Abstract
Background and purpose
Patients with acute ischemic stroke are at high‐risk for contracting COVID‐19 infection. Additionally, healthcare professionals including neurovascular ultrasound providers are also at risk of being infected by SARS‐CoV‐2 virus. Yet, preparedness to continue to guarantee hyperacute treatment is vital for patients outcome. In light of this situation, the European Society of Neurosonology and Cerebral Hemodynamic (ESNCH) appointed a task force to provide consensus recommendations for the performance of neurovascular ultrasound investigations in acute ischemic stroke during the COVID‐19 pandemic with the aim of protecting both patients and ultrasound providers.
Methods
The “ultrasound in acute stroke working group” of the ESNCH examined literature articles and reviews using the following key words: “corona virus” or “COVID‐19” or “SARS‐CoV‐2 virus”, and “acute stroke” or “cerebrovascular disease”, and “ultrasound”. Thereafter, a thorough discussion was conducted with the “education and guidelines working group” of the ESNCH.
Results
We propose rapid up‐to‐date recommendations for healthcare personnel involved in the pre‐hospital and intra‐hospital assessment of stroke patients, with a particular attention to neurovascular ultrasound performance.
Conclusion
The ESNCH provides a guidance summary for the performance of neurovascular ultrasound investigations in acute ischemic stroke in the time of COVID‐19.
Keywords: COVID‐19, stroke, ultrasound
Introduction
Since the COVID‐19 pandemic stormed into healthcare systems worldwide, protected stroke pathways have been suggested, in order not to spread the viral infection and ensure hyper‐acute treatment [1, 2. Noteworthy, patients with acute ischaemic stroke are at high risk for contracting SARS‐CoV‐2 virus, particularly the severe form, because COVID‐19 and cerebrovascular diseases share common risk factors [3]. Conversely, amongst patients hospitalized with SARS‐CoV‐2 respiratory distress, about 5% might suffer a stroke [4]. During the acute stages of the pandemic, thousands of healthcare professionals have already contracted COVID‐19 infection [5, 6, 7, although the actual number is likely to be higher because healthcare workers are not always tested and protection measures at hospitals are not always readily available. This is the setting in which neurovascular ultrasound providers (physicians, sonographers) should expect to be involved in the care of stroke patients. In light of this situation, effective safety and prevention strategies need to be implemented not only during the lockdown period but especially afterwards when healthcare workers and patients will tend to let their guard down. Therefore, the European Society of Neurosonology and Cerebral Hemodynamics (ESNCH) is providing the following recommendations for the performance of neurovascular ultrasound investigations with the aim of protecting both patients and ultrasound providers. To develop this document, members of the Ultrasound in Acute Stroke Working Group of the ESNCH examined literature articles and reviews using the following keywords: ‘corona virus’ or ‘COVID‐19’ or ‘SARS‐CoV‐2 virus’, and ‘acute stroke’ or ‘cerebrovascular disease’, and ‘ultrasound’. The World Health Organization and the Center for Disease Control and Prevention (CDC) recommendations on the COVID‐19 pandemic were also consulted. After a thorough discussion with the Education and Guidelines Working Group of the ESNCH, a final consensus was reached (Table 1 and Fig. 1). The successful care of COVID‐19‐positive or presumed positive stroke patients will depend also on these prevention strategies [8].
Table 1.
ESNCH recommendations for neurovascular ultrasound in acute stroke during the COVID‐19 pandemic
| Pre‐hospital assessment |
| Field personnel should screen all acute stroke patients for COVID‐19 infection |
| If the screening is positive or medical history is not available, patients should be considered as possibly COVID‐19 infected |
| A COVID‐19 known positive or presumed positive patient should wear a surgical mask |
| Healthcare providers should use appropriate protection according to COVID status (see below) |
| Clinically oriented extracranial and intracranial ultrasound evaluation should be performed during transport to reveal large vessel occlusion strokes and indicate the most suitable destination for the patient |
| Intra‐hospital assessment |
| All stroke patients should undergo a fast track COVID‐19 nasopharyngeal test |
| COVID‐19‐negative patients |
| The ESNCH recommends that all ultrasound providers wear standard PPE: surgical mask, latex‐free disposable gloves and a disposable gown |
| COVID‐19‐positive or presumed positive patients |
| The ESNCH recommends that all ultrasound providers wear enhanced PPE: respirators (N95 or FFP2 masks), surgical cap, goggles, face shield, full‐sleeved gown, two pairs (internal and external) of extended cuff gloves and outer short cuff gloves, boot‐type shoe covers, and proper donning/doffing hygiene |
| Therapeutic decisions on systemic or endovascular reperfusion therapies may rely on ultrasound to avoid potential time delays related to computed tomography or magnetic resonance angiography |
| Organization of neurosonology laboratory |
| It is recommended to have one laboratory designated as a ‘COVID room’, stocked with enhanced PPE and with at least one dedicated ultrasound machine for COVID‐19‐positive or presumed positive patients |
| A room adjacent to the angiography suite should be designated as post‐thrombectomy ultrasound monitoring |
Figure 1.
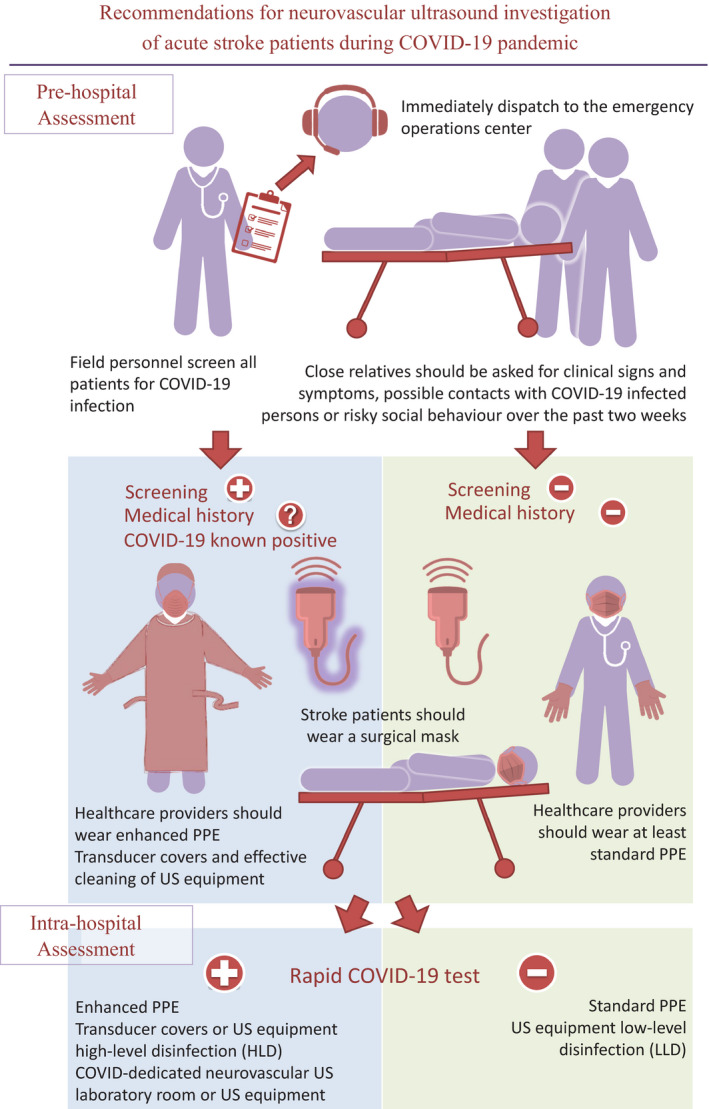
Recommendations for neurovascular ultrasound investigation of acute stroke patients during COVID‐19 Pandemic. PPE, personal protective equipment; US, ultrasound.
Pre‐hospital assessment
Field personnel should screen all patients for COVID‐19 infection using standardized checklists of clinical signs and symptoms (i.e. fever, cough, chest pain, dyspnoea, anosmia, ageusia, headache, myalgias and gastrointestinal symptoms including vomiting and diarrhoea) or history of close contact exposure with a COVID‐positive patient, and immediately dispatch these data to the emergency operations centre for preparedness. Stroke patients might not be able to provide a full history due to neurological impairment; in this case, close relatives should be asked for clinical signs and symptoms, possible contacts with COVID‐19‐infected persons or risky social behaviour over the past 2 weeks. If the screening is positive or medical history is not available, patients should be considered as possibly COVID‐19 infected. For safety reasons, a COVID‐19 known positive or presumed positive patient should wear a surgical mask, whilst healthcare providers should use appropriate protection according to COVID status (see below). Furthermore, clinically oriented extracranial and intracranial ultrasound evaluation, if available, should be performed during transport to reveal large vessel occlusion strokes and indicate the most suitable destination for the patient [9, 10. Eligibility for hyper‐acute therapies is not modified by the COVID‐19 pandemic; consequently current guidelines should be implemented [11, 12 as a denial of these therapies might increase the social burden of stroke, creating also a greater drain on healthcare resources [13].
Intra‐hospital assessment
If resources are available, all stroke patients should undergo a fast track COVID‐19 nasopharyngeal test in order to prevent in‐hospital transmission and indicate the most appropriate safety measures for ultrasound providers.
COVID‐19‐negative patients
As the sensitivity of nasopharyngeal swabs is about 70%, the ESNCH recommends wearing standard personal protective equipment (PPE): surgical mask, latex‐free disposable gloves and a disposable gown. Before and after direct patient contact, appropriate hand hygiene, even with a sanitizer, is imperative.
COVID‐19‐positive or presumed positive patients
Therapeutic decisions on systemic or endovascular reperfusion therapies for COVID‐19‐positive and presumed positive patients presenting with acute stroke symptoms may rely on ultrasound to avoid potential time delays related to computed tomography or magnetic resonance angiography [14]. Patients with COVID‐19‐positive documentation or those presumed positive should be treated with maximum safety precautions. Therefore, all ultrasound providers should wear enhanced PPE at all times: respirators (N95 or FFP2 masks), surgical cap, goggles, face shield, full‐sleeved gown, two pairs (internal and external) of extended cuff gloves and outer short cuff gloves, boot‐type shoe covers, and proper donning/doffing hygiene. In the case of resource limitations, a standard surgical mask can be worn over the N95 or FFP2 mask for preservation of the latter.
If resources are not available, stroke patients presumed to be COVID‐19 negative on a clinical and anamnestic basis should wear a surgical mask, whilst healthcare providers should wear at least standard PPE.
Ultrasound equipment
Since many patients who undergo neurovascular ultrasound will be COVID‐19 positive or presumed positive in the current environment, effective cleaning of ultrasound equipment is compulsory. Transducers and ultrasound equipment must be cleaned with a compatible disinfectant after each patient, also in accordance with local guidelines. For external procedures, such as extracranial and intracranial ultrasound, low‐level disinfection (LLD) is effective in agreement with CDC guidelines [15]. Common LLD agents include quaternary ammonium compounds, alcohols and phenols available as sprays and disinfectant wipes. Ultrasound providers should ensure that the chosen LLD method is compatible with the transducer; for example, alcohols are often contraindicated due to material incompatibility. If LLD agents are depleted, soap and water are a valid alternative. Noteworthy, external transducers that come into contact with contaminated skin, such as skin infections, should be covered with a single‐use transducer cover. If transducer covers are not available, medical gloves or other physical barriers as compatible medical dressings should be used. If the transducer comes in contact with mucous membranes or any body fluids, or a transducer cover is required and becomes compromised, the transducer must undergo high‐level disinfection (HLD). HLD agents include hydrogen peroxide, hypochlorite/hypochlorous acid, glutaraldehyde, peracetic acid, phenol/phenolate, chlorhexidine gluconate [16]. At the end of the day, the ultrasound room should also undergo appropriate cleaning and disinfection.
Organization of neurosonology laboratory
For hospitals with multiple neurovascular ultrasound laboratories, it is recommended to have one laboratory designated as a ‘COVID room’, stocked with enhanced PPE to minimize risk and with at least one dedicated ultrasound machine for COVID‐19‐positive or presumed positive patients, since COVID‐19 can survive on plastic surfaces for up to 72 h [17]. Concerning post‐thrombectomy ultrasound monitoring [18, 19, 20, 21, if possible a room adjacent to the angiography suite should be designated for this purpose, to minimize exposure of any at‐risk group of patients to potential COVID‐19 contact in the hospital environment.
Disclosure of conflict of interest
The authors declare no financial or other conflicts of interest.
See commentary by D. Školoudík and M. Mijajlović on page 1774
[Correction added on 18 August 2020, after first online publication: The corresponding author listed has been amended from A. Pieroni to C. Baracchini at the request of the authors, the co‐author name G. Tsvigoulis has been amended to G. Tsivgoulis in this version.]
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
References
- 1. Khosravani H, Rajendram P, Notario L, et al. Protected Code Stroke: Hyperacute stroke management during the coronavirus disease 2019 (COVID‐19) pandemic. Stroke 2020; 51: 1891–1895. 10.1161/STROKEAHA.120.029838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baracchini C, Pieroni A, Viaro F, et al. Acute stroke management pathway during Coronavirus‐19 pandemic. Neurol Sci 2020;41 :1003–1005. 10.1007/s10072-020-04375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol 2020; 109: 531–538. 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 2020. https://doi.org:10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Z, McGoogan JM, Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 6. Minder R, Peltier E. Virus knocks thousands of health workers out of action in Europe. The New York Times, 24 March 2020.
- 7. Coronavirus Disease 2019 (COVID‐19), Cases, Data, and Surveillance . Centers for Disease Control and Prevention. www.cdc.gov/coronavirus/2019‐ncov/cases‐updates (accessed 14 April 2020).
- 8. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid‐19. N Engl J Med 2020; 382: 2049–2055. 10.1056/NEJMsb2005114 [DOI] [PubMed] [Google Scholar]
- 9. Schlachetzki F, Herzberg M, Hölscher T, et al. Transcranial ultrasound from diagnosis to early stroke treatment: part 2: Prehospital neurosonography in patients with acute stroke: the Regensburg stroke mobile project. Cerebrovasc Dis 2012; 33: 262–271. 10.1159/000334667. [DOI] [PubMed] [Google Scholar]
- 10. Herzberg M, Boy S, Hölscher T, et al. Prehospital stroke diagnostics based on neurological examination and transcranial ultrasound. Crit Ultrasound J 2014; 6: 3. 10.1186/2036-7902-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kayan Y, Meyers PM, Prestigiacomo CJ, et al. Current endovascular strategies for posterior circulation large vessel occlusion stroke: report of the Society of NeuroInterventional Surgery Standards and Guidelines Committee. J Neurointerv Surg 2019; 11: 1055–1062. 10.1136/neurintsurg-2019-014873. [DOI] [PubMed] [Google Scholar]
- 12. Mokin M, Ansari SA, McTaggart RA, et al. Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): report of the SNIS Standards and Guidelines Committee. J Neurointerv Surg 2019; 11: 215–220. 10.1136/neurintsurg-2018-014640. [DOI] [PubMed] [Google Scholar]
- 13. Lekander I, Willers C, von Euler M, et al. Relationship between functional disability and costs one and two years post stroke. PLoS One 2017; 12: e0174861. 10.1371/journal.pone.0174861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chernyshev OY, Garami Z, Calleja S, et al. Yield and accuracy of urgent combined carotid/transcranial ultrasound testing in acute cerebral ischemia. Stroke 2005; 36: 32–37. 10.1161/01.STR.0000150496.27584.e3. [DOI] [PubMed] [Google Scholar]
- 15. Coronavirus Disease 2019 (COVID‐19). Centers for Disease Control and Prevention. www.cdc.gov/coronavirus/2019‐ncov (accessed 26 April 2020)
- 16. Agency USEP.List N: Disinfectants for use against SARS‐CoV‐2. https://www.epa.gov/pesticide‐registration/list‐n‐disinfectants‐use‐against‐sars‐cov‐2 (accessed 26 April 2020)
- 17. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med 2020; 382: 1564–1567. 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rubiera M, Cava L, Tsivgoulis G, et al. Diagnostic criteria and yield of real‐time transcranial Doppler monitoring of intra‐arterial reperfusion procedures. Stroke 2010; 41: 695–699. 10.1161/STROKEAHA.109.565762. [DOI] [PubMed] [Google Scholar]
- 19. Kneihsl M, Niederkorn K, Deutschmann H, et al. Increased middle cerebral artery mean blood flow velocity index after stroke thrombectomy indicates increased risk for intracranial hemorrhage. J Neurointerv Surg 2018; 10: 882–887. 10.1136/neurintsurg-2017-013617. [DOI] [PubMed] [Google Scholar]
- 20. Baracchini C, Farina F, Palmieri A, et al. Early hemodynamic predictors of good outcome and reperfusion injury after endovascular treatment. Neurology 2019; 92: e2774–e2783. 10.1212/WNL.0000000000007646. [DOI] [PubMed] [Google Scholar]
- 21. Sheriff F, Diz‐Lopes M, Khawaja A, et al. Microemboli after successful thrombectomy do not affect outcome but predict new embolic events. Stroke 2020; 51: 154–161. 10.1161/STROKEAHA.119.025856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


