Abstract
Background and purpose
Stroke assistance is facing changes and new challenges since COVID‐19 became pandemic. A variation on the patient influx might be one of the greater concerns, due to fewer people coming to emergency departments or coming too late. However, no data quantifying this have been published until now. The aim was to analyse the impact of the COVID‐19 epidemic outbreak on hospital stroke admissions and their characteristics in our region.
Methods
The data of every patient admitted to any hospital of our healthcare system with a diagnosis of ischaemic stroke between 30 December 2019 and 19 April 2020 were reviewed. Demographic and clinical data were recorded and compared between periods before and after the setting of the state of emergency secondary to the COVID‐19 outbreak.
Results
In total, 354 patients with ischaemic stroke were admitted in our study period. There was a weekly average of 27.5 cases before the setting of the state of emergency against 12 afterwards (P < 0.001). This drop in stroke cases occurred progressively from week 11, persisting in time despite the decrease in confirmed cases of COVID‐19. No differences in the proportion of intravenous thrombolysis (21.1% vs. 21.5%, P = 0.935) or endovascular therapy (12.4% vs. 15.2%, P = 0.510) were found, nor in other demographic or clinical characteristics except for median onset‐to‐door time (102 vs. 183 min, P = 0.015).
Conclusions
This observational study offers the perspective of a whole region in one of the countries more heavily stricken by the SARS‐CoV‐2 epidemic and shows that the decrease of stroke events, since the beginning of the COVID‐19 outbreak, happened globally and without any specific patient distribution.
Keywords: COVID‐19, stroke
Introduction
Each year, between 1.1 and 1.5 million Europeans have a stroke [1]. Two to three out of 10 patients die as a consequence of it and about one‐third remain functionally dependent [2]. As is known, the likelihood of a favourable outcome in this disease relies heavily on patients presenting promptly after symptom onset and on hospitals providing immediate access to optimized stroke care [3, 4].
Since the first reported case in early December 2019, severe acute respiratory coronavirus 2 (SARS‐CoV‐2) infection, known as COVID‐2019, has become pandemic so rapidly that healthcare systems are overwhelmed [5, 6, 7]. In Spain, by 19 April, 195 944 cases and 20 453 deaths had been confirmed [8]. With our health system on the brink of collapse, there is a risk that the remaining ‘non‐COVID‐19’ pathologies lag behind and, unfortunately, it seems stroke is not an exception.
Stroke assistance is facing changes and new challenges. In a survey, amongst 426 stroke care providers from 55 countries, only one in five reported that stroke patients are currently receiving the standard acute and post‐acute care [9]. Furthermore, it seems that fewer people are coming to emergency departments or are coming too late. However, data quantifying this are lacking.
The aim was to analyse the impact of the COVID‐19 epidemic outbreak on hospital stroke admissions and patient baseline characteristics in our region. Aragón represents one of the 17 first‐level political and administrative divisions of Spain, one of the countries with a greater number of confirmed SARS‐CoV‐2 cases and deaths. It has 1 319 291 inhabitants and around 2000 cases of stroke admissions per year. According to our regional health system [Servicio Aragonés de Salud (SALUD)], public hospitals are the only hospitals where reperfusion therapies for ischaemic stroke are available.
Methods
The data of every patient admitted to any hospital of SALUD with a diagnosis of ischaemic stroke between 30 December 2019 and 19 April 2020 were reviewed. Demographic and clinical data were recorded including age, sex, modified Rankin Scale score prior to qualifying stroke, patient location, time of onset, Code Stroke activation, National Institutes of Health Stroke Scale (NIHSS) score, Alberta Stroke Program Early Computed Tomography Score (ASPECTS), intravenous thrombolysis (IVT) and/or endovascular therapy (EVT), onset‐to‐door time (ODT), door‐to‐needle time, door‐to‐puncture time and COVID‐19 diagnostic test results. The number of stroke patients and confirmed COVID‐19 cases were quantified per week (depicted according to ISO 8601:2019 standards). Weekly national policies carried out on the COVID‐19 pandemic were taken into account.
Descriptive statistics were used to compare the incidence of stroke admissions before and after the setting of the state of emergency in our country (14 March 2020) expressed in strokes per week and the differences between the baseline characteristics and demographics of patients attended in those periods. Quantitative variables were reported as median with interquartile range (IQR) and tested for differences with the Mann–Whitney U and Kruskal–Wallis tests. Chi‐squared tests were used to test for differences in dichotomous variables.
Ethical approval for this study was obtained from the regional Research Ethics Committee, Comité de Ética de la Investigación de la Comunidad Autónoma de Aragón.
Results
In total, 354 patients with ischaemic stroke (185 men, 52.3%), median age 79 years (IQR 68–86), were admitted to a hospital in SALUD between 30 December 2019 and 19 April 2020. There was a weekly average of 27.5 cases before the setting of the state of emergency against 12 afterwards (P < 0.001). This drop in stroke cases occurred progressively from week 11 (9–15 March), persisting in time despite the decrease in confirmed cases of COVID‐19 (Fig. 1). This also happened in the number of patients who received reperfusion therapies, IVT and/or EVT, per week (8.5 vs. 4, P = 0.011).
Figure 1.
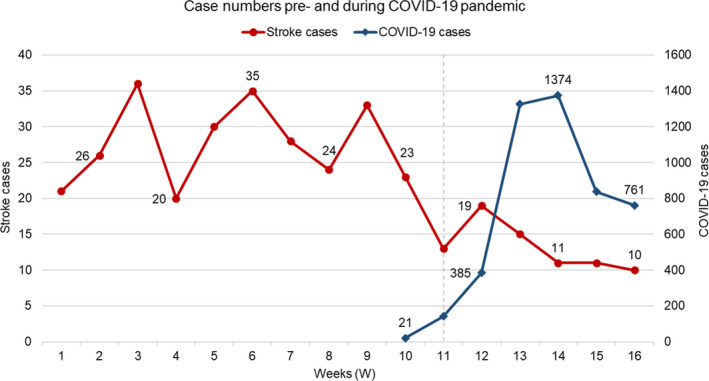
Case numbers pre‐COVID‐19 and during the COVID‐19 pandemic in Aragón (Spain), from 30 December 2019 (W1) to 19 April (W16) [22]. The dashed line represents the establishment of the state of emergency in W11 (14 March). The first SARS‐CoV‐2 confirmed case in the Peninsula was in W9 (Barcelona, 25 February).
There were no differences in the proportion of IVT (21.1% vs. 21.5%, P = 0.935) or EVT (12.4% vs. 15.2%, P = 0.510) in the total amount of ischaemic strokes of each period, nor in the other demographic or clinical characteristics except for median ODT (102 vs. 183 min, P = 0.015) (Table 1). Only 28 stroke patients were tested for COVID‐19 according to clinical suspicion; six of them were positive (21.4%).
Table 1.
Demographic and clinical characteristics of stroke admissions in Aragón before (pre‐CoV) and after (p‐CoV) the setting of the state of emergency in Spain
| pre‐CoV (n = 275) | p‐CoV (n = 79) | P | |
|---|---|---|---|
| Age, median (IQR) | 80 (68.0–86.0) | 78 (67.0–85.0) | 0.298 |
| >80 years, n (%) | 130 (47.3) | 32 (40.5) | 0.287 |
| Male, n (%) | 143 (52.0) | 42 (53.2) | 0.855 |
| In‐hospital stroke, n (%) | 15 (5.5) | 5 (6.3) | 0.783 |
| Strokes per week, median (IQR) | 27.5 (22.5–33.5) | 12 (10.8–16) | <0.001 |
| TP per week, median (IQR)a | 8.5 (6.3–9.3) | 4 (2.5–5.3) | 0.011 |
| IVT, n (%) | 58 (21.1) | 17 (21.5) | 0.935 |
| EVT, n (%) | 34 (12.4) | 12 (15.2) | 0.510 |
| >24 h from SO, n (%) | 59 (21.5) | 10 (12.7) | 0.082 |
| Unknown‐onset stroke, n (%) | 129 (46.9) | 35 (44.3) | 0.682 |
| ODT, min, median (IQR)b | 102 (65.3–196.8) | 183 (80.0–532.0) | 0.015 |
| mRS > 2, n (%) | 39 (14.2) | 15 (19.0) | 0.295 |
| Patients from retirement homes, n (%) | 18 (6.5) | 4 (5.1) | 0.794 |
| Brought in ambulance | 90 (32.7) | 32 (40.5) | 0.200 |
| ASPECTS, median (IQR)c | 10 (9–10) | 10 (9–10) | 0.309 |
| ASPECTS < 6, n (%)c | 9 (4.0) | 4 (5.9) | 0.508 |
| NIHSS, median (IQR) | 5 (2–11) | 6 (3–12) | 0.156 |
| NIHSS < 5, n (%) | 143 (52.0) | 38 (48.1) | 0.541 |
| NIHSS > 15, n (%) | 39 (14.2) | 13 (16.5) | 0.615 |
| DNT, min, median (IQR) | 50 (35.0–70.3) | 64 (38.5–89.0) | 0.112 |
| DPT, min, median (IQR) | 59.5 (37.3–108.5) | 68 (41.8–126.8) | 0.388 |
ASPECTS, Alberta Stroke Program Early Computed Tomography Score; DNT, door‐to‐needle time; DPT, door‐to‐puncture time; EVT, endovascular therapy; IQR, interquartile range; IVT, intravenous thrombolysis; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; ODT, onset‐to‐door time; SO, symptom onset; TP, treated patients. Bold font indicates statistical significance.
aTreated patients are the ones who received IVT and/or EVT; bout‐of‐hospital patients with less than 24 h from symptom onset; conly patients with anterior circulation strokes.
Discussion
Aragón is the fourth largest administrative division in Spain and the eleventh most populated. Amongst its major demographic characteristics are the dispersion and elderly population (285 599 people older than 65 years, which represents 21.65%). The population is very unevenly distributed, with the main province gathering 964 693 inhabitants (Zaragoza) but an average population density for the rest of the territory fewer than 20 inhabitants/km2. SALUD is the entity responsible for healthcare in the region, assisting 97% of its citizens. The entire territory is covered by eight district health boards, each one with a referral hospital. Three of the districts correspond to the city of Zaragoza, whilst the rest provide care for less inhabited areas. There is one tertiary referral hospital for stroke EVT in the whole region, and all the other hospitals can offer IVT. The whole SALUD uses the same electronic medical record and has common stroke entry/discharge reports, allowing exploitation of stroke hospital data from all over Aragón in real time.
On 14 March 2020, the Government of Spain implemented extraordinary measures to control viral transmission, restricting free mobility over the entire country. This was reinforced from 31 March to 11 April, essential workers being the only ones allowed to leave their homes. Until now, the state of emergency is still on. It is believed that the significant reduction in current stroke admissions might be related to fewer people going to the emergency department due to fear of being infected and in response to the measures previously described. This might make sense with minor non‐disabling strokes. However, it is hard to assume that people with a severely disabling stroke, such as those secondary to large‐vessel occlusion, did not come to the hospital, even if they were late for reperfusion treatment. Having said that, although there was a decrease in the number of patients who received reperfusion therapies per week, no changes in the proportion of IVT and EVT patients were found in our region when a comparison was made with the total number of ischaemic strokes for each period. There was also a similar number of low ASPECTS or high NIHSS scores and the same proportion of patients with strokes of more than 24 h of evolution or those with unknown time of symptom onset.
It seems unlikely that COVID‐19 is directly related to the decrease in the influx of ischaemic stroke patients to our emergency departments. Furthermore, possible mechanisms by which SARS‐CoV‐2 may increase the risk of stroke have been described: angiotensin‐converting enzyme 2 receptor may act as an entry for SARS‐CoV‐2 causing neurological complications such as stroke through direct and indirect mechanisms [10]. The high level of D‐dimer, low platelet count and production of antiphospholipid antibodies predispose to a hypercoagulative state [11, 12, 13]. Besides, SARS‐CoV‐2 is reported to cause cytokine storm syndrome, which could contribute to stroke. Hypoxia in the central nervous system deriving from alveolar gas exchange disorder, cardioembolism due to virus‐related cardiac injury, or direct virus nervous system invasion are other proposed mechanisms. Nevertheless, these associations remain to be determined [14].
Considering frailty and strict social isolation, older patients could be at higher risk of dying at home from this or other diseases if they do not seek emergency services. In fact, it is the age group with higher mortality from COVID‐19 and also the one with more ischaemic stroke rates. However, this would have increased the proportion of strokes in young adults, which did not happen. Another hypothesis is that some stroke patients could be in a different in‐patient area and would not have been transferred to the neurology ward. Considering the actual hospital organization derived from COVID‐19, patients hospitalized with other diseases could be in isolation units where stroke might not be the major issue or were not being paid enough attention to make the diagnosis, which would be a serious inconvenience. As a result, some patients might not be receiving the necessary stroke standard of care.
On the other hand, it seems that around 29% of the global burden of stroke may be attributed to air pollution [15]. The measures adopted to control viral transmission in Spain have led to a drastic and general reduction in urban transportation, industrial activity and other sources of environmental pollution. There has been a 77% decrease in road traffic in main Spanish cities, with a maximum near 90% on weekends. In Zaragoza, this reduction has contributed to a 45% decrease in the NO2 levels registered in the city [16]. It would be tempting to assume that isolation might have contributed to better control of some modifiable stroke risk factors, making it plausible that the decrease in stroke admissions in this period might reflect a real reduction in stroke incidence. However, more studies are needed to make this statement.
Questions might arise whether the seasonal pattern of stroke occurrence may play a role in our results, but evidence is conflicting. Some studies report that ischaemic stroke events are significantly higher during spring and autumn than in summer [17, 18] and, in our case, the drop in stroke admissions has occurred during the beginning of spring. However, another study stated that there was a fairly even distribution of ischaemic stroke over all four seasons [19] and a recent meta‐analysis showed very little seasonal variation [20].
The higher ODT observed in our sample could be secondary to the collapse of the emergency services, focused on the transfers of COVID‐19 patients. On the other hand, it is possible that fear could also delay stroke activation by patients or their families.
To the best of our knowledge, this is the first study that quantifies the impact of the COVID‐19 outbreak in stroke hospital admissions in an entire region. However, some limitations should be noted. First, the way records were obtained from the SALUD database limits stroke events to the ones where cerebrovascular disease was the main diagnosis during hospitalization. This did not allow us to thoroughly assess the characteristics of the whole COVID‐19 population suffering from ischaemic stroke, but other series analysing these aspects found that the incidence of acute ischaemic stroke in this population is no more than 5% [21]. Secondly, the time frame to analyse how SARS‐CoV‐2 infection and policies affect stroke care is short, but due to the urgent need for some advice to other countries which are in a previous phase of the epidemic this registered month was considered significant enough to obtain interesting data that could help stroke centres.
Conclusions
This observational study offers the perspective of a whole region in one of the countries most heavily stricken by the SARS‐CoV‐2 epidemic. It shows that the decrease of stroke events, since the beginning of the COVID‐19 outbreak, happened globally and without any specific patient distribution. This forces us to stay alert and make a thorough investigation of ischaemic strokes. It might be useful to remind the general population and other health services that Code Stroke has not suffered from new policies and diversion of resources and to highlight the importance of alert activation in order to provide potential patients with the care that they deserve.
Conflict of interest
All the authors declare that there is no conflict of interest regarding the publication of this article.
Members of Grupo de Seguimiento y Mejora del Programa de Atención al Ictus en Aragón (PAIA)
Olalla Alberti González, Juan Carlos Aragües Bravo, Jorge Artal Roy, Laura Ballester Marco, María Rosario, Barrena Caballo, María Bestué Cardiel, Isabel Campello Morer, Carolina García Arguedas, María José Gimeno Peribáñez, Antonio Goméz Peligros, Belen Gros Bañeres, Natalia Hernando Quintana, María José Lafuente González, Javier Marta Moreno, Gloria Martínez Borobio, Marta Palacín Larroy, Cristina Pérez Lázaro, Pilar Ruiz Palomino, Marta Sampériz Murillo, Marta Serrano Ponz.
Contributor Information
H. Tejada Meza, Email: htmeza@gmail.com.
Grupo de Seguimiento y Mejora del Programa de Atención al Ictus en Aragón:
Olalla Alberti González, Juan Carlos Aragües Bravo, Jorge Artal Roy, Laura Ballester Marco, María Rosario Barrena Caballo, María Bestué Cardiel, Isabel Campello Morer, Carolina García Arguedas, María José Gimeno Peribáñez, Antonio Goméz Peligros, Belen Gros Bañeres, Natalia Hernando Quintana, María José Lafuente González, Javier Marta Moreno, Gloria Martínez Borobio, Marta Palacín Larroy, Cristina Pérez Lázaro, Pilar Ruiz Palomino, Marta Sampériz Murillo, and Marta Serrano Ponz
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Truelsen T, Piechowski‐Jóźwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol 2006; 13: 581–598. [DOI] [PubMed] [Google Scholar]
- 2. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006; 367: 1747–1757. [DOI] [PubMed] [Google Scholar]
- 3. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 4. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: 344–418.30626290 [Google Scholar]
- 5. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed 2020; 91: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fauci AS, Lane HC, Redfield RR. Covid‐19 – Navigating the uncharted. N Engl J Med 2020; 382: 1268–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahase E, Kmietowicz Z. Covid‐19: doctors are told not to perform CPR on patients in cardiac arrest. BMJ 2020; 368: m1282. [DOI] [PubMed] [Google Scholar]
- 8. Situación COVID‐19 en España [Internet] . Madrid: Ministerio de Sanidad, Gobierno de España; February 2020 [access 19 April 2020]. Available at: https://covid19.isciii.es.
- 9. ESO Executive Committee . Likely increase in the risk of death or disability from stroke during the COVID‐19 pandemic. European Stroke Organization [Internet]. April 8th 2020;4053.
- 10. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 2020; 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu K, Pan M, Xiao Z, Xu X. Neurological manifestations of the coronavirus (SARS‐CoV‐2) pandemic 2019–2020. J Neurol Neurosurg Psychiatry 2020; 91: 669–670. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med 2020; 382: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 2020; pii:S0049‐3848:30120‐30121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Markus HS, Brainin M. COVID‐19 and stroke—A global World Stroke Organization perspective. Int J Stroke. 2020; 15: 361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016; 15: 913–924. [DOI] [PubMed] [Google Scholar]
- 16. Ceballos MA,coordinator. Efectos de la crisis de la COVID‐19 en la calidad del aire urbano en España. Resultados provisionales a 30 de abril de 2020 para las 26 principales ciudades. Propuestas para una desescalada con aire limpio. Madrid: Ecologistas en acción; 2020. Available at: https://www.ecologistasenaccion.org/140177.
- 17. Sipilä JO, Ruuskanen JO, Kauko T, Rautava P, Kytö V. Seasonality of stroke in Finland. Ann Med 2017; 49: 310–318. [DOI] [PubMed] [Google Scholar]
- 18. Palm F, Dos Santos M, Urbanek C, et al. Stroke seasonality associations with subtype, etiology and laboratory results in the Ludwigshafen Stroke Study (LuSSt). Eur J Epidemiol 2013; 28: 373–381. [DOI] [PubMed] [Google Scholar]
- 19. Toyoda K, Koga M, Yamagami H, et al. Seasonal variations in neurological severity and outcomes of ischemic stroke: 5‐year single‐center observational study. Circ J 2018; 82: 1443–1450. [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Zhou Z, Chen N, He L, Zhou M. Seasonal variation in the occurrence of ischemic stroke: a meta‐analysis. Environ Geochem Health 2019; 41: 2113–2130. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Wang M, Zhou Y, Chang J, Mao XY, Li M, et al.Acute cerebrovascular disease following COVID‐19: a single center, retrospective, observational study. Available at SSRN: doi: 10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed]
- 22. Situación epidemiológica del coronavirus COVID‐19 en Aragón [Internet] . Zaragoza: Gobierno de Aragón; March 4th 2020. [access April 19th 2020]. Available at: https://www.aragon.es/coronavirus/situacion‐actual.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


