Abstract
Extracellular vesicles (EVs) were initially characterized as “garbage bags” with the purpose of removing unwanted material from cells. It is now becoming clear that EVs mediate intercellular communication between distant cells through a transfer of genetic material, a process important to the systemic adaptation in physiological and pathological conditions. Although speculative, it has been suggested that the majority of EVs that make it into the bloodstream would be coming from skeletal muscle, since it is one of the largest organs in the human body. Although it is well established that skeletal muscle secretes peptides (currently known as myokines) into the bloodstream, the notion that skeletal muscle releases EVs is in its infancy. Besides intercellular communication and systemic adaptation, EV release could represent the mechanism by which muscle adapts to certain stimuli. This review summarizes the current understanding of EV biology and biogenesis and current isolation methods and briefly discusses the possible role EVs have in regulating skeletal muscle mass.
Keywords: extracellular vesicles, microRNA, skeletal muscle
INTRODUCTION
Extracellular vesicles (EVs) were first observed more than 50 years ago when Peter Wolf detected the presence of minute particulate material in plasma, which was described as “platelet dust” (90). Since then, it has been well established that virtually all cells in the body release distinct types of vesicles that are conserved throughout evolution, from bacteria to humans. During the years, these vesicles have received heterogeneous classifications, and, despite the debatable nomenclature that has circulated within the scientific community, it has currently adopted the term “EVs.” Additionally, based on the new position statement of the International Society for Extracellular Vesicles (80), if physical characteristics, such as size or density, are assessed the term “small EVs” (<150 nm) or “medium/large EVs” (>200 nm) is preferable. To date, three main subtypes of EVs have been described based on size and the mechanism of release: exosomes (30–150 nm in diameter), microvesicles (100–1,000 nm), and apoptotic bodies (>1 μm). Exosomes, the best-characterized subtype, are made by an endocytic pathway and released into the extracellular space upon fusion with the plasma membrane (10); microvesicles (also referred to as ectosomes or microparticles) are formed by outward blebbing of the plasma membrane and subsequent scission of the plasma membrane blebs; and apoptotic bodies are released by outward protrusion from the plasma membrane in dying cells (36). Although exosomes and microvesicles are by far the most-studied EV subtype, recent studies have postulated that apoptotic bodies are involved across many aspects of immunity and disease settings, which challenges the idea that they are mere by-products of apoptosis (reviewed in Ref. 6). For the purpose of this review, the adopted generic term “EVs” is used to refer to exosomes and microvesicles, unless otherwise stated.
Interest in the field was sparked in the early 1980s when two independent research groups observed that reticulocytes were able to produce and secrete vesicles enriched in transferrin receptors (26, 59). Although interesting, at that time EVs were described as a means of discarding unwanted cellular material (e.g., RNA, DNA, protein), and not much attention was given to this area of research. The “trash can” concept has been challenged in recent literature with the observation that EVs can be secreted and taken up by nearby and/or distant cells, thereby impacting physiological and pathological conditions. Raposo and colleagues (65) conducted a pioneering study in which they demonstrated that antigen-presenting cells secreted exosomes able to deliver antigen peptides to T cells. This breakthrough, followed by three important studies (2, 66, 83), has stimulated new interest in what was a dormant field and has since drawn new attention to the investigation of EVs as potential biological vehicles for different cell types. In more recent years, the number of publications involving EV biology has increased considerably, further illustrating their importance and their ability to facilitate intercellular communication.
In the cell-to-cell communication context, skeletal muscle has gained considerable attention because of accumulating evidence demonstrating that, during contraction, skeletal muscle acts as an important endocrine organ. Furthermore, it has been confirmed that skeletal muscle is able to release EVs containing different cargoes into the extracellular environment (23). Although the idea of muscle contributing to whole body adaptation is interesting and intriguing, the release of EVs could also be related to the process of skeletal muscle adaptation. This review highlights the current understanding of EV biology and biogenesis and current isolation methods and discusses the possible role of EVs in skeletal muscle adaptation and regulation of muscle mass.
EXTRACELLULAR VESICLE BIOGENESIS
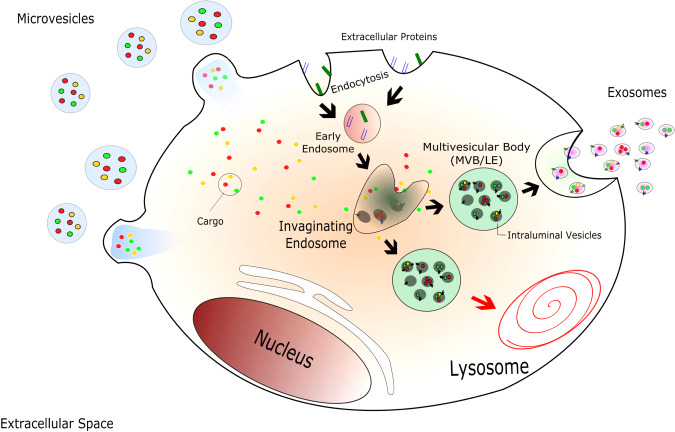
EV biology is not completely understood; however, a lot of progress has been made in understanding the biogenesis process in the last few years. It is well established that the mechanisms that govern EV biogenesis are different between microvesicles and exosomes. Microvesicle formation occurs through the budding of the plasma membrane; in exosomes, an inward invagination of late endosome/multivesicular bodies (MVBs) forms what is known as intraluminal vesicles (ILVs). Upon fusion with the plasma membrane, the ILVs are released into the extracellular environment, thus being called exosomes (Fig. 1). Interestingly, although the biogenesis process occurs through distinct processes for microvesicles and exosomes, evidence suggests that some mechanisms are shared.
Fig. 1.
Extracellular vesicle biogenesis and secretion. Microvesicles are released by outward budding of the plasma membrane due to changes in the membrane composition. After endocytosis and the formation of the early endosome, an inward budding of the endosomal membrane originates intraluminal vesicles into late endosome (LE)/multivesicular body (MVB) formation. MVBs have 2 fates: fuse with the lysosome, where its content is degraded, or fuse with the plasma membrane and have its content released into the extracellular environment, being called exosomes. [Modified from Kalra et al. (36) under Creative Commons by Attribution (CCBY) 4.0 license. Copyright 2016 Kalra et al.]
Microvesicle Biogenesis
Microvesicles, or shedding microvesicles, are released via a direct outward budding of the plasma membrane, ranging from 100 to 1,000 nm in diameter (36, 82). The budding process and formation of microvesicles are preceded by changes in the plasma membrane components, such as proteins and lipids, that modulate the curvature of the plasma membrane and mimic a vesicular shape (40). For example, Stachowiak and colleagues (72) have shown that protein crowding at the cell periphery causes a lateral pressure generated by collisions between bound proteins, driving membrane bending. Interestingly, the authors demonstrated that even the accumulation of proteins unrelated to membrane curvature, such as green fluorescent protein, could also stimulate membrane curvature formation.
Despite the fact that microvesicles are formed through an outward rather than inward budding of the plasma membrane (exosomes), some key proteins are involved in the biogenesis of both subtypes. For example, tumor susceptibility gene 101 protein (TSG101), involved in MVB formation, has been shown to participate in localized changes in membrane curvature, favoring the direct budding of microvesicles (57). Muralidharan-Chari and colleagues (56) demonstrated that the GTP-binding protein ADP-ribosylation factor 6 (ARF6) plays a critical role in the release of microvesicles. They demonstrated that ARF6 initiates a signaling cascade that recruits extracellular signal-regulated kinase and molecules (e.g., myosin light chain kinase) that affect vesicle curvature. It is worth mentioning that there are several other described mechanisms involved in microvesicle biogenesis, such as an increase in Ca2+ concentration, activation of acid sphingomyelinase, p38 mitogen-activated protein kinase, protein kinase C, and purinergic ATP receptors (3, 8, 12, 69).
Exosome Biogenesis
Exosomes are the smallest population of EVs and, unlike microvesicles, are formed primarily through an endocytosis process followed by exocytosis. During the maturation of early endosomes into late endosomes, ILVs are accumulated in their lumen after an inward budding of the endosomal membrane. The accumulation of ILVs in the endosomal lumen gives rise to MVBs. MVBs can have two fates, fusion with lysosomes (degradation pathway) or fusion with the plasma membrane (releasing ILVs into the extracellular environment, where they are called exosomes) (10). How the cells decide the fate of MVBs is not completely understood, but evidence suggests that the presence or absence of cholesterol in ILVs can determine the fate of MVBs (54). Although several mechanisms have already been described to participate in exosome biogenesis, the molecular machinery that governs these mechanisms is still not completely understood. Currently, they are classified as endosomal sorting complexes required for transport (ESCRT)-dependent or -independent pathways.
The best-described mechanism involved in the exosome biogenesis process is the ESCRT-dependent pathway. There are studies showing the presence of several ESCRT components within exosomes (9, 34, 79), suggesting the participation of this machinery in exosome biogenesis. ESCRT is composed of four protein complexes (ESCRT-0, -I, -II, -III) in conjunction with several associated proteins that are involved in bud formation, sorting, and scission (9, 25). Briefly, hepatocyte growth factor-associated tyrosine kinase substrate (Hrs) (ESCRT-0 subunit) recognizes and binds to ubiquitinated proteins that recruit the ESCRT-I complex (TSG101 and Vps28) to the endosomal membrane, forming the ESCRT-0-ESCRT-I complex. The activation of ESCRT-II (Vps22) by the ESCRT-I complex or by the associate protein programmed cell death 6 interacting protein (PDCD6IP, also called ALIX) initiates the budding process of nascent ILVs within MVBs, as well as the sorting of cargoes (e.g., RNAs and proteins). The sorting process is completed after the removal of the ubiquitin tag from the cargo proteins by deubiquitinating enzymes. Finally, the ESCRT-II complex recruits ESCRT-III (Snf7 and CHMP4) inside the neck of nascent ILVs, promoting closure of the cargo-containing vesicles and driving vesicle scission (9, 59, 65, 68). Aside from linking ESCRT-I to ESCRT-III, ALIX has been shown to interact directly with syntenin, a cytoplasmic adaptor of syndecan heparan sulfate proteoglycans, which promote intraluminal budding of ILVs (1).
Although the ESCRT-dependent pathways are widely accepted as the key mechanism for exosome biogenesis, there has been accumulating evidence demonstrating an ESCRT-independent mechanism during exosome biogenesis. In 2008, Trajkovic and colleagues demonstrated the possibility of a different mechanism in exosome biogenesis when they injected GW4869 (a neutral sphingomyelinase inhibitor that inhibits the conversion of sphingomyelin into ceramide) on mouse oligodendroglial cells and showed a decrease in exosome number (81). Besides neutral sphingomyelinases, lipids, such as phospholipase D2 (PLD2) (42) and ARF6 (22), participate in exosome biogenesis. Perhaps the most striking study indicating that exosomes could be made in an ESCRT-independent manner was published by Stuffers and colleagues (75). The authors demonstrated exosome formation in human epithelial type 2 (HEp-2) cells following depletion of components of all four ESCRT complexes. Tetraspanins (transmembrane proteins) have also been shown to participate in the biogenesis process through an ESCRT-independent machinery. For example, there is a significant decrease in the secretion of exosomes when knocking down CD9 (7) and CD63 (41). Finally, there is evidence that the small integral membrane protein of the lysosome/late endosome (SIMPLE) plays a role in exosome biogenesis (97). The existence of several mechanisms in the exosome biogenesis process does not preclude the possibility of all pathways working simultaneously or preferentially, depending on cell type and conditions.
EXTRACELLULAR VESICLE ISOLATION
One of the leading ongoing challenges in the field is the ability to define methods that are able to distinguish EV subtypes. Although much improvement has occurred in that regard, it is currently not possible to isolate a specific population of EVs with just one approach (39). Table 1 summarizes some of the features of EV subtypes. To date, many different methods have been described; however, each has its own specific advantages and disadvantages, which, depending on the methodology used, can differentially affect the properties of the isolated vesicles greatly (85). This is caused in part by each technique exploiting a particular feature of EVs in the isolation process, such as density, shape, size, and surface proteins. Although numerous isolation techniques have recently been published, this review focuses on those among them that are most used.
Table 1.
Main features of EV subtypes
| Features | Exosomes | Microvesicles |
|---|---|---|
| Biogenesis | MVB | Plasma membrane |
| Size | 30–150 nm | 100–1,000 nm |
| Ultracentrifugation sedimentation | 100,000 g | 10,000 g |
| Density in gradient ultracentrifugation | 1.08–1.14 g/mL | 1.12–1.20 g/mL |
EV, extracellular vesicle; MVB, multivesicular body.
Ultracentrifugation (UC) is still one of the most commonly used methods in the field and until recently was considered the gold standard technique for EV isolation. Initially used by Johnstone in 1987 (33) to isolate EVs from reticulocytes, this method has seen many improvements (47) and now consists of differential centrifugation steps to eliminate bigger vesicles and decrease the risk of contamination. However, UC does not permit complete separation among EV populations, allowing for protein complexes, lipoprotein particles, and other contaminants to be present in the final sedimentation. Additionally, Brisson’s group (49) has shown that high-speed centrifugation induces the formation of EV aggregates composed of a mixture of vesicle subtypes.
UC followed by floatation into a sucrose gradient/cushion (78) is another way to isolate EVs. The addition of the density gradient does not allow protein complexes (sedimented with UC) to float in the sucrose but permits lipid-containing vesicles to float upward. Although this method allows for a more favorable separation between particles (based on density), the process is time consuming (i.e., 16–90 h). Additionally, it might change the osmotic property of the EVs (48) and allow for high-density lipoproteins (HDL) to be coisolated (93). One consideration might be to employ the use of an isosmotic iodixanol gradient (OptiPrep gradient), which preserves the size of EVs while in the gradient, improving on some of the limitations of the sucrose gradient (5, 14, 45, 48). Although this method provides high purity and greater yield of exosomes (all the while preserving their structure and function), it still allows the coisolation of HDL.
Another popular isolation method for isolating EVs is size exclusion chromatography (SEC) (74). The principle of this technique is to separate suspended particles on the basis of their size or molecular weight. SEC is a type of ultrafiltration and is considered to be faster than UC. However, the action and process of forcing suspended particles to pass through a filter may break up larger vesicles, causing contamination in the preparation. Additionally, SEC only permits the isolation of EVs larger than the pore size of the matrix used (i.e., 70 nm for CL-2B Sepharose); moreover, particles of the same size (e.g., chylomicrons and VLDL lipoproteins) would be coisolated (71).
Various companies have manufactured commercially available methods claiming to swiftly and simply isolate exosomes; however, the technology used in these kits is based on immunocapture and/or polymer-based precipitation. These kits allow for low-volume input and a high-yield recovery of EVs, yet they also present some notable limitations. The first kit technology to be discussed is based on immunoaffinity interactions between proteins in the membrane of EVs and antibody-coated membranes or beads (16, 76, 96). The success of this technique relies entirely on protein markers that are specific to the EV subtype. Although some markers have been identified (39), it is currently not known whether EVs from different cell types would also have those markers. The second kit technology utilizes a water-excluding polymer (polyethylene glycol) (31, 87), a standard method used for virus isolation (91), to capture small EVs based on the difference in their solubility (ExoQuick and Total Exosome Isolation Reagent). Basically, these water-excluding polymers bind water molecules and force the less soluble particles (i.e., smaller vesicles) out of solution, recovering them by low-speed centrifugation (i.e., 1,500 g). Although the precipitation method is relatively quick and easy and does not require any special equipment, the purity of this isolation is a concern in the field, since coprecipitation of ribonucleoproteins and lipoproteins has been demonstrated (32, 43). The main advantages and disadvantages of all these methods are highlighted in Table 2.
Table 2.
Advantages and disadvantages of currently available methods for EV isolation
| Approaches | Advantages | Disadvantages | References |
|---|---|---|---|
| Ultracentrifugation | Decreases the risk of contamination with bigger vesicles | Expensive equipment; contamination with protein complex and lipoproteins; disrupts EV membrane because of high force; low yield | (47, 49) |
| Density gradient ultracentrifugation | Increases the purity of EV | Time-consuming; does not separate HDL from EV; low yield | (45, 48, 78, 93) |
| Size exclusion chromatography | Fast; separates EV from HDL; reproducibility and purity; preserves vesicle integrity | Vesicles of the same size would be co-isolated with EV | (71, 74) |
| Polyethylene glycol | Recovery of a higher yield; does not require expensive equipment; low volume necessary | Contamination with proteins, protein complexes, lipoproteins, and nucleoproteins as well as viral and other particles | (31, 32, 87) |
| Immunocapture | Isolation of homogeneous population; does not require special equipment; decreases contamination with other vesicles | Markers for different cell types are currently unknown; high selectivity and cost; nonspecific binding | (16, 77, 96) |
EV, extracellular vesicle; HDL, high-density lipoproteins.
Although remarkable progress has been made in regard to EV isolation, the ability to isolate more pure populations is only possible with a combination of techniques. For example, the combination of utilizing a density gradient with immunocapture-based technique (39) or with SEC (37) has been shown to improve the purity of exosomes, whereby lipoprotein particles (usually coisolated with most current techniques) are separated from exosomes. Regardless of the protocol employed, each technique must be validated to confirm the identity of the purified EV. Therefore, in an attempt to standardize the ongoing studies in the EV field, the International Society for Extracellular Vesicles has proposed the use of at least three different methods of EV analysis and a description in detail of the methodology used for the isolation procedures (51, 89).
Among those methods, there are optical (fluorescence-activated cell sorter, dynamic light scattering, nanoparticle tracking analysis, etc.) and nonoptical (transmission electron microscopy, atomic force microscopy, etc.) approaches to analyze EV size and morphology. Although they are all good options for characterization of EV preparation, they also present limitations. Table 3 presents the main methods for EV detection.
Table 3.
Main optical and nonoptical methods for EV analysis
| Methods | Principle | Limitations | Reference |
|---|---|---|---|
| Fluorescence-activated cell sorter | Laser-interrogated particle fluorescence method with a nondestructive and quantitative manner | Beads with known size necessary; low refractive signal of EVs; high background; nonoptimum for particles below 300 nm | (64) |
| Dynamic light scattering | Reflects scattering light intensity distribution under Brownian motion of suspended particles, providing size from those particles | Less accurate with heterogeneous size population; biases toward larger particles | (21, 44) |
| Nanoparticle tracking analysis | Dark-field microscope that combines laser light scattering with charge-coupled device for detection of particles smaller than 1,000 nm; calculates diameter of each vesicle with Stokes-Einstein equation | Need a “right” dilution to not underestimate smaller vesicles; low sensitivity to fluorescence signals | (15, 20) |
| Transmission electron microscopy | High-resolution microscope with resolution down to 1 nm; image is created by electron interference when the electron beam crosses the sample | Not applied for high-throughput profiling of EV; high quality of EV preparation is necessary; preparation could change EV morphology | (50, 67, 92) |
| Atomic force microscopy (AFM) | Super high resolution that can provide size, distribution, morphology and map mechanical properties with nanometric precision; a technique that detects and records interactions between the probing tip and the sample surface | Slow speed and limited imaging area; influenced by AFM probes | (94) |
EV, extracellular vesicle.
SKELETAL MUSCLE MASS AND EXTRACELLULAR VESICLES
Skeletal muscle seems to have a significant contribution in this exciting and growing field of intercellular communication. It is well established that skeletal muscle fibers secrete peptides (currently termed myokines), which might have an influence on whole body adaptation (17, 60–63, 73). Additionally, it has also been demonstrated that muscle fibers can secrete EVs in the bloodstream (24). Although not validated, the majority of EVs that make it into the bloodstream could be coming from skeletal muscle, because it is one of the largest organ systems in the human body. Therefore, it seems plausible to speculate that muscle-derived EVs would have an impact on whole body homeostasis through intercellular communication. This hypothesis has been shown to be valid in both in vitro (52) and in vivo (58, 88) studies, demonstrating that muscle-derived EVs can regulate distant cells by delivering functional cargo.
Although skeletal muscle seems to contribute to intercellular communication, the release and the uptake of EVs themselves could represent a new mechanism by which skeletal muscle adapts to certain stimuli. For example, Hudson and colleagues (29), showed that microRNA (miR)-23a, which targets atrogenes, was rapidly decreased in C2C12 myotubes after dexamethasone-induced atrophy. Interestingly, this rapid decrease was associated with its increase in exosomes. This mechanism allowed for the increase in the atrogenes MuRF1 and MAFbx, leading to myotube atrophy. In cancer-associated cachexia, lung cancer- and pancreatic cancer-derived EVs induce catabolism in both mouse and human myoblast cells (27). In particular, miR-21 within cancer-derived EV activates toll-like receptor (TLR7 in mice and TLR8 in humans) and promotes apoptosis through c-Jun NH2-terminal kinase (JNK) activity.
Mobley and colleagues (55) observed that hydrolyzed whey protein-derived exosomes promoted an increase in myotube protein synthesis and growth in C2C12 myotubes. Although the authors did not investigate a potential mechanism, they speculated that the delivery of whey-derived bovine miR-214 could repress phosphatase and tensin homolog (PTEN) expression, modulating mechanistic target of rapamycin (mTOR) and promoting skeletal muscle hypertrophy. Such a mechanism more broadly suggests that during muscle hypertrophy EV-delivered miR could modulate important pathways in the regulation of muscle mass. Our group has demonstrated that skeletal muscle myogenic progenitor cells secrete miR-206 containing-EVs that contribute to skeletal muscle growth (18). Specifically, miR-206 regulates fibrogenic cell collagen expression through repression of ribosome binding protein 1 (Rrbp1), facilitating appropriate remodeling of the extracellular matrix and preventing fibrosis.
Given the paucity of studies that have directly investigated the role of EVs in skeletal muscle hypertrophy, we are left with only speculation on the potential role of EVs in this process. D’Souza and colleagues (13), reported an increase in abundance of a muscle-specific miR (myomiR-1) in EVs after high-intensity interval exercise in human subjects. The increase in exosomal miR-1 abundance was associated with a decrease in skeletal muscle miR-1 expression but not until 4 h after exercise. Although the focus of this study was not muscle hypertrophy, changes in myomiR expression have been associated with muscle growth. McCarthy and Esser (53), showed a decrease of myomiRs (miR-1 and -133a) during skeletal muscle hypertrophy. Based on the target predictions, the authors suggest that the decrease in the expression of these myomiRs might promote an increase in the expression of genes known to participate in muscle hypertrophy such as c-Met, HGF, IGF-I, and SRF. Despite the decrease in the mature form, interestingly, the authors found an increase in the precursor levels, suggesting that this decrease was not associated with an impaired biogenesis process. Although not verified in this study, the discrepancy between the mature form and the precursor levels during muscle growth could represent an adaptive process mediated by EV release. All together, these studies provide support for the idea that the release or uptake of miRs by EVs by muscle fibers represents a novel mechanism involved in the control of skeletal muscle mass.
Even though the majority of studies have focused on miRNAs, it is well established that EVs also transfer lipids, protein, DNA, and a substantial amount of different RNA species (mRNA, rRNA, yRNA, vault RNA, tRNA, scaRNA, snoRNA, snRNA, and piRNA) that could have an impact on the host and/or the recipient cells (35, 46, 84, 95). Lipids for instance, one of the least studied cargoes within EVs, might have an impact on muscle mass. For example, cholesterol, one of the most abundant lipids present on EVs (70), increases membrane fluidity (11) and thickness (19) and is important for lipid raft formation (28). These lipid rafts function as platforms for the assembly of components of signaling pathways, such as IGF-I receptor (30), phosphoinositide kinase-3 (PI3K)-AKT (4), protein kinase C (PKC) (86), and interleukin-6 (IL-6) (38), all important for skeletal muscle growth. Although speculative, EV release and/or uptake could favor those changes in the lipid composition of the plasma membrane, stimulating the regulation of muscle mass by increasing signaling pathways that are important for muscle growth.
Therefore, it seems reasonable to speculate that EVs have a big impact on muscle mass adaptation due to their broad content. Cargo in EVs could have, upon delivery, an impact on molecular pathways involved in promoting muscle mass or, at the same time, work as a means to get rid of unwanted material, removing the break and allowing the muscle to adapt.
FINAL CONSIDERATIONS
The EV field is still in its infancy, and even though important advances were achieved during the last decade there are numerous uncertainties regarding EV biology. For example, although the importance of EVs for intercellular communication has been intensively investigated, the effects on the donor cells after EV release have not been explored at this point. The ability of EVs in packaging different cargoes could represent a mechanism by which cells would export a specific cargo and be able to adapt to different stimuli. Earliest studies (26, 59) suggest that EVs act as a “garbage bag” for the cell, removing unwanted cellular material. The demonstration that skeletal muscle can package unwanted miRs during skeletal muscle adaptation (29) suggests that, although speculative, the release of EV-contained unwanted material could represent a faster and more direct response to a stimulus. Nonetheless, why cells decide to send specific unwanted material to the extracellular space via EV export if the content could be degraded upon fusion with the lysosome is unknown. Perhaps the export of the cargo is faster than lysosome degradation, or there exists a two-way communication where the recipient cells and the donor cells work together for a more specific adaptation. Regardless, more studies are needed to better understand this new and exciting biological process.
GRANTS
The study on EVs is being supported by National Institutes of Health Grant DK-119619 to John McCarthy and Charlotte Peterson.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
I.J.V. conceived and designed research; prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Dr. C. Brooks Mobley for input on this manuscript.
REFERENCES
- 1.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14: 677–685, 2012. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 2.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Brański P, Ratajczak MZ, Zembala M. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother 55: 808–818, 2006. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J 28: 1043–1054, 2009. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calay D, Vind-Kezunovic D, Frankart A, Lambert S, Poumay Y, Gniadecki R. Inhibition of Akt signaling by exclusion from lipid rafts in normal and transformed epidermal keratinocytes. J Invest Dermatol 130: 1136–1145, 2010. doi: 10.1038/jid.2009.415. [DOI] [PubMed] [Google Scholar]
- 5.Cantin R, Diou J, Bélanger D, Tremblay AM, Gilbert C. Discrimination between exosomes and HIV-1: purification of both vesicles from cell-free supernatants. J Immunol Methods 338: 21–30, 2008. doi: 10.1016/j.jim.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Caruso S, Poon IK. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol 9: 1486, 2018. doi: 10.3389/fimmu.2018.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol 190: 1079–1091, 2010. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocucci E, Racchetti G, Podini P, Meldolesi J. Enlargeosome traffic: exocytosis triggered by various signals is followed by endocytosis, membrane shedding or both. Traffic 8: 742–757, 2007. doi: 10.1111/j.1600-0854.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 9.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126: 5553–5565, 2013. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 10.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289, 2014. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 11.Cooper RA. Influence of increased membrane cholesterol on membrane fluidity and cell function in human red blood cells. J Supramol Struct 8: 413–430, 1978. doi: 10.1002/jss.400080404. [DOI] [PubMed] [Google Scholar]
- 12.Curtis AM, Wilkinson PF, Gui M, Gales TL, Hu E, Edelberg JM. p38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticles. J Thromb Haemost 7: 701–709, 2009. doi: 10.1111/j.1538-7836.2009.03304.x. [DOI] [PubMed] [Google Scholar]
- 13.D’Souza RF, Woodhead JS, Zeng N, Blenkiron C, Merry TL, Cameron-Smith D, Mitchell CJ. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am J Physiol Endocrinol Metab 315: E723–E733, 2018. doi: 10.1152/ajpendo.00138.2018. [DOI] [PubMed] [Google Scholar]
- 14.Dettenhofer M, Yu XF. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J Virol 73: 1460–1467, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, Carr B, Redman CW, Harris AL, Dobson PJ, Harrison P, Sargent IL. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 7: 780–788, 2011. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enderle D, Spiel A, Coticchia CM, Berghoff E, Mueller R, Schlumpberger M, Sprenger-Haussels M, Shaffer JM, Lader E, Skog J, Noerholm M. Characterization of RNA from exosomes and other extracellular vesicles isolated by a novel spin column-based method. PLoS One 10: e0136133, 2015. doi: 10.1371/journal.pone.0136133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53: 1643–1648, 2004. doi: 10.2337/diabetes.53.7.1643. [DOI] [PubMed] [Google Scholar]
- 18.Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20: 56–69, 2017. doi: 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallová J, Uhríková D, Islamov A, Kuklin A, Balgavý P. Effect of cholesterol on the bilayer thickness in unilamellar extruded DLPC and DOPC liposomes: SANS contrast variation study. Gen Physiol Biophys 23: 113–128, 2004. [PubMed] [Google Scholar]
- 20.Gardiner C, Ferreira YJ, Dragovic RA, Redman CW, Sargent IL. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles 2: 19671, 2013. doi: 10.3402/jev.v2i0.19671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gercel-Taylor C, Atay S, Tullis RH, Kesimer M, Taylor DD. Nanoparticle analysis of circulating cell-derived vesicles in ovarian cancer patients. Anal Biochem 428: 44–53, 2012. doi: 10.1016/j.ab.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, Slavík J, Machala M, Zimmermann P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun 5: 3477, 2014. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 23.Guescini M, Canonico B, Lucertini F, Maggio S, Annibalini G, Barbieri E, Luchetti F, Papa S, Stocchi V. Muscle releases alpha-sarcoglycan positive extracellular vesicles carrying miRNAs in the bloodstream. PLoS One 10: e0125094, 2015. doi: 10.1371/journal.pone.0125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guescini M, Guidolin D, Vallorani L, Casadei L, Gioacchini AM, Tibollo P, Battistelli M, Falcieri E, Battistin L, Agnati LF, Stocchi V. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res 316: 1977–1984, 2010. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol 28: 337–362, 2012. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 26.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97: 329–339, 1983. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He WA, Calore F, Londhe P, Canella A, Guttridge DC, Croce CM. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc Natl Acad Sci USA 111: 4525–4529, 2014. doi: 10.1073/pnas.1402714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helms JB, Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic 5: 247–254, 2004. doi: 10.1111/j.1600-0854.2004.0181.x. [DOI] [PubMed] [Google Scholar]
- 29.Hudson MB, Woodworth-Hobbs ME, Zheng B, Rahnert JA, Blount MA, Gooch JL, Searles CD, Price SR. miR-23a is decreased during muscle atrophy by a mechanism that includes calcineurin signaling and exosome-mediated export. Am J Physiol Cell Physiol 306: C551–C558, 2014. doi: 10.1152/ajpcell.00266.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K. Lipid rafts/caveolae are essential for insulin-like growth factor-1 receptor signaling during 3T3-L1 preadipocyte differentiation induction. J Biol Chem 278: 11561–11569, 2003. doi: 10.1074/jbc.M211785200. [DOI] [PubMed] [Google Scholar]
- 31.Iverius PH, Laurent TC. Precipitation of some plasma proteins by the addition of dextran or polyethylene glycol. Biochim Biophys Acta 133: 371–373, 1967. doi: 10.1016/0005-2795(67)90079-7. [DOI] [PubMed] [Google Scholar]
- 32.Izzo C, Grillo F, Murador E. Improved method for determination of high-density-lipoprotein cholesterol I. Isolation of high-density lipoproteins by use of polyethylene glycol 6000. Clin Chem 27: 371–374, 1981. [PubMed] [Google Scholar]
- 33.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262: 9412–9420, 1987. [PubMed] [Google Scholar]
- 34.Juan T, Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol 74: 66–77, 2018. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Kalluri R, LeBleu VS. Discovery of double-stranded genomic DNA in circulating exosomes. Cold Spring Harb Symp Quant Biol 81: 275–280, 2016. doi: 10.1101/sqb.2016.81.030932. [DOI] [PubMed] [Google Scholar]
- 36.Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci 17: 170, 2016. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karimi N, Cvjetkovic A, Jang SC, Crescitelli R, Hosseinpour Feizi MA, Nieuwland R, Lötvall J, Lässer C. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell Mol Life Sci 75: 2873–2886, 2018. doi: 10.1007/s00018-018-2773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Adam RM, Solomon KR, Freeman MR. Involvement of cholesterol-rich lipid rafts in interleukin-6-induced neuroendocrine differentiation of LNCaP prostate cancer cells. Endocrinology 145: 613–619, 2004. doi: 10.1210/en.2003-0772. [DOI] [PubMed] [Google Scholar]
- 39.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 113: E968–E977, 2016. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozlov MM, Campelo F, Liska N, Chernomordik LV, Marrink SJ, McMahon HT. Mechanisms shaping cell membranes. Curr Opin Cell Biol 29: 53–60, 2014. doi: 10.1016/j.ceb.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamb TD. Spontaneous quantal events induced in toad rods by pigment bleaching. Nature 287: 349–351, 1980. doi: 10.1038/287349a0. [DOI] [PubMed] [Google Scholar]
- 42.Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent-Schneider H, Lankar D, Salles JP, Bonnerot C, Perret B, Record M. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett 572: 11–14, 2004. doi: 10.1016/j.febslet.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 43.Laurent LC, Abdel-Mageed AB, Adelson PD, Arango J, Balaj L, Breakefield X, Carlson E, Carter BS, Majem B, Chen CC, Cocucci E, Danielson K, Courtright A, Das S, Abd Elmageed ZY, Enderle D, Ezrin A, Ferrer M, Freedman J, Galas D, Gandhi R, Huentelman MJ, Van Keuren-Jensen K, Kalani Y, Kim Y, Krichevsky AM, Lai C, Lal-Nag M, Laurent CD, Leonardo T, Li F, Malenica I, Mondal D, Nejad P, Patel T, et al. Meeting report: discussions and preliminary findings on extracellular RNA measurement methods from laboratories in the NIH Extracellular RNA Communication Consortium. J Extracell Vesicles 4: 26533, 2015. doi: 10.3402/jev.v4.26533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrie AS, Albanyan A, Cardigan RA, Mackie IJ, Harrison P. Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox Sang 96: 206–212, 2009. doi: 10.1111/j.1423-0410.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 45.Li K, Wong DK, Hong KY, Raffai RL. Cushioned-density gradient ultracentrifugation (C-DGUC): a refined and high performance method for the isolation, characterization, and use of exosomes. Methods Mol Biol 1740: 69–83, 2018. doi: 10.1007/978-1-4939-7652-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M, Zeringer E, Barta T, Schageman J, Cheng A, Vlassov AV. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc Lond B Biol Sci 369: 20130502, 2014. doi: 10.1098/rstb.2013.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics 7: 789–804, 2017. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Donowitz M. Fractionation of subcellular membrane vesicles of epithelial and non-epithelial cells by OptiPrep density gradient ultracentrifugation. Methods Mol Biol 1174: 85–99, 2014. doi: 10.1007/978-1-4939-0944-5_6. [DOI] [PubMed] [Google Scholar]
- 49.Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles 4: 29509, 2015. doi: 10.3402/jev.v4.29509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linares R, Tan S, Gounou C, Brisson AR. Imaging and quantification of extracellular vesicles by transmission electron microscopy. Methods Mol Biol 1545: 43–54, 2017. doi: 10.1007/978-1-4939-6728-5_4. [DOI] [PubMed] [Google Scholar]
- 51.Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3: 26913, 2014. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madison RD, McGee C, Rawson R, Robinson GA. Extracellular vesicles from a muscle cell line (C2C12) enhance cell survival and neurite outgrowth of a motor neuron cell line (NSC-34). J Extracell Vesicles 3: 22865, 2014. doi: 10.3402/jev.v3.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarthy JJ, Esser KA. MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J Appl Physiol (1985) 102: 306–313, 2007. doi: 10.1152/japplphysiol.00932.2006. [DOI] [PubMed] [Google Scholar]
- 54.Möbius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, Slot JW, Geuze HJ. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic 4: 222–231, 2003. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 55.Mobley CB, Mumford PW, McCarthy JJ, Miller ME, Young KC, Martin JS, Beck DT, Lockwood CM, Roberts MD. Whey protein-derived exosomes increase protein synthesis and hypertrophy in C2C12 myotubes. J Dairy Sci 100: 48–64, 2017. doi: 10.3168/jds.2016-11341. [DOI] [PubMed] [Google Scholar]
- 56.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 19: 1875–1885, 2009. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci USA 109: 4146–4151, 2012. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y, Ochi M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett 589: 1257–1265, 2015. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 59.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33: 967–978, 1983. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 60.Pedersen BK, Fischer CP. Beneficial health effects of exercise—the role of IL-6 as a myokine. Trends Pharmacol Sci 28: 152–156, 2007. doi: 10.1016/j.tips.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Pedersen BK, Fischer CP. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr Opin Clin Nutr Metab Care 10: 265–271, 2007. doi: 10.1097/MCO.0b013e3280ebb5b3. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol 536: 329–337, 2001. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pedersen L, Hojman P. Muscle-to-organ cross talk mediated by myokines. Adipocyte 1: 164–167, 2012. doi: 10.4161/adip.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pospichalova V, Svoboda J, Dave Z, Kotrbova A, Kaiser K, Klemova D, Ilkovics L, Hampl A, Crha I, Jandakova E, Minar L, Weinberger V, Bryja V. Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J Extracell Vesicles 4: 25530, 2015. doi: 10.3402/jev.v4.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183: 1161–1172, 1996. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20: 847–856, 2006. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 67.Romancino DP, Paterniti G, Campos Y, De Luca A, Di Felice V, d’Azzo A, Bongiovanni A. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Lett 587: 1379–1384, 2013. doi: 10.1016/j.febslet.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt O, Teis D. The ESCRT machinery. Curr Biol 22: R116–R120, 2012. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene 23: 956–963, 2004. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- 70.Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res 60: 9–18, 2019. doi: 10.1194/jlr.R084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sódar BW, Kittel Á, Pálóczi K, Vukman KV, Osteikoetxea X, Szabó-Taylor K, Németh A, Sperlágh B, Baranyai T, Giricz Z, Wiener Z, Turiák L, Drahos L, Pállinger É, Vékey K, Ferdinandy P, Falus A, Buzás EI. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci Rep 6: 24316, 2016. doi: 10.1038/srep24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stachowiak JC, Schmid EM, Ryan CJ, Ann HS, Sasaki DY, Sherman MB, Geissler PL, Fletcher DA, Hayden CC. Membrane bending by protein-protein crowding. Nat Cell Biol 14: 944–949, 2012. doi: 10.1038/ncb2561. [DOI] [PubMed] [Google Scholar]
- 73.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529: 237–242, 2000. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stranska R, Gysbrechts L, Wouters J, Vermeersch P, Bloch K, Dierickx D, Andrei G, Snoeck R. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J Transl Med 16: 1, 2018. doi: 10.1186/s12967-017-1374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10: 925–937, 2009. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 76.Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 56: 293–304, 2012. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 87: 3–10, 2015. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 78.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 30: 3.22.1–3.22.29, 2006. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 79.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 166: 7309–7318, 2001. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 80.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750, 2018. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247, 2008. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 82.Tricarico C, Clancy J, D’Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases 8: 220–232, 2017. doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 84.van Balkom BW, Eisele AS, Pegtel DM, Bervoets S, Verhaar MC. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J Extracell Vesicles 4: 26760, 2015. doi: 10.3402/jev.v4.26760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O, Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles 3: 24858, 2014. doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wehmeyer L, Du Toit A, Lang DM, Hapgood JP. Lipid raft- and protein kinase C-mediated synergism between glucocorticoid- and gonadotropin-releasing hormone signaling results in decreased cell proliferation. J Biol Chem 289: 10235–10251, 2014. doi: 10.1074/jbc.M113.544742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weng Y, Sui Z, Shan Y, Hu Y, Chen Y, Zhang L, Zhang Y. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst 141: 4640–4646, 2016. doi: 10.1039/C6AN00892E. [DOI] [PubMed] [Google Scholar]
- 88.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JF, Febbraio MA. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab 27: 237–251.e4, 2018. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2: 20360, 2013. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 13: 269–288, 1967. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto KR, Alberts BM, Benzinger R, Lawhorne L, Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 40: 734–744, 1970. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- 92.Yuana Y, Koning RI, Kuil ME, Rensen PC, Koster AJ, Bertina RM, Osanto S. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J Extracell Vesicles 2: 21494, 2013. doi: 10.3402/jev.v2i0.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuana Y, Levels J, Grootemaat A, Sturk A, Nieuwland R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J Extracell Vesicles 3: 23262, 2014. doi: 10.3402/jev.v3.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia Rodriguez P, Bertina RM, Osanto S. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J Thromb Haemost 8: 315–323, 2010. doi: 10.1111/j.1538-7836.2009.03654.x. [DOI] [PubMed] [Google Scholar]
- 95.Zakharova L, Svetlova M, Fomina AF. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol 212: 174–181, 2007. doi: 10.1002/jcp.21013. [DOI] [PubMed] [Google Scholar]
- 96.Zarovni N, Corrado A, Guazzi P, Zocco D, Lari E, Radano G, Muhhina J, Fondelli C, Gavrilova J, Chiesi A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 87: 46–58, 2015. doi: 10.1016/j.ymeth.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 97.Zhu H, Guariglia S, Yu RY, Li W, Brancho D, Peinado H, Lyden D, Salzer J, Bennett C, Chow CW. Mutation of SIMPLE in Charcot-Marie-Tooth 1C alters production of exosomes. Mol Biol Cell 24: 1619–1637, 2013. doi: 10.1091/mbc.e12-07-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]