alone, without protein expression studies. However, Leung et al. (11) showed that increased COPD bronchial ACE2 gene expression was replicated in protein expression studies, providing confidence that the ACE2 gene expression results reported relate to protein levels.
We did not observe any differences in ACE2 expression in hypertensive compared with non‐hypertensive patients. Simonnet et al. (6) previously showed increased COVID‐19 severity in patients with obesity independent of hypertension. Increased ACE2 expression may provide one mechanism whereby SARS‐CoV‐2 is more virulent, and other complex mechanisms may exist in hypertensive individuals.
In summary, we have shown increased ACE2 expression in the bronchial epithelium of patients with COPD who are overweight compared with those who are not overweight. This may provide increased opportunities for SARS‐CoV‐2 infection of the respiratory tract. These individuals may be at greater risk for developing severe COVID‐19.
Funding agencies
This research was supported by the NIHR Manchester Biomedical Research Centre and the North West Lung Centre Charity, Manchester. This report is independent research and the views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Disclosure
AH has received personal fees from Chiesi. DS has received personal fess from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Glenmark, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Therevance, and Verona.
References
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med 2020;201:1380‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID‐19: evidence from meta‐analysis. Aging 2020;12:6049‐6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID‐19 hospital admission. Clin Infect Dis 2020;71:896‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simonnet A, Chetboun M, Poissy J, et al.; LICORN and the Lille COVID‐19 and Obesity study group . High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with COVID‐19. Obesity (Silver Spring) 2020;28:1200‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leung JM, Yang CX, Tam A, et al. ACE‐2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID‐19. Eur Respir J 2020;55:2000688. doi:10.1183/13993003.00688‐2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vanfleteren L, Spruit MA, Wouters EFM, Franssen FME. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med 2016;4:911‐924. [DOI] [PubMed] [Google Scholar]
- 13. Pinheiro TA, Barcala‐Jorge AS, Andrade JMO, et al. Obesity and malnutrition similarly alter the renin‐angiotensin system and inflammation in mice and human adipose. J Nutr Biochem 2017;48:74‐82. [DOI] [PubMed] [Google Scholar]
- 14. Kolsum U, Damera G, Pham TH, et al. Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J Allergy Clin Immunol 2017;140:1181‐1184.e7. [DOI] [PubMed] [Google Scholar]
- 15. Dietz W, Santos‐Burgoa C. Obesity and its implications for COVID‐19 mortality. Obesity (Silver Spring) 2020;28:1005. [DOI] [PubMed] [Google Scholar]
- 16. Cai G, Bosse Y, Xiao F, Kheradmand F, Amos CI. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS‐CoV‐2. Am J Respir Crit Care Med 2020;201:1557‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]


