This article refers to ‘Myocardial localization of coronavirus in COVID‐19 cardiogenic shock’ by G. Tavazzi et al., published in Eur J Heart Fail 2020;22:911–915.
The current pandemic coronavirus disease 2019 (COVID‐19) is primary a respiratory infection caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Epidemiological data from China show that approximately 20% of COVID‐19 patients have concomitant established cardiovascular diseases and are more likely to develop life‐threatening complications in the course of infection. 1 In some cases, the development of myocarditis, a cardiac disorder characterized by inflammatory cell infiltration of the heart and greater risk of deterioration of cardiac function, 2 has been clinically suggested. However, endomyocardial biopsy (EMB) analysis as the gold standard diagnostic tool to verify the clinical diagnosis 3 and to understand the underlying pathomechanisms at the cellular level, had rarely been used.
To the best of our knowledge, only 11 single cases, including the case of Tavazzi et al., 4 have been reported so far of probable SARS‐CoV‐2‐associated myocarditis (Table 1 ). 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 In most of the cases, a SARS‐CoV‐2‐related myocarditis was clinically suspected based on cardiac biomarkers, combined with echocardiography and electrocardiogram, and in five cases combined with magnetic resonance imaging findings. One group also provided a case in the absence of any clinical symptoms or cardiac functional limitations. 12 In a post‐mortem examination, endothelial inflammation was found in a patient with suspected myocarditis. 14 In the same case series, global endotheliitis was associated with viral inclusion structures in endothelial cells in severe COVID‐19‐diseased patients. This indicates that the findings and definitions are not completely consistent and that several clinical‐based ‘myocarditis‐like syndromes’ are described. Thus, in most of these reports, no pathophysiological confirmation of the suspected diagnosis occurred. Only in one report, EMB analysis was performed, describing a mild lymphocytic myocarditis in the absence of myocardial SARS‐CoV‐2 RNA presence. 9
Table 1.
Case reports of myocarditis‐like syndromes
| Study | COVID‐19 nasopharyngeal swab | MC diagnosis after clinical onset | Clinical parameters | Cardiac biomarker | MRI | EMB | EMB | Diagnosis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Histology | Immunohistology | Viral PCR or EM | ||||||||
| Inciardi et al. 5 | Positive | 7 days |
No respiratory involvement EF 35% |
Troponin: elevated NT‐proBNP: elevated |
Yes (T1/T2 pos) | No | _ | _ | _ | COVID‐19 myo‐pericarditis |
| Zeng et al. 6 | Positive (Cycle threshold) | ? |
ARDS, shock LVEDD 61 mm EF 32% |
Troponin: elevated NT‐proBNP: elevated |
No | No | _ | _ | _ | COVID‐19‐complicated fulminant myocarditis |
| Hu et al. 7 | Positive | 3 days |
Pneumonia, shock LVEDD 58 mm EF 27% |
Troponin: ? BNP elevated |
No | No | _ | _ | _ | Coronavirus infection‐caused fulminant myocarditis |
| Kim et al. 8 | Positive | ? | Pneumonia, severe LV systolic dysfunction |
Troponin: elevated NT‐proBNP: elevated |
Yes (T1/T2 pos) | No | _ | _ | _ | COVID‐19‐related myocarditis |
| Sala et al. 9 | Positive | 3 days |
Pneumonia, no LV dilatation EF 43% |
Troponin: elevated NT‐proBNP: elevated |
Yes (T1/T2 pos) | Yes | x | Mild inflammation | Negative for SARS‐CoV‐2 | Acute virus‐negative lymphocytic myocarditis associated with SARS‐CoV‐2 respiratory infection |
| Tavazzi et al. 4 | Positive | 4 days |
ARDS, shock LVEDD 56 mm EF 34% |
Troponin: elevated NT‐proBNP: ? |
No | Yes | Dallas criteria negative | Low‐grade inflammation | SARS‐CoV‐2 particle detection in macrophages, no myocyte necrosis in EM PCR not provided | Low grade myocarditis in COVID‐19 cardiogenic shock |
| Irabien‐Ortiz et al. 10 | Positive | 5 days | Shock, severe biventricular failure |
Troponin: elevated NT‐proBNP: elevated |
No | No | _ | _ | _ | Fulminant COVID‐19 myocarditis |
| Hua et al. 11 | Positive | ? |
Shock due to LV perforation Normal EF |
Troponin: elevated NT‐proBNP: ? |
No | No | _ | _ | _ | COVID‐19 myo‐pericarditis |
| Paul et al. 12 | Positive | ? |
Absolutely no clinical signs Normal EF |
Troponin: elevated NT‐proBNP: ? |
Yes | No | _ | _ | _ | COVID‐19 myocarditis |
| Doyen et al. 13 | positive | ? |
ARDS Normal EF |
Troponin: elevated NT‐proBNP: ? |
Yes | No | _ | _ | _ | COVID‐19 myocarditis |
| Varga et al. 14 | Positive | N/A |
Severe hypoxic failure Preserved EF |
Troponin: elevated NT‐proBNP: elevated |
No | Post‐mortem tissue | Endothelial inflammation in heart, small bowel lung | _ | SARS‐CoV‐2 particle detection in renal endothelial cells in EM PCR not provided | Global endotheliitis in COVID‐19 |
| Positive | 16 days |
ARDS Normal EF |
Troponin: elevated NT‐proBNP:? |
No | Post‐mortem tissue | Endothelial inflammation in lung, heart, kidney and liver | _ | _ | ||
_, not performed; ARDS, acute respiratory distress syndrome; COVID‐19, coronavirus disease 2019; EF, ejection fraction; EM, electron microscopy; EMB, endomyocardial biopsy; LV, left ventricular; LVEDD, left ventricular end‐diastolic dimension; MC, myocarditis; MRI, magnetic resonance imaging; N/A, not applicable; NT‐proBNP, N‐terminal pro brain natriuretic peptide; PCR, polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
In the previous issue of the Journal, Tavazzi et al. 4 provide the first case of EMB‐proven localization of viral particles in the heart with morphology and size typical of SARS‐CoV‐2 in a COVID‐19 patient presenting with cardiogenic shock. Viral particles are observed in interstitial macrophages and their surroundings, but not in cardiomyocytes or endothelial cells, excluding the direct damage of the heart due to virus replication. In contrast to the clinical presentation suggestive for severe and necrotizing acute myocarditis, only low‐grade myocardial inflammation and absence of myocyte necrosis was observed. This is in agreement with Xu et al. 15 who also only found low‐grade inflammatory responses in cardiac autopsy species of a COVID‐19 patient with acute respiratory distress syndrome. Furthermore, cardiac myocytes showed only non‐specific features such as myofibrillar lysis, and no signs of myocyte hypertrophy. There were no indications of vasculitis or thrombosis, and fibrosis was mainly perivascular.
This case report is of important value since it demonstrates for the first time the cardiac presence of SARS‐CoV‐2 in a COVID‐19 patient with cardiogenic shock. Despite the tropism of SARS‐CoV‐2 for angiotensin‐converting enzyme 2 (ACE2), which is expressed by cardiomyocytes, fibroblasts, endothelial cells and particularly by pericytes, 16 viral particles were interestingly not found in these cells, but in macrophages, which may have reached the heart during transient viremia or by migration, e.g. from the infected lung. The cellular serine proteases TMPRSS2, which is co‐expressed on lung cells expressing ACE2, and essential for viral entry, 17 is not co‐expressed in cardiomyocytes, pericytes and fibroblasts, 18 suggesting also its relevance for viral uptake in cardiac cells, without excluding the potential need of other co‐receptors like cathepsin B and L, for spike protein priming. Though, the absence of viral particles in cardiomyocytes, pericytes and fibroblasts seen in this patient may not be generalized, not knowing the cardiac (cellular)‐specific expression levels of ACE2 in this patient, the clinical history of this patient — ACE2 is increased in heart failure patients, 19 — and the SARS‐CoV‐2 viral load in the heart or other organs (lung). Investigations in model systems are needed to assess correlations between ACE2 and co‐receptor expression and viral uptake in specific cardiac cells and to evaluate whether SARS‐CoV‐2 can damage cardiac cells dependent or independent of virus replication in the myocardium.
The low‐grade cardiac inflammation on the one hand, and the presence of viral particles only in macrophages, as source of inflammation on the other hand, suggest that virus presence in the heart of COVID‐19 patients is rare. This advocates that the clinical presentation of fulminant myocarditis is rather caused by an indirect, e.g. via a cytokine storm, than a direct SARS‐CoV‐2‐mediated viral effect, or even by other concomitant diseases/infections. It further supports the relevance to exclude SARS‐CoV‐2 in the blood and the need to quantify SARS‐CoV‐2 copy numbers in EMB, allowing comparison with other cases and primary with virus load in nasopharyngeal swab material, to enable interpretation of the clinical and laboratory observations and to assess the severity of the infection and viremic response.
Despite the clinical manifestation similar to this of fulminant myocarditis, no lymphocytic myocarditis in heart tissue was diagnosed. This resembles the clinical presentation by influenza‐associated septic shock, which also mimics fulminant myocarditis, but occurs in the absence of inflammatory cell infiltration or necrosis of cardiomyocytes. 20 Acute respiratory infections as well as sepsis are often associated with a rise in troponin, indicative for cardiac injury 21 and in frame with the present case.
This case report underpins the importance of performing EMB analysis for quantitative SARS‐CoV‐2 RNA evaluation and specific immune cell quantification by immunohistological staining to enable correct diagnosis and insights in the potential association, or not, between SARS‐CoV‐2 and myocarditis. Since myocarditis is a cardiac disorder with a heterogeneous aetiology, associations between SARS‐CoV‐2 and myocarditis based on sole cardiac imaging should be interpreted with caution. At the same time, this case as an example of a SARS‐CoV‐2‐induced septic cardiomyopathy does not exclude the occurrence of SARS‐CoV‐2‐associated cardiac inflammation. Beyond in vitro and in vivo models enabling further insights related to direct or indirect (cytokine storm, autoantibody production) SARS‐CoV‐2‐mediated injury and/or cardiac immune cell presence, profound characterization and follow‐up of COVID‐19 patients are needed. In patients with suspected SARS‐CoV‐2‐associated myocarditis, this includes quantitative evaluation of SARS‐CoV‐2 RNA in nasopharyngeal swab, and in EMB in combination with immunohistological analysis of EMB according to European Society of Cardiology guidelines 3 and biomarker analysis. In COVID‐19 patients without any signs of cardiovascular involvement, SARS‐CoV‐2 copy numbers in nasopharyngeal swab and the collection of plasma/serum for potential subsequent (antibody) analyses are needed, to rule out the potential occurrence of myocarditis due to a SARS‐CoV‐2‐triggered autoantibody production, which might take place 3 to 4 weeks post‐infection.
In conclusion, this case reports for the first time cardiac presence of SARS‐CoV‐2 in a COVID‐19 patient with cardiogenic shock mimicking fulminant myocarditis. Many questions about COVID‐19‐related heart disease remain unanswered, calling for profound COVID‐19 patient characterization and model systems, stressing also basic virology studies enabling to study the pathogenesis of multifaced SARS‐CoV‐2‐related heart disease and to differentiate cardiac inflammatory‐like pathologies triggered by SARS‐CoV‐2 (Figure 1 ). These insights are needed to allow appropriate therapeutic regimens (antiviral vs. immunosuppressive/immunomodulatory).
Figure 1.
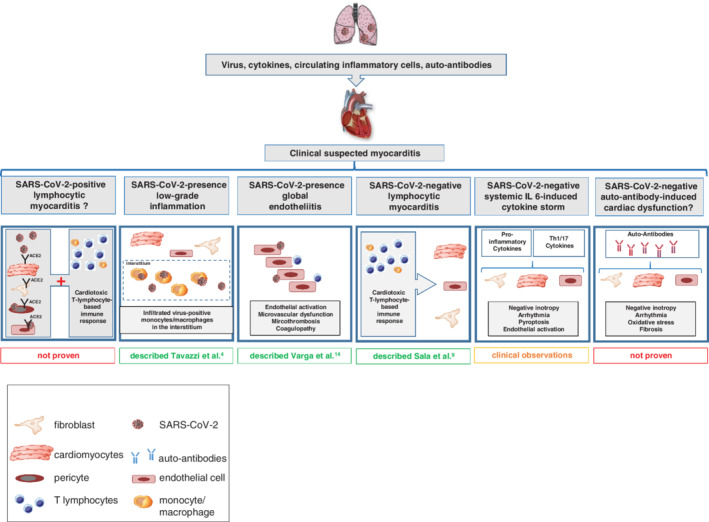
Hypothetical cardiac inflammatory‐like pathomechanisms triggered by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). So far, different cardiac inflammatory‐like pathomechanisms triggered by SARS‐CoV‐2 have been suggested: (i) lymphocytic myocarditis induced by SARS‐CoV‐2 infection of cardiac cells (SARS‐CoV‐2‐positive lymphocytic myocarditis), (ii) cardiac SARS‐CoV‐2 presence with low‐grade inflammation, (iii) cardiac SARS‐CoV‐2 presence and global endotheliitis, (iv) lymphocytic myocarditis in the absence of cardiac infection of SARS‐CoV‐2 (SARS‐CoV‐2‐negative lymphocytic myocarditis), (v) cardiac injury elicited by a SARS‐CoV‐2 triggered interleukin (IL)‐6‐induced cytokine storm, and (vi) cardiac dysfunction due to auto‐antibodies in the absence of SARS‐CoV‐2. Differentiation of cardiac inflammatory‐like pathologies triggered by SARS‐CoV‐2 is required to allow specific treatment strategies (antiviral vs. immunosuppressive/modulating). Therefore, biomarkers are needed and model systems, including basic virology studies.
Conflicts of interest: none declared.
The opinions expressed in this article are not necessarily those of the Editors of the European Journal of Heart Failure or of the European Society of Cardiology. doi: 10.1002/ejhf.1828
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020. Mar 27. 10.1001/jamacardio.2020.1286 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 4. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail 2020;22:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020. Mar 27. 10.1001/jamacardio.2020.1096 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, Wang LF, Gao H, Wang Y, Dong CF, Li YJ, Xie XJ, Feng C, Liu L. First case of COVID‐19 complicated with fulminant myocarditis: a case report and insights. Infection 2020. Apr 10. 10.1007/s15010-020-01424-5 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020. Mar 16. 10.1093/eurheartj/ehaa190 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim IC, Kim JY, Kim HA, Han S. COVID‐19‐related myocarditis in a 21‐year‐old female patient. Eur Heart J 2020;41:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C, Esposito A. Acute myocarditis presenting as a reverse Tako‐Tsubo syndrome in a patient with SARS‐CoV‐2 respiratory infection. Eur Heart J 2020;41:1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irabien‐Ortiz A, Carreras‐Mora J, Sionis A, Pamies J, Montiel J, Tauron M. Fulminant myocarditis due to COVID‐19. Rev Esp Cardiol (Engl Ed) 2020;73:503–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hua A, O'Gallagher K, Sado D, Byrne J. Life‐threatening cardiac tamponade complicating myo‐pericarditis in COVID‐19. Eur Heart J 2020;41:2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paul JF, Charles P, Richaud C, Caussin C, Diakov C. Myocarditis revealing COVID‐19 infection in a young patient. Eur Heart J Cardiovasc Imaging 2020;21:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID‐19: a cause of raised troponin and ECG changes. Lancet 2020;395:1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, Holubec T, Walther T, Zeiher AM, Dimmeler S. Cell type‐specific expression of the putative SARS‐CoV‐2 receptor ACE2 in human hearts. Eur Heart J 2020;41:1804–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffmann M, Kleine‐Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tschöpe C, Ammirati E, Bozkurt B, Caforio AL, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, Kelle S, Klingel K, Maatz H, Parwani AS, Spillmann F, Starling RC, Tsutsui H, Seferovic P, Van Linthout S. Inflammatory cardiomyopathy: current evidence and future directions. With special reference to virus‐induced and virus‐associated myocarditis. Nat Rev Cardiol 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS‐CoV‐2. Cardiovasc Res 2020;116:1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujioka M, Suzuki K, Iwashita Y, Imanaka‐Yoshida K, Ito M, Katayama N, Imai H. Influenza‐associated septic shock accompanied by septic cardiomyopathy that developed in summer and mimicked fulminant myocarditis. Acute Med Surg 2019;6:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. Elevated troponin in patients with coronavirus disease 2019 (COVID‐19): possible mechanisms. J Card Fail 2020;26:470‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]


