Abstract
Coronavirus disease 2019 (COVID‐19) outbreak is an ongoing pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) with considerable mortality worldwide. The main clinical manifestation of COVID‐19 is the presence of respiratory symptoms, but some patients develop severe cardiovascular and renal complications. There is an urgency to understand the mechanism by which this virus causes complications so as to develop treatment options. Curcumin, a natural polyphenolic compound, could be a potential treatment option for patients with coronavirus disease. In this study, we review some of the potential effects of curcumin such as inhibiting the entry of virus to the cell, inhibiting encapsulation of the virus and viral protease, as well as modulating various cellular signaling pathways. This review provides a basis for further research and development of clinical applications of curcumin for the treatment of newly emerged SARS‐CoV‐2.
Keywords: acute respiratory distress syndrome, curcuminoids, pulmonary fibrosis, viral infection
1. INTRODUCTION
Coronaviruses (COVs) have been the cause behind severe acute respiratory diseases in humans in the 21st century. The severe acute respiratory coronavirus (SARS‐CoV) in 2003 was associated with 10% fatality (Lee et al., 2003) while the Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) had a fatality of 35% (de Groot et al., 2013). The present pandemic crippling the world is in SARS‐CoV‐2, officially named as COVID19 by WHO. The SARS‐CoV‐2 is an enveloped, β‐coronavirus, with a large, positive‐sense, single‐stranded RNA genome ranging from 26–32 kilobases. The Spike (S) protein, envelope (E) protein, membrane (M) protein, and nucleocapsid (N) protein are the four structural proteins found in the virus. The spike protein interacts with host cell membrane to enable entry of the virus during infection (Y. Chen, Liu, & Guo, 2020).
Combating the newly emerging viruses has always been a challenge. Due to the lack of proofreading ability, viral RNA polymerase has a high rate of mutation (Elena & Sanjuán, 2005). This feature helps viruses with an RNA genome to develop resistance against pre‐existing antiviral medications (Bolken & Hruby, 2008; Sahin et al., 2020).
The preliminary step in the CoV infection is the interaction of human cells with viral spike protein. Angiotensin‐converting enzyme (ACE) 2 present in the lower respiratory tract of humans, is a receptor recognized by the spike protein of SARS‐CoV‐2 The viral genome RNA is released into the cytoplasm (Jia et al., 2005), following membrane fusion and the RNA codes for immediate proteins required for the replication and transcription complex (de Wilde, Snijder, Kikkert, & van Hemert, 2017). After entering the cell, CoV facilitates the gene expression and genome encoding occurs. This will, in turn, encode accessory proteins, which facilitate the adaptation of CoVs to their human host (ViralZone, 2019). There is a high recombination rate of CoVs since the RNA dependent RNA polymerase (RdRP) jumps and develops transcription errors consistently (Drexler et al., 2010). Due to its high mutation rates, CoVs are zoonotic pathogens that can infect various animals and humans resulting in a wide range of clinical features, ranging from asymptomatic course to multi‐organ failure (Yin & Wunderink, 2018). At present, there are no effective therapeutic strategies for managing COVID‐19 infection and there is no sufficient research in this area to guide the treatment (Rodríguez‐Morales, MacGregor, Kanagarajah, Patel, & Schlagenhauf, 2020). Potential anti‐coronavirus therapies target the human cells or the virus itself. Human immune system is known to play an important role in eliminating the virus and studies have shown the antiviral activity of type I and type II interferons. Interferon‐beta (IFN‐β) was reported to reduce the in vitro replication of MERS‐CoV (Hui et al., 2020). Blocking the cell surface receptors, for binding of coronavirus, and the cell signaling pathways, which help in viral replication, are the other targets in human cells. Angiotensin‐converting enzyme 2 (ACE2) is one of the proposed candidates for targeting drug target therapy to prevent virus infection since the virus gains entry to the cell through ACE2 receptors (H. Xu et al., 2020; Yan et al., 2020). This can be achieved by anti‐ACE2 monoclonal antibodies, anti‐SARS‐CoV‐2 neutralizing monoclonal antibodies, peptidic fusion inhibitors and anti‐proteases (Shanmugaraj, Siriwattananon, Wangkanont, & Phoolcharoen, 2020).
Broad‐spectrum antiviral drugs are being evaluated to treat the pandemic infection. Some preliminary research explored the potential combinations for the management of COVID‐19‐infected patients including the combination of protease inhibitors, ritonavir and lopinavir (anti‐HIV drugs). Other reported antiviral therapeutic agents for human pathogenic CoVs include remdesivir, nucleoside analogues, umifenovir (arbidol), neuraminidase inhibitors, lamivudine (3TC), and tenofovir disoproxil (TDF) (Lu, 2020). For containing the spread of the virus, rapid public health initiatives with antibodies, antiviral agents, and novel vaccines are highly important. Passive antibody therapy can be considered as one of the potential strategies to limit COVID‐19 pandemic. However, these are preliminary findings and none of these agents is not yet approved for therapeutic use for the management of COVID‐19‐infected patients (Yan et al., 2020).
There is growing evidence on the antiviral potential of herbal compounds (Praditya et al., 2019). In this regard, the use of phytochemicals has been noticed owing to their background efficacy and safety in light of the ethnomedicinal reports. Moreover, modern pharmacological investigations and clinical trials have unraveled numerous pharmacological activities for selected phytochemicals. Curcumin, the bioactive ingredient of turmeric (Abdollahi, Momtazi, Johnston, & Sahebkar, 2018; Iranshahi, Sahebkar, Takasaki, Konoshima, & Tokuda, 2009; Mollazadeh et al., 2019; Panahi et al., 2016, 2017; Rezaee, Momtazi, Monemi, & Sahebkar, 2017; Sahebkar, 2010), is a good example of phytochemicals with multi‐mechanistic mode of action. Curcumin is already approved by the US food and drug administration (FDA). Over 300 clinical trials have reported the beneficial protective effects of curcumin against various diseases including inflammatory diseases, neurological diseases, cardiovascular diseases, pulmonary disease, metabolic diseases, liver diseases, and cancers (Jäger et al., 2014). Curcumin has shown antiviral activities against several different viruses, and could be a therapeutic option for the management of COVID‐19 infection. All of the hypotheses mentioned in this review are based on the premise that the immune responses against COVID‐19 are similar to that of other coronaviruses, which should be confirmed by future insights on SARSCoV‐2.
2. AN OVERVIEW OF CURCUMIN AS AN ANTIVIRAL AGENT
Evidence suggests that curcumin has an inhibitory potential against various viral infections. The antiviral effects of curcumin were observed against viruses including vesicular stomatitis virus, parainfluenza virus type 3, vesicular stomatitis virus, flock house virus, herpes simplex virus, and respiratory syncytial virus (Zorofchian Moghadamtousi et al., 2014).
The pleiotropic effects of curcumin against viruses arise from its ability to interact with various molecular targets, thereby triggering cellular signaling pathways such as apoptosis and inflammation. Previous research has shown that curcumin interacts directly with around 30 proteins, including DNA polymerase, thioredoxin reductase, focal adhesion kinase (FAK), protein kinase (PK), tubulin, and lipoxygenase (LOX). Moreover, curcumin modulates intercellular signaling cascades which are essential for efficient virus replication such as attenuation of NF‐κB and PI3K/Akt signaling. It also affects cellular post‐transcriptional and post‐translational modifications, thereby limiting the viral multiplication by interfering with crucial steps in their replication cycle, including genome replication, and viral attachment (Ahn, Sethi, Jain, Jaiswal, & Aggarwal, 2006; D. Mathew & Hsu, 2018; Praditya et al., 2019; Puar et al., 2018).
It has been shown that curcumin treatment can modify the structure of the surface protein in viruses, thereby blocking entry of virus and virus budding. Furthermore, curcumin has an effect on membrane proteins by modulating the characteristics of the host lipid bilayer (T.‐Y. Chen et al., 2013). Utomo et al. used molecular docking with target receptors including SARS‐CoV‐2 protease, spike glycoprotein‐RBD, and PD‐ACE2, which are believed to participate in virus infection in comparison with the known ligand or drugs as references. Their result demonstrated that several compounds such as curcumin could bind to the target receptors (Utomo & Meiyanto, 2020).
3. CURCUMIN CAN POTENTIALLY TARGET CRITICAL STEPS OF THE VIRAL REPLICATION CYCLE
A virus does not have all the enzymes needed for its replication as a single unit. The virus uses cellular machinery for its metabolic processes and reproduction. The antiviral agents should prevent the growth of viruses in infected cells without harming the healthy cells. The processes of the replication of the viruses, including attachment, penetration, uncoating, genome replication, and gene expression are potential therapeutic targets. Some of the known effects of curcumin include impeding the viral infection by targeting the penetration of virus and attacking the components required for viral replication (D. Mathew & Hsu, 2018).
3.1. Viral attachment/penetration
When curcumin was applied to the cells before or after infection, it attenuated the infectivity of certain viruses, enveloped viruses, including members of poxvirus, flavivirus, herpesvirus, and orthomyxovirus (T.‐Y. Chen et al., 2013). Du et al. evaluated the influence of curcumin on the virus entry. They showed that curcumin could alter the surface protein structure in viruses and block the entry of viruses to the cell. In addition, the positively charged curcumin on the surface is subjected to electrostatic interactions with PEDV or cell membranes and competing with the virus to bind with the cells (Ting et al., 2018).
Recently, a molecular ducking study indicated that curcumin possesses better binding capability to the receptors and may inhibit the entry of COVID‐19 virus. ACE2 is the receptor that binds with SARS‐CoV‐2 spike glycoprotein which facilitates membrane fusion and viral infection occurs through endocytosis. Therefore, spike glycoprotein is a potential candidate for drug targeting to inhibit the entry of virus (Utomo & Meiyanto, 2020) that in silico docking studies revealed that curcumin could potentially inhibit ACE2 to suppress COVID19 entry to the cell (Figure 1).
FIGURE 1.
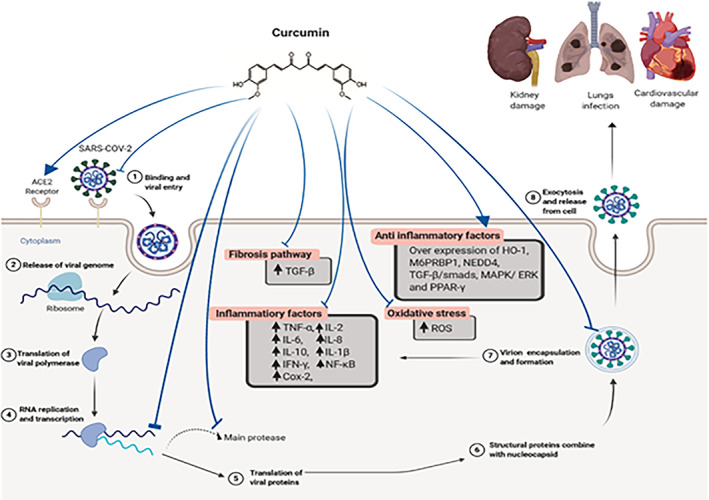
Multi‐site inhibitory effects of curcumin in the pathogenesis of COVID‐19 [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Viral replication
One of the potential therapeutic strategies for inhibiting the virus is based on the use of agents that can potentially inhibit the replication of virus (Praditya et al., 2019; X. X. Yang, Li, Li, Wang, & Huang, 2017). Wen et al. have studied the effect of curcumin on viral replication by quantification of the number of spike proteins present in cultures of Vero E6 cells infected with SARS‐CoV. Their result demonstrated that the inhibitory effect of curcumin in EC50 values was higher than 10 μM on SARS‐CoV replication (Wen et al., 2007).
Furthermore, Ting Du et al. studied the effects of curcumin on negative‐strand RNA synthesis by using PEDV as a coronavirus model. They demonstrated that curcumin could inhibit PEDV at the replication step. The plaque numbers were reduced when exposed to curcumin. The reduction in plaque numbers and virus titers showed that curcumin could inhibit viral replication (Ting et al., 2018). This evidence supports the potential role of curcumin as a promising antiviral agent.
4. POTENTIAL INHIBITORY EFFECT OF CURCUMIN ON VIRAL PROTEASE
SARS‐CoV and MERS‐CoV encode papain‐like proteases (PLPs) that can impede the immune response (L. Sun et al., 2012). The drugs that are currently tried for the management of COVID‐19 are protease inhibitors that primarily act on the main protease (Mpro). Beta CoV applies protease to cleave the essential structural proteins of the host cells during viral formation. The protease inhibitors have been developed to impede the proliferation of viruses such as HIV‐AIDS, MERS, and SARS (Zumla, Chan, Azhar, Hui, & Yuen, 2016). Various candidate protease inhibitor drugs have been tested with promising result to treat SARS‐CoV‐2, such as lopinavir (HIV medication) (Harrison, 2020; Senathilake, Samarakoon, & Tennekoon, 2020; Wang, 2020).
Khaerunnisa et al. examined the role of several phytochemical compounds such as curcumin that may have the potential to inhibit the COVID‐19 infection by molecular docking. Curcumin showed relatively low binding energies and inhibition constants. They suggested that curcumin could have a potential inhibitory effect on COVID‐19 Mpro and could potentially act as a therapeutic agent (Khaerunnisa, Kurniawan, Awaluddin, Suhartati, & Soetjipto, 2020).
5. POTENTIAL EFFECT OF CURCUMIN ON INTERFERONS
Interferons play a pivotal role in the defense against CoV infection. These viruses could hinder the induction of interferon in humans. In addition, the virus antagonizes STAT1, which is a key protein in the interferon‐mediated immune response. This may explain the increased immune cell response thresholds to IFNs during CoV infections (Kindler, Thiel, & Weber, 2016). All types of IFNs play a role in preventing viral infections (Samuel, 2001).
Differences in the dynamics of the innate immune responses associated with interferons in children, adults, and elderly may describe the reported variation in rates of fatality. The higher mortality rates in older people can be explained by the higher interferon‐mediated immune response threshold (Shahabi nezhad et al., 2020).
The key to success in reducing the fatality of SARA‐CoV may be the activation of innate immune responses to trigger IFN production at the very early stages of this disease. This could be achieved through the administration of agents that can increase the synthesis of IFNs such as poly ICLC (Kumaki, Salazar, Wandersee, & Barnard, 2017; Zhao et al., 2012). Despite the positive results of pre‐clinical studies on the efficacy of IFNs in treating CoV‐induced infections, the suitable dosing and optimal timing for such interventions need to be verified in clinical trials. Moreover, IFN‐γ along with IFN‐I as combination therapy is strongly proposed (Shahabi nezhad et al., 2020).
There is growing evidence on the effect of curcumin on IFNs in different viral diseases (Jasso‐Miranda et al., 2019; Mounce, Cesaro, Carrau, Vallet, & Vignuzzi, 2017; Sordillo & Helson, 2015). Viruses can stimulate NF‐κB and interferon‐regulatory factors to produce numerous antiviral cytokines. The antiviral IFNs via the JAK/STAT pathway induces the synthesis of a variety of IFN‐stimulated genes (ISGs). The antiviral IFNs also directly stimulate IFN‐independent pathways to halt various stages of viral replication (Schoggins & Rice, 2011). Ting Du et al. have shown that treatment with cationic carbon dots based on curcumin can suppress the PEDV model of coronavirus reproduction by stimulating the production of interferon‐stimulating genes (ISGs) and the cytokines (IL8 and IL6) of Vero cells by triggering the innate immunity of the host (Ting et al., 2018).
6. THE POTENTIAL EFFECT OF CURCUMIN IN THE TREATMENT OF PULMONARY INFLAMMATION, EDEMA, AND FIBROSIS
Coronaviruses can induce various inflammatory cytokines. They trigger “cytokine cascade” or “cytokine storm” which results in various organ damage. CoVs stimulate the immune cells to secrete various inflammatory cytokines into pulmonary vascular endothelial cells (Jiang et al., 2020).
6.1. Pulmonary inflammation
There is growing evidence on the inhibitory actions of curcumin on inflammatory cytokines. Curcumin blocks the essential signals regulating the expression of various pro‐inflammatory cytokines including nuclear factor‐κB and MAPK pathways (Ferreira, Nazli, Dizzell, Mueller, & Kaushic, 2015). Curcumin has anti‐inflammatory and anti‐fibrotic effects by reducing the expression of crucial chemokines and cytokines involved in lung infection such as IFNγ, MCP‐1, IL‐6, and IL‐10 (Avasarala et al., 2013). Curcumin has an inhibitory effect against the human respiratory syncytial virus (RSV) infection by preventing RSV replication, the release of TNF‐alpha and downregulating phospho‐NF‐κB (Obata et al., 2013).
6.2. Pulmonary fibrosis
Pulmonary fibrosis is a devastating outcome of COVID‐19 infection associated with an almost universally terminal acute respiratory distress syndrome (ARDS) in around 32% of patients infected with COVID‐19 (Rodriguez‐Morales, Cardona‐Ospina et al., 2020). When SARS‐CoV‐2 affects the upper and lower respiratory tract, it results in varying degrees of ARDS, releasing pro‐inflammatory cytokines. The attachment of SARS‐CoV‐2 to the toll‐like receptor results in the release of pro‐IL‐1β that is cleaved by caspase‐1, leading to the activation of inflammasome and generation of active mature IL‐1β, which mediates pulmonary inflammation and fibrosis (Conti et al., 2020).
TGF‐ß and its signaling pathways are involved in lung fibrosis and TGF‐ß overexpression is correlated with poorer prognosis in ARDS (Budinger et al., 2005; Dhainaut, Charpentier, & Chiche, 2003; Fahy et al., 2003; Gauldie, Bonniaud, Sime, Ask, & Kolb, 2007; Scotton & Chambers, 2007). Curcumin has been shown to inhibit the cellular inflammatory response by attenuating the cytokine/chemokine expression through the NF‐кB pathway and fibrotic response during the regeneration phase of the disease via attenuating of the TGF‐ß pathway in a mouse model of viral‐induced ARDS. Curcumin can also inhibit apoptosis pathways mediated by the p38 MAPK pathway (Avasarala et al., 2013). Furthermore, curcumin has been shown to reduce collagen in experimental models of pulmonary fibrosis induced by whole‐body irradiation, bleomycin, and cyclophosphamide (B. Chen, Zhang, & Gao, 2008; Cutroneo, White, Phan, & Ehrlich, 2007; Punithavathi, Venkatesan, & Babu, 2000, 2003; Tourkina et al., 2004; Venkatesan, 2000; Venkatesan & Chandrakasan, 1995; M. Xu, Deng, Chow, Zhao, & Hu, 2007).
6.3. Pulmonary oedema
The histopathological examination of some patients with COVID‐19 showed pulmonary oedema along with inflammatory clusters consisting of fibrinoid material and multinucleated giant cells (Tian et al., 2020). Pulmonary oedema results from the accumulation of fluid in the lungs (Bärtsch, Mairbäurl, Maggiorini, & Swenson, 2005; Maggiorini, 2006). Studies have shown that in SARS‐CoV infection, the activation of protein kinase C (PKC) by SARS‐CoV envelope (E) protein, results in reduced activity of epithelial sodium channels at the apical surface of pulmonary epithelial cells, and the ion channel activity of E protein leads to pulmonary oedema (DeDiego et al., 2014).
Recently, evidence shows that prophylactic application of curcumin decreased the inflammation resulting in a reduced influx of fluid in lungs of rats under hypoxia. This was through downregulation of the pro‐inflammatory cytokines and cell adhesion molecules by modulation of NF‐кB activity and stabilizing hypoxia‐inducible factor 1‐alpha (HIF1‐α), leading to downregulation of angiogenic molecules such as VEGF followed by a reduction in pulmonary oedema and albumin extravasation in the bronchoalveolar lavage fluid of rats (T. Mathew & Sarada, 2015; Sagi, Mathew, & Patir, 2014; Titto, Ankit, Saumya, Gausal, & Sarada, 2020).
7. THE POTENTIAL EFFECT OF CURCUMIN IN THE TREATMENT OF COVID‐19 ASSOCIATED CARDIOVASCULAR DAMAGE
Angiotensin‐converting enzyme 2 (ACE2) is involved in cardiovascular function as well as have a role in the development of diabetes mellitus and hypertension (Turner, Hiscox, & Hooper, 2004). SARS‐CoV‐2 infection is initiated by the binding of the virus spike protein to ACE2. ACE2 receptors are highly expressed in various organs including the heart, lungs, and kidney. SARS‐CoV‐2 causes respiratory symptoms by infecting alveolar epithelial cells. These symptoms are more pronounced in patients having cardiovascular disease. This could be potentially due to the fact that ACE2 is expressed more in patients with cardiovascular disease compared with people without cardiovascular disease. Since ACE2 acts like a receptor for SARS‐CoV‐2, the safety and potential effects of antihypertension therapy with angiotensin‐receptor blockers or ACE inhibitors in COVID‐19 infected patients should be studied (Zheng, Ma, Zhang, & Xie, 2020). However, the reduction of ACE2 activity is detrimental to the heart, since it contributes to cardiac dysfunction, partly due to increased stimulation of the AT1 receptor by angiotensin II (Yamamoto et al., 2006). Pang et al. demonstrated that curcumin significantly decreases mean arterial blood pressure and improves cardiac fibrosis in rats through upregulation of angiotensin II type II receptor, down‐regulation of angiotensin II type I receptor, and increase of ACE2 in the myocardium (Pang et al., 2015).
In patients with COVID‐19 infection, cardiovascular symptoms occur because of the systemic inflammatory response triggered by the imbalance response of type 1 and type 2 T helper cells (Huang et al., 2020). It has been shown that curcumin reduces inflammation and necrotic tissue in the myocardial ischemia–reperfusion model in the rat by inhibition of early growth response‐1 and reduction of tumor necrosis factor‐alpha and interleukin‐6 (Salabei & Conklin, 2013). Curcumin reduces myocardial ischemia–reperfusion injury through a reduction of c‐Jun N‐terminal kinase (JNK) and NF‐κB nuclear translocation phosphorylation (Sahebkar & Henrotin, 2016). Moreover, curcumin reduced the infiltration of immune cells and the expression of adhesion molecules and pro‐inflammatory mediators in vascular cells (X. Li et al., 2017).
8. THE EFFECT OF CURCUMIN IN COVID‐19 ASSOCIATED KIDNEY DAMAGE
There is an increased incidence of acute kidney injury following infection with COVID‐19, which may be due to the presence of SARS‐CoV‐2, the inflammatory response, or a synergistic impact of both these factors on kidneys. It has been shown that patients with acute renal injury have a higher mortality rate (Cheng et al., 2020). ACE2 is highly expressed in the kidneys (Ye et al., 2006). A reduction in ACE2 and an increase in ACE expression could potentially result in renal damage in diabetes (Ye et al., 2004). Angiotensin II reduction can facilitate the development of glomerular sclerosis and proteinuria. This suggests that ACE inhibitors could have a potential adverse effect during the management of COVID‐19 infection (Ahmad, Siddiqui, & Ahmad, 1997).
Xu et al. have shown that curcumin could potentially upregulate the ACE2 and ACE2 mRNA, resulting in improved renal blood flow, and have a potential anti‐fibrotic effect in kidneys in type 2 diabetic rat models (X. Xu, Cai, & Yu, 2018). Curcumin potentially reduces renal fibrosis at priming and activation stages by suppressing the inflammation caused by reduced MCP‐1, NF‐κB, TNF‐α, IL‐1𝛽, COX‐2, and cav‐1 levels. Curcumin also increases the expression of anti‐inflammatory factors such as neural precursor cell expressed developmentally down‐regulated protein 4 (NEDD4), mannose‐6‐phosphate receptor binding protein 1(M6PRBP1), and heme oxygenase‐1 (HO‐1). Curcumin also targets MAPK/ERK, TGF‐𝛽/smads, and PPAR‐γ pathways in animal models of kidney disease (X. Sun et al., 2017). Thus, curcumin could be potentially beneficial for the treatment of COVID‐19 associated renal inflammation.
9. THE POTENTIAL EFFECT OF CURCUMIN IN INHIBITION OF OXIDATIVE STRESS IN VIRAL INFECTION
Oxidative stress is present in all severe lung injuries including ARDS caused by COVs and influenza virus infections. This has been attributed to the initiation and maintenance of chronic low‐grade inflammation (Imai et al., 2008). SARS‐CoV papain‐like protease (PLpro) significantly induces the generation of reactive oxygen species (ROS) and activates TGF‐β1‐mediated pro‐fibrotic response (S. W. Li et al., 2016). Curcumin has the electron transfer capability to scavenge various intracellular small oxidative molecules (Barzegar & Moosavi‐Movahedi, 2011). Curcumin can up‐regulate the expression of glutathione (GSH), and inhibits the generation of reactive oxygen species (ROS) and malondialdehyde (MDA) (Rong et al., 2012).
Curcumin can reduce the infection with influenza A virus and influenza pneumonia by activating Nrf2 signaling and inducing the generation of various antioxidants. Curcumin also suppresses influenza A virus‐mediated oxidative stress and indirectly inhibits influenza A virus‐induced activation of TLR2/4, MAPK, and NF‐κB pathways. The above processes may suppress the influenza A virus‐mediated inflammation and replication (Dai et al., 2018). Therefore, curcumin potentially has beneficial antioxidant properties in the treatment of SARS‐COV‐2 mediated oxidative stress in the lungs.
10. CONCLUSION AND CHALLENGES
In this review, we have attempted an overview of the potential antiviral effects of curcumin that can be helpful for researchers to further investigate the potency of curcumin against the new emerging SARS‐CoV‐2 infection. The ability of curcumin to modulate a wide range of molecular targets makes it a suitable candidate for the management of coronavirus infection.
Curcumin may have beneficial effects against COVID‐19 infection via its ability to modulate the various molecular targets that contribute to the attachment and internalization of SARS‐CoV‐2 in many organs, including the liver, cardiovascular system, and kidney. Curcumin could also modulate cellular signaling pathways such as inflammation, apoptosis, and RNA replication. Curcumin may also suppress pulmonary edema and fibrosis‐associated pathways in COVID‐19 infection. Despite the potential beneficial effects and safety profile of curcumin against various diseases, the limited bioavailability of this turmeric‐derived compound, especially via oral administration may be a problematic issue (Anand, Kunnumakkara, Newman, & Aggarwal, 2007). Yang et al. demonstrated that intravenous administration of curcumin (10 mg/kg) resulted in better bioavailability in comparison to oral administration with a higher dose (500 mg/kg) (K. Y. Yang, Lin, Tseng, Wang, & Tsai, 2007). Several clinical trials have shown that the issue regarding the bioavailability of curcumin can be mitigated by administering higher concentrations within non‐toxic limits (Kunnumakkara et al., 2019). In addition, many studies have suggested various ways to improve the bioavailability of curcumin such as manipulation and encapsulation of curcumin into micelles, liposomes, phospholipid complexes, exosomes, or polymeric nanocarrier formulation and also utilization of curcumin in combination with cellulosic derivatives, natural antioxidants, and a hydrophilic carrier (Jäger et al., 2014; Moballegh Nasery et al., 2020). Moreover, several studies have reported the synergistic therapeutic effects of curcumin in combination with other natural or synthetic compounds (Singh et al., 2013). Overall, the well‐documented anti‐inflammatory and immunomodulatory effects of curcumin along with the evidence on the anti‐fibrotic and pulmonoprotective effects of this phytochemical on the lung tissue make it a promising candidate for the treatment of COVID‐19. Since curcumin is known to have strong inhibitory effects on NF‐κB and several pro‐inflammatory cytokines, it can be particularly helpful as an adjunct in reversing the fatal cytokine storm that occurs in serious cases of COVID‐19.
To sum up, this review shows that curcumin as an antiviral and anti‐inflammatory agent can be helpful for both prevention and treatment of new emerging coronavirus. However, well‐designed clinical trials are needed to demonstrate the potential efficacy of curcumin against SARS‐CoV‐2 infection and its ensuing complications.
CONFLICT OF INTEREST
The authors have no other conflicting interests to disclose.
ACKNOWLEDGEMENTS
MB has served on the speaker's bureau and as an advisory board member for Amgen, Sanofi, Aventis and Lilly. NK has given talks, attended conferences and participated in trials sponsored by Amgen, Angelini, Astra Zeneca, Boehringer Ingelheim, Galenica, MSD, Novartis, Novo Nordisk, Sanofi, and WinMedica. KR received a research grant from Sanofi, and served on the speaker's bureau and as an advisory board member for Sanofi, Astra Zeneca and Pfizer. Muhammed Majeed is the founder of Sabinsa Corporation and Sami Labs Ltd.
Zahedipour F, Hosseini SA, Sathyapalan T, et al. Potential effects of curcumin in the treatment of COVID‐19 infection. Phytotherapy Research. 2020;34:2911–2920. 10.1002/ptr.6738
REFERENCES
- Abdollahi, E. , Momtazi, A. A. , Johnston, T. P. , & Sahebkar, A. (2018). Therapeutic effects of curcumin in inflammatory and immune‐mediated diseases: A nature‐made jack‐of‐all‐trades? Journal of Cellular Physiology, 233(2), 830–848. 10.1002/jcp.25778 [DOI] [PubMed] [Google Scholar]
- Ahmad, J. , Siddiqui, M. A. , & Ahmad, H. (1997). Effective postponement of diabetic nephropathy with enalapril in normotensive type 2 diabetic patients with microalbuminuria. Diabetes Care, 20(10), 1576–1581. 10.2337/diacare.20.10.1576 [DOI] [PubMed] [Google Scholar]
- Ahn, K. S. , Sethi, G. , Jain, A. K. , Jaiswal, A. K. , & Aggarwal, B. B. (2006). Genetic deletion of NAD(P)H:Quinone oxidoreductase 1 abrogates activation of nuclear factor‐kappaB, IkappaBalpha kinase, c‐Jun N‐terminal kinase, Akt, p38, and p44/42 mitogen‐activated protein kinases and potentiates apoptosis. The Journal of Biological Chemistry, 281(29), 19798–19808. 10.1074/jbc.M601162200 [DOI] [PubMed] [Google Scholar]
- Anand, P. , Kunnumakkara, A. B. , Newman, R. A. , & Aggarwal, B. B. (2007). Bioavailability of curcumin: Problems and promises. Molecular Pharmaceutics, 4(6), 807–818. 10.1021/mp700113r [DOI] [PubMed] [Google Scholar]
- Avasarala, S. , Zhang, F. , Liu, G. , Wang, R. , London, S. D. , & London, L. (2013). Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral‐induced acute respiratory distress syndrome. PLoS One, 8(2), e57285. 10.1371/journal.pone.0057285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bärtsch, P. , Mairbäurl, H. , Maggiorini, M. , & Swenson, E. R. (2005). Physiological aspects of high‐altitude pulmonary edema. Journal of Applied Physiology (Bethesda, MD: 1985), 98(3), 1101–1110. 10.1152/japplphysiol.01167.2004 [DOI] [PubMed] [Google Scholar]
- Barzegar, A. , & Moosavi‐Movahedi, A. A. (2011). Intracellular ROS protection efficiency and free radical‐scavenging activity of curcumin. PLoS One, 6(10), e26012. 10.1371/journal.pone.0026012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolken, T. C. , & Hruby, D. E. (2008). Discovery and development of antiviral drugs for biodefense: Experience of a small biotechnology company. Antiviral Research, 77(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budinger, G. R. , Chandel, N. S. , Donnelly, H. K. , Eisenbart, J. , Oberoi, M. , & Jain, M. (2005). Active transforming growth factor‐beta1 activates the procollagen I promoter in patients with acute lung injury. Intensive Care Medicine, 31(1), 121–128. 10.1007/s00134-004-2503-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B. , Zhang, D. P. , & Gao, W. (2008). Effect of curcumin on the expression of collagen type I protein and transforming growth factor‐beta1 mRNA in pulmonary fibrosis rats]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, 26(5), 257–261. [PubMed] [Google Scholar]
- Chen, T.‐Y. , Chen, D.‐Y. , Wen, H.‐W. , Ou, J.‐L. , Chiou, S.‐S. , Chen, J.‐M. , … Hsu, W.‐L. (2013). Inhibition of enveloped viruses infectivity by curcumin. PLoS One, 8(5), e62482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Liu, Q. , & Guo, D. (2020). Emerging coronaviruses: Genome structure, replication, and pathogenesis. Journal of Medical Virology, 92, 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y. , Luo, R. , Wang, K. , Zhang, M. , Wang, Z. , Dong, L. , … Xu, G. (2020). Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney International, 97, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, P. , Ronconi, G. , Caraffa, A. , Gallenga, C. E. , Ross, R. , Frydas, I. , & Kritas, S. K. (2020). Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): Anti‐inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents, 34(2), 1. 10.23812/conti-e [DOI] [PubMed] [Google Scholar]
- Cutroneo, K. R. , White, S. L. , Phan, S. H. , & Ehrlich, H. P. (2007). Therapies for bleomycin induced lung fibrosis through regulation of TGF‐beta1 induced collagen gene expression. Journal of Cellular Physiology, 211(3), 585–589. 10.1002/jcp.20972 [DOI] [PubMed] [Google Scholar]
- Dai, J. , Gu, L. , Su, Y. , Wang, Q. , Zhao, Y. , Chen, X. , … Li, K. (2018). Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF‐κB pathways. International Immunopharmacology, 54, 177–187. 10.1016/j.intimp.2017.11.009 [DOI] [PubMed] [Google Scholar]
- de Groot, R. J. , Baker, S. C. , Baric, R. S. , Brown, C. S. , Drosten, C. , Enjuanes, L. , … Memish, Z. A. (2013). Commentary: Middle East respiratory syndrome coronavirus (MERS‐CoV): Announcement of the Coronavirus Study Group. Journal of Virology, 87(14), 7790–7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde, A. H. , Snijder, E. J. , Kikkert, M. , & van Hemert, M. J. (2017). Host factors in coronavirus replication. In Tripp R., Tompkins S. (eds), Roles of host gene and non‐coding RNA expression in virus infection (pp. 1–42). New York: Springer. [Google Scholar]
- DeDiego, M. L. , Nieto‐Torres, J. L. , Jimenez‐Guardeño, J. M. , Regla‐Nava, J. A. , Castaño‐Rodriguez, C. , Fernandez‐Delgado, R. , … Enjuanes, L. (2014). Coronavirus virulence genes with main focus on SARS‐CoV envelope gene. Virus Research, 194, 124–137. 10.1016/j.virusres.2014.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhainaut, J. F. , Charpentier, J. , & Chiche, J. D. (2003). Transforming growth factor‐beta: A mediator of cell regulation in acute respiratory distress syndrome. Critical Care Medicine, 31(4 Suppl), S258–S264. 10.1097/01.Ccm.0000057901.92381.75 [DOI] [PubMed] [Google Scholar]
- Drexler, J. F. , Gloza‐Rausch, F. , Glende, J. , Corman, V. M. , Muth, D. , Goettsche, M. , … Yordanov, S. (2010). Genomic characterization of severe acute respiratory syndrome‐related coronavirus in European bats and classification of coronaviruses based on partial RNA‐dependent RNA polymerase gene sequences. Journal of Virology, 84(21), 11336–11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena, S. F. , & Sanjuán, R. (2005). Adaptive value of high mutation rates of RNA viruses: Separating causes from consequences. Journal of Virology, 79(18), 11555–11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy, R. J. , Lichtenberger, F. , McKeegan, C. B. , Nuovo, G. J. , Marsh, C. B. , & Wewers, M. D. (2003). The acute respiratory distress syndrome: A role for transforming growth factor‐beta 1. American Journal of Respiratory Cell and Molecular Biology, 28(4), 499–503. 10.1165/rcmb.2002-0092OC [DOI] [PubMed] [Google Scholar]
- Ferreira, V. H. , Nazli, A. , Dizzell, S. E. , Mueller, K. , & Kaushic, C. (2015). The anti‐inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV‐1 and HSV‐2. PLoS One, 10(4), e0124903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie, J. , Bonniaud, P. , Sime, P. , Ask, K. , & Kolb, M. (2007). TGF‐beta, Smad3 and the process of progressive fibrosis. Biochemical Society Transactions, 35(Pt 4), 661–664. 10.1042/bst0350661 [DOI] [PubMed] [Google Scholar]
- Harrison, C. (2020). Coronavirus puts drug repurposing on the fast track. Nature Biotechnology, 38, 379–381. [DOI] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395(10223), 497–506. 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui, D. S. , Azhar, E. I. , Madani, T. A. , Ntoumi, F. , Kock, R. , Dar, O. , … Drosten, C. (2020). The continuing 2019‐nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. International Journal of Infectious Diseases, 91, 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y. , Kuba, K. , Neely, G. G. , Yaghubian‐Malhami, R. , Perkmann, T. , van Loo, G. , … Penninger, J. M. (2008). Identification of oxidative stress and Toll‐like receptor 4 signaling as a key pathway of acute lung injury. Cell, 133(2), 235–249. 10.1016/j.cell.2008.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranshahi, M. , Sahebkar, A. , Takasaki, M. , Konoshima, T. , & Tokuda, H. (2009). Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. European Journal of Cancer Prevention, 18(5), 412–415. 10.1097/CEJ.0b013e32832c389e [DOI] [PubMed] [Google Scholar]
- Jäger, R. , Lowery, R. P. , Calvanese, A. V. , Joy, J. M. , Purpura, M. , & Wilson, J. M. (2014). Comparative absorption of curcumin formulations. Nutrition Journal, 13, 11. 10.1186/1475-2891-13-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasso‐Miranda, C. , Herrera‐Camacho, I. , Flores‐Mendoza, L. K. , Dominguez, F. , Vallejo‐Ruiz, V. , Sanchez‐Burgos, G. G. , … Reyes‐Leyva, J. (2019). Antiviral and immunomodulatory effects of polyphenols on macrophages infected with dengue virus serotypes 2 and 3 enhanced or not with antibodies. Infection and Drug Resistance, 12, 1833–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, H. P. , Look, D. C. , Shi, L. , Hickey, M. , Pewe, L. , Netland, J. , … McCray, P. B. (2005). ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. Journal of Virology, 79(23), 14614–14621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, F. , Deng, L. , Zhang, L. , Cai, Y. , Cheung, C. W. , & Xia, Z. (2020). Review of the clinical characteristics of coronavirus disease 2019 (COVID‐19). Journal of General Internal Medicine, 35, 1545–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaerunnisa, S. , Kurniawan, H. , Awaluddin, R. , Suhartati, S. , & Soetjipto, S. (2020). Potential inhibitor of COVID‐19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints, 2020030226. 10.20944/preprints202003.0226.v1 [DOI]
- Kindler, E. , Thiel, V. , & Weber, F. (2016). Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Advances in Virus Research, 96, 219–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaki, Y. , Salazar, A. M. , Wandersee, M. K. , & Barnard, D. L. (2017). Prophylactic and therapeutic intranasal administration with an immunomodulator, Hiltonol® (Poly IC:LC), in a lethal SARS‐CoV‐infected BALB/c mouse model. Antiviral Research, 139, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara, A. B. , Harsha, C. , Banik, K. , Vikkurthi, R. , Sailo, B. L. , Bordoloi, D. , … Aggarwal, B. B. (2019). Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opinion on Drug Metabolism & Toxicology, 15(9), 705–733. 10.1080/17425255.2019.1650914 [DOI] [PubMed] [Google Scholar]
- Lee, N. , Hui, D. , Wu, A. , Chan, P. , Cameron, P. , Joynt, G. M. , … To, K . (2003). A major outbreak of severe acute respiratory syndrome in Hong Kong. New England Journal of Medicine, 348(20), 1986–1994. [DOI] [PubMed] [Google Scholar]
- Li, S. W. , Wang, C. Y. , Jou, Y. J. , Yang, T. C. , Huang, S. H. , Wan, L. , … Lin, C. W. (2016). SARS coronavirus papain‐like protease induces Egr‐1‐dependent up‐regulation of TGF‐β1 via ROS/p38 MAPK/STAT3 pathway. Scientific Reports, 6, 25754. 10.1038/srep25754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Fang, Q. , Tian, X. , Wang, X. , Ao, Q. , Hou, W. , … Bai, S. (2017). Curcumin attenuates the development of thoracic aortic aneurysm by inhibiting VEGF expression and inflammation. Molecular Medicine Reports, 16(4), 4455–4462. 10.3892/mmr.2017.7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H. (2020). Drug treatment options for the 2019‐new coronavirus (2019‐nCoV). Bioscience Trends, 14(1), 69–71. [DOI] [PubMed] [Google Scholar]
- Maggiorini, M. (2006). High altitude‐induced pulmonary oedema. Cardiovascular Research, 72(1), 41–50. 10.1016/j.cardiores.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Mathew, D. , & Hsu, W.‐L. (2018). Antiviral potential of curcumin. Journal of Functional Foods, 40, 692–699. [Google Scholar]
- Mathew, T. , & Sarada, S. (2015). Attenuation of NFkB activation augments alveolar transport proteins expression and activity under hypoxia. International Journal of Science and Research, 4, 2230–2237. [Google Scholar]
- Moballegh Nasery, M. , Abadi, B. , Poormoghadam, D. , Zarrabi, A. , Keyhanvar, P. , Khanbabaei, H. , … Sethi, G. (2020). Curcumin delivery mediated by bio‐based nanoparticles: A review. Molecules, 25(3), 689. 10.3390/molecules25030689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollazadeh, H. , Cicero, A. F. G. , Blesso, C. N. , Pirro, M. , Majeed, M. , & Sahebkar, A. (2019). Immune modulation by curcumin: The role of interleukin‐10. Critical Reviews in Food Science and Nutrition, 59(1), 89–101. 10.1080/10408398.2017.1358139 [DOI] [PubMed] [Google Scholar]
- Mounce, B. C. , Cesaro, T. , Carrau, L. , Vallet, T. , & Vignuzzi, M. (2017). Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Research, 142, 148–157. 10.1016/j.antiviral.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Obata, K. , Kojima, T. , Masaki, T. , Okabayashi, T. , Yokota, S. , Hirakawa, S. , … Tanaka, S. (2013). Curcumin prevents replication of respiratory syncytial virus and the epithelial responses to it in human nasal epithelial cells. PLoS One, 8(9), e70225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panahi, Y. , Hosseini, M. S. , Khalili, N. , Naimi, E. , Simental‐Mendía, L. E. , Majeed, M. , & Sahebkar, A. (2016). Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post‐hoc analysis of a randomized controlled trial. Biomedicine and Pharmacotherapy, 82, 578–582. 10.1016/j.biopha.2016.05.037 [DOI] [PubMed] [Google Scholar]
- Panahi, Y. , Kianpour, P. , Mohtashami, R. , Jafari, R. , Simental‐Mendía, L. E. , & Sahebkar, A. (2017). Efficacy and safety of phytosomal curcumin in non‐alcoholic fatty liver disease: A randomized controlled trial. Drug Research, 67(4), 244–251. 10.1055/s-0043-100019 [DOI] [PubMed] [Google Scholar]
- Pang, X. F. , Zhang, L. H. , Bai, F. , Wang, N. P. , Garner, R. E. , McKallip, R. J. , & Zhao, Z. Q. (2015). Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Design, Development and Therapy, 9, 6043–6054. 10.2147/dddt.S95333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praditya, D. , Kirchhoff, L. , Brüning, J. , Rachmawati, H. , Steinmann, J. , & Steinmann, E. (2019). Anti‐infective properties of the golden spice curcumin. Frontiers in Microbiology, 10, 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puar, Y. R. , Shanmugam, M. K. , Fan, L. , Arfuso, F. , Sethi, G. , & Tergaonkar, V. (2018). Evidence for the involvement of the master transcription factor NF‐κB in cancer initiation and progression. Biomedicine, 6(3), 82. 10.3390/biomedicines6030082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punithavathi, D. , Venkatesan, N. , & Babu, M. (2000). Curcumin inhibition of bleomycin‐induced pulmonary fibrosis in rats. British Journal of Pharmacology, 131(2), 169–172. 10.1038/sj.bjp.0703578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punithavathi, D. , Venkatesan, N. , & Babu, M. (2003). Protective effects of curcumin against amiodarone‐induced pulmonary fibrosis in rats. British Journal of Pharmacology, 139(7), 1342–1350. 10.1038/sj.bjp.0705362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaee, R. , Momtazi, A. A. , Monemi, A. , & Sahebkar, A. (2017). Curcumin: A potentially powerful tool to reverse cisplatin‐induced toxicity. Pharmacological Research, 117, 218–227. 10.1016/j.phrs.2016.12.037 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Morales, A. J. , Cardona‐Ospina, J. A. , Gutiérrez‐Ocampo, E. , Villamizar‐Peña, R. , Holguin‐Rivera, Y. , Escalera‐Antezana, J. P. , … Sah, R. (2020). Clinical, laboratory and imaging features of COVID‐19: A systematic review and meta‐analysis. Travel Medicine and Infectious Disease, 34, 101623. 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Morales, A. J. , MacGregor, K. , Kanagarajah, S. , Patel, D. , & Schlagenhauf, P. (2020). Going global–Travel and the 2019 novel coronavirus. Travel Medicine and Infectious Disease, 33, 101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, S. , Zhao, Y. , Bao, W. , Xiao, X. , Wang, D. , Nussler, A. K. , … Liu, L. (2012). Curcumin prevents chronic alcohol‐induced liver disease involving decreasing ROS generation and enhancing antioxidative capacity. Phytomedicine, 19(6), 545–550. 10.1016/j.phymed.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Sagi, S. , Mathew, T. , & Patir, H. (2014). Prophylactic administration of curcumin abates the incidence of hypobaric hypoxia induced pulmonary edema in rats: A molecular approach. Journal of Pulmonary & Respiratory Medicine, 4, 1000164. [Google Scholar]
- Sahebkar, A. (2010). Molecular mechanisms for curcumin benefits against ischemic injury. Fertility and Sterility, 94(5), e75–e76. 10.1016/j.fertnstert.2010.07.1071 [DOI] [PubMed] [Google Scholar]
- Sahebkar, A. , & Henrotin, Y. (2016). Analgesic efficacy and safety of curcuminoids in clinical practice: A systematic review and meta‐analysis of randomized controlled trials. Pain Medicine, 17(6), 1192–1202. 10.1093/pm/pnv024 [DOI] [PubMed] [Google Scholar]
- Sahin, A. R. , Erdogan, A. , Agaoglu, P. M. , Dineri, Y. , Cakirci, A. Y. , Senel, M. E. , … Tasdogan, A. M. (2020). 2019 novel coronavirus (COVID‐19) outbreak: A review of the current literature. EJMO, 4(1), 1–7. [Google Scholar]
- Salabei, J. K. , & Conklin, D. J. (2013). Cardiovascular autophagy: Crossroads of pathology, pharmacology and toxicology. Cardiovascular Toxicology, 13(3), 220–229. 10.1007/s12012-013-9200-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, C. E. (2001). Antiviral actions of interferons. Clinical Microbiology Reviews, 14(4), 778–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins, J. W. , & Rice, C. M. (2011). Interferon‐stimulated genes and their antiviral effector functions. Current Opinion in Virology, 1(6), 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotton, C. J. , & Chambers, R. C. (2007). Molecular targets in pulmonary fibrosis: The myofibroblast in focus. Chest, 132(4), 1311–1321. 10.1378/chest.06-2568 [DOI] [PubMed] [Google Scholar]
- Senathilake, K. , Samarakoon, S. , & Tennekoon, K. (2020). Virtual screening of inhibitors against spike glycoprotein of 2019 novel Corona virus: A drug repurposing approach. Preprints, 2020030042. 10.20944/preprints202003.0042.v1 [DOI]
- Shahabi nezhad, F. , Mosaddeghi, P. , Negahdaripour, M. , Dehghani, Z. , Farahmandnejad, M. , Moghadami, M. , Nezafat, N. , & Masoompour, S. M. (2020). Therapeutic approaches for COVID‐19 based on the dynamics of interferon‐mediated immune responses. Preprints, 2020030206. 10.20944/preprints202003.0206.v1 [DOI]
- Shanmugaraj, B. , Siriwattananon, K. , Wangkanont, K. , & Phoolcharoen, W. (2020). Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease‐19 (COVID‐19). Asian Pacific Journal of Allergy and Immunology. 38(1), 10–18. [DOI] [PubMed] [Google Scholar]
- Singh, M. , Ramos, I. , Asafu‐Adjei, D. , Quispe‐Tintaya, W. , Chandra, D. , Jahangir, A. , … Gravekamp, C. (2013). Curcumin improves the therapeutic efficacy of Listeriaat‐Mage‐b vaccine in correlation with improved T‐cell responses in blood of a triple‐negative breast cancer model 4T1. Cancer Medicine, 2(4), 571–582. 10.1002/cam4.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordillo, P. P. , & Helson, L. (2015). Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with ebola and other severe viral infections. In Vivo, 29(1), 1–4. [PubMed] [Google Scholar]
- Sun, L. , Xing, Y. , Chen, X. , Zheng, Y. , Yang, Y. , Nichols, D. B. , … Baker, S. C. (2012). Coronavirus papain‐like proteases negatively regulate antiviral innate immune response through disruption of STING‐mediated signaling. PLoS One, 7(2), e30802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Liu, Y. , Li, C. , Wang, X. , Zhu, R. , Liu, C. , … Li, Y. (2017). Recent advances of curcumin in the prevention and treatment of renal fibrosis. BioMed Research International, 2017, 2418671. 10.1155/2017/2418671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, S. , Hu, W. , Niu, L. , Liu, H. , Xu, H. , & Xiao, S. Y. (2020). Pulmonary pathology of early‐phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. Journal of Thoracic Oncology, 15, 700–704. 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting, D. , Dong, N. , Fang, L. , Lu, J. , Bi, J. , Xiao, S. , & Han, H. (2018). Multisite inhibitors for enteric coronavirus: Antiviral cationic carbon dots based on curcumin. ACS Applied Nano Materials, 1(10), 5451–5459. [DOI] [PubMed] [Google Scholar]
- Titto, M. , Ankit, T. , Saumya, B. , Gausal, A. , & Sarada, S. (2020). Curcumin prophylaxis refurbishes alveolar epithelial barrier integrity and alveolar fluid clearance under hypoxia. Respiratory Physiology & Neurobiology, 274, 103336. [DOI] [PubMed] [Google Scholar]
- Tourkina, E. , Gooz, P. , Oates, J. C. , Ludwicka‐Bradley, A. , Silver, R. M. , & Hoffman, S. (2004). Curcumin‐induced apoptosis in scleroderma lung fibroblasts: Role of protein kinase cepsilon. American Journal of Respiratory Cell and Molecular Biology, 31(1), 28–35. 10.1165/rcmb.2003-0354OC [DOI] [PubMed] [Google Scholar]
- Turner, A. J. , Hiscox, J. A. , & Hooper, N. M. (2004). ACE2: From vasopeptidase to SARS virus receptor. Trends in Pharmacological Sciences, 25(6), 291–294. 10.1016/j.tips.2004.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utomo, R. Y. , & Meiyanto, E. (2020). Revealing the potency of citrus and galangal constituents to Halt SARS‐CoV‐2 infection. Preprints, 2020030214. 10.20944/preprints202003.0214.v1 [DOI]
- Venkatesan, N. (2000). Pulmonary protective effects of curcumin against paraquat toxicity. Life Sciences, 66(2), PL21–PL28. 10.1016/s0024-3205(99)00576-7 [DOI] [PubMed] [Google Scholar]
- Venkatesan, N. , & Chandrakasan, G. (1995). Modulation of cyclophosphamide‐induced early lung injury by curcumin, an anti‐inflammatory antioxidant. Molecular and Cellular Biochemistry, 142(1), 79–87. 10.1007/bf00928916 [DOI] [PubMed] [Google Scholar]
- ViralZone . (2019). Coronavirinae in ViralZone. Retrieved from https://viralzone.expasy.org/785
- Wang, J. (2020). Fast identification of possible drug treatment of Coronavirus disease‐19 (COVID‐19) through computational drug repurposing study. J Chem Inf Model. 10.1021/acs.jcim.0c00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, C.‐C. , Kuo, Y.‐H. , Jan, J.‐T. , Liang, P.‐H. , Wang, S.‐Y. , Liu, H.‐G. , … Lee, S.‐S. (2007). Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. Journal of Medicinal Chemistry, 50(17), 4087–4095. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Zhong, L. , Deng, J. , Peng, J. , Dan, H. , Zeng, X. , … Chen, Q. (2020). High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science, 12(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , Deng, B. , Chow, Y. L. , Zhao, Z. Z. , & Hu, B. (2007). Effects of curcumin in treatment of experimental pulmonary fibrosis: A comparison with hydrocortisone. Journal of Ethnopharmacology, 112(2), 292–299. 10.1016/j.jep.2007.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Cai, Y. , & Yu, Y. (2018). Effects of a novel curcumin derivative on the functions of kidney in streptozotocin‐induced type 2 diabetic rats. Inflammopharmacology, 26(5), 1257–1264. 10.1007/s10787-018-0449-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, K. , Ohishi, M. , Katsuya, T. , Ito, N. , Ikushima, M. , Kaibe, M. , … Ogihara, T. (2006). Deletion of angiotensin‐converting enzyme 2 accelerates pressure overload‐induced cardiac dysfunction by increasing local angiotensin II. Hypertension, 47(4), 718–726. 10.1161/01.HYP.0000205833.89478.5b [DOI] [PubMed] [Google Scholar]
- Yan, R. , Zhang, Y. , Li, Y. , Xia, L. , Guo, Y. , & Zhou, Q. (2020). Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science. 367(6485), 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K. Y. , Lin, L. C. , Tseng, T. Y. , Wang, S. C. , & Tsai, T. H. (2007). Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC‐MS/MS. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 853(1‐2), 183–189. 10.1016/j.jchromb.2007.03.010 [DOI] [PubMed] [Google Scholar]
- Yang, X. X. , Li, C. M. , Li, Y. F. , Wang, J. , & Huang, C. Z. (2017). Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale, 9(41), 16086–16092. [DOI] [PubMed] [Google Scholar]
- Ye, M. , Wysocki, J. , Naaz, P. , Salabat, M. R. , LaPointe, M. S. , & Batlle, D. (2004). Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: A renoprotective combination? Hypertension, 43(5), 1120–1125. 10.1161/01.Hyp.0000126192.27644.76 [DOI] [PubMed] [Google Scholar]
- Ye, M. , Wysocki, J. , William, J. , Soler, M. J. , Cokic, I. , & Batlle, D. (2006). Glomerular localization and expression of angiotensin‐converting enzyme 2 and angiotensin‐converting enzyme: Implications for albuminuria in diabetes. Journal of the American Society of Nephrology, 17(11), 3067–3075. 10.1681/asn.2006050423 [DOI] [PubMed] [Google Scholar]
- Yin, Y. , & Wunderink, R. G. (2018). MERS, SARS and other coronaviruses as causes of pneumonia. Respirology, 23(2), 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Wohlford‐Lenane, C. , Zhao, J. , Fleming, E. , Lane, T. E. , McCray, P. B. , & Perlman, S. (2012). Intranasal treatment with Poly(I·C) protects aged mice from lethal respiratory virus infections. Journal of Virology, 86(21), 11416–11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y. Y. , Ma, Y. T. , Zhang, J. Y. , & Xie, X. (2020). COVID‐19 and the cardiovascular system. Nature Reviews. Cardiology, 17, 259–260. 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorofchian Moghadamtousi, S. , Abdul Kadir, H. , Hassandarvish, P. , Tajik, H. , Abubakar, S. , & Zandi, K. (2014). A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Research International, 2014, 186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla, A. , Chan, J. F. , Azhar, E. I. , Hui, D. S. , & Yuen, K.‐Y. (2016). Coronaviruses—Drug discovery and therapeutic options. Nature Reviews Drug Discovery, 15(5), 327–347. [DOI] [PMC free article] [PubMed] [Google Scholar]


