1.
Introduction: Since its discovery in December 2019, the novel coronavirus disease 2019 (COVID‐19) has caused several clinical presentations: mainly respiratory, rarely gastrointestinal, and exceptionally neurological. The neuroinvasive mechanism of this virus is poorly described. We report two recent observations of altered mental status that inaugurate an infection with SARS‐CoV‐2.
1.1.
Case Report 1: On March 8th, a 68‐year‐old male patient with no significant medical history (except obesity) presented to emergency department with altered consciousness since few days. Physical examination revealed blood pressure at 140/80 mmHg, regular heartbeat at 86/min. The patient was afebrile with normal pulmonary auscultation, neurological examination showed a patient with a confused verbal response and a Glasgow Coma Scale score of 14/15 (E = 4, M = 5, and V = 4). The patient didn't complain about headache, vomiting, or seizure. He was drowsy without neck stiffness or focal abnormalities. Serology for HIV, Syphilis and Borrelia burgdorferi were negative. Chest X‐ray was normal. Brain magnetic resonance imaging (MRI) with angio‐MRI was normal. Treatment with high dose of ceftriaxone and aciclovir was empirically initiated, stopped after the receipt of the results of lumbar puncture (LP) (Table 1). On March 11th, he presented fever measured at 39.5C° with cough and bilateral crackling sounds on pulmonary auscultation. Chest X‐ray showed a bilateral interstitial infiltrates. Urinary Legionella pneumophila antigen, serology for L. pneumophila, Mycoplasma pneumonia, Chlamydophila pneumonia, and Coxiella burnetii were negatives. Real‐time polymerase chain reaction (RT‐PCR) on nasopharyngeal swab and sputum confirmed the diagnosis of infection with SARS‐CoV‐2. On March 13th, the patient was admitted in intensive care unit (ICU) for acute respiratory failure to be mechanically ventilated. In addition to that, the patient was treated with antibiotics and lopinavir/ritonavir. The clinical course was favorable with successful extubation, on April 2nd.
Table 1.
Demographic characteristics, laboratory findings, and imaging features of the two patients infected by COVID‐19 with a presentation of encephalopathy
| Patient 1 | Patient 2 | |
|---|---|---|
| Demographic characteristics/medical history | ||
| Age (y) | 68 | 39 |
| Comorbidities | No | No |
| Treatment | No (no medication or illegal drugs) | No (no medication or illegal drugs) |
| Laboratory findings | ||
| Date of the blood sample | On admission (March 8th) | On admission (March 24th) |
| CBC | ||
| White‐cell count (per mm3) | 5160 | 7380 |
| Lymphocytes (per mm3) | 1590 | 2410 |
| Platelets count (per mm3) | 109,000 | 312,000 |
| Hemoglobin (g/L) | 13.3 | 15.5 |
| Albumin (g/L) | 31.1 | 40 |
| Alanine aminotransferase (U/L) | 37 | 86 |
| Aspartate aminotransferase (U/L) | 21 | 88 |
| Lactate dehydrogenase (U/L) | 518 | 157 |
| Blood glucose (mmol/L) | 6.9 | 5.3 |
| Creatinine (µmol/L) | 97 | 76 |
| Electrolytes | ||
| Na + (mmol/L) | 136 | 140 |
| K+ (mmol/L) | 4.4 | 3.7 |
| Ca2+ (mmol/L) | 2.51 | 2.26 |
| Mg2+ (mmol/L) | 0.79 | 0.83 |
| Creatine kinase (U/L) | 559 | 64 |
| High‐sensitivity cardiac troponin I (pg/ml) | 19.7 | 2.9 |
| Prothrombin time (s) | 11.1 | 11.4 |
| Fibrinogen (g/L) | ND | 3.1 |
| d‐dimer (mg/L) | ND | 315 |
| Serum ferritin (µg/L) | 1730 | 362 |
| C‐reactive protein (mg/L) | 156 | 102 |
| Procalcitonin (ng/ml) | 0.13 | ND |
| Lumbar puncture (CSF analysis) | ||
| Macroscopic examination of CSF | Clear | Clear |
| WBC count (/mm3) | 4 | 1 |
| Protein concentration (<0.45 g/L) | 0.23 | 0.37 |
| Glucose concentration (2.5–4 mmol/L) | 4.4 | 3.5 |
| Ratio CSF glucose/blood glucose (>0.5) | >0.5 | >0.5 |
| Bacterial culture | No growth | No growth |
| RT‐PCR HSV, VZV | Negative | Negative |
| RT‐PCR enterovirus | Negative | Negative |
| RT‐PCR (Listeria monocytognenes) | Negative | Negative |
| RT‐PCR (Mycobacterium tuberculosis) | ND | Negative |
| RT‐PCR Covid‐19 | ||
| CSF | Negative | Negative |
| Respiratory specimen | Nasopharyngeal swab and sputum | Nasopharyngeal swab |
| Result | Positive | Positive |
| Viral load in respiratory specimen (log copies/ml) (normal average: 5.5 log copies/ml)a | 7.2 | 7.0 |
| Imaging features | ||
| Thoracic imaging | Bilateral pulmonary infiltrates | Bilateral ground‐glass opacity |
| Brain MRI with vascular sequences (2D‐TOF, 3D‐TOF, and 3D T1 sequence with gadolinium enhancement) | Normal | Normal |
Abbreviations: ANOVA, analysis of variance; CBC, complete blood count; CSF, cerebrospinal fluid; HSV, herpes simplex virus; MRI, magnetic resonance imaging; ND, not determined; RT‐PCR, real‐time polymerase chain reaction; VZV, varicella zoster virus; WBC, white blood cell.
In Nord Franche‐Comté Hospital, between March 1st and March 14th; 68 patients were diagnosed with COVID‐19, without any neurologic symptoms; the average load in respiratory sample was 5.5 log copies/ml for these 68 patients versus 7.1 log copies/ml for these two patients (p < 0.001, ANOVA test). The cycle numbers (semi‐quantitative viral counts) are not determined.
1.2.
Case Report 2: On March 15th, a 39‐year‐old male patient with no significant medical history had frontal headache and fever followed by anosmia and dysgeusia 4 days after. He had just come back few days ago from a cruise on the Caribbean Sea. On March 24th, he presented cough with dyspnea, diarrhea. He developed also dysarthria, inattention, progressive drowsiness, and decreased consciousness. On admission, he was febrile at 38.8C°, neurological examination showed a Glasgow Coma Scale score of 12/15 (E = 4, M = 5, and V = 3) with nonfluent aphasia. There was no neck stiffness or other neurological physical sign. Altered consciousness still remains at emergency department during the period without fever. Routine laboratory findings showed elevated CRP (102 mg/l). The brain MRI and the LP were normal (Table 1). In the epidemic context of COVID‐19, the patient was isolated immediately and a thoracic computed tomography (CT) scan was performed, revealing bilateral ground‐glass opacities (Figure 1). COVID‐19 was diagnosed based on RT‐PCR microbiologic (positive RT‐PCR on nasopharyngeal swab) and CT thoracic imaging results. It has not been possible to perform a test for the presence of SARS‐CoV‐2 in the cerebrospinal fluid (CSF), for this patient. The initial treatment was supportive, associated to hydroxychloroquine for 10 days. The clinical course was rapidly favorable with resolution of neurological symptoms after 3 days and all symptoms on 29th March. The patient was discharged on 30th March.
Figure 1.
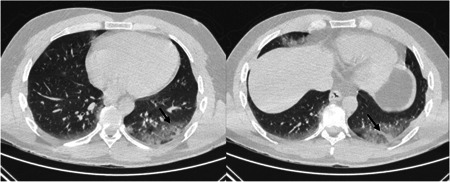
Computed tomography scan showed subpleural ground‐glass opacities with a basal distribution in the left lower lobe (arrow in the left image) and alveolar consolidation in linear atelectasis (arrow in the right image)
2.
Discussion: The description of our two cases suggests several hypotheses in the potential neuroinvasive mechanism of COVID‐19.
In addition to the usual symptoms (general, respiratory, and otorhinolaryngological) of the infection with SARS‐CoV‐2, several authors have described neurological manifestations as headache, nausea, and vomiting. It is known that the entry of SARS‐CoV‐2 into human host cells is mediated mainly by a cellular receptor: angiotensin‐converting enzyme 2 (ACE2), which is expressed in human airways epithelia, lung parenchyma, but also in small intestine cells, which explains this clinical features. 1 , 2 , 3 In our opinion, nausea and vomiting are more often related to gastrointestinal than central nervous system (CNS) invasion by SARS‐CoV‐2. Encephalopathy occurs frequently with severe infections, and since here there is no other culprit than COVID‐19, this infection is indeed the most likely. On the contrary, some experts describe disseminated intravascular coagulation and venous thromboembolism induced by this virus. 4 This coagulation dysfunction may probably explain cerebrovascular manifestations of COVID‐19 like cerebral venous thrombosis or intracerebral hemorrhage. 5 , 6 In our cases, cerebral MRI with vascular sequences has been performed, the normality of MRI doesn't sustain this hypothesis.
Eventually, the neuroinvasive propensity has been demonstrated as a common feature of coronaviruses (CoVs) that can cause nerve damage via diverse pathways 7 It is known that CoVs may enter the CNS through two distinct routes: hematogenous dissemination or neuronal retrograde dissemination. 8 Some CoVs have been demonstrated to be able to spread via a synapse‐connected route to the medullary cardiorespiratory center from the mechanoreceptors and chemoreceptors in the lungs and lower respiratory airways. These viruses can invade brainstem via a synapse‐connected route from the lungs and airways. 9 Considering the high similarity between SARS‐CoV‐2 and others CoVs, 10 it is still not clearly known whether the potential neuro‐invasion of SARS‐CoV2 is partially responsible for respiratory failure in patients with COVID‐19. 9 , 11 , 12 We don't know the exact physiopathology of the neurological signs in our two patients. No thrombosis, bleeding, signs of cerebral edema, or inflammation were found on brain MRIs. LP was normal with negative RT‐PCR COVID‐19 in CSF. There is no sufficient proof that the symptoms of the patients described are caused by direct CNS involvement.
To our knowledge, only one case of meningitis/encephalitis associated with SARS‐CoV‐2 has been described in the literature, with the detection of SARS‐CoV‐2 RNA in CSF. 10 In our patients, the LP was performed the second week after the onset of symptoms, which could explain viral load (VL) decreasing in the CSF and its nondetection.
As the second patient initially described anosmia and dysgeusia, we wonder if there was invasion of the olfactory receptors of the first cranial nerves in the nasal cavity cell membrane, as described with other viruses. 13 , 14 , 15 , 16 , 17 , 18 Our final assumption is that there is a possibility of correlation between the VL level in respiratory samples and these neurological features. In the literature, it has been shown that VL in respiratory samples is higher during the first week of symptoms or during the second week in severe cases with acute respiratory distress syndrome. 19 In our two patients we notice that the mean VL was clearly higher (7.1 log copies/ml) than the mean VL of our COVID population without neurological symptoms (5.5 log copies/ml).
Finally, no other treatment (as immunoglobulin therapy, anti‐IL6‐R or steroids) than lopinavir/ritonavir and hydroxychloroquine has been administrated to our patients. No recommendations to date have been published to our knowledge for the treatment of COVID‐19 with neurologic manifestations.
Conclusion: The neuroinvasive potential of COVID‐19 remains uncertain but possible. Therefore, in the context of COVID pandemic, it would be reasonable to perform a thoracic CT and a RT‐PCR for SARS‐CoV‐2 in case of encephalopathy with normal lumbar puncture and brain imaging. This will help to prevent the transmission of the virus in hospital settings, especially to health care workers and to not delay the management of patients with neurological presentation.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Collected clinical data and drafted the manuscript: Souheil Zayet and Timothee Klopfenstein. Described radiologic data: Yousri Ben Abdallah. Revised the final manuscript: Lynda Toko, Pierre‐Yves Royer, Vincent Gendrin, and Timothee Klopfenstein.
ACKNOWLEDMENT
We thank especially Dr Zahra Hajer for her help.
REFERENCES
- 1. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with covid‐19. N Engl J Med. 2020;382:1653‐1659. 10.1056/NEJMsr2005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS‐CoV‐2 spike glycoprotein with ACE‐2 receptor homologs and human TLRs. J Med Virol. 2020;92:2105‐2113. 10.1002/jmv.25987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094‐1099. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;296(2):201187. 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharifi‐Razavi A, Karimi N, Rouhani N. COVID 19 and intra cerebral hemorrhage: causative or coincidental. New Microbes New Infect. 2020;35:100669. 10.1016/j.nmni.2020.100669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain Behav Immun. 2020;87:18‐22. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou L, Zhang M, Gao J, Wang J. Sars‐Cov‐2: underestimated damage to nervous system. Travel Med Infect Dis. 2020;36:101642. 10.1016/j.tmaid.2020.101642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y‐C, Bai W‐Z, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92:552‐555. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019‐nCoV) originating in China. Cell Host Microbe. 2020;27(3):325‐328. 10.1016/j.chom.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Bai W‐Z, Hashikawa T. Response to commentary on: “the neuroinvasive potential of SARS‐CoV‐2 may play a role in the respiratory failure of COVID‐19 patients”. J Med Virol. 2020;92(7):707‐709. 10.1002/jmv.25824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nataf S. An alteration of the dopamine synthetic pathway is possibly involved in the pathophysiology of COVID‐19. J Med Virol. 2020;92:1743‐1744. 10.1002/jmv.25826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki M, Saito K, Min W‐P, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272‐277. 10.1097/01.mlg.0000249922.37381.1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zayet S, N'dri Juliette K‐O, Royer P‐Y, Toko L, Gendrin V, Klopfenstein T. Coronavirus disease 2019: new things to know!. J Med Virol. 2020;92:1767‐1768. 10.1002/jmv.25874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klopfenstein T, Kadiane‐Oussou NJ, Toko L, et al. Features of anosmia in COVID‐19. Med Mal Infect. 2020;50:436‐439. 10.1016/j.medmal.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finsterer J, Stollberger C. Causes of hypogeusia/hyposmia in SARS‐CoV2 infected patients. J Med Virol. 2020;92:1793‐1794. 10.1002/jmv.25903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Maria A, Varese P, Dentone C, Barisione E, Bassetti M. High prevalence of olfactory and taste disorder during SARS‐CoV‐2 infection in outpatients. J Med Virol. 2020. 10.1002/jmv.25995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251‐2261. 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. To KK‐W, Tsang OT‐Y, Leung W‐S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20:565‐574. 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


