Abstract
Coronavirus disease 2019 (COVID‐19) infection has the potential for targeting the central nervous system, and several neurological symptoms have been described in patients with severe respiratory distress. Here, we described the case of a 60‐year‐old patient with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection but only mild respiratory abnormalities who developed an akinetic mutism attributable to encephalitis. Magnetic resonance imaging was negative, whereas electroencephalography showed generalized theta slowing. Cerebrospinal fluid analyses during the acute stage were negative for SARS‐CoV‐2, positive for pleocytosis and hyperproteinorrachia, and showed increased interleukin‐8 and tumor necrosis factor‐α concentrations. Other infectious or autoimmune disorders were excluded. A progressive clinical improvement along with a reduction of cerebrospinal fluid parameters was observed after high‐dose steroid treatment, thus arguing for an inflammatory‐mediated brain involvement related to COVID‐19. ANN NEUROL 2020;88:423–427.
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is characterized by respiratory tract symptoms, with possible severe outcome mostly related to pneumonia and severe acute respiratory distress syndrome (SARS). 1 Data regarding specific neurological involvement attributable to SARS‐CoV‐2 infections are lacking. Neurological symptoms, including headache and delirium, have been described in epidemiological studies in ≤30% of patients. 2 Although previous coronavirus infections have been associated with involvement of the central nervous system (CNS), 3 the neurotropism of COVID‐19 is still under debate. 4 , 5 , 6
The present report describes a case of encephalitis in a patient with COVID‐19. During his hospitalization, he never developed SARS but had mild respiratory symptoms.
Patient and Methods
The patient provided informed written consent for the report, and the diagnostic/therapeutic procedures were conducted in accordance with institutional and international guidelines for protection of human subjects. The study was approved by the local ethics committee of the ASST Spedali Civili di Brescia Hospital, and the requirement for informed consent was waived by the Ethics Commission (NP 4067, approved May 8, 2020).
Results
An otherwise healthy 60‐year‐old man presented to the Emergency Department with a severe alteration of consciousness. According to the relatives, the symptoms started 5 days earlier with the development of progressive irritability, confusion, and asthenia, followed 2 days later by fever, cough, and cognitive fluctuations. At admission, his vital parameters were within the normal ranges and his body temperature was 36.8°C. Oxygen saturation was within normal limits, with a slight reduction of the arterial partial pressure of oxygen (73mmHg).
The patient showed a severe akinetic syndrome associated with mutism; he was uncooperative and unable to carry out even simple orders if not stimulated. Positive palmomental and glabella reflexes with moderate nuchal rigidity were detected, with no focal signs at the neurological examination.
Blood analyses revealed normal blood cell counts, increased D‐dimer (968ng/ml) but normal concentrations of C‐reactive protein, fibrinogen, and ferritin. Chest X‐ray showed moderate bilateral interstitial pneumonia. Based on the history, the radiological findings, and the COVID‐19 outbreak in the region, a real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) assay on a nasopharyngeal swab was performed and confirmed a SARS‐CoV‐2 infection. Antiviral therapy with lopinavir/ritonavir 400/100mg twice daily and hydroxychloroquine 200mg twice daily was started.
A brain computed tomography scan was unremarkable, and the patient was hospitalized. At admission, a lumbar puncture was performed, and cerebrospinal fluid (CSF) showed inflammatory findings, with mild lymphocytic pleocytosis (18/μl) and a moderate increase of CSF protein (69.6mg/dl). The analysis of neurotropic viruses (herpes simplex virus [HSV]‐1, HSV‐2, HSV‐6, HSV‐8, Epstein‐Barr virus, varicella‐zoster virus, adenovirus, and enterovirus) was negative. CSF RT‐PCR for SARS‐CoV‐2 was also negative. An electroencephalogram (EEG) exhibited generalized slowing, with decreased reactivity to acoustic stimuli (Figure 1). The same day, an empirical treatment of ampicillin and acyclovir was introduced. The day after, magnetic resonance imaging (MRI) with gadolinium contrast did not reveal any significant alterations or contrast‐enhanced areas within the brain and/or meninges (Figure 1). Three days after admission, given the persistence of clinical symptoms, high‐dose intravenous steroid treatment was started (methylprednisolone 1g/day for 5 days). CSF analyses, carried out 1 day after steroid administration, showed lymphocytic pleocytosis (18/μl), hyperproteinorrachia (127.2mg/dl), and normal Link index without oligoclonal bands. CSF RT‐PCR for SARS‐CoV‐2 was still negative.
FIGURE 1.
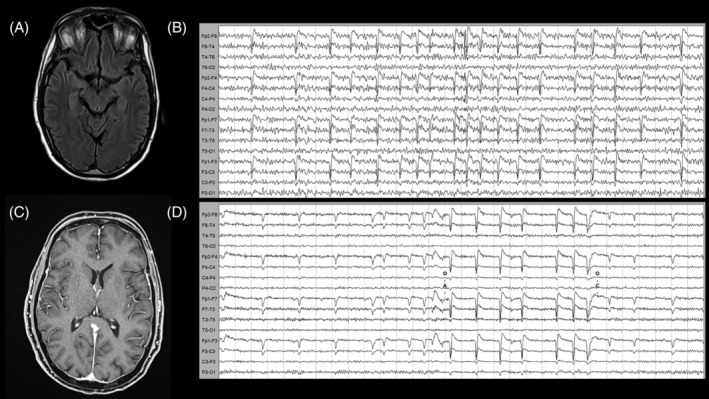
Magnetic resonance imaging (MRI) and electroencephalography (EEG) findings. (A,C) Normal MRI findings on T2 (A) and T1 with gadolinium (C). (B,D) EEG alterations at admission during the akinetic mutism state with global mid‐amplitude theta slowing without reactivity to eye opening (B) and reorganization of the background alpha rhythm and reactivity after 5 days of high‐dose steroid treatment (D). EEG acquisition settings: 10–20 system, longitudinal montage. Recording speed: 30s/page; Sensitivity: 7μV/mm; time constant: 0.1s, high‐frequency filter: 15Hz.
At this time, CSF analysis revealed a slightly increased concentration of interleukin‐6 (IL‐6) of 2.36pg/ml, a strongly increased concentration of interleukin‐8 (IL‐8, >1100pg/ml), and increased concentrations of tumor necrosis factor alpha (TNF‐α, 1.31pg/ml) and β2‐microglobulin (β2M, 3.06mg/l) (Figure 2). Biomarkers for neuronal injury (Tau and neurofilament light chain [NfL]) were normal (Supplementary Table 1). After the first infusion, the patient improved. He became alert and able to execute simple tasks on command and to repeat single words. During the following days, the patient was able to talk and to answer simple questions. Once the 5 days of high‐dose steroid therapy ended, he displayed only mild disinhibition and somewhat fluctuating alertness.
FIGURE 2.
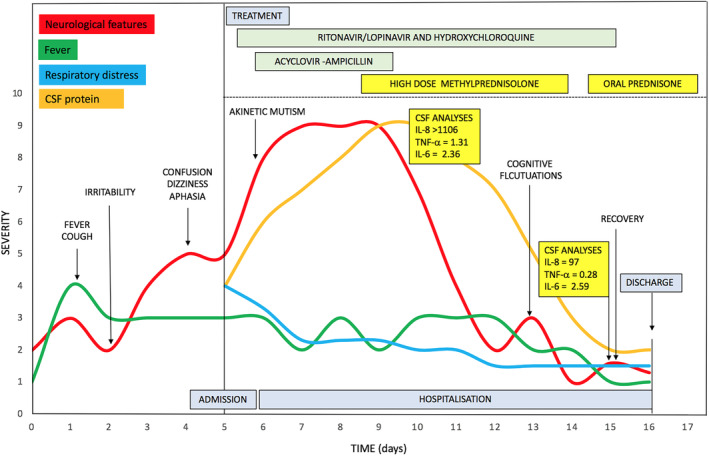
Clinical features, treatment, and respiratory/inflammatory profile during the disease course. Abbreviations: CSF = cerebrospinal fluid; IL‐6 = interleukin 6; IL‐8 = interleukin 8; TNF‐α = tumor necrosis factor alpha.
A follow‐up MRI (9 days after admission) was normal. Serial EEG revealed improvement and normalization of reactivity and slowing (Fig 1D). A wide immunological screening of immune‐mediated encephalitis (antibodies against NMDAR, LGI1, CASPR2, , GABAB1R, GABAB2R, AMPA1, AMPA2, Ri, Yo, Ma2, CV2, Hu, and amphiphysin) was negative, as were anti‐thyroglobulin, anti‐thyroid peroxidase, and anti‐MOG antibodies (Supplementary details).
Ten days after hospital admission, CSF protein concentrations decreased (91.4mg/dl), but lymphocytic pleocytosis (38 cells/μl) increased. CSF IL‐8 and TNF‐α decreased to 97pg/ml and 0.28pg/ml, respectively, whereas IL‐6 and β2‐microglobulin values were stable (Supplementary table 1). CSF Tau and NfL concentrations remained normal. Given the remaining neuroinflammatory changes, oral prednisone was started, with progressive tapering. At discharge, 11 days after admission, the patient presented with normal neurological examination.
Discussion
The potential neurotropism of SARS‐CoV‐2 with a direct or autoimmune‐mediated response is still debated. 4 , 5 , 6 , 7 Here, we have described a case of steroid‐responsive encephalitis in a patient with confirmed SARS‐CoV‐2 infection.
After the first reports describing severe pneumonia cases in Wuhan, China, COVID‐19 rapidly spread worldwide, presenting critical challenges for the health‐care systems globally. 8 , 9 The World Health Organization has thus declared COVID‐19 a public health emergency of international concern, with >200,000 deaths by April 25, 2020. Brescia, situated in the Lombardy region, northern Italy, was one of the provinces most severely affected by COVID‐19, accounting for >2100 deaths by April 25, 2020.
Epidemiological studies showed that COVID‐19 presents, in most cases, with fever and upper respiratory symptoms. 2 Recently, the study performed by Mao and colleagues 6 reported neurological manifestations of COVID‐19 in the outbreak in China in ≤36.4% of patients hospitalized, including alteration of consciousness, headache, dizziness, and delirium. 6 Neurological manifestations were also reported in previous epidemiological studies on larger samples. 2 Neuro‐infectivity has been described for other coronaviruses but is still questioned for SARS‐CoV‐2. Support for a hypothesis of CNS infection through a nasopharyngeal route is provided by clinical observations of frequent and persistent anosmia/dysgeusia.
Several authors have reported neurological symptoms in severe cases, which supports the concept that CNS symptoms might be secondary to severe respiratory failure. 7
Another important aspect is the hyperinflammation state secondary to SARS‐CoV‐2 infection, with a massive release of cytokines and chemokines that could alter the permeability of the blood–brain barrier. This phenomenon could result in activation of the neuroinflammation cascade. 5 Hypothetically, SARS‐CoV‐2 could also induce, by molecular mimicry‐related mechanisms, the production of antibodies against neural or glial cells, as demonstrated for HSV‐1, Epstein‐Barr virus, and Japanese encephalitis. 10
The COVID‐19 case described here is of interest because the patient presented with encephalitis but with only mild respiratory alterations. Meningeal signs at presentation suggested meningoencephalitis, and the patient commenced combined antibiotic and antiviral treatment, according to current guidelines for CNS infections. 11 Lack of presence of COVID‐19 in two different CSF samples could not definitely exclude CNS infection because the presence of the virus in CSF could be transient or not detectable, such as in the cases of 40% of West Nile virus and ≤69% of enterovirus infections. 12 In our case, the blood–brain barrier alterations supported by CSF analyses argue for a potential CNS invasion of SARS‐CoV‐2 via the blood route. 3 Of interest, a peculiar presentation of akinetic mutism has been associated with cases of Epstein–Barr encephalitis. 13 However, the normal MRI findings, with no meningeal enhancement or brain alterations, and the response to steroid treatment support alternative pathophysiological mechanisms. In this case, we excluded the most common causes of autoimmune encephalitis, akinetic mutism, and psychosis. 14 , 15 , 16 Another possible explanation of transitory akinetic mutism would be an abnormal neuroinflammatory response induced by SARS‐CoV‐2, 17 , 18 better fitting with the prompt response to steroid treatment.
To support this hypothesis, we measured CSF concentrations of IL‐6, IL‐8, TNF‐α, and β2‐microglobulin at two time points. At the time of presentation with akinetic mutism, we observed pleocytosis and a severe increase of CSF inflammatory proteins, IL‐8 in particular, but also TNF‐α and β2‐microglobulin. After clinical improvement, the inflammatory biomarkers decreased, whereas β2‐microglobulin and CSF cell counts remained abnormal. This suggests a possible cytokine‐mediated hyperinflammatory response. The role of steroid treatment in the resolution of symptoms of this patient, however, definitely requires further observations to be confirmed. Indeed, an unpublished work posted on a pre‐print server described two cases of suspected encephalitis associated with COVID‐19, in which improvement occurred in a similar time frame independent of treatment. 19 Of interest, alterations in CSF cytokines were not associated with severe abnormalities of peripheral markers of inflammation or severe respiratory involvement, 19 arguing for a CNS‐specific abnormal inflammatory response leading to neurological manifestation. 20 This fits with the normal serial imaging and the absence of biomarker evidence of neuronal injury, suggesting a functional rather than a destructive impairment of the neuronal network.
Conclusions
The case described highlights that COVID‐19 might involve severe neurological alterations independently from respiratory function. Despite the lack of a clear pathophysiological mechanism, the apparently positive response to steroid treatment together with the normalization of CSF cytokines suggests that the encephalitis in our patient was mediated by a hyperinflammatory mechanism. Nevertheless, although there was no evidence of SARS‐CoV‐2 in the CSF by RT‐PCR, a direct CNS infection cannot be excluded. We suggest that it might be useful to investigate the therapeutic effects of corticosteroid administration in COVID‐19‐related encephalitis.
Author Contributions
A.P., S.O., S.M., and A.P. contributed to the conception and design of the study; A.P., S.O., S.M., A.C., I.V., S.G., S.N., A.P., E.F., A.C., M.L., M.P.P., R.G., F.C., N.J.A., K.B., H.Z., and A.P. contributed to the acquisition and analyses of data; A.P., S.O., S.M., S.G., and A.P. contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest
Nothing to report.
Supporting information
Supplementary Table 1 Blood and cerebrospinal fluid analyses
Acknowledgments
We thank the patient for his participation in the study.
References
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. China medical treatment expert Group for Covid‐19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2019;12:14. 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chem Nerosci 2020;11:995–998. [DOI] [PubMed] [Google Scholar]
- 5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol 2020. 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao L, Jin H, Wang M, et al. Neurological manifestations of hospitalized patients with COVID‐19 in Wuhan, China: a retrospective case series study. JAMA Neurol 2020. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou L, Zhang M, Gao J, Wang J. Sars‐Cov‐2: underestimated damage to nervous system. Travel Med Infect Dis 2020;101642. 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lancet Infectious disease . COVID‐19, a pandemic or not? Lancet Infect Dis 2020;20:383. 10.1016/S1473-3099(20)30180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kupferschmidt K, Cohen J. Can China's COVID‐19 strategy work elsewhere? Science 2020;367:1061–1062. 10.1126/science.367.6482.1061. [DOI] [PubMed] [Google Scholar]
- 10. Dorcet G, Benaiteau M, Bost C, et al. Two cases of late‐onset anti‐NMDAr auto‐immune encephalitis after herpes simplex virus 1 encephalitis. Front Neurol 2020;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sigfrid L, Perfect C, Rojek A, et al. A systematic review of clinical guidelines on the management of acute, community‐acquired CNS infections. BMC Med 2019;17:170. 10.1186/s12916-019-1387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis 2013;57:1114–1128. 10.1093/cid/cit458.Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodrigo‐Armenteros P, Kapetanovic‐García S, Antón‐Méndez L, et al. Akinetic mutism and status epilepticus due to Epstein Barr virus encephalitis. Clin Neurol Neurosurg 2019;185:105492. [DOI] [PubMed] [Google Scholar]
- 14. Dale RC, Church AJ, Surtees RA, et al. Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain 2004;127:21–33. [DOI] [PubMed] [Google Scholar]
- 15. Pollak TA, Lennox BR, Müller S, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry 2020;7:93–108. 10.1016/S2215-0366(19)30290-1. [DOI] [PubMed] [Google Scholar]
- 16. Linnoila JJ, Rosenfeld MR, Dalmau J. Neuronal surface antibody mediated autoimmune encephalitis. Semin Neurol 2014. Sep;34:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehta P, McAuley DF, Brown M, et al. HLH across Speciality collaboration, UK. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034. 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moore BJB, June CH. Cytokine release syndrome in severe COVID‐19. Science 2020;368:473–474. 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 19. Bernard‐Valnet R, Pizzarotti B, Anichini A, et al. Two patients with acute meningo‐encephalitis concomitant to SARS‐CoV2 infection. Eur J Neurol 2020. 10.1111/ene.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sajjad MU, Blennow K, Knapskog AB, et al. Erebrospinal fluid levels of Interleukin‐8 in delirium, dementia, and cognitively healthy patients. J Alzheimers Dis 2020;73:1363–1372. 10.3233/JAD-190941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Blood and cerebrospinal fluid analyses


