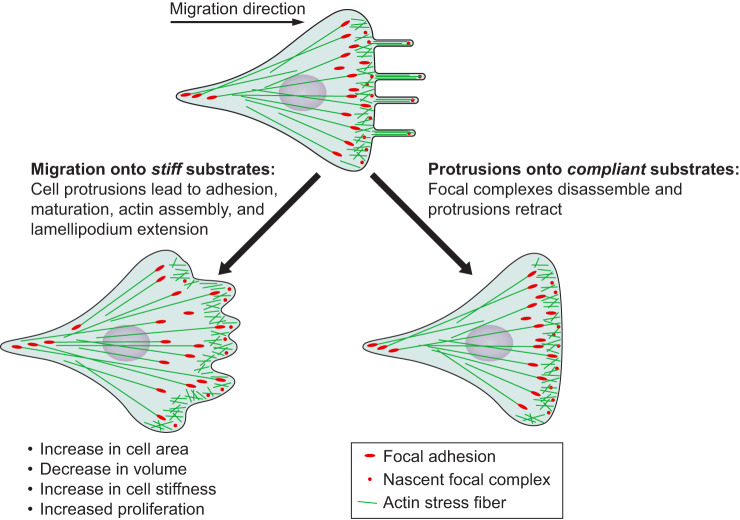
Keywords: cell mechanics, cytoskeleton, mechanobiology, mechanotransduction, substrate stiffness, tissue mechanics, viscoelasticity
Abstract
Physical stimuli are essential for the function of eukaryotic cells, and changes in physical signals are important elements in normal tissue development as well as in disease initiation and progression. The complexity of physical stimuli and the cellular signals they initiate are as complex as those triggered by chemical signals. One of the most important, and the focus of this review, is the effect of substrate mechanical properties on cell structure and function. The past decade has produced a nearly exponentially increasing number of mechanobiological studies to define how substrate stiffness alters cell biology using both purified systems and intact tissues. Here we attempt to identify common features of mechanosensing in different systems while also highlighting the numerous informative exceptions to what in early studies appeared to be simple rules by which cells respond to mechanical stresses.
Biological systems are driven and characterized by both biochemistry and mechanical properties. Tissues change their stiffness during normal development and as the result of pathologies such as those that can be diagnosed by palpation, ultrasound, or other forms of elastography. Cells respond to the stiffness of their environment by changing morphology, motility, or proliferation or by altering their differentiation state. Signaling pathways elicited by mechanical stimuli are beginning to be identified, and mechanobiology has the potential to reveal new targets for diagnosis and therapy.
I. BACKGROUND AND INTRODUCTION
Evidence that mechanical signals elicit specific responses in cells has existed nearly as long as cells have been identified as the building blocks of tissues, and the effect of mechanical stresses on tissue formation and function have been studied for centuries. Plant development is shaped by the gravitational field, bones and muscles grow when they are subjected to forces and atrophy when they are not, and endothelial monolayers reorganize when vascular fluid flow changes. Our senses of touch, hearing, and sight are responses to physical stimuli, and only the senses of taste and smell are primarily chemical. Abnormal mechanical properties of tissues have been recognized for millennia as evidence for disease. For example, the word sclerosis derives from the Greek for “harden” and in Middle English was used to denote a stiff tumor.
Transduction of mechanical stimuli to biological signals and their ultimate responses can be roughly separated into two distinct aspects. One is the active or passive response of cells to exogenously applied forces such as exist when fluid shear stresses impinge on the endothelium due to blood flow through the vasculature or when gravity compresses joints, bones, or adipose tissues. These kinds of stimuli are the basis of Wolff's law for the remodeling of bone, the relation of altered blood flow to development of atherosclerosis, or solid stress developed by growing tumors, and they are the subject of several excellent recent reviews (181, 190, 207, 214, 246). The other aspect is the cell and tissue responses that arise from the forces generated by the cell itself that are resisted by the viscoelastic or active properties of the extracellular matrix or the surrounding cells. This review focuses on this latter aspect: how cells respond when they adhere to surfaces and matrices that resist deformation when cells pull or push on them or on each other. Changes in the mechanical properties of the tissues in which cells reside are a common feature of normal development as well as injury and disease. Interest in mechanobiology has been stimulated by the idea that these mechanical changes are not only the consequences of development or disease, but also significant contributors to these biological processes. For example, increased liver stiffness, regardless of its cause, is a stronger risk factor for later development of hepatocellular carcinoma than nearly any other risk factor for any type of cancer (66, 198), although the links between changes in stiffness and development of disease are not yet defined. The mechanisms by which cells sense differences in viscoelasticity are only beginning to be revealed, but their responses to the mechanical environment are well characterized and often remarkably large and complex.
Systematic studies of the response of cells to the mechanical properties of their environment have grown substantially since the pioneering work using hydrogel or elastomer substrates of varying stiffness to show how fibroblast morphology and motility are altered as the elastic modulus of the substrate was changed (92, 93). Many different cell types attached by numerous different receptors to surfaces with a wide range of chemistry, topography, and viscoelasticity have now been studied. Within this large and expanding set of data, some general rules appear to apply. But there are also many, often highly informative, exceptions to general trends. As with any developing interdisciplinary field, a set of terminology and rules has developed in mechanobiology. The basic terms and principles of materials science and cell biology useful to interpret studies of mechanosensing are briefly summarized in TABLES 1 and 2 (see also FIGURE 1). In the body of this review, we attempt to state the most important general rules or hypotheses about mechanosensing, often derived from the first study that reported them, and then fact check these claims and the extent of their applicability by examining how well they have been verified by other studies and identify cell type-, context-, or substrate-dependent exceptions. The field of mechanobiology has grown enormously in the last decade, and it is no longer feasible to attempt a fully comprehensive review of the field that a decade ago was in its early stages. We apologize for inevitably not being able to include all of the important advances in the past, and we hope that our selection of works is not unduly biased and represents as much as possible an accurate summary of the field at present.
Table 1.
Glossary of mechanobiology terms
| Mechanobiology Term | Definition |
|---|---|
| Elasticity | The property of a material to deform to a defined extent in response to a force and then return to its original state when the force is removed. Elasticity per se is not a measure of stiffness; for this, what is usually meant is the elastic modulus, the ratio of stress to strain for a purely elastic solid. |
| Viscosity | Measure of resistance of a fluid to deformation in response to shear stress. The viscosity η is the ratio of stress to the rate of strain for a liquid. |
| Viscoelasticity | A combination of elastic and viscous responses to applied stress. Most biological materials are viscoelastic: when they are deformed, the degree of their resistance decays with time, usually to a stable baseline (viscoelastic solid) but sometimes to zero at long times (viscoelastic fluid). |
| Stress | Force per unit area: σ = F/A, SI unit is N/m2 |
| Shear stress | Force parallel to a material’s axis per unit area |
| Axial stress | Force perpendicular to a material’s axis per unit area |
| Strain | Unitless parameter quantifying the extent of deformation after application of stress |
| Shear strain (γ) | Unitless parameter quantifying the extent of deformation after application of shear stress. For a cube, shear strain is ratio of lateral displacement over sample height. For other shapes, the form factor relates measured displacement to unitless strain. |
| Elongational strain (ε) | Fractional change in length or elongation: ε = δ/L |
| Young's modulus (E) | A constant describing a material’s resistance to deformation in extension: E = σ/ε |
| Shear modulus (G) | A constant describing a material’s resistance to deformation in shear: G = σ/γ |
| Compliance (J) | The relative extent to which a body yields to deflection by force, usually measured by time-dependent strain divided by constant stress |
| Linear elasticity | Young’s or shear modulus constant over range of strains; equivalently stress is proportional to strain |
| Newtonian viscosity | Viscosity independent of shear strain rate; linear relationship between stress and strain rate |
| Nonlinear elasticity | Young’s or shear modulus that changes with strain |
| Non-Newtonian viscosity | Viscosity dependent on shear strain rate; nonlinear relationship between stress and strain rate (i.e., shear thickening or thinning) |
| Poisson’s ratio (ν) | The ratio of transverse to axial strain when a material is deformed in stretch or compression. A material that conserves volume under strain has a Poisson’s ratio of 0.5. Materials with a Poisson’s ratio <0.5 lose volume when compressed and gain volume when stretched. For linear elastic materials at small strain, Poisson's ratio relates shear and Young's moduli by the expression E = 2G(1 + ν) |
| Dynamic viscoelasticity | Many time-dependent rheological measurements are made by applying a sinusoidally varying stress or a sinusoidally varying strain to a sample and measuring its strain or stress response, respectively, as a function of frequency. For linear materials, the result is two sinusoidal functions, and both the elastic and dissipative properties of the material are computed from the amplitudes and phase shifts of the sinusoidal functions (see FIGURE 1). |
| Elastic or storage modulus (G') | Measure of energy stored during a strain cycle; under sinusoidal conditions, the part of shear stress in phase with shear strain divided by shear strain, often expressed as the real part of the complex modulus: G′ = (σo/γo)*cos(δ) (see FIGURE 1) |
| Viscous or loss modulus (G'') | Measure of energy lost during a strain cycle, often expressed as the imaginary part of the complex modulus: G″ = (σo/γo)*sin(δ), where the phase angle (δ) is the shift between the sinusoidally varying stress and strain in an oscillatory measurement. The value of δ is zero for a purely elastic solid and 90 degrees for a purely viscous liquid (see FIGURE 1). |
Table 2.
Glossary of cell biology terms
| Cell Biology Term | Definition |
|---|---|
| Cell-matrix junction | Anchors the cell through integrin-mediated binding. The junction itself is composed of both extracellular portion and intracellular portion, linking the internal cellular cytoskeleton to the extracellular matrix. Junction associate molecules can have a structural role in anchoring the cell and/or a signaling role. |
| Cell-cell junction | Adhesion complex linking cells to each other. Cell-cell junctions connect the cytoskeleton of one cell to the cytoskeleton of another cell. There are three major functions of cell-cell junctions: anchoring (adherens junctions, desmosomes, and hemidesmosomes), communication (gap junctions), and barrier formation (tight junctions). Similar to cell-matrix junctions, the proteins associated with cell-cell junctions can have a structural and/or signaling role. |
| Focal adhesion | Dynamic, integrin-based cluster of signaling and structural proteins that mediates contact between the cell and the extracellular matrix. They have a mechanosensory function and can grow with force. They are located at the end of stress fibers. |
| Focal complex | A smaller, less mature precursor to a focal adhesion that is generally more dynamic and less stable than a focal adhesion. Like a focal adhesion, it also has structural and signaling capabilities. |
| Stress fiber | Large bundles of actomyosin that are anchored at focal adhesions to provide an anchor to the matrix and exert tension through their association with integrins. They are composed of bundled actin, crosslinking proteins including α-actinin and filamin, fascin, caldesmon, and signaling proteins including myosin light-chain kinase. |
| Cytoskeleton | The architectural scaffolding of the cell which serves both a structural and signaling role. It gives the cell shape and mediates migration behaviors. It is composed of three primary types of filaments: microfilaments, microtubules, and intermediate filaments. |
| Microfilaments | 5–7 nm diameter filaments composed of actin. They are responsible for cell shape changes and membrane extension as well as cell contraction and migration. |
| Microtubules | Microtubules are largely composed of tubulin and have two major functions: they are the primary components in the mitotic spindle which organizes chromosomes during division, and they are responsible for the organization of flagella and cilia in eukaryotes. |
| Intermediate filaments | Primarily responsible for structure and support within the cell. They also provide structural integrity to the nucleus and contribute to linking cells to each other. |
FIGURE 1.
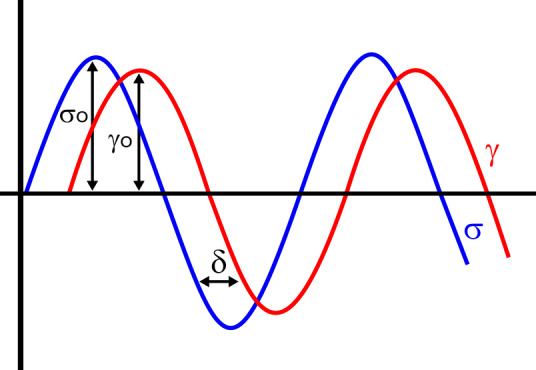
Stress σ0 and strain γ0 amplitudes against time in an oscillatory deformation. The stress and strain signals are phase shifted by an angle δ.
II. DEFINITION OF TERMS FOR MECHANOBIOLOGY
Most mechanobiology studies summarized here refer to properties such as substrate or cell stiffness, or rigidity. The term stiffness, although commonly used to denote the material properties of both cells and substrates, has no unambiguous definition in soft matter physics or rheology. Intuitively, stiffness is a measure of the resistance of a material to deformation as mechanical forces are applied to it. TABLE 1 presents a short glossary of the most commonly used terms in many of the references cited here. Deformation is defined by the unitless value of strain and force is normalized by area to compute stress, but the ratio of stress to strain, which defines what is perceived as stiffness, can depend on the geometry of deformation, the time scale, the degree of deformation, the rate at which forces are applied, and other factors (see also FIGURE 1).
In addition, most rheological quantities such as shear modulus, Young’s modulus, or Poisson’s ratio assume continuous materials and often a limit of small deformations. Even for a relatively isotropic material like a hydrogel, an elastomer, or a collagen network, the rheology can be complex, especially for fibrous networks for which the basic physics is still being defined (90, 182). In addition, issues of heterogeneity and spatial asymmetry complicate efforts to compare one type of measurement to another. A striking example of this difficulty is seen in a comparison of different methods used to measure cell stiffness, all using identical cell preparations and cell culture media supplied from a single source (239). In this study, measures of stiffness quantified by Young’s modulus for MCF-7 breast cancer cells ranged from 18 Pa measured by optical stretching of suspended cells to ~100 Pa by intracellular particle microrheology, to 1–14 kPa by atomic force microscopy (AFM) depending on the size of the AFM probe. These differences probably reflect more the heterogeneous structure of the cell and the noncontinuous nature of its cytoskeleton than inherent uncertainty in measurements by any single method. Therefore, changes in cell stiffness measured using the same method for cells on different substrates appear to be reliable, but quantitative comparison between stiffness measured by different methods is limited by the details of how stiffness is measured by the technique.
The stiffness of the substrate to which a cell responds is also sometimes not simply defined by the macroscopic elastic modulus of the material measured in simple shear or uniaxial deformation. Cells apply both tangential and vertical forces at the sites by which they attach to the substrate. If a substrate is very thin, then the strain field generated by the cell can extend to the rigid material on which the soft substrate is attached, and therefore cellular response might in some sense be “measuring” a stiffness different from that of the rheometer or AFM. These issues have been carefully analyzed in a series of studies to define cell-generated strain fields and the thickness of substrate sufficient to ensure that the effective stiffness to which the cell responds is the macroscopic stiffness of the substrate (27, 28, 141). A qualitative rule seems to be that substrates need to be thicker than the diameter of the cell in order that the cell not feel the rigid surface underneath the soft substrate.
III. CHANGES IN CELL MORPHOLOGY
A. Cell Area Increases with Substrate Stiffness
The adherent area of a cell on a flat substrate is probably the simplest and most accurate metric to determine experimentally by light microscopy and is the most frequently reported quantity in studies of cell responses to substrates of different stiffness. Some early studies of cells grown in soft or stiff matrices formed by diluted blood clots (231) showed that cells and their nuclei became more elongated as the concentration of the clot increased, but interpretation of this result as due to differences in substrate mechanics is confounded by the differences in protein density and fiber formation as blood and fibrinogen concentrations are altered. More direct evidence that cells exert tension on their substrate through specific adhesion sites and do not adhere well if the substrate is too compliant came from observations that fibroblasts could bend thin glass needles of similar diameter to collagen fibers, but bound poorly to films at the surface of the medium if the film was too soft (147).
Perhaps the earliest quantitative study of substrate stiffness effects in which elasticity was isolated from protein density differences was a study of cells on films of different elastic moduli (116). In this work, protein films were formed at the interface of a fluorocarbon oil and water. When fibroblasts in cell culture medium were added to the protein film, they were not able to adhere and spread unless the protein film was crosslinked (77). By varying the crosslinking using pentafluorobenzoyl chloride, which partitions to the oil-water interface, films of albumin or gelatin could be formed with the same protein density but varying surface shear moduli. This study reported that the spread area of cells was larger on the stiffer films and interpreted this effect as due to fibroblasts exerting a traction stress on the film. If the film was sufficiently rigid, the cell elastically deformed the substrate to a limited extent, in which case the cell continued to spread, but if the film was weak, the cell exceeded a breaking force, in which case spreading ceased. An important observation that is echoed in subsequent studies of mechanosensing (219) was that transformed fibroblasts were able to spread on softer films that did not support the spreading of normal fibroblasts (116).
There is no physical reason why a cell adhering to a surface must spread more on a stiffer surface, nor does it need the work done by motor proteins to do so. In general, the shape of a liquid or viscoelastic drop or cell adhering to a surface is determined by a balance of forces due to the surface tension of the drop, the elastic modulus of the cell, the elastic modulus of the substrate, and the adhesion energy between cell and substrate (185). Some early criticism of the hypothesis that substrate stiffness directly controlled cell phenotype focused on skepticism that changes in ligand density due to different coupling of integrin ligands to gels with different amounts of crosslinking or polymer density were the true cause of differences in cell spread area on different substrates. To address this issue, Engler et al. (59) systematically varied the surface density of collagen on substrates of different stiffness (FIGURE 2). Ligand density had a significant effect on cell area, especially on glass substrates, but under no conditions were cells cultured on soft gels as well spread as they were on glass. Increasing the collagen density led to a plateau or maximum in cell area at densities around 100 ng/cm2 regardless of the substrate stiffness.
FIGURE 2.
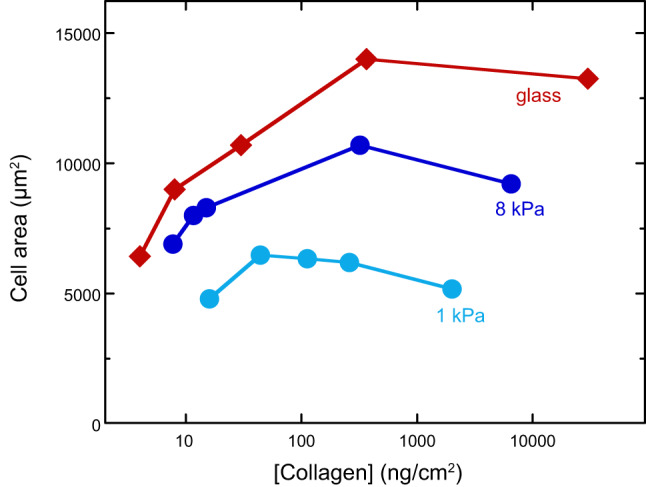
Spread cell area as a function of ligand density on soft, stiff, and rigid substrates 4 h after plating. [From Engler et al. (59), with permission from Elsevier.]
Effects of substrate stiffness on cell morphology have been documented using a large number of different materials, including silicone films (6, 94, 136, 250); rubber-like elastomers such as polydimethylsiloxane (PDMS) (25, 131, 210) or polyurethanes (5, 31); needle-like pillar arrays made from PDMS (75, 204, 210), silicon (170), or glass (148); and hydrogels formed by crosslinked polyacrylamide (14, 109), polyethylene glycol (PEG) (16, 103, 153, 172), agarose (7, 164, 189, 248), alginate (12, 70), hyaluronic acid (3, 9, 46, 53, 59, 117, 126), DNA (103, 104, 108), and other flexible water-soluble polymers.
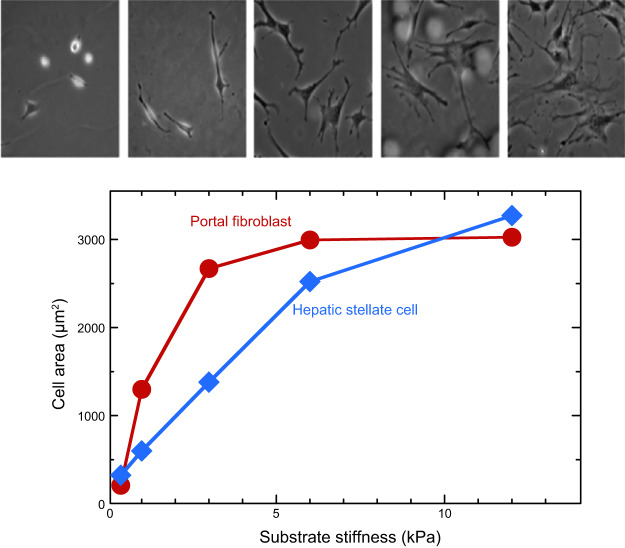
The first systematic study of stiffness effects using hydrogels (169) expanded on a method developed in the 1970s (227, 228) to use polyacrylamide hydrogels, to which most cells do not adhere, but to which specific cell adhesion molecules can be covalently attached. Altering the concentrations and ratios of acrylamide monomers and bis-acrylamide crosslinker produces gels with controllable shear storage moduli, and nearly no mechanical loss. When NIH-3T3 fibroblasts were cultured on such gels laminated with collagen, their area increased monotonically and reached a saturating level similar to that of the area of cells on tissue culture plastic on gels with shear moduli >30 kPa (169). Later studies using primary hepatic fibroblasts and hepatic stellate cells showed how dramatically these cells changed their area and morphology (FIGURE 3) as the stiffness of the substrates varied from the stiffness of a normal liver (<1 kPa) to that of a fibrotic liver (>10 kPa) (71). Similar effects were seen with primary hepatic stellate cells, and the stiffness-mediated changes in spread area coincided with initiation of profibrotic function in these cells (137, 166).
FIGURE 3.
Adherent area of primary murine portal fibroblasts and hepatic stellate cells on collagen I-coated polyacrylamide gels of different stiffness. The panel above shows images of fibroblasts on each of the stiffnesses. [From Li et al. (137), with permission from John Wiley and Sons.]
Several other cell types, including endothelial cells (4, 8, 22, 23, 33, 37, 40, 44, 67, 80, 112, 202, 213, 223, 245, 249), macrophages (16, 63, 85, 100, 134, 164), hepatic stellate cells (39, 68, 71, 137, 166), platelets (135, 178), and myocytes (15, 18, 24, 25, 36, 45, 47, 51, 58, 172, 242) have been shown to similarly increase area with substrate stiffness but, as discussed below, other cell types appear to be insensitive to stiffness when spread area is the metric for response or spread more on soft substrates. These cell types include central nervous system neurons (64, 73, 106, 121, 122, 153), murine embryonic stem cells (176), and several cancer cell lines (138, 240). In several cases, whether cells change area with substrate stiffness depends on the protein linked to the substrate and therefore the adhesion receptor by which the cell binds. Similarly, chemical stimuli or withdrawal of stimuli such as serum starvation can increase the spread area of cells such as fibroblasts on a soft substrate to levels otherwise seen only on rigid substrates (84).
The substrate elastic modulus and the density of adhesion molecules on the substrates can be controlled experimentally and are sufficient to determine the shape of a drop of known elastic modulus and surface tension, but a cell can change its elastic modulus, surface tension, and adhesion molecule density by altering its cytoskeleton, changing motor activity, and delivering or retrieving transmembrane protein complexes (62, 159). Therefore, it is expected that some cell types have a different response to substrate stiffness and that chemical manipulation of the cell can alter its response to substrate stiffness.
Perhaps the clearest example of cells that do not follow the paradigm of increased spreading on stiff substrates are neurons derived from the central nervous system. Cortical brain neurons extend processes faster and farther on soft substrates resembling the elastic modulus of the brain and lose the ability to extend and branch as substrate stiffness increases from <1 kPa to >10 kPa (7, 64, 122). The enhanced neurite extension on soft substrates is also seen in three-dimensional (3D) gels formed by fibrin (72, 73, 106), collagen (232), PEG (153), and a dynamically changeable DNA-crosslinked gel (103). Changes in stiffness can be used to steer axonal growth in intact tissue with neurons diverting from areas of increased stiffness (121).
Glial cells have the opposite response to stiffness as neurons, and like fibroblasts they spread to larger areas on stiffer substrates. Astrocytes, like fibroblasts, increase spread area with increasing stiffness, and the magnitude of the response depends on the adhesive coating of the gel (72, 152, 174, 175). Microglia also spread more on stiff substrates especially when they are coated with laminin (179). The opposite preference of neurons and glia extends beyond their morphological repose, and tuning substrate stiffness in two dimensions (2D) and 3D can selectively promote either neuronal or glial cell growth or survival from an initially mixed cortical culture (73).
The relation of area to substrate stiffness depends on the type of adhesive protein on the substrate, and therefore on the type of adhesion complex by which the cell binds. A repeated finding with glial cells and other cell types is that the spread area they reach on superphysiologically stiff hydrogels coated with a single adhesive ligand is often not as great as that of the same cell preparations on glass coated with the same ligand. The example in FIGURE 4 shows that LBC3 cells, derived from a human brain tumor unusually rich in collagen I, are much more responsive to substrate stiffness when the gels are coated with collagen than with laminin. At stiffnesses of ~20 kPa, the cells on collagen-coated gels have the same area as they do on glass, but those on laminin-coated gels are much smaller (173). This result suggests that adhesion to glass or plastic, probably like adhesion to a native extracellular matrix (ECM), involves a more chemically complex binding interface that engages not only the integrin targeted by the adhesive ligand used, but also the many serum and cell-derived factors that adhere to glass or plastic but not to inert hydrogels like polyacrylamide or PEG. Engagement of multiple receptors can also override the relation between area and stiffness. For example, soft (300 Pa) hydrogels containing both hyaluronic acid (HA) plus an integrin ligand can promote spread areas of myocytes, fibroblasts, and some other cells types, to the same extent as 30 kPa polyacrylamide gels that contain only an integrin ligand (45, 46, 53, 117, 146, 173). HA does not eliminate cells’ sensitivity to substrate stiffness (117), but rather it appears to shift morphological response to lower elastic moduli (46).
FIGURE 4.
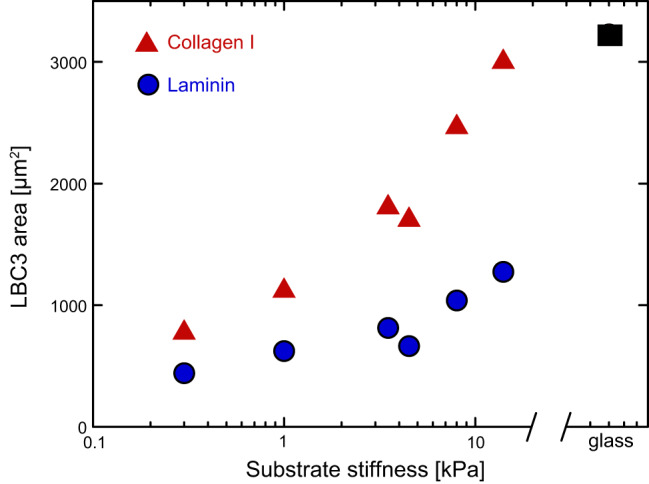
Integrin ligand dependence of response to substrate stiffness. Area of LBC3 human glioma cells on polyacrylamide gels coated with collagen I or laminin compared with area on glass after 24 h. [From Pogoda et al. (173).]
Enough evidence has now accrued to reveal that there is no simple rule relating adherent area to substrate stiffness. The physical factors that govern a liquid drop adhering to a surface of course apply to cells, and the energies involved in adhesion and surface tension are significant. In addition, however, the molecular control of cytoskeletal assembly, adhesion protein expression, vesicle trafficking, and other processes that control cell shape can be highly distinct between different cell types or even for the same cell type on chemically distinct substrates. The integration of physical effects with biochemical signaling can lead to a wide range of responses to substrate stiffness that will require more knowledge of molecular detail to make reliable predictions.
B. Stiffness Sensing and Cell Spreading Are Triggered by Nano- or Microscale Mechanisms
Currently there appears to be no consensus on either the molecular mechanism of stiffness sensing or the length scale over which the relevant probing of the substrate is done. There is a limit to the size of a rigid particle that a human finger can sense, but the area over which a cell probes its interface is not yet clear and likely to be different in different contexts. The elastic modulus of the substrate, the control parameter used in any study of mechanosensing in vitro, is a macroscopic concept assuming a continuous material property, which raises the issue of the length scale over which cells measure stiffness. Several lines of evidence suggest that even though the adhesion receptors such as integrins are nanoscale protein complexes, the scale of the substrate to which the cell responds mechanically is much larger. For example, pillar arrays made from stiff materials such as PDMS (75, 133) with E = MPa, or glass (148, 170) with E >GPa are sensed as soft substrates as long as the pillars are tall enough so that the traction force needed to bend them is low enough for the cellular machinery to move the pillar. Even on PDMS pillars with diameters of microns, the signals elicited by stiff substrates such as continuous PDMS are not engaged. A study that placed variably sized islands of rigid polymer on the surface of hydrogels showed that the island needed to be on the order of 4 μm before large focal adhesions or myosin activation characteristic of a stiff substrate were triggered (238). This study led to a model for spreading on soft or stiff substrates in which filopodia extend from an adherent cell body and contact adhesion proteins on the island surface. If the island is small enough to allow only a single nascent adhesion to form and then moves when actomyosin is activated, the adhesion complex dissociates, and the cell does not spread. If the island is large enough to allow two adhesion sites to pull against each other, then the adhesion sites mature and the cell spreads. A model in which the stiffness sensor consists of a single myosin minifilament of length 150 nm that pulls on adhesion sites on opposite sides of the filament has also been shown to mediate the signals that lead to cell spreading (236).
C. Cell Volume Decreases with Substrate Stiffness
Volume is much more difficult to measure by light microscopy than is area, and a relation between cell volume and substrate stiffness is only beginning to emerge. A study using confocal microscopy to determine the volume of mesenchymal stem cells concluded that the volume of a cell decreases as it spreads to greater adherent area on stiffer substrates due to water efflux (86). As the water leaves, the cytoplasm becomes more crowded and the cell stiffness also thereby increases on stiff substrates. The change in volume is similar in magnitude to what can be achieved by changing the osmotic pressure in the medium. Decreased substrate stiffness also leads to an increase in chondrocyte volume as measured by confocal microscopy of fluorescently labeled cells (111). A similar study using confocal microscopy of breast cancer-derived MCF-7 cells came to the opposite conclusion, that the volume of MCF-7 cells increases as the substrate stiffness increases. This volume change could be blocked by inhibition of cell membrane anion permeability or the dopamine receptor (221).
Because the area of the cell, the state of the cytoskeleton, and many other molecular effects are triggered as substrate stiffness changes, the change in volume might not be directly linked to the increased tension of cells on stiff substrates. The intuitively simple idea that the tension within the cell squeezes cytosol out to reduce volume is very likely not physically realistic, since the magnitudes of traction stresses (100s to 1,000s of Pa) are orders of magnitude lower than the osmotic difference that would arise if water were to leave the cell. Earlier studies suggesting that the cytoskeleton might resist osmotic shock directly by its elastic modulus were similarly determined not to be likely, and any effect of the cytoskeleton is more likely due to its effect on membrane tension and the activity of ion channels. A recent study using NIH 3T3 fibroblasts and HeLa cells similarly concluded that the volume of the cell decreases exponentially with its spread area, regardless of the cause of the area limitation. Changing the spread area of the cell by altering substrate stiffness had the same quantitative effect on volume as changing size by microprinting-limited adhesive area, changing the adhesivity of a continuous surface, drug treatment, or time after an initially suspended cell binds to a surface (241). Therefore, as for other effects of substrate stiffness, the changes in volume are likely not to be predicted by analogy with inactive soft materials but rather are determined by the secondary effects of signals that arise at the interface of the cell and its substrate and are mediated by adhesion receptors, second messengers, and the cellular changes they initiate.
Discrepancies between the relation of substrate stiffness to cell volume might result from differences in cell type, the nature of the adhesive surface, or uncertainties related to quantifying volume from stacks of confocal microscopy images. An alternative and reportedly more accurate method to measure cell volume is by the fluorescence exclusion method in which cells displace a fluorescent marker from a known volume that is imaged by optical microscopy (20, 32). With the use of this method, the relation between cell volume and substrate stiffness is not simple and not even monotonic. In this study, cell volume is positively correlated with the size of the cell adhesion to the substrate and related to the cells’ membrane tension. Cells with greater apical tension tended to have larger cell volume as well as higher levels of nuclear Yes-associated protein and transcriptional coactivator with PDZ-binding motif (YAP/TAZ) (82).
The relation between cell volume and substrate stiffness is reported to be different for cells in 3D matrices. When cells were grown in microfabricated 3D niches of various volumes in different materials, processes such as nuclear localization of YAP/TAZ and formation of actin stress fibers was determined by the cell volume, regardless of the stiffness of the 3D niche (9).
IV. CHANGES IN CELL MECHANICS
The mechanical properties of the cell often described by the term stiffness result from the combination of multiple factors and depend on time and the magnitude of deformation. The term stiffness itself is ill-defined, but generally derives from a measurement of the relation of a force applied somewhere in or on the cell and the resulting deformation of the cell. Analysis of the system-dependent force-displacement relation by an appropriate model leads to computation of elastic or viscous moduli that are true, material properties that generally depend on time and strain, but they should not depend on the method used to measure them (TABLE 1 and FIGURE 1). Nonetheless, efforts to measure the shear or Young's modulus of the same cell type by multiple different methods leads to estimates that differ by orders of magnitude, depending on the type of measurement and on the site in the cell where the measurement is made (239). One reason for this disparity is the obvious fact that cells are structurally heterogeneous, but an additional important factor is that the structures dominating cell mechanics are generally far from equilibrium. The pressures and internal tensions generated by molecular motors, osmotic pressures, and surface tensions at membranes alter force-displacement measurements as strongly as do the concentrations and crosslinking density of the cytoskeleton, which is often presumed to determine cell stiffness. As a result, measurement of elastic moduli by microrheology of beads in the cell interior generally report values much lower than those obtained from cell surface indentation or twisting of membrane-bound beads. The former measurements are relatively unaffected by internal pressures or surface tension, but the latter two are sensitive to membrane tension as well as the structure of the cytoskeleton. In addition, computation of elastic moduli from force-indentation curves obtained by surface indentation or twisting are model dependent, and the value depends strongly on whether the cell is modeled as an elastic continuum or a thin elastic shell surrounding a fluid interior (26, 38, 161, 184). Despite these complications, many studies have shown that the stiffness as measured by numerous different methods can be strongly altered by the stiffness of the substrate, in a cell- and ligand-dependent manner.
A. Traction Stress Increases with Substrate Stiffness
An early discussion of traction stresses in 3D involved the hypothesis that forces generated by a single cell within a matrix depended on the structure and mechanical properties of the matrix, and that this force could stimulate a nearby but not contacting cell to move toward the source of traction and thereby guide morphogenesis and wound repair (115). Whether the target cell responds to the force per se or to the cell-dependent alignment of fibers within the matrix (114, 230) leads to a vigorous debate that is not yet resolved. As the substrate becomes stiffer and the cells spread more, it seems reasonable to infer that cells also pull more strongly on the stiffer substrates, and this feature is predicted by a theoretical model for the mechanism by which cell membrane tension is generated (206). The source of the traction stress is not exclusively dependent on contraction by the actomyosin network or any other cytoskeleton, and significant traction stresses have also been generated by liposomes adhering to compliant substrates. The traction stress in this case arises from internal pressures developed as the liposomes adhere to the surface and change shape (157).
A study of fibroblasts and epithelial cells on pillar arrays showed that both cell types increased the force they applied to the pillar as the pillar became stiffer. At relatively low pillar rigidity, both cell types increased force linearly with the pillar rigidity and eventually reached a saturation plateau for the largest rigidities (75) (FIGURE 5). An earlier study of fibroblasts reaches the opposite conclusion that fibroblast contractile force is independent of substrate stiffness and is force limited, not displacement limited. These studies raise the interesting question about whether cells sense and respond to substrate stiffness by strain-controlled or stress-controlled mechanisms (140). The linear force increase with pillar rigidity suggests that cells apply the same strain to the pillars, at least in the range where they exert sufficient force to deform all pillars to the same extent. What this strain-controlled mechanism might be is still not clear, but such studies to define the basic length, force, and time scales of mechanosensing are important for differentiating among different proposed molecular mechanisms. Alternatively, cells might be programmed to exert a set degree of stress on their extracellular environment, a concept termed tensional homeostasis (167) that depends on contractile motor activity, the assembly state of the cytoskeleton, and transcriptional programs that regulate signals to the force-generating mechanisms. Defects in tensional homeostasis have been proposed as part of the malignant transformation of cancer cells (167).
FIGURE 5.
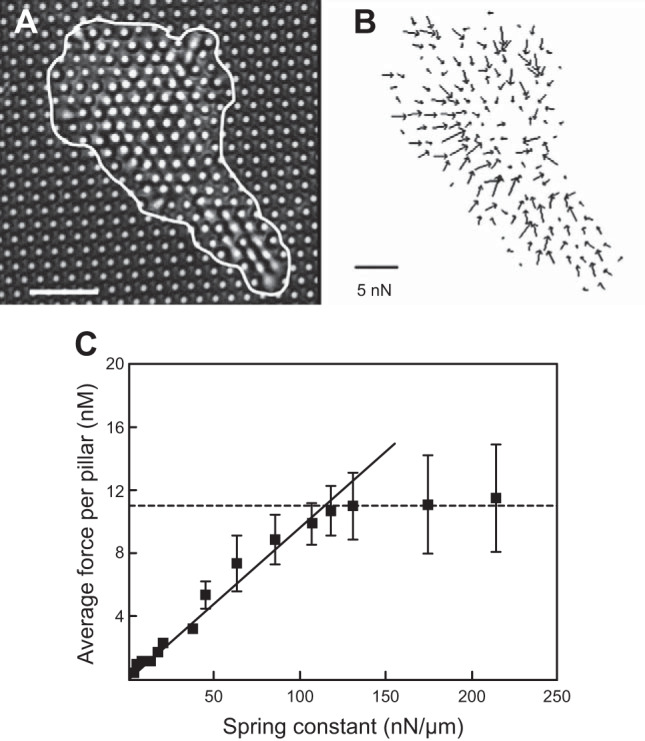
Force measurements on individual 3T3 fibroblast cells using a fibronectin-coated microfabricated pillar array. A: transmission light microscopy image of a cell deforming pillars. The cell border is represented by the white line. Scale bar = 10 μm. B: force field exerted by the cell obtained from the deflection of the underlying pillars. C: mean traction forces exerted by individual fibroblasts as a function of substrate rigidity. [From Ghibaudo et al. (75).]
The ability to dynamically respond to stiffness changes may depend on whether cells have reached steady state or not. When a fibroblast was allowed to attach and spread simultaneously on an AFM cantilever and glass surface, both coated with fibronectin, and reach a steady-state contractile force between the AFM and surface, transient changes in effective stiffness across the cell, created by changing the force-displacement response of the AFM, did not alter the contractile force or spread area at steady state (226). A similar AFM measurement of contraction and spreading by platelets revealed an increase in final contractile force and loading rate by the platelet with increasing stiffness across the platelet (127), possibly contributing to more uniform contraction of mechanically heterogeneous clots.
B. Membrane Tension Is Increased by Traction Stress on Stiff Substrates
Membrane tension has emerged as a critical mechanical constraint on cell movements, shape change, and volume regulation (206). Increases in membrane tension were found to increase the crawling speed of osmotically shocked Caenorhabditis elegans sperm cells by limiting extraneous protrusions (11) and to act as a long-range inhibitor of actin assembly that helps neutrophils maintain polarity and limit the number of pseudopods to one (95). Membrane tension has also been shown to control the placement of adhesions at the leading edge of mouse embryonic fibroblasts by causing the buckling of lamellipodial actin in a myosin II-independent manner (177), and it can limit the assembly of actin at the leading edge of crawling neutrophils through a negative-feedback loop involving phospholipase D2 (PLD2) and the mammalian target of rapamycin complex 2 (mTORC2) (55). All of these studies were done on stiff substrates, so how might a softer substrate affect membrane tension?
One recent study measured membrane tension of hepatocellular carcinoma cells on soft HA and stiff polyacrylamide substrates by pulling membrane tethers using optical tweezers (146). Membrane tensions observed on soft 300 Pa HA substrates were comparable to those measured on 30 kPa polyacrylamide substrates, and the magnitude of membrane tension measured was not correlated with the traction forces exerted by the cells on the substrates. This suggests that forces exerted by cells on their extracellular matrix may not significantly alter membrane tension.
Substrate stiffness has been shown to impact the ability of cells to carry out endocytosis, where membrane tension has long been considered to influence the ability of endocytic pits to form high curvatures, and actin assembly at endocytic pits is thought to counteract the effect of membrane tension (21). A study using polyacrylamide nanoparticles found that bovine aortic endothelial cells plated on stiff substrates took up fewer nanoparticles through endocytosis per unit area, although they took up more nanoparticles overall due to a larger spread area than soft substrates (96). For neuroblastoma cells on polyacrylamide gels of different stiffnesses, cellular uptake of amyloid-β protein increased when the cells were on soft substrates compared with stiff, both per unit area and overall (124).
Overall, there does not appear to be a universal relation between cytoskeletal-dependent traction stress and membrane tension. Evidence that traction stress at the cell’s basal surface is transferred to stretch the cell’s membrane and cortex is observed in some cases, but in others, basal traction stresses can change without significant changes in apical membrane tension, a result that is consistent with evidence that even within the membrane, tensions can be spatially different and not immediately related to locally applied forces (195).
C. Cells Become Stiffer on Stiffer Substrates: Stiffness is Different in Different Places and Different Scales: Global to Local
Measurements of cell stiffness have employed both local and global measures that range from either a local stiffness (e.g., AFM with sharp tip, magnetic twisting cytometry) or an average global stiffness (e.g., AFM with large bead, pipette aspiration). Early reports of the relation between cell and substrate stiffness showed that human mesenchymal stem cells altered their stiffness, as measured by micropipette aspiration (60), as the stiffness of their collagen-coated gel substrate was varied while keeping the chemical composition, and in particular the density of integrin ligands, constant (FIGURE 6). This finding was confirmed by AFM measurements (207) using gel substrates that were coated with fibronectin rather than collagen. In this case, cell stiffening was greater than that observed for the same cell type bound only to collagen receptors. The cell stiffness reached a plateau of 7 kPa when the substrate stiffness was ~20 kPa, and the cells retained this stiffness even on essentially infinitely rigid substrates like glass. A similar increase in cell stiffness as a function of substrate stiffness was observed with NIH 3T3 fibroblasts, and here again cells cultured on 20 kPa substrates had a cortical stiffness that was indistinguishable from that on glass (200). That study also showed that cell stiffness correlated with cell area, but no causal relation was suggested. Also, most of the stiffening in cells on fibronectin-coated hydrogels occurred over a range of stiffness where the cells made few if any stress fibers, suggesting that the stiffness detected by AFM, and by micropipette aspiration, was determined mostly by the cortical actin shell rather than by the denser fibers attached to focal adhesions on the cell's ventral surface. The changes in actin assembly that occur as substrate stiffness is varied have recently been summarized (88). Cells that generally lack actin stress fibers, such as neurons, can also alter stiffness on variable stiffness substrates. A study of glial and neural cells cultured on PDMS substrates showed that the internal cell stiffness as measured by magnetic tweezers decreased when cells were cultured on softer substrates (43).
FIGURE 6.
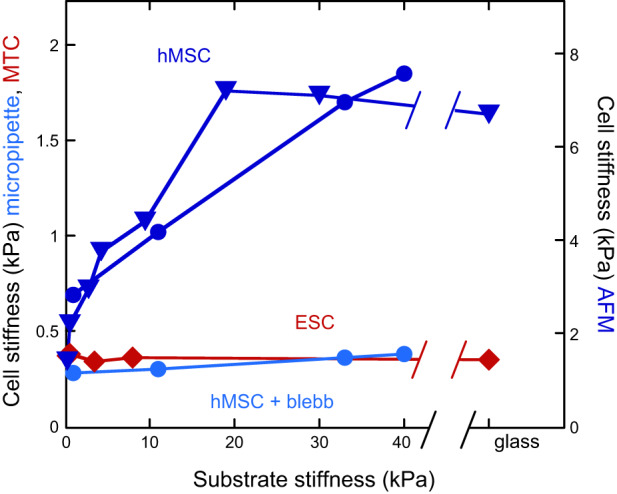
Cell stiffness tracks substrate stiffness for some but not all cell types. Cortical cell stiffness measured by micropipette aspiration (circles), magnetic bead twisting cytometry (MTC; diamonds), or atomic force microscopy (AFM; triangles). Cell cortical stiffness measured by micropipette aspiration or AFM scales with substrate stiffness for adult human bone marrow stromal cells (hMSC; dark blue) but not for murine embryonic stem cells (ESC; red). Inhibition of myosin by blebbistatin abrogates stiffness response of hMSC (light blue). [From Engler et al. (60), with permission from Elsevier; and Poh et al. (176), with permission from Elsevier.]
Many experiments have shown that increased cell spreading is accompanied by increased cell stiffness, although there is in general no physical principle by which this must occur, and exceptions to this pattern have also been observed. If a cell spreading on a substrate is analogous to a drop wetting an adhesive surface, then a simple mechanism to increase the adherent area on surfaces with constant adhesive strength would be to decrease the cortical tension, allowing the adhesion energy to overcome the elastic resistance of the cell. This mechanism would predict that as cells spread more their cortical stiffness, as measured by AFM or magnetic bead twisting rheometry, would decrease or remain unchanged as the cytoskeletal fluidization and adhesion energy change the cell's area-to-volume ratio. Increased cortical stiffness during cell spreading would appear to require a stretching of the cortex or an active upregulation of cytoskeletal assembly and internal stress. An early study in which cell spreading was increased by increasing the surface density of fibronectin on a rigid surface showed that cell cortical stiffness as measured by twisting a surface-bound magnetic bead increased as the cells increased spread area (222). AFM measurements of fibroblasts (200) also reveal that as substrate stiffness increases on fibronectin-coated polyacrylamide gels, both the spread area and the cell stiffness increase approximately proportionally to each other (FIGURE 7). Evidence that the relation between area and stiffness is coincident and not causal is shown in FIGURE 7, by a similar experiment done with LBC3 glioma cells on collagen-coated gels. In this case, except for the cells on the softest substrate (300 Pa) which are both very small and very soft, there is no detectable difference in stiffness for cells that vary in area by nearly a factor of four (173).
FIGURE 7.
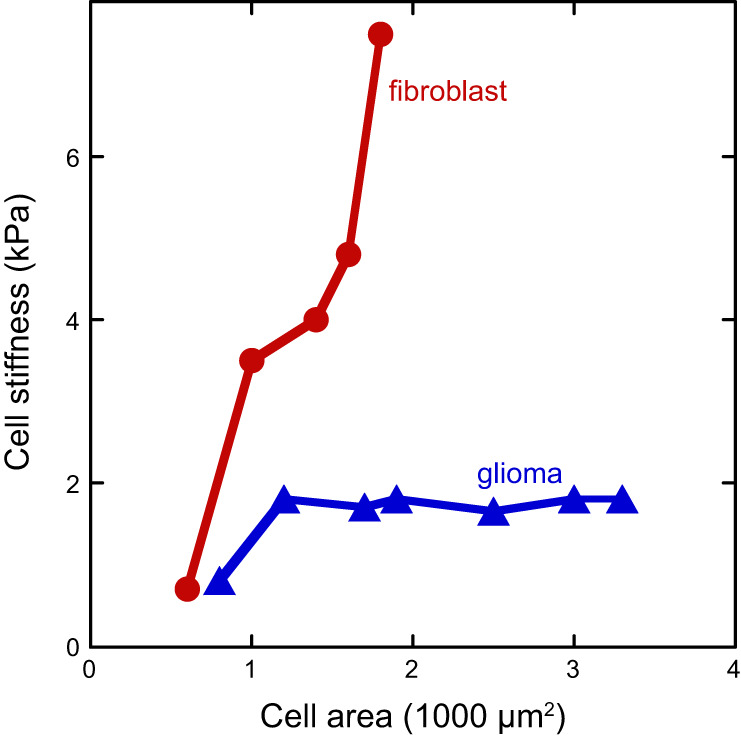
Relation of cell stiffness to spread area for NIH 3T3 fibroblasts and LBC3 glioma cells grown on gel substrates of different stiffness. [Adapted from Solon et al. (200), with permission from Elsevier; and Pogoda et al. (173).]
Not all cell types change stiffness in response to substrate stiffness; mouse embryonic stem cells (ESC), in contrast to adult human bone-marrow derived stem cells, maintain a constant stiffness (FIGURE 6), as measured by magnetic twisting cytometry (176). In this study, tripeptide Arg-Gly-Asp (RGD)- or fibronectin-coated magnetic beads were attached to the cell surface by fibronectin receptors, and the authors raise the possibility that beads coupled to collagen receptors might respond differently. Nevertheless, substrate stiffness did affect the magnitude of traction stress that the ESCs exerted on their substrate. This is one of the first reports that traction stress exerted on the bottom of a cell can be decoupled from the cortical stiffness on the top of the cell.
D. Dependence on Adhesion Receptors and Actin Binding Proteins
The capacity of cells to change stiffness in response to substrate stiffness depends on both the type of adhesion receptor by which the cell attaches, and the complement of cytoskeletal proteins that can respond to adhesion receptor-based signals. For example, FIGURE 8 compares the stiffness response of two melanoma cell lines that differ in the expression levels of the actin crosslinker filamin A (30). A7 cells, which express filamin A, avidly change their stiffness as substrate stiffness changes when they are bound to the substrate through collagen receptors, but they do so weakly if at all on the same gels when bound only through fibronectin receptors. Coating the gels with both types of integrin ligands leads to an intermediate level of stiffness response. M2 cells, which lack filamin A, do not alter their stiffness on gels coated with either type of integrin ligand, even though they have the same stiffness as A7 cells on soft substrates. A similar result was seen using magnetic twisting cytometry rather than AFM: A7 cells were stiffer on stiffer collagen-coated gels, but M2 cells did not stiffen (113). Analogous results have been reported for other cell types with different subsets of integrins. The glioma cell line LBC3 monotonically changes its stiffness with substrate stiffness when bound to soft gels coated with laminin, but when the same gels are coated with collagen I, there is no change in cell stiffness when substrate stiffness is varied from 1 to 10 kPa, or when the cells are grown on glass (173).
FIGURE 8.
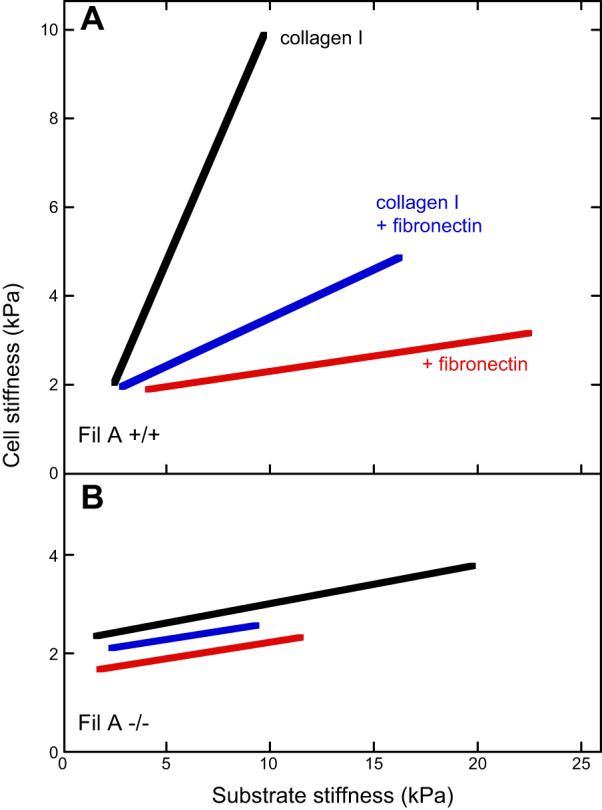
Melanoma stiffness measured by atomic force microscopy. The effective Young’s moduli of A7 (A) and M2 (B) melanoma cells cultured for 24 h on polyacrylamide gels laminated with collagen I (black), fibronectin (red), or mixture of collagen I and fibronectin (blue). [From Byfield et al. (30), with permission from Elsevier.]
In most cases, even when cells alter their stiffness in response to substrate stiffness, the elastic modulus of the cell cortex is less than that of the substrate, and in nearly all of these studies, cells adhere to their substrates primarily through integrins. The results appear to be different when soft substrates are made not from an inert hydrogel like polyacrylamide or an elastomer like PDMS but are made from a soft hydrogel formed by crosslinked HA to which an integrin ligand is attached. In this case, the apparent Young's modulus of the cell can be an order of magnitude greater than that of the substrate, and the cell stiffness on a 200 Pa HA-fibronectin gel can be the same as on a stiff gel or glass (46, 173).
E. Changes in Cell Viscosity
The first studies of cell stiffening focused on the elastic modulus of the cell, but subsequent studies showed that the time-dependent viscoelastic response of cells, and in particular the viscous component of the cells' rheology, was also affected by substrate stiffness, even when the substrate was an almost purely elastic hydrogel such as crosslinked polyacrylamide. More sophisticated AFM methods in which the indenting force was applied at different rates enabled measurements of the shear storage and loss moduli as a function of frequency (1). These measurements showed that not only the magnitude of the shear storage modulus changed with substrate stiffness but so did its frequency dependence, as well as the magnitude and frequency dependence of the shear loss modulus (1). As substrates became stiffer, the elastic and modulus increased more than the viscous modulus (FIGURE 9), showing that cells on stiffer substrates are not only stiffer, but also less dissipative. This study also accounted for the possible influence of the substrate stiffness itself on the apparent cortical stiffness measured on the cells apical surface, because under some conditions the strains generated on top of the cell can propagate through it and deform the substrate beneath it (215). These quantitative corrections are not large, and they do not change the central observation that stiffer substrates lead to stiffer cells, at least under these conditions (1). Endothelial cells also alter both elastic and viscous properties in a similar manner: they increase both cell stiffness and apparent viscosity in response to increasing substrate rigidity (101).
FIGURE 9.
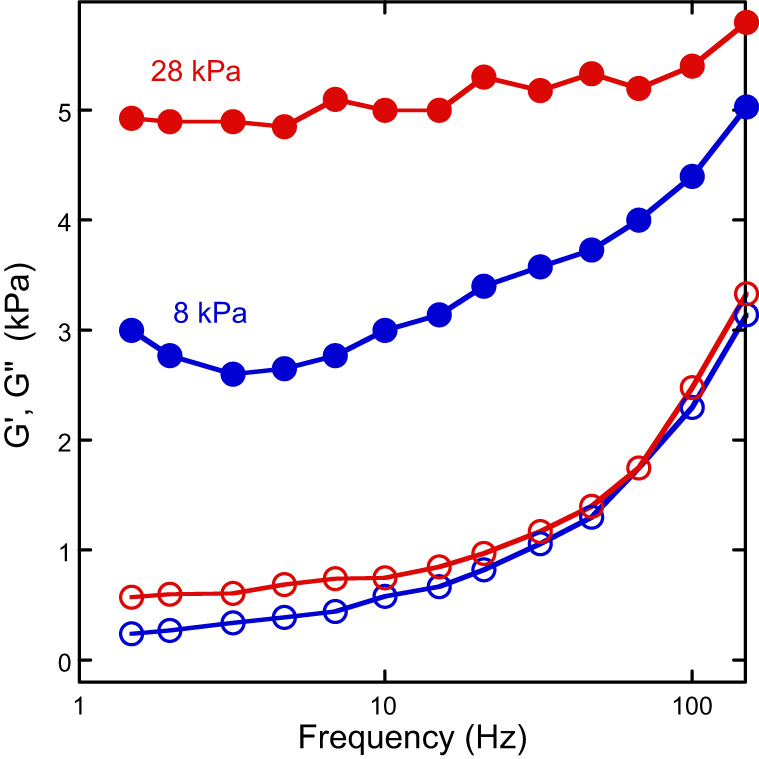
Both viscous and elastic moduli change with substrate stiffness. Shear storage and loss moduli of T24 cells measured in the perinuclear region on gels with effective Young's moduli = 8 and 28 kPa. [From Abidine et al. (1), with permission from Elsevier.]
F. Micropost Arrays and 3D Cultures
Cells also modulate their cortical stiffness when cultured on micro-pillar arrays of different heights, rather than on homogeneous hydrogels. This finding is significant because the Young's modulus of the PDMS pillars is itself much higher than the elastic modules of the cell, and AFM indentation force cannot deform the pillar underneath. Only the shear forces that cells apply to adjacent pillars are able to deform the pillars, to an extent that depends on the pillar height. PDMS pillar arrays can produce substrates with effective shear elastic moduli equivalent to those of soft hydrogels, and the cells cultured on them respond similarly (75). A confounding factor in relating substrate stiffness to cell stiffness is the large variance in cell area when an ensemble of cells is grown on a uniform surface, because the area of a cell might be an independent determinant of cell contractility and stiffness. FIGURE 10 shows how the stiffness of the cell increases with increased pillar array stiffness (decreasing pillar height) when different adhesive areas are printed on the pillar array (207). When cells of 2,500 or 5,000 µm2 are selected, there is a clear increase in cell stiffness on stiffer pillar arrays, but if cells are confined to very small areas, the stiffness differences are no longer significant. At the other extreme, when cells that are able to spread to 10,000 µm2 are selected, there is no significant difference in stiffness on the stiffer arrays, but no such cells can be found on the softest arrays.
FIGURE 10.
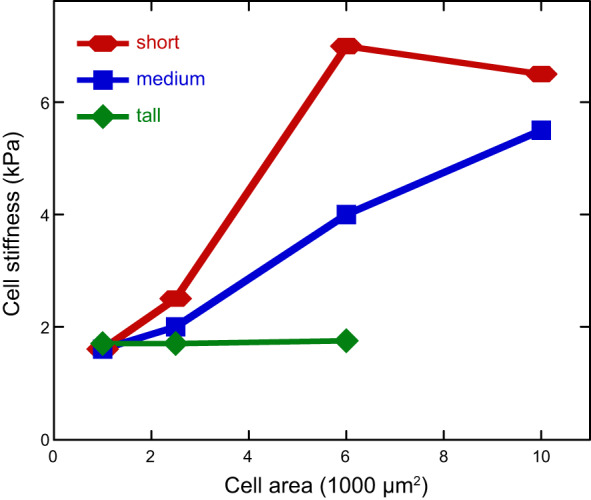
Stiffness of human bone marrow stromal cells (hMSCs) as functions of projected area and substrate stiffness. hMSCs are plated on microposts of different spring constants with various sizes of fibronectin patterns. For tall posts (3.8 nN/mm), cell stiffness remains constantly soft; for medium (18 nN/mm) and short (1,500 nN/mm) posts, cell stiffness increases with projected area. [From Tee et al. (207), with permission from Elsevier.]
Even cells that grow in three-dimensional matrices acquire stiffnesses that respond to the stiffness of the matrix in which they are embedded (29). The effective stiffness of fibroblasts that had been grown in collagen gels of different elastic moduli, and then released from those gels by treatment with collagenase, depended on the stiffness of the matrix from which they were released. Cells grown in 500 Pa collagen gels were more than twice as stiff as those released from 125 Pa gels. The magnitude of the stiffness difference is at least as great as the difference between cells cultured on the surface of gels with different stiffnesses. Prolonged incubation of cells removed from collagen gels showed that their cortical stiffness equalized once they were removed from the matrices of different stiffness and cultured on surfaces with the same elastic properties. These studies show that the structures that determine cell stiffness can reorganize to match that of a new environment in which it is placed.
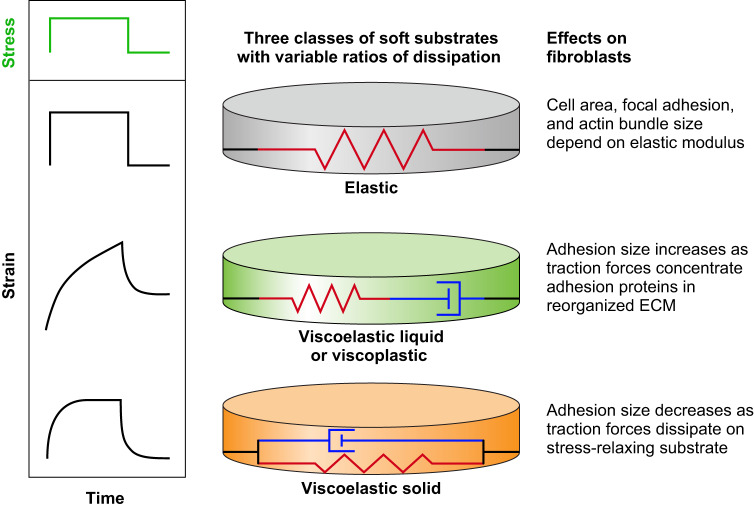
G. Stiffness Responses to Nonlinear Viscoelastic or Viscoplastic Substrates
The native ECM and intact tissues are not purely linear elastic materials, and increasing efforts have been made to produce controllable viscoelastic substrates with apparent stiffness that depends on both time scale and magnitude of deformation (FIGURE 11). Two major differences between a native fibrous ECM and standard hydrogel elastomers used to mimic it are nonlinear strain-stiffening and dissipation due to viscoelasticity or plasticity. For example, mesenchymal stem cells and fibroblasts respond differently to matrices of the same shear modulus measured in the limit of small strain, depending on whether the matrix stiffens at larger strains. Mesenchymal stem cells (MSCs) develop a soft cell phenotype on fibrinogen-coated linearly elastic polyacrylamide gels but become more similar to their morphology on rigid substrates on fibrin gels of the same low-strain stiffness (233), and the traction stress they exert on their substrate strains the substrate and makes it stiffer (233). When cells are bound on or within fibrous networks that strain-stiffen, they transmit forces hundreds of microns away from their surface in a nonuniform manner that also involves fiber alignment (90). As a result, neighboring cells hundreds of microns away respond to these forces and the resulting matrix change, whereas on linear elastic hydrogels cell-derived displacements decay more rapidly, and long-range mechanical signaling is no longer possible.
FIGURE 11.
Different classes of soft substrates with variable elastic and viscous properties. The simplest type of soft material is purely elastic, made from polymer chains that are covalently attached to each other to make a continuous network and which are embedded in a solvent of low viscosity. A common example is the polyacrylamide gel, which has a shear storage modulus much larger than its loss modulus and can be modeled as a simple spring (top). As a result, when such materials are subjected to a sudden stress (shown in green), they immediately respond to reach a time-independent level of strain; when the stress is removed, they recover their initial state before stress was applied. If the gel network is only partially connected by covalent bonds or is crosslinked by covalent and noncovalent bonds placed in series, the rheological response can be modeled as a spring in series with a dissipative dashpot (middle). The material continues to deform (creep) in response to a constant stress, like a viscoelastic liquid, and when the stress is removed it does not recover its initial shape and exhibits a plastic deformation. If the dissipative or labile links are placed in parallel with the covalent bonds, the material is a viscoelastic solid (bottom). The material deforms slowly in response to a sudden stress but eventually reaches a constant deformation; when the stress is removed, the sample slowly recovers and eventually reaches its initial unstrained state.
Viscoelastic mimics of the ECM have been made from purified components such as a mixture of HA and fibronectin to show that effects such as stiffening of cells on stiffer substrates are also observed with such materials (76). Different PDMS formulations have been developed to produce substrates with different viscous and elastic properties, with resulting mechanical changes in NIH/3T3 fibroblasts and MDCK cells (149) as well as in the correlated movement of epithelial sheets (156).
The first systematic work to produce nonbiological controlled viscoelastic gels employed polyacrylamide that was crosslinked to just beyond the gel point, with the incomplete crosslinking allowing for creep: the continued deformation of a material under a constant stress (35). MSCs on these viscoelastic gels increased proliferation and spread area compared with their response to a more completely crosslinked purely elastic gel. One consequence of substrate creep is upregulation of the small GTPase Rac-1, a regulator of actin assembly and cortical cytoskeletal dynamics (36). Another approach to address the dissipative properties of the ECM is to produce hydrogels in which covalent crosslinks are placed in series with noncovalent dynamic crosslinks, for example, by combining Ca2+-mediated and covalent crosslinking in alginate gels to allow the gel to creep under constant stress while maintaining partial elastic resistance (42). MSCs on such compliant substrates increase the size of their adhesion sites compared with those on purely elastic substrates, because forces exerted by the cell on the substrate can cluster adhesion molecules. An alternative method to make viscoelastic materials that have significant dissipation but no creep is to produce networks with static and dynamic crosslinks in parallel rather than in series. Formation of a crosslinked polyacrylamide network around a preexisting viscous solution of unreactive linear polyacrylamide allows formation of gels that have the same elastic resistance at long times but different resistance at short times. On these dissipative gels, fibroblasts lose large focal adhesions even with the shear modulus being high, and differentiation of hepatic stellate cells to a fibrotic phenotype can be prevented and reversed (41). The relaxation times of these viscoelastic solids enable testing of models for mechanosensing in which the differing time constants of molecular motors, clutches, and adhesion receptors are proposed to regulate the mechanoresponse (61, 81, 125).
V. CHANGES IN CELL MOTILITY AND FUNCTION
A. Cell Motility Is Faster on Soft Elastic Substrates
One of the earliest studies of cell response to substrate stiffness by Pelham and Wang (169) showed the remarkable change in the motility of kidney epithelial cells moving on polyacrylamide gels coated with collagen I. The speed of these cells increased by a factor of five as the substrate stiffness was decreased by reducing the amount of crosslinker in the gel. Increased speed was correlated with increased lamellipodial ruffling, lower total tyrosine phosphorylation, and smaller, more irregular adhesion sites. When a polyacrylamide gel coated in collagen I is made with regions having two different substrate stiffnesses, by spatially varying the amount of crosslinker in the gel, cells approaching the interface between the stiffnesses will behave differently depending on which side they originate. In experiments with NIH 3T3 fibroblasts, Lo et al. (143) found that cells approaching from the soft substrate side would continue moving to the stiff side, but cells approaching from the stiff substrate side would turn at the interface and remain on the stiff side. The substrate stiffness in this experiment changed by a factor of two, and the migration speed of the 3T3 fibroblasts was twice as fast on the soft compared with the stiff substrate. This preference of migrating cells to move toward stiff substrates or away from soft substrates, which they named ‟durotaxis,ˮ is observed in multiple cell types.
The migration speed of cells does not decrease monotonically with substrate stiffness but rather appears to have a maximum speed at a particular stiffness. As a result, speed can increase or decrease with substrate stiffness depending on which side of the speed peak the cell is on. Peyton and Putnam (171) found that smooth muscle cells migrating on polyacrylamide gels coated with fibronectin increased their speed up to a maximum between 50 and 300 kPa and then decreased at higher stiffness. However, this biphasic speed profile as a function of stiffness was dependent on ligand density; decreasing the fibronectin density by an order of magnitude shifted the maximum speed to stiffer substrates. Neutrophils migrating on polyacrylamide were similarly found to exhibit a biphasic speed profile with substrate stiffness, and to vary with fibronectin concentration, but the maximum speed occurred at a much lower stiffness (~4 kPa) for these cells (202). Similar biphasic migration behaviors with extracellular matrix stiffness and ligand density are seen for mammary epithelial cells, although with different stiffness and density resulting in maximum cell motility (194).
The ability of cells to move from one place to another is dependent not only on speed but also on their persistence of motion in a given direction. Oakes et al. (163) showed that neutrophils on soft polyacrylamide substrates (5 kPa) moved quickly but turned frequently, while those on stiff substrates (100 kPa) moved more slowly but turned less frequently. This increase in persistence with substrate stiffness was quantified by calculating the mean square displacement (MSD) of neutrophil tracks over different time intervals, with an increased MSD slope indicating greater persistence. Such an increase in MSD with stiffness is also seen in endothelial cells on polyacrylamide gels coated with RGD, where substrate stiffnesses that were too low hindered coordination of endothelial cells and prevented tissue formation (180).
While migration speed depends on substrate stiffness and ligand density in two dimensions, other factors can play a role in three-dimensional gels. For example, glioblastoma cells on polyacrylamide gels with collagen I exhibit increased migration speed with increasing stiffness of the gel (211), but those same cells in 3D agarose gels with collagen I migrate more slowly with increasing stiffness of the gel (212). Using electron microscopy, the authors show that small pore sizes imposed by agarose gels at high stiffnesses restrict movement in 3D, indicating that steric barriers can play a role in modulating speed independent of stiffness.
B. Effects of Viscosity on Motility and Adhesion
Not only does the viscoelasticity of the solid substrate alter cell morphology and motility, but the viscosity of the medium also can influence features such as the rate and extent of cell spreading. In planar culture, where cells are surrounded by medium with a viscosity near that of water, addition of inert macromolecules to produce large non-Newtonian viscosity in the medium tends to increase the rate of cell spreading and the steady-state area of HepG2 cells on fibronectin-coated glass (FIGURE 12), but does not alter spreading on nonspecific adhesive polylysine-coated glass (83). The effect of extracellular fluid viscosity depends on the stiffness of the substrate and can alter the motility of HepG2 cells in a manner that might relate to motility differences in mucin-rich tumors.
FIGURE 12.
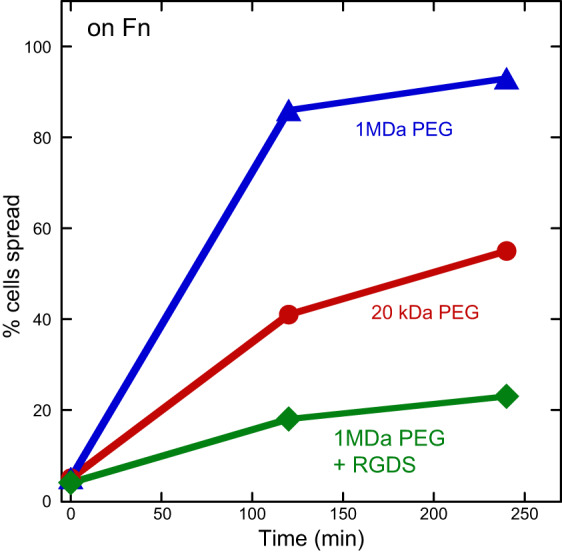
Effect of medium viscosity on cell spreading. Addition of high-molecular-weight polyethylene glycol (PEG) increases medium viscosity and the rate and extent of cell spreading. Blocking integrins with the tripeptide Arg-Gly-Asp (RGD) blocks spreading on fibronectin (Fn). [From Gonzalez-Molina et al. (83), with permission from Elsevier.]
Fluid viscosity is also known to partially govern the forces experienced by leukocytes in blood vessels as they adhere and roll along endothelial cells, before transmigration in response to inflammation (209). However, whether wall shear rate or wall shear stress is most important for determining rolling velocity was not clear. In experiments with neutrophils on P-selectin-coated surfaces, Smith et al. (199) found that doubling fluid viscosity and, hence, wall shear stress had no effect on rolling velocity but instead correlated with cell deformation. In contrast, beads coated with a ligand for P-selectin showed increases in rolling velocity with doubling of fluid viscosity, suggesting that deformability of the neutrophils offset potential increases in rolling velocity with increases in fluid viscosity.
C. Cells Divide Faster on Stiff Substrates
Substrate stiffness affects not only short-timescale behaviors such as spreading and motility but also long-timescale processes including division and differentiation. In one study using an extracellular matrix mimic of hyaluronan and fibronectin, primary adult dermal fibroblasts were found to migrate faster on softer substrates but proliferate more rapidly on stiffer substrates (76). Cells grown on a soft substrate with a stiffness of 95 Pa showed only a ~10% increase in number of cells after 3 days, while cells grown on a substrate with a stiffness of 4,300 Pa doubled in number after 3 days (76).
This increase in proliferation with stiffness was also seen with primary myoblasts cultured on 2D alginate hydrogels, with cells grown for 7 days on a 45 kPa substrate showing approximately triple the growth of cells grown on a 1 kPa substrate (17). This study also found that adhesion of the primary myoblasts was increased with increasing stiffness, as was expression of a marker of myoblast differentiation. Varying substrate stiffness from the range of normal tissues to those of a breast tumor or an atherosclerotic artery increased the proliferation rate of mammary epithelial cells, vascular smooth muscle cells, and mouse embryonic fibroblasts. The fraction of cells incorporating BrdU increased from near zero at 1 kPa to nearly 100% at 30 kPa for all three cell types. The increase in proliferation was related to increased focal adhesion kinase (FAK) activation on stiff substrates (118). Glioblastoma cells grown on polyacrylamide substrates coated with fibronectin also showed increased growth with substrate stiffness, with proliferation increasing by a factor of five between substrate stiffnesses of 0.08 and 119 kPa (211). The mechanisms that control mechanotransduction during cell division are still the subject of investigation, but work in PtK2 epithelial cells on polyelectrolyte multilayer films with substrate stiffnesses ranging from 0 to 500 kPa has pointed to abnormal chromosome segregation on soft substrates as a reason for reduced division (119).
As with cell motility, the stimulating effects of stiffness do not continue indefinitely. Instead, increased division rates observed of cells on stiff substrates reach a maximum at some substrate stiffness, and then they decrease with further increases in substrate stiffness. This biphasic division behavior was observed for human aortic endothelial cells cultured on gelatin scaffolds of different stiffnesses, where differentiation rate increased on substrates up to a modulus of ~500 Pa and then decreased with further increases of stiffness (155). Similarly, myeloid leukemia cells embedded in 3D alginate hydrogels containing RGD peptides showed biphasic proliferation as a function of hydrogel stiffness, peaking at a stiffness of ~300 Pa (196). These observations of ‟preferredˮ stiffnesses suggests that cell growth, and especially differentiation, may be tuned to specific mechanical environments, which is the focus of the next section.
D. Cells Differentiate Best on Substrates with the Stiffness of the Target Tissue
One of the most significant findings from studies investigating the effect of substrate stiffness on cell behavior has been the connection with cell differentiation. Multiple studies have now shown that differentiation toward a particular lineage is highest on the substrate stiffness of the target tissue. Early work with myoblasts found that fusion into myotubes was independent of substrate stiffness but formation of striated acto-myosin structures in the myotubes only occurred at substrate stiffnesses of typical muscles (58). Further work by the Discher laboratory found that mesenchymal stem cells could be differentiated into neurogenic, myogenic, or osteogenic cell lineages based on matching the substrate stiffness to the target tissue–soft for the brain, stiffer for muscles, and very stiff for bone (60).
In addition to differentiation, which has been studied extensively and reviewed recently (216), stem cell renewal has also been shown to depend on substrate stiffness. Experiments with muscle stem cells show that cells cultured on hydrogels that mimic the stiffness of typical muscles self-renew and support muscle regeneration when implanted into mice, something muscle stem cells cultured on plastic dishes will not do (78). Recent work has shown that differentiation depends on substrate stiffness as well as other factors that include spatial patterning of elasticity (243) and on viscous dissipation (41).
E. Matrix Stiffness Increases Cellular Metabolism
Our knowledge of the relationship between the mechanical microenvironment and cellular metabolism is still in a relatively early stage. Already, however, there is potentially conflicting evidence on the relationship between the mechanical microenvironment and cellular metabolic activity. Recent work using single cell measurements of glucose uptake and ATP found that glucose uptake, ATP/ADP, and ATP hydrolysis all increase in stiffer, denser matrices (247). Work using 2D polyacrylamide has shown that metabolism, protein synthesis, and cell proliferation all decrease on more compliant substrates (208), which correlates with the first study using 3D collagen and single cell measurements. However, there are other reports that when matrix stiffness is increased using collagen gels of increasing density, oxygen consumption and glucose metabolism decrease without changes to glucose uptake, cell viability, or reactive oxygen species (ROS) (151). In stiffer, more dense matrices, cells become more reliant on glutamine, suggesting that matrix density causes a shift in the metabolic pathways utilized by cells. These potentially conflicting results could indicate cell-type specific differences. It is also possible that single cell measurements reveal different results from population level measurements due to the ability of single cell measurements to focus on specific cell phenotypes. So, while we can conclude that the mechanical microenvironment affects metabolism, it is not yet possible to make general statements on the nature of that relationship.
Outside of the few studies published on cellular metabolism and matrix stiffness, there are several clues about how the two are linked. Importantly, many fundamental metabolically demanding functions, including proliferation, require anchorage of a cell to a substrate. As such, if the mechanical properties of that substrate change, it is likely the metabolic requirements of a cell change. There is evidence in the literature to substantiate this connection between ECM, integrins, and metabolic profile. AMP-activated protein kinase (AMPK) is the metabolic housekeeper in most cells (91). It is activated when AMP levels are elevated, leading to the generation of ATP. However, there is evidence to suggest there is a feedback loop between AMPK, integrin activation, ECM remodeling, and intracellular stiffness (74). Specifically, knockout of AMPK results in increased fibronectin assembly and intracellular stiffness in fibroblasts. It has been suggested that knockdown of AMPK increases the binding capacity of integrins, thereby facilitating ECM remodeling. While it has not yet been shown directly, these data suggest a feedback loop between cellular ATP levels and cell-mediated ECM remodeling.
FAK, which is known to activate in response to matrix stiffness, is also linked to glucose uptake, insulin signaling, and phosphatidylinositol 3-kinase/AKT stimulation of glycolysis (249). There is evidence to suggest that FAK mediates expression of the insulin receptor. FAK activation can stimulate the activation of k-Ras (102) and Myc which are known to stimulate glutamine metabolic enzymes (234). Importantly, glutamine addiction is a hallmark of rapidly proliferating cancer cells (235). FAK activation has also been shown to be correlated with increased expression of lipid enzymes, suggesting a role of FAK in lipid metabolism (249). It is important to note however that there are exceptions to the general idea that FAK activation leads to enhanced metabolism. FAK in neurons has been shown to negatively regulate metabolism, where FAK inactivation leads to increased glucose uptake (87). As such, since the role of FAK in metabolism is likely cell type specific, it is also likely that the effects of stiffness on metabolism are also cell-type specific.
VI. MECHANISMS OF MECHANOSENSING AND RELATION TO CELL DYSFUNCTION
A. Cancer Cells Respond (or Not) to Stiffness Differently from Normal Cells
One of the most striking changes in cells infected with transforming viruses such as simian virus 40 (SV40) is that they lose their requirement for adhesion to a surface to grow. Harvesting cells that have acquired this ability to grow in suspension after transformation became a standard method of purifying such transformed cells (145). The ability of cells to grow in suspension was shown to be the single most accurate predictor of the ability to produce tumors when injected into mice (48, 197). SV40-transformed cells were shown to spread more on softer substrates than normal cells in one of the first systematic studies of substrate stiffness (116). Overexpression of the activity of the oncogene Ras, which codes for a small GTPase associated with regulating cell adhesion, was shown to be sufficient to induce anchorage-independent cell growth (110). Just as SV40-infected cells displayed altered stiffness sensing, H-ras-transformed NIH 3T3 fibroblasts lost their capacity to distinguish between soft and stiff substrates (219).
As more cell types are studied on more kinds of deformable substrates, it is abundantly evident that the response of cells to substrate stiffness is highly cell-type specific, as well as dependent on the type of adhesion ligands to which the cell adheres (179). One of the most important questions in mechanobiology is whether cancer cells respond differently to substrate stiffness or other mechanical stimuli than normal cells, because if they do, then manipulation of the mechanical properties of diseased tissues might have therapeutic potential for cancer as well as other diseases such as fibrosis or atherosclerosis when stiffness changes are a characteristic of the disease. An important early study showed that mammary epithelial cells, like fibroblasts, increased spread area on stiffer substrates, and multicellular acini of mammary epithelial cells lost structural integrity as individual mammary cells began to invade the surrounding matrix (167). Similarly, human colon carcinoma (HCT-8) cells, which initially form an epithelial sheet on collagen, begin to dissociate, spread, and become motile as single cells after prolonged culture on stiff (21 kPa) gels, but not when they are grown on 1 kPa gels (205). In contrast, tumorigenic mammary epithelial cells grown in 3D gels that are transiently compressed, changing both force on the acini and local mechanical properties, become less tumorigenic and invasive, possibly because of increased mechanical interactions with the surrounding matrix (183). These studies and other reports that cancer cells derived from breast or colorectal tumors increased area on stiffer substrates suggested that the stiffness of tumors might produce an environment in which cancer cells have a growth advantage. Alternatively, the stiff environment could activate stromal cells, by analogy to the formation of myofibroblasts in fibrotic disease, to promote cancer cell survival growth and motility.
An augmented response to stiffness, or preferential growth in stiff environments, appears, however, to be inconsistent with the hallmark of a malignant cell types as capable of growth on soft agar. Although numerous cancer cell types are clearly responsive to substrate stiffness, many are not, and recent studies have emphasized the loss of stiffness sensing in numerous malignant cell types.
There appears to be no universal pattern to whether cancer cells respond to substrate stiffness. FIGURE 13 shows how four different cancer cell lines proliferate when these cells are cultured on substrates with elastic moduli that span the range from that of normal brain or fat to those of stiffer tissues or of some tumors. A549 and MDA-MB-231 cells, derived from lung and breast tumors, respectively, proliferate increasingly rapidly as substrate stiffness increases. In contrast, PC-3 and mPanc96 cells, which are derived from prostate and pancreatic cancers, respectively, proliferate faster on the softest substrates and show little change or a slight decrease in proliferation on stiffer substrates. Other metrics such as area and speed also depend on substrate stiffness in opposite ways for these cell lines (208).
FIGURE 13.
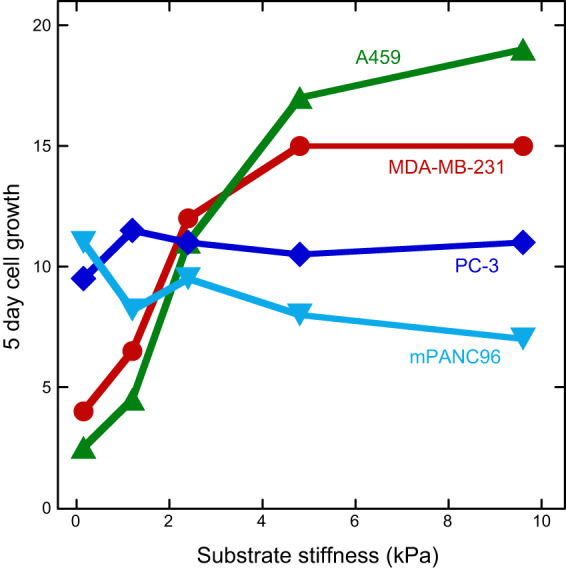
Rigidity-dependent changes in proliferation of diverse cancer cell lines. [From Tilghman et al. (208).]
B. Reversing Altered Stiffness Responses in Cancer Cells
Ras transformed fibroblasts (154, 219) lose the change in spread area as substrate stiffness increases that occurs in the untransfected cells. Similarly, NIH3T3 fibroblasts, which strongly alter their cortical stiffness when substrate stiffness changes (200), lose this ability when Ha-RasV12 is overexpressed in them to produce 7–4 cells (138). This study also made the important observation that stiffness-insensitive Ras-transformed fibroblasts regain their stiffening response after treatment with the ras inhibitor farnesylthiosalicylic acid (FTA) or the downstream MEK1/2 inhibitor U0126 (138). A related study of cancer cells derived from breast, bladder, cervix, and pancreatic tumors concluded that these cells, which were all softer than their normal counterparts, had lost response to substrate stiffness by a mechanism involving decreased caveolin-1 (138).
The viscoelastic responses of cancer cells to collagen gel stiffness have also been investigated by measurements of both intracellular rheology using an optical trap, and whole cell stiffness by cell deformation when cells are released from the collagen matrix and forced through a microfluidic channel (240). This study also shows changes in both the time-dependent intracellular viscoelasticity and the whole cell stiffness of some cell lines. By comparison of multiple cancer cell lines, this report concludes that cancer cells with high invasive capacity can alter their stiffness in response to matrix stiffness, but those that are less invasive lack stiffness response.
C. Substrate Stiffness Can Lead to Heritable Epigenetic Changes
A fundamental complication in nearly all studies of mechanosensing in vitro is that the cell preparation that is used to define a response to changes in substrate stiffness is generally a population of cells that has been grown and passaged in plastic culture flasks to obtain enough cells for quantitative measurements. As a result, the cell that is probed for response to substrate stiffness has already acquired the cellular contents, cell and nuclear morphology, and chromatin structure appropriate to a rigid substrate coated with the serum components or other solutes that are added to the culture flask. Because many proteins have half-lives greater than that of the time over which mechanosensing is probed, and because transcriptional reprogramming often requires more time than the experimental study, the typical responses documented are the acute response of a cell to perturbation of its substrate stiffness away from the rigid environment in which it was initially grown. Resuspension of the cell by trypsinization or other detachment methods from the rigid surface removes some of the markers of a rigid environment, but most of the cellular machinery by which the cell responds are remnants of the former environment. To determine the duration or extent to which a cell retains a memory of its past mechanical state, some recent studies have examined what happens when cells are cultured for long periods and allowed to divide repeatedly on substrates of controlled stiffness. This approach is often difficult or not feasible, because cell proliferation is often retarded or nearly eliminated on soft substrates that resemble a healthy tissue in which cell division times are generally much longer than they are in vitro (118).
To study how prolonged incubation on different stiffness substrates affects slow processes such as conversion of resting fibroblasts to contractile myofibroblasts, normal fibroblasts were grown 3 wk on stiff substrates to elicit the myofibroblast phenotype, characterized by increased proliferation, contractility, α-smooth muscle actin, extracellular matrix, and active transforming growth factor (TGF)-β. This phenotype was maintained for at least 2 wk after moving the cells to a soft substrate (6). FIGURE 14 shows the large change in area and actin expression of rat lung fibroblasts grown on soft (5 kPa) or stiff (100 kPa) gels and tissue culture plastic. When cells were first grown on soft gels and then transferred to stiff gels (5 kPa to 100 kPa), they retain the soft substrate phenotype after four cell divisions, whereas cells primed on a stiff gel retain the stiff phenotype after four passages on a soft gel. This result suggests that the mechanically determined phenotype can survive after multiple cell divisions after the mechanical stimulus is removed. Consistent with this hypothesis, when mesenchymal cells that are capable of differentiating into myofibroblasts are grown on soft substrates, they resist mechanical activation in vitro and when implanted into a mouse model (136). Prolonged growth on stiff substrates upregulates the microRNA miR-21 through a mechanosensitive myocardin-related transcription factor-A (MRTF-A)-dependent mechanism that persists 2 wk after removal from the stiff substrate; reducing miR-21 levels restored the ability of these cells to adapt to a soft environment (136).
FIGURE 14.
Mechanical priming of cells. Rat lung fibroblasts were seeded at 2,500 cells/cm2 on collagen-coated silicone substrates and cultured for four passages to model healthy lung tissue (5 kPa), mature fibrotic tissue (100 kPa), and unphysiologically stiff tissue culture plastic (TCP). The cells were then migrated from soft to fibrotic-stiff zones (5T100), or out of stiff fibrotic zones to soft substrates (100T5). Fourteen days (2 passages) later, the myofibroblast phenotype was assaying immunostaining for α-smooth muscle actin (green). Scale bar = 100 mm. [From Balestrini et al. (6), with permission from Oxford University Press.]
Another major potential contributor to mechanical memory is the transcriptional regulation controlled by the Hippo pathway activators YAP and TAZ, which transit from the cytoplasm of multiple cells types to their nucleus on stiff substrates. When epithelial cells are first cultured on a stiff substrate and then move to an adjacent soft area, they have higher levels of actomyosin activation, form larger focal adhesions, and retain more YAP in their nuclei compared with cells that have been on the soft surface continuously. When cells move from a soft to a stiff area of the same substrate, they express lower levels of the markers that are characteristic of growth on stiff substrates. Exogenous downregulation of YAP reduces this apparent memory (158). Cancer cells also appear to have a mechanical memory. When the breast cancer-derived cell lines MDA-MB-231 and MCF-7 were cultured for long times on compliant polyacrylamide substrates, their response to subsequent growth on gels of different stiffness was reduced compared with the response of these cell types obtained directly from standard tissue culture plates (203). The mechanisms by which cells retain memory of their mechanical environments are just beginning to be defined, and the extent to which this memory represents an epigenetic change similar to those produced by other stimuli remains to be determined.
D. Stiff Substrates Induce an Inflammatory Phenotype
Inflammation is characterized by the release of cytokines by inflammatory cells including macrophages and endothelial cells; the expression of inflammatory markers on the surface of cells including vascular cell adhesion molecule, intracellular adhesion molecule (ICAM), and selectins; increased vascular permeability; and increased recruitment of leukocytes. Substrate stiffness has been shown to modulate several of these hallmarks of inflammation, and it is associated with several inflammatory diseases, including most notably atherosclerosis. Atherosclerosis is more commonly called hardening of the arteries largely due to the characteristic formation of plaques and calcifications in the arterial wall in later stages of the disease. However, even early in disease progression, the arterial wall can stiffen due to mutations in Apo-E (123) or the presence of advanced glycation end products (AGEs) (192). AGEs are proteins that become glycated due to sugars present in tissue. AGE crosslinks in collagen, for instance, result in stiffer matrix. AGEs themselves, outside of their ability to stiffen tissue, can induce a proinflammatory response through their ability to bind RAGEs (receptors of advanced glycation end products) on the surface of cells. AGEs are prevalent in diabetes, and thought to be causative of diabetic vascular complications, and have also been linked to Alzheimer’s progression. Outside of the RAGE-AGE interaction that is known to stimulate inflammation, a stiffer matrix, like that which can occur through the action of AGEs, can also induce an inflammatory phenotype.
Stiffer matrix, regardless of the mechanism of stiffening, has been shown to enhance leukocyte-endothelial interactions in the inflammatory cascade. On stiffer substrates seeded with a confluent monolayer of endothelial cells, leukocytes more easily transmigrate through the monolayer than when the monolayer is on a compliant substrate (98, 202). This increase in transmigration has largely been attributed to disruption of endothelial cell-cell junctions, rather than a change in inflammatory marker expression in endothelial cells; however, there is increasing evidence to suggest that some cells are more sensitive to inflammatory cytokines when on stiffer substrates. For instance, endothelial cells exposed to tumor necrosis factor-α (TNF-α) and thrombin exhibit a greater increase in traction forces on stiff substrates compared with more compliant substrates (213). Fibroblasts have also been shown to be more sensitive to TNF-α on stiff substrates (142, 201), increasing their spreading and traction force generation. The exact mechanism by which this enhanced sensitivity occurs is not clear but is likely due to crosstalk with integrin-related pathways.
Macrophages have also recently been shown to have a significant response to substrate stiffness. Notably, bone marrow-derived macrophages secrete more TNF-α on compliant substrates than on stiff substrates when Toll-like receptor 4 (TLR4) is stimulated (85) (FIGURE 15). Inhibition of cell contractility through ROCK1/2 resulted in an increase of TNF-α release. This result is notable as it is somewhat counter to the typical trend found in cells with regard to substrate stiffness, where increased stiffness results in an increase in a particular response. Here, increased stiffness results in a decrease in TNF-α release. However, it is important to note that these results appear to be cell type and stimulus specific. The macrophage-like murine cell line RAW264.7 (FIGURE 15) and monocyte-like human THP-1 cells do not exhibit the same response (85, 100), and differentiated human U937 macrophages stimulated with lipopolysaccharide appear to have the opposite response: they increase TNF-α production on stiff substrates (168).
FIGURE 15.
Tumor necrosis factor (TNF)-α release by macrophages is regulated by substrate stiffness. Bone marrow-derived macrophages (BMMs) (A) and RAW264.7 cells (B) were plated on fibronectin-coated 1, 20, and 150 kPa polyacrylamide gels for 18 h and stimulated for 24 h with media (−) or 3 μM CpG DNA (+). *Statistically significant; ns, not significant. [From Gruber et al. (85), with permission from Oxford University Press.]
Matrix stiffness has also been shown to affect monocyte attachment to selectins. Notably, adhesion under flow to E-selectin but not P-selectin has been shown to increase with increasing substrate stiffness at stiffnesses above 1 kPa (144). Additional work has demonstrated that a biphasic response may exist where adhesion also increases on matrices that are less than 1 kPa (191). This response was shown not to be due to changes in inflammatory receptor expression in endothelial cells, but rather it is due to ICAM clustering mediated by Rho activity. It is worth noting, however, that there is some evidence to suggest that long-term culture on stiff substrates can alter ICAM expression in endothelial cells (244). Together, this work has clear implications in atherosclerosis and diabetic vascular diseases, where the vessel wall stiffens. However, it also underscores the need to pursue this work in vivo where endothelial cells have been exposed long term to both flow and matrix stiffness.
More recent work has investigated the role of stiffness in inflammatory and foreign body response in vivo. In a murine muscle loss model, PEG gels of varying stiffness were implanted. The data suggest that increased scaffold stiffness results in increased neutrophil recruitment to an implant. Moreover, the duration of time in which neutrophils were present at the site increased with stiffness, suggesting stiff scaffolds induce chronic recruitment (186).
E. Matrix Stiffness Disrupts Cell-Cell Junctions
Cell-cell junctional integrity is an essential structural element in numerous tissues including the gut, skin, other epithelial tissues, and the endothelium within the blood vessel wall. Cell-cell junctional integrity is maintained primarily by two classes of junctions: tight junctions and adherens junctions. Tight junctions are primarily associated with maintaining and controlling the barrier properties between cells, whereas adherens junctions are largely associated with anchoring cells to each other through the family of cadherin proteins. Disruption of cell-cell junctions can result in malformations during development as well as impaired barrier function in epithelial tissues and cancer.
Early work investigating the effects of substrate stiffness on cell behavior identified that cells tend to adhere more to their neighbors, rather than spreading onto the substrate, when seeded on compliant matrices. Cells often aggregate or form network structures on compliant substrates (54). It has been hypothesized that the driving force for cell-cell adhesion on compliant matrices is based on a balance of forces between the cells compared with that between the cells and their substrate that contribute to providing mechanical resistance required for cytoskeletal maintenance. On stiffer substrates, cell-matrix adhesion increases and cell-cell adhesion decreases.
The physiological ramifications of matrix stiffening on cell-cell adhesion and tissue function are becoming increasingly evident. Recent data suggest that increased substrate stiffness, mimicking the stiffening that occurs with age in large arteries, can drive increased permeability in endothelium (98, 213). It is possible that this increase in permeability contributes to cholesterol permeation into the vessel wall and the initiation of atherosclerosis. Notably, this effect has been shown to be mediated by contractility, as inhibition of contractility mediators increases cell-cell adhesion on stiff substrates. These findings extend into the microvasculature as well. Tumor vasculature is notably leakier than vasculature in healthy tissue, a trait that has been largely attributed to various growth factors present in the tumor microenvironment. However, recent evidence now suggests that tissue stiffness also contributes to vessel morphology and permeability (19). Vessels in stiff matrices in vitro and in stiff tumors in vivo are more permeable than those in more compliant matrices and tumor tissue. FAK activation and relocalization of Src due to increased matrix stiffness are thought to mediate VE-cadherin disengagement and increased permeability (224).
A role for substrate stiffness in the maintenance and disruption of cell-cell contacts has also been demonstrated in epithelial cells, where more compliant substrates lead to epithelial cell aggregation and stiffer substrates contribute to scattering (52, 205). Stiff substrates induce an increase in integrin-mediated traction stresses that leads to the disruption of E-cadherin bonds between cells. Cell scattering is a component of epithelial-to-mesenchymal transition (EMT), contributing to cell invasion in metastasis. These results suggest that substrate stiffness can contribute to EMT and cancer metastasis by promoting cell-cell dissociation.
The mechanisms by which substrate stiffness alters cell-cell adhesions are likely twofold. First, changes in cell contractility clearly mediate cell-cell/cell-matrix interactions. Increased substrate stiffness causes an increase in acto-myosin contractility which may physically disrupt cell-cell adhesion. Second, changes in cell-matrix adhesion can result in the recruitment and likely a change in distribution of key structural and signaling proteins between cell-matrix adhesions and cell-cell adhesions. There is separate evidence to show that both vinculin and Src change their localization relative to integrin-mediated adhesions and cadherin-mediated adhesions as a function of substrate stiffness (52, 224). Disruption of cell-cell adhesions therefore may occur in part due to the disassembly of the adhesion sites.
While matrix stiffness is known to increase cell contractility, which can increase tension at cell-cell junctions, numerous physiological forces (tension, fluid shear stress, etc.) that cells are exposed to can also alter cell contractility and cell-cell junctions. How these forces affect each other remains unclear. Interestingly, engagement and tension on VE-cadherin through magnetic beads can disrupt cell-cell junctions up to three cell diameters from the source of tension (10). These data suggest that there is an integrated mechanism by which cells transfer information regarding tension to affect global monolayer integrity. When substrate stiffness is also introduced as a variable, cell-cell adhesion and barrier integrity in response to applied tension are altered. Cells become more sensitive to imposed forces when cells are on stiffer substrates. This manifests in numerous pathologically relevant changes: barrier integrity is more disrupted when force is applied to cells on stiffer substrates (4). Endothelial cells release less nitric oxide and elongate less in response to flow when seeded on stiffer substrates (120). The ramifications of these findings could be significant. The data suggest that areas of matrix stiffening may make cells more susceptible to a disease-causing signal by altering their response to mechanical forces and subsequent mechanotransduction pathways.
F. Matrix Stiffness Results in a Feed-Forward Loop of Increased Matrix Deposition/Degradation
Cells can deposit, rearrange, and degrade matrix, and their ability to do so is in part dictated by their microenvironment. There exists a feedback loop between the matrix the cell encounters, the matrix it deposits, and a cell’s remodeling efforts. In many cases, matrix stiffening can trigger pathways associated with increased matrix deposition, thereby further increasing the stiffness of the matrix. Fibroblasts, for example, respond to matrix stiffening through the activation of the transcription regulators YAP/TAZ (142, 162). In response to increased tension, YAP/TAZ localizes to the nucleus to promote transcriptional activity that increases ECM production. Notably, YAP/TAZ activation alone is not sufficient to increase matrix production (105). Cells must also be seeded on a stiff matrix, suggesting that cells require additional signals from stiff matrices beyond those mediated by YAP/TAZ. Stiffness has been shown to increase matrix deposition in numerous cell types beyond fibroblasts. In vascular smooth muscle cells, both ECM deposition and matrix crosslinking increase in response to stiffness (123). Collagen and fibronectin expression increase as well as the expression of the collagen crosslinker lysyl oxidase (Lox). This work has been validated in vivo, where TAZ expression has been shown to be elevated in fibrosis in the lungs (79), kidney (193), liver (225), and skin (132).
Matrix stiffening can occur through crosslinking and deposition; however, it may also occur through changes in alignment. It has been suggested that aligned matrix fibers are stiffer than unaligned fiber networks (69). Interestingly, cell and fiber alignment alter the orientation of cell-deposited fibers, likely through alterations to cellular tractions, and increased fiber alignment increases matrix deposition (187, 220). There is likely a feedback loop between fiber orientation and subsequent fiber deposition and orientation. This is notable as fiber alignment has been implicated as a strong prognostic indicator of tumor formation (50).
Fibroblasts are a major mediator of ECM remodeling, and in the tumor microenvironment, cancer-associated fibroblasts (CAFs) deposit, reorganize, and degrade matrix to promote tumor growth and metastasis. Notably, matrix stiffening increases stress fiber formation and cell contractility, hallmarks of the CAF phenotype, and matrix stiffening can aid in the maintenance of CAFs, again contributing to a feed-forward loop between cells, matrix stiffening, and matrix remodeling (FIGURE 16). Stiffness not only affects the relative expression levels of ECM proteins, there is also evidence that it mediates splicing events to cause the differential expression of different isoforms of those proteins. More specifically, matrix stiffness can induce the production of EDB-fibronectin, an isoform typically only associated with development and tumors (19). This suggests a previously unidentified role for stiffness in mediating protein splicing which contributes to the diversity of proteins expressed by cells. Notably, this loop is again at least partly mediated by YAP/TAZ, which has been shown to be required for the maintenance of the CAF phenotype (34). Stiff substrates are also thought to be necessary for fibroblast activation through TGF-β, which then triggers cell contractility and matrix remodeling (188). This remodeling can then result in further increases in matrix stiffness. It may be possible to revert the contractile myofibroblastic phenotype by softening the matrix (218) or increasing viscous dissipation (41) providing further evidence of the feedback loop between matrix stiffness and cell-mediated matrix remodeling.
FIGURE 16.
Reciprocal relation between substrate and cell. As a cell encounters a substrate with specific physical properties, it responds by reorganizing its morphology but also by changing processes such as matrix deposition or activation of matrix remodeling enzymes that alter the chemical and physical properties of the substrate.
Matrix stiffness is also linked to matrix degradation. Cells degrade matrix primarily through a family of enzymes called matrix metalloproteinases (MMPs). Stiffness can affect both the expression of MMPs and their activity. Endothelial cells in stiff collagen gels increase MT1-MMP expression and MT1-MMP activity compared with endothelial cells in more compliant gels (19). Pancreatic cells upregulate MMP activity in response to matrix stiffness, which is mediated through cell contractility (89). In loose, compliant matrices, cells show little benefit from increased MMP expression (65). Likewise, cells in aligned fibers also have decreased need for MMP compared with cells in disorganized fiber ECM. The ability of a cell to tune its MMP expression and activity depending on the stiffness, density, and alignment of the fibers within the ECM may explain how cells are able to navigate in a variety of microenvironments.
MMPs can be secreted in discrete locations by cellular structures called invadopodia. Invadopodia are actin-rich structures that localize to the membrane and facilitate local secretion of MMPs. While their mechanisms of formation are still under investigation, it has been shown that matrix stiffness increases invadopodia activity (2). A similar relationship between activity and stiffness has also been shown for podosomes, a similar structure also responsible for matrix degradation (49, 97, 107) in numerous cell types. Together, these data suggest that cells have the ability to tune their protease production and secretion dependent on their microenvironment. Confinement during migration can trigger localization of invadopodia ahead of the nucleus to generate local MMP release that facilitates migration (99), further evidence that cells can direct matrix degradation on an as-needed basis.
Given this feedback loop between matrix stiffening and matrix deposition, the question has been raised about which comes first in pathological conditions. There is evidence to suggest in liver fibrosis and cardiovascular disease that tissue stiffening precedes changes in matrix deposition and disease progression (71, 229). In the case of hypertension, it has been long thought that stiffness in arteries was simply an adaptive mechanism to increased load due to higher blood pressures. However, more recent evidence suggests stiffening precedes hypertension and may actually cause it, and genetic deletion of Apo-E in a mouse model led to stiffening of arteries before any sign of atherosclerotic lesions could be detected (123). Similarly, in a rat model of liver fibrosis, stiffening precedes fibrosis as assessed by changes in cell structure or matrix deposition (71). The stiffening is partially due to crosslinking of the preexisting matrix by lysyl oxidase. The cause and effect relationship of stiffening and liver fibrosis is not yet clear, although it has been suggested that stiffening may promote early fibrosis, and liver stiffness is the strongest known risk factor for later development of hepatocellular carcinoma (66).
VII. CONCLUSIONS AND FUTURE DIRECTIONS
Cell phenotype is inextricably linked to matrix and tissue stiffness. Almost every cell type and cell function has been shown to be affected by matrix stiffness. Therefore the ‟stateˮ of a cell can only be defined if the mechanical microenvironment is also known. However, creating generalizable rules the apply across cell types and physiological or pathological conditions is likely not possible. Cells probe the mechanical state of their matrix and receive feedback based on the strains imposed in the matrix (FIGURE 17). While most cells increase their spreading, stiffness, and proliferation and decrease their volume on stiffer substrates, there are cells that respond by doing the opposite. Cell response to matrix stiffness is both cell-type specific and ligand specific. We know now that there are almost always exceptions to the rules governing cell-matrix mechanobiology.
FIGURE 17.
Cartoon of cell sensing and response to matrix stiffness. As a cell encounters a matrix, it uses actin-rich filopodia to probe the mechanical environment, establishing nascent adhesions at the leading edge. Resistance from a stiff matrix promotes the maturation of nascent adhesions into focal adhesions and spreading of the lamellipodium. If a cell, instead, encounters a compliant matrix, the adhesions disassemble and the filipodia retract. Importantly, these responses are cell-type and ligand-type specific.
The number of papers published on matrix stiffness and cellular mechanosensing of stiffness has grown exponentially over the past two decades, yet numerous challenges remain. Fundamentally, there is a significant gap in our knowledge of how cells sense mechanical stimuli and how that sensing is translated into phenotypic changes. Moreover, since the rules for how cells respond to stiffness are not generalizable, it is not clear that the intracellular sensing mechanism will necessarily be universal across cell types. The development of governing parameters for the design of a new biomaterial for therapeutic treatments, as one example, will need to be tailored for a given cell type and tissue.
Numerous challenges exist in our fundamental understanding of matrix mechanobiology, limited in part by available platforms and technologies to probe and manipulate matrix stiffness and cellular mechanosensing. As just one example, the development of novel materials that permit control over the plastic and viscous response are fundamental to understand the key features of the mechanical properties of a material that contribute to cell response. Additionally, there remains a gap in our ability to handle strain to stress relations when the material is nonlinear. This limits our ability to develop a complete understanding of stress/strain relationships in most native biomaterials (collagen, laminin, etc.) where the materials are not linearly elastic.
As we transition this work into additional in vivo models, there is a need to control and measure mechanics and mechanical response in vivo. While it is possible to remove a tissue and measure the mechanical properties using AFM or large-scale mechanical testing, these are end-point measurements and cannot be made nondestructively. Ultrasound, magnetic resonance elastography (181), and optical coherence elastography (130) potentially offer the ability to monitor stiffness in vivo in real-time; however, they are still relatively semiquantitative, and there is a need to compare and convert the stiffness metrics they obtain into the metrics typically reported with more conventional mechanical testing platforms. There also remains the challenge of monitoring cellular response in real-time. Recently, a biosensor of RhoA was reported, where the biosensor detects activated RhoA in a number of different tissues in vivo (160). As additional biosensors are developed, and the signal-to-noise ratio is improved, mechanotransduction will be able to be monitored in real-time, nondestructively in tissues.
One of the biggest challenges that remains is the ability to translate findings in mechanobiology to the clinic. The field of mechanomedicine has been born from the need to bring findings from the mechanics laboratory to patient care. But the underlying question is, How does one target ‟mechanicsˮ? In the case of matrix stiffening, one can imagine either targeting the mechanisms leading to changes in matrix stiffness or targeting the intracellular signaling pathways responsible for transmitting information regarding matrix mechanical properties to the cell. Phenyl thiazolium bromide analogs, like alagebrium, have been developed to break a subset of AGE-based crosslinks. They have shown some efficacy in restoring vascular compliance in diabetic rodent models (237) and in patients with systolic hypertension (251). However, off-target toxicity has halted their further development. Additionally, they do not target all mechanisms of tissue stiffening, including glucosapene crosslinks which are a major pathological crosslink occurring with age and diabetes (56) that do not yet have an inhibitor. Matrix stiffening also occurs through myofibroblast-mediated matrix deposition and reorganization. Targeting myofibroblasts is difficult due to the lack of specific markers; however, there is a microRNA, MRG-201, that is in clinical trials for the treatment of fibrosis. Due to the multiple mechanisms by which matrix stiffening occurs, it is likely that treating or preventing matrix stiffening clinically will likely require multiple drugs to address the full landscape of stiffening mechanisms.
Rather than targeting stiffening, targeting cellular signaling response to stiffening may be a potential avenue to disrupt aberrant mechanobiology in disease. Interestingly, there are numerous drugs in the pipeline that target well-known mechanotransduction and stiffening pathways (129). Many of the drugs have already shown promise, like those targeting FAK and TGF-β. Statins have been suggested to be one family of drugs that may be repurposed for mechanobiology. Clinically, statins are prescribed due to their cholesterol-lowering effects; however, they have also been shown to interfere with Rho localization and activation at clinically relevant doses. Rho and Rho kinase are key mechanotransduction molecules involved in translating changes in matrix stiffness into changes in cell contractility, and they have been implicated in a number of diseases. Recent data suggest that statins may have beneficial effects in endothelial barrier function in response to vessel stiffening (128), maintaining the phenotype of valvular interstitial cells in heart valves (150), and as part of a cocktail used against numerous cancers through the inhibition of Rho (13).
As we continue to define the relevant pathways, with an eye towards identifying specific targets to minimize off-target effects, mechanomedicine will expand. Matrix stiffness is clearly a factor in the progression of numerous diseases, but it is unlikely that we will be able to identify generalizable parameters that define its role across organs, cells, and disease states. The current challenge in the field is identifying its relative importance in specific disease treatments. As the field matures and new methods are developed for measuring stiffness, it is possible that mechanomedicine will also impact personalized medicine: where treatment approach will depend on the mechanical state of a particular patient’s disease.
GRANTS
This work was supported by National Institutes of Health Grants EB017753, CA193417, and GM096971 and by National Science Foundation Grant CMMI-154857.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Present addresses: D. A. Fletcher, Dept. of Bioengineering, Univ. of California–Berkeley, 648 Stanley Hall, MC 1762, Berkeley, CA 94720; and C. A. Reinhart-King, Dept. of Biomedical Engineering, Vanderbilt University, PMB 351631, Nashville, TN 351631–1631.
Address for reprint requests and other correspondence: P. Janmey, Dept. of Physiology, Univ. of Pennsylvania, 415 Curie Blvd., Philadelphia, PA 19104–6085 (e-mail: janmey@mail.med.upenn.edu).
REFERENCES
- 1.Abidine Y, Constantinescu A, Laurent VM, Sundar Rajan V, Michel R, Laplaud V, Duperray A, Verdier C. Mechanosensitivity of Cancer Cells in Contact with Soft Substrates Using AFM. Biophys J 114: 1165–1175, 2018. doi: 10.1016/j.bpj.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol 18: 1295–1299, 2008. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananthanarayanan B, Kim Y, Kumar S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials 32: 7913–7923, 2011. doi: 10.1016/j.biomaterials.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andresen Eguiluz RC, Kaylan KB, Underhill GH, Leckband DE. Substrate stiffness and VE-cadherin mechano-transduction coordinate to regulate endothelial monolayer integrity. Biomaterials 140: 45–57, 2017. doi: 10.1016/j.biomaterials.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriani Y, Chua JM, Chua BY, Phang IY, Shyh-Chang N, Tan WS. Polyurethane acrylates as effective substrates for sustained in vitro culture of human myotubes. Acta Biomater 57: 115–126, 2017. doi: 10.1016/j.actbio.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol 4: 410–421, 2012. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 7.Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials 22: 1077–1084, 2001. doi: 10.1016/S0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 8.Banyard DA, Sarantopoulos CN, Borovikova AA, Qiu X, Wirth GA, Paydar KZ, Haun JB, Evans GR, Widgerow AD. Phenotypic Analysis of Stromal Vascular Fraction After Mechanical Shear Reveals Stress-Induced Progenitor Populations. Plast Reconstr Surg 138: 237e–247e, 2016. doi: 10.1097/PRS.0000000000002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao M, Xie J, Katoele N, Hu X, Wang B, Piruska A, Huck WTS. Cellular volume and matrix stiffness direct stem cell behavior in a 3D microniche. ACS Appl Mater Interfaces 11: 1754–1759, 2019. doi: 10.1021/acsami.8b19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barry AK, Wang N, Leckband DE. Local VE-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers. J Cell Sci 128: 1341–1351, 2015. doi: 10.1242/jcs.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batchelder EL, Hollopeter G, Campillo C, Mezanges X, Jorgensen EM, Nassoy P, Sens P, Plastino J. Membrane tension regulates motility by controlling lamellipodium organization. Proc Natl Acad Sci USA 108: 11429–11434, 2011. doi: 10.1073/pnas.1010481108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer A, Gu L, Kwee B, Li WA, Dellacherie M, Celiz AD, Mooney DJ. Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts. Acta Biomater 62: 82–90, 2017. doi: 10.1016/j.actbio.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckwitt CH, Brufsky A, Oltvai ZN, Wells A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res 20: 144, 2018. doi: 10.1186/s13058-018-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beningo KA, Lo CM, Wang YL. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol 69: 325–339, 2002. doi: 10.1016/S0091-679X(02)69021-1. [DOI] [PubMed] [Google Scholar]
- 15.Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng 105: 1148–1160, 2010. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 16.Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A 100: 1375–1386, 2012. doi: 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boontheekul T, Hill EE, Kong HJ, Mooney DJ. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng 13: 1431–1442, 2007. doi: 10.1089/ten.2006.0356. [DOI] [PubMed] [Google Scholar]
- 18.Boothe SD, Myers JD, Pok S, Sun J, Xi Y, Nieto RM, Cheng J, Jacot JG. The Effect of Substrate Stiffness on Cardiomyocyte Action Potentials. Cell Biochem Biophys 74: 527–535, 2016. doi: 10.1007/s12013-016-0758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordeleau F, Mason BN, Lollis EM, Mazzola M, Zanotelli MR, Somasegar S, Califano JP, Montague C, LaValley DJ, Huynh J, Mencia-Trinchant N, Negrón Abril YL, Hassane DC, Bonassar LJ, Butcher JT, Weiss RS, Reinhart-King CA. Matrix stiffening promotes a tumor vasculature phenotype. Proc Natl Acad Sci USA 114: 492–497, 2017. doi: 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottier C, Gabella C, Vianay B, Buscemi L, Sbalzarini IF, Meister JJ, Verkhovsky AB. Dynamic measurement of the height and volume of migrating cells by a novel fluorescence microscopy technique. Lab Chip 11: 3855–3863, 2011. doi: 10.1039/c1lc20807a. [DOI] [PubMed] [Google Scholar]
- 21.Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol 13: 1124–1131, 2011. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandes G, Reale E, Messina A. Microfilament system in the microvascular endothelium of the palmar fascia affected by mechanical stress applied from outside. Virchows Arch 429: 165–172, 1996. doi: 10.1007/BF00192439. [DOI] [PubMed] [Google Scholar]
- 23.Bravo B, García de Durango C, González Á, Gortázar AR, Santos X, Forteza-Vila J, Vidal-Vanaclocha F. Opposite Effects of Mechanical Action of Fluid Flow on Proangiogenic Factor Secretion From Human Adipose-Derived Stem Cells With and Without Oxidative Stress. J Cell Physiol 232: 2158–2167, 2017. doi: 10.1002/jcp.25712. [DOI] [PubMed] [Google Scholar]
- 24.Broughton KM, Russell B. Cardiomyocyte subdomain contractility arising from microenvironmental stiffness and topography. Biomech Model Mechanobiol 14: 589–602, 2015. doi: 10.1007/s10237-014-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown XQ, Ookawa K, Wong JY. Evaluation of polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials 26: 3123–3129, 2005. doi: 10.1016/j.biomaterials.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Brückner BR, Janshoff A. Elastic properties of epithelial cells probed by atomic force microscopy. Biochim Biophys Acta 1853, 11 Pt B: 3075–3082, 2015. doi: 10.1016/j.bbamcr.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Butler JP, Tolić-Nørrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 28.Buxboim A, Rajagopal K, Brown AE, Discher DE. How deeply cells feel: methods for thin gels. J Phys Condens Matter 22: 194116, 2010. doi: 10.1088/0953-8984/22/19/194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byfield FJ, Reen RK, Shentu TP, Levitan I, Gooch KJ. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J Biomech 42: 1114–1119, 2009. doi: 10.1016/j.jbiomech.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, Janmey PA. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J 96: 5095–5102, 2009. doi: 10.1016/j.bpj.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cacopardo L, Guazzelli N, Nossa R, Mattei G, Ahluwalia A. Engineering hydrogel viscoelasticity. J Mech Behav Biomed Mater 89: 162–167, 2019. doi: 10.1016/j.jmbbm.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 32.Cadart C, Zlotek-Zlotkiewicz E, Venkova L, Thouvenin O, Racine V, Le Berre M, Monnier S, Piel M. Fluorescence eXclusion Measurement of volume in live cells. Methods Cell Biol 139: 103–120, 2017. doi: 10.1016/bs.mcb.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Califano JP, Reinhart-King CA. Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact. Cell Mol Bioeng 3: 68–75, 2010. doi: 10.1007/s12195-010-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15: 637–646, 2013. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron AR, Frith JE, Cooper-White JJ. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32: 5979–5993, 2011. doi: 10.1016/j.biomaterials.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Cameron AR, Frith JE, Gomez GA, Yap AS, Cooper-White JJ. The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials 35: 1857–1868, 2014. doi: 10.1016/j.biomaterials.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Canver AC, Ngo O, Urbano RL, Clyne AM. Endothelial directed collective migration depends on substrate stiffness via localized myosin contractility and cell-matrix interactions. J Biomech 49: 1369–1380, 2016. doi: 10.1016/j.jbiomech.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Cartagena-Rivera AX, Logue JS, Waterman CM, Chadwick RS. Actomyosin Cortical Mechanical Properties in Nonadherent Cells Determined by Atomic Force Microscopy. Biophys J 110: 2528–2539, 2016. doi: 10.1016/j.bpj.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan EP, Bradshaw C, Gaca MD, Marcinkiewicz C, Wells RG. Integrins function as mechanosensors to mediate hepatic stellate cell activation in response to substrate stiffness. Hepatology 44: 264A, 2006. [Google Scholar]
- 40.Chang H, Liu XQ, Hu M, Zhang H, Li BC, Ren KF, Boudou T, Albiges-Rizo C, Picart C, Ji J. Substrate Stiffness Combined with Hepatocyte Growth Factor Modulates Endothelial Cell Behavior. Biomacromolecules 17: 2767–2776, 2016. doi: 10.1021/acs.biomac.6b00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charrier EE, Pogoda K, Wells RG, Janmey PA. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat Commun 9: 449, 2018. doi: 10.1038/s41467-018-02906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 15: 326–334, 2016. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Li W, Maybeck V, Offenhäusser A, Krause HJ. Statistical study of biomechanics of living brain cells during growth and maturation on artificial substrates. Biomaterials 106: 240–249, 2016. doi: 10.1016/j.biomaterials.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 44.Cheng S, Wang N, Larson MG, Palmisano JN, Mitchell GF, Benjamin EJ, Vasan RS, Levy D, McCabe EL, Vita JA, Wang TJ, Shaw SY, Cohen KS, Hamburg NM. Circulating angiogenic cell populations, vascular function, and arterial stiffness. Atherosclerosis 220: 145–150, 2012. doi: 10.1016/j.atherosclerosis.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chopra A, Lin V, McCollough A, Atzet S, Prestwich GD, Wechsler AS, Murray ME, Oake SA, Kresh JY, Janmey PA. Reprogramming cardiomyocyte mechanosensing by crosstalk between integrins and hyaluronic acid receptors. J Biomech 45: 824–831, 2012. doi: 10.1016/j.jbiomech.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, Raz-Ben Aroush D, Galie PA, Pogoda K, Bucki R, Marcinkiewicz C, Prestwich GD, Zarembinski TI, Chen CS, Puré E, Kresh JY, Janmey PA. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials 35: 71–82, 2014. doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chopra A, Tabdanov E, Patel H, Janmey PA, Kresh JY. Cardiac myocyte remodeling mediated by N-cadherin-dependent mechanosensing. Am J Physiol Heart Circ Physiol 300: H1252–H1266, 2011. doi: 10.1152/ajpheart.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colburn NH, Bruegge WF, Bates JR, Gray RH, Rossen JD, Kelsey WH, Shimada T. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res 38: 624–634, 1978. [PubMed] [Google Scholar]
- 49.Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N. Self-organized podosomes are dynamic mechanosensors. Curr Biol 18: 1288–1294, 2008. doi: 10.1016/j.cub.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 178: 1221–1232, 2011. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dasbiswas K, Majkut S, Discher DE, Safran SA. Substrate stiffness-modulated registry phase correlations in cardiomyocytes map structural order to coherent beating. Nat Commun 6: 6085, 2015. doi: 10.1038/ncomms7085. [DOI] [PubMed] [Google Scholar]
- 52.De Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol 171: 153–164, 2005. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deegan DB, Zimmerman C, Skardal A, Atala A, Shupe TD. Stiffness of hyaluronic acid gels containing liver extracellular matrix supports human hepatocyte function and alters cell morphology. J Mech Behav Biomed Mater 55: 87–103, 2016. doi: 10.1016/j.jmbbm.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143, 2005. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 55.Diz-Muñoz A, Thurley K, Chintamen S, Altschuler SJ, Wu LF, Fletcher DA, Weiner OD. Membrane Tension Acts Through PLD2 and mTORC2 to Limit Actin Network Assembly During Neutrophil Migration. PLoS Biol 14: e1002474, 2016. doi: 10.1371/journal.pbio.1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Draghici C, Wang T, Spiegel DA. Concise total synthesis of glucosepane. Science 350: 294–298, 2015. doi: 10.1126/science.aac9655. [DOI] [PubMed] [Google Scholar]
- 58.Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166: 877–887, 2004. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engler AJ, Richert L, Wong JY, Picart C, Discher DE. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: correlations between substrate stiffness and cell adhesion. Surf Sci 570: 142–154, 2004. doi: 10.1016/j.susc.2004.06.179. [DOI] [Google Scholar]
- 60.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 61.Étienne J, Fouchard J, Mitrossilis D, Bufi N, Durand-Smet P, Asnacios A. Cells as liquid motors: mechanosensitivity emerges from collective dynamics of actomyosin cortex. Proc Natl Acad Sci USA 112: 2740–2745, 2015. doi: 10.1073/pnas.1417113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan H, Li S. Modeling universal dynamics of cell spreading on elastic substrates. Biomech Model Mechanobiol 14: 1265–1280, 2015. doi: 10.1007/s10237-015-0673-1. [DOI] [PubMed] [Google Scholar]
- 63.Féréol S, Fodil R, Labat B, Galiacy S, Laurent VM, Louis B, Isabey D, Planus E. Sensitivity of alveolar macrophages to substrate mechanical and adhesive properties. Cell Motil Cytoskeleton 63: 321–340, 2006. doi: 10.1002/cm.20130. [DOI] [PubMed] [Google Scholar]
- 64.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport 13: 2411–2415, 2002. doi: 10.1097/00001756-200212200-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fraley SI, Wu PH, He L, Feng Y, Krisnamurthy R, Longmore GD, Wirtz D. Three-dimensional matrix fiber alignment modulates cell migration and MT1-MMP utility by spatially and temporally directing protrusions. Sci Rep 5: 14580, 2015. doi: 10.1038/srep14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fung J, Lai CL, Seto WK, Wong DK, Yuen MF. Prognostic significance of liver stiffness for hepatocellular carcinoma and mortality in HBeAg-negative chronic hepatitis B. J Viral Hepat 18: 738–744, 2011. doi: 10.1111/j.1365-2893.2010.01355.x. [DOI] [PubMed] [Google Scholar]
- 67.Galie PA, van Oosten A, Chen CS, Janmey PA. Application of multiple levels of fluid shear stress to endothelial cells plated on polyacrylamide gels. Lab Chip 15: 1205–1212, 2015. doi: 10.1039/C4LC01236D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ganesan M, Dagur RS, Makarov E, Poluektova LI, Kidambi S, Osna NA. Matrix stiffness regulate apoptotic cell death in HIV-HCV co-infected hepatocytes: Importance for liver fibrosis progression. Biochem Biophys Res Commun 500: 717–722, 2018. doi: 10.1016/j.bbrc.2018.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gehler S, Ponik SM, Riching KM, Keely PJ. Bi-directional signaling: extracellular matrix and integrin regulation of breast tumor progression. Crit Rev Eukaryot Gene Expr 23: 139–157, 2013. doi: 10.1615/CritRevEukarGeneExpr.2013006647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Genes NG, Rowley JA, Mooney DJ, Bonassar LJ. Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch Biochem Biophys 422: 161–167, 2004. doi: 10.1016/j.abb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 71.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol 293: G1147–G1154, 2007. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 72.Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol (1985) 98: 1547–1553, 2005. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- 73.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J 90: 3012–3018, 2006. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Georgiadou M, Lilja J, Jacquemet G, Guzmán C, Rafaeva M, Alibert C, Yan Y, Sahgal P, Lerche M, Manneville JB, Mäkelä TP, Ivaska J. AMPK negatively regulates tensin-dependent integrin activity. J Cell Biol 216: 1107–1121, 2017. doi: 10.1083/jcb.201609066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghibaudo M, Saez A, Trichet L, Xayaphoummine A, Browaeys J, Silberzan P, Buguin A, Ladoux B. Traction forces and rigidity sensing regulate cell functions. Soft Matter 4: 1836–1843, 2008. doi: 10.1039/b804103b. [DOI] [Google Scholar]
- 76.Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, Ren XD, Rafailovich M, Clark RA. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials 28: 671–679, 2007. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giaever I, Keese CR. Behavior of cells at fluid interfaces. Proc Natl Acad Sci USA 80: 219–222, 1983. doi: 10.1073/pnas.80.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329: 1078–1081, 2010. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gokey JJ, Sridharan A, Xu Y, Green J, Carraro G, Stripp BR, Perl AT, Whitsett JA. Active epithelial Hippo signaling in idiopathic pulmonary fibrosis. JCI Insight 3: e98738, 2018. doi: 10.1172/jci.insight.98738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goli-Malekabadi Z, Tafazzoli-Shadpour M, Tamayol A, Seyedjafari E. Time dependency of morphological remodeling of endothelial cells in response to substrate stiffness. Bioimpacts 7: 41–47, 2017. doi: 10.15171/bi.2017.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gong Z, Szczesny SE, Caliari SR, Charrier EE, Chaudhuri O, Cao X, Lin Y, Mauck RL, Janmey PA, Burdick JA, Shenoy VB. Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc Natl Acad Sci USA 115: E2686–E2695, 2018. doi: 10.1073/pnas.1716620115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gonzalez NP, Tao J, Rochman ND, Vig D, Chiu E, Wirtz D, Sun SX. Cell tension and mechanical regulation of cell volume. Mol Biol Cell 29: 2509–2601, 2018. doi: 10.1091/mbc.E18-04-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzalez-Molina J, Zhang X, Borghesan M, Mendonça da Silva J, Awan M, Fuller B, Gavara N, Selden C. Extracellular fluid viscosity enhances liver cancer cell mechanosensing and migration. Biomaterials 177: 113–124, 2018. doi: 10.1016/j.biomaterials.2018.05.058. [DOI] [PubMed] [Google Scholar]
- 84.Grinnell F, Ho CH. The effect of growth factor environment on fibroblast morphological response to substrate stiffness. Biomaterials 34: 965–974, 2013. doi: 10.1016/j.biomaterials.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gruber E, Heyward C, Cameron J, Leifer C. Toll-like receptor signaling in macrophages is regulated by extracellular substrate stiffness and Rho-associated coiled-coil kinase (ROCK1/2). Int Immunol 30: 267–278, 2018. doi: 10.1093/intimm/dxy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo M, Pegoraro AF, Mao A, Zhou EH, Arany PR, Han Y, Burnette DT, Jensen MH, Kasza KE, Moore JR, Mackintosh FC, Fredberg JJ, Mooney DJ, Lippincott-Schwartz J, Weitz DA. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc Natl Acad Sci USA 114: E8618–E8627, 2017. doi: 10.1073/pnas.1705179114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gupta A, Bisht B, Dey CS. Focal adhesion kinase negatively regulates neuronal insulin resistance. Biochim Biophys Acta 1822: 1030–1037, 2012. doi: 10.1016/j.bbadis.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 88.Gupta M, Sarangi BR, Deschamps J, Nematbakhsh Y, Callan-Jones A, Margadant F, Mège RM, Lim CT, Voituriez R, Ladoux B. Adaptive rheology and ordering of cell cytoskeleton govern matrix rigidity sensing. Nat Commun 6: 7525, 2015. doi: 10.1038/ncomms8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haage A, Schneider IC. Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. FASEB J 28: 3589–3599, 2014. doi: 10.1096/fj.13-245613. [DOI] [PubMed] [Google Scholar]
- 90.Hall MS, Alisafaei F, Ban E, Feng X, Hui CY, Shenoy VB, Wu M. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc Natl Acad Sci USA 113: 14043–14048, 2016. doi: 10.1073/pnas.1613058113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature 290: 249–251, 1981. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- 93.Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science 208: 177–179, 1980. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 94.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12: 2730–2741, 2001. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, Altschuler SJ, Wu LF, Weiner OD. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell 148: 175–188, 2012. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang C, Butler PJ, Tong S, Muddana HS, Bao G, Zhang S. Substrate stiffness regulates cellular uptake of nanoparticles. Nano Lett 13: 1611–1615, 2013. doi: 10.1021/nl400033h. [DOI] [PubMed] [Google Scholar]
- 97.Huynh J, Bordeleau F, Kraning-Rush CM, Reinhart-King CA. Substrate Stiffness Regulates PDGF-Induced Circular Dorsal Ruffle Formation Through MLCK. Cell Mol Bioeng 6: 138–147, 2013. doi: 10.1007/s12195-013-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med 3: 112ra122, 2011. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Infante E, Castagnino A, Ferrari R, Monteiro P, Agüera-González S, Paul-Gilloteaux P, Domingues MJ, Maiuri P, Raab M, Shanahan CM, Baffet A, Piel M, Gomes ER, Chavrier P. LINC complex-Lis1 interplay controls MT1-MMP matrix digest-on-demand response for confined tumor cell migration. Nat Commun 9: 2443, 2018. doi: 10.1038/s41467-018-04865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Irwin EF, Saha K, Rosenbluth M, Gamble LJ, Castner DG, Healy KE. Modulus-dependent macrophage adhesion and behavior. J Biomater Sci Polym Ed 19: 1363–1382, 2008. doi: 10.1163/156856208786052407. [DOI] [PubMed] [Google Scholar]
- 101.Jannatbabaei A, Tafazzoli-Shadpour M, Seyedjafari E, Fatouraee N. Cytoskeletal remodeling induced by substrate rigidity regulates rheological behaviors in endothelial cells. J Biomed Mater Res A 107: 71–80, 2019. doi: 10.1002/jbm.a.36533. [DOI] [PubMed] [Google Scholar]
- 102.Jeon J, Lee H, Park H, Lee JH, Choi S, Hwang J, Han IO, Oh ES. Phosphorylation of focal adhesion kinase at Tyrosine 407 negatively regulates Ras transformation of fibroblasts. Biochem Biophys Res Commun 364: 1062–1066, 2007. doi: 10.1016/j.bbrc.2007.10.134. [DOI] [PubMed] [Google Scholar]
- 103.Jiang FX, Yurke B, Schloss RS, Firestein BL, Langrana NA. Effect of dynamic stiffness of the substrates on neurite outgrowth by using a DNA-crosslinked hydrogel. Tissue Eng Part A 16: 1873–1889, 2010. doi: 10.1089/ten.tea.2009.0574. [DOI] [PubMed] [Google Scholar]
- 104.Jiang FX, Yurke B, Schloss RS, Firestein BL, Langrana NA. The relationship between fibroblast growth and the dynamic stiffnesses of a DNA crosslinked hydrogel. Biomaterials 31: 1199–1212, 2010. doi: 10.1016/j.biomaterials.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 105.Jorgenson AJ, Choi KM, Sicard D, Smith KM, Hiemer SE, Varelas X, Tschumperlin DJ. TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. Am J Physiol Cell Physiol 312: C277–C285, 2017. doi: 10.1152/ajpcell.00205.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ju YE, Janmey PA, McCormick ME, Sawyer ES, Flanagan LA. Enhanced neurite growth from mammalian neurons in three-dimensional salmon fibrin gels. Biomaterials 28: 2097–2108, 2007. doi: 10.1016/j.biomaterials.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Juin A, Planus E, Guillemot F, Horakova P, Albiges-Rizo C, Génot E, Rosenbaum J, Moreau V, Saltel F. Extracellular matrix rigidity controls podosome induction in microvascular endothelial cells. Biol Cell 105: 46–57, 2013. doi: 10.1111/boc.201200037. [DOI] [PubMed] [Google Scholar]
- 108.Kahn JS, Hu Y, Willner I. Stimuli-Responsive DNA-Based Hydrogels: From Basic Principles to Applications. Acc Chem Res 50: 680–690, 2017. doi: 10.1021/acs.accounts.6b00542. [DOI] [PubMed] [Google Scholar]
- 109.Kandow CE, Georges PC, Janmey PA, Beningo KA. Polyacrylamide hydrogels for cell mechanics: steps toward optimization and alternative uses. Methods Cell Biol 83: 29–46, 2007. doi: 10.1016/S0091-679X(07)83002-0. [DOI] [PubMed] [Google Scholar]
- 110.Kang JS, Krauss RS. Ras induces anchorage-independent growth by subverting multiple adhesion-regulated cell cycle events. Mol Cell Biol 16: 3370–3380, 1996. doi: 10.1128/MCB.16.7.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karim A, Hall AC. Chondrocyte Morphology in Stiff and Soft Agarose Gels and the Influence of Fetal Calf Serum. J Cell Physiol 232: 1041–1052, 2017. doi: 10.1002/jcp.25507. [DOI] [PubMed] [Google Scholar]
- 112.Karki P, Birukova AA. Substrate stiffness-dependent exacerbation of endothelial permeability and inflammation: mechanisms and potential implications in ALI and PH (2017 Grover Conference Series). Pulm Circ 8: 2045894018773044, 2018. doi: 10.1177/2045894018773044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kasza KE, Nakamura F, Hu S, Kollmannsberger P, Bonakdar N, Fabry B, Stossel TP, Wang N, Weitz DA. Filamin A is essential for active cell stiffening but not passive stiffening under external force. Biophys J 96: 4326–4335, 2009. doi: 10.1016/j.bpj.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Katzberg AA. Attraction fields between growing tissue cultures - Reply. Science 115: 295–296, 1952. [DOI] [PubMed] [Google Scholar]
- 115.Katzberg AA. Distance as a factor in the development of attraction fields between growing tissue in culture. Science 114: 431–432, 1951. doi: 10.1126/science.114.2965.431. [DOI] [PubMed] [Google Scholar]
- 116.Keese CR, Giaever I. Substrate mechanics and cell spreading. Exp Cell Res 195: 528–532, 1991. doi: 10.1016/0014-4827(91)90406-K. [DOI] [PubMed] [Google Scholar]
- 117.Kim Y, Kumar S. CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. Mol Cancer Res 12: 1416–1429, 2014. doi: 10.1158/1541-7786.MCR-13-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol 19: 1511–1518, 2009. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kocgozlu L, Rabineau M, Koenig G, Haikel Y, Schaaf P, Freund JN, Voegel JC, Lavalle P, Vautier D. The control of chromosome segregation during mitosis in epithelial cells by substrate elasticity. Biomaterials 33: 798–809, 2012. doi: 10.1016/j.biomaterials.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 120.Kohn JC, Zhou DW, Bordeleau F, Zhou AL, Mason BN, Mitchell MJ, King MR, Reinhart-King CA. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys J 108: 471–478, 2015. doi: 10.1016/j.bpj.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, Svoboda H, Viana M, Costa LD, Guck J, Holt CE, Franze K. Mechanosensing is critical for axon growth in the developing brain. Nat Neurosci 19: 1592–1598, 2016. doi: 10.1038/nn.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kostic A, Sap J, Sheetz MP. RPTPalpha is required for rigidity-dependent inhibition of extension and differentiation of hippocampal neurons. J Cell Sci 120: 3895–3904, 2007. doi: 10.1242/jcs.009852. [DOI] [PubMed] [Google Scholar]
- 123.Kothapalli D, Liu SL, Bae YH, Monslow J, Xu T, Hawthorne EA, Byfield FJ, Castagnino P, Rao S, Rader DJ, Puré E, Phillips MC, Lund-Katz S, Janmey PA, Assoian RK. Cardiovascular protection by ApoE and ApoE-HDL linked to suppression of ECM gene expression and arterial stiffening. Cell Rep 2: 1259–1271, 2012. doi: 10.1016/j.celrep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kruger TM, Bell K, Lansakara TI, Tivanski AV, Doorn JA, Stevens LL. Reduced extracellular matrix stiffness prompts SH-SY5Y cell softening and actin turnover to selectively increase Aβ(1–42) endocytosis. ACS Chem Neurosci 10: 1284–1293, 2019. doi: 10.1021/acschemneuro.8b00366. [DOI] [PubMed] [Google Scholar]
- 125.Kurzawa L, Vianay B, Senger F, Vignaud T, Blanchoin L, Théry M. Dissipation of contractile forces: the missing piece in cell mechanics. Mol Biol Cell 28: 1825–1832, 2017. doi: 10.1091/mbc.e16-09-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ladam G, Vonna L, Sackmann E. Protrusion force transmission of amoeboid cells crawling on soft biological tissue. Acta Biomater 1: 485–497, 2005. doi: 10.1016/j.actbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 127.Lam WA, Chaudhuri O, Crow A, Webster KD, Li TD, Kita A, Huang J, Fletcher DA. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater 10: 61–66, 2011. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lampi MC, Faber CJ, Huynh J, Bordeleau F, Zanotelli MR, Reinhart-King CA. Simvastatin Ameliorates Matrix Stiffness-Mediated Endothelial Monolayer Disruption. PLoS One 11: e0147033, 2016. doi: 10.1371/journal.pone.0147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lampi MC, Reinhart-King CA. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci Transl Med 10: eaao0475, 2018. doi: 10.1126/scitranslmed.aao0475. [DOI] [PubMed] [Google Scholar]
- 130.Leartprapun N, Iyer RR, Untracht GR, Mulligan JA, Adie SG. Photonic force optical coherence elastography for three-dimensional mechanical microscopy. Nat Commun 9: 2079, 2018. doi: 10.1038/s41467-018-04357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee JN, Jiang X, Ryan D, Whitesides GM. Compatibility of mammalian cells on surfaces of poly(dimethylsiloxane). Langmuir 20: 11684–11691, 2004. doi: 10.1021/la048562+. [DOI] [PubMed] [Google Scholar]
- 132.Lee MJ, Byun MR, Furutani-Seiki M, Hong JH, Jung HS. YAP and TAZ regulate skin wound healing. J Invest Dermatol 134: 518–525, 2014. doi: 10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- 133.Lee S, Hong J, Lee J. Cell motility regulation on a stepped micro pillar array device (SMPAD) with a discrete stiffness gradient. Soft Matter 12: 2325–2333, 2016. doi: 10.1039/C5SM00649J. [DOI] [PubMed] [Google Scholar]
- 134.Leifer CA, Gruber E, Stelzer S, Erlich E, Sinha S. Macrophage lipid accumulation is regulated by substrate stiffness. J Immunol 196 Suppl 1: 57.5, 2016. [Google Scholar]
- 135.Leiva O, Leon C, Kah Ng S, Mangin P, Gachet C, Ravid K. The role of extracellular matrix stiffness in megakaryocyte and platelet development and function. Am J Hematol 93: 430–441, 2018. doi: 10.1002/ajh.25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li CX, Talele NP, Boo S, Koehler A, Knee-Walden E, Balestrini JL, Speight P, Kapus A, Hinz B. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater 16: 379–389, 2017. doi: 10.1038/nmat4780. [DOI] [PubMed] [Google Scholar]
- 137.Li Z, Dranoff JA, Chan EP, Uemura M, Sévigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology 46: 1246–1256, 2007. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 138.Lin HH, Lin HK, Lin IH, Chiou YW, Chen HW, Liu CY, Harn HI, Chiu WT, Wang YK, Shen MR, Tang MJ. Mechanical phenotype of cancer cells: cell softening and loss of stiffness sensing. Oncotarget 6: 20946–20958, 2015. doi: 10.18632/oncotarget.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lin S, Lampi MC, Reinhart-King CA, Tsui G, Wang J, Nelson CA, Gu L. Eigenstrain as a mechanical set-point of cells. Biomech Model Mechanobiol 17: 951–959, 2018. doi: 10.1007/s10237-018-1004-0. [DOI] [PubMed] [Google Scholar]
- 141.Lin YC, Tambe DT, Park CY, Wasserman MR, Trepat X, Krishnan R, Lenormand G, Fredberg JJ, Butler JP. Mechanosensing of substrate thickness. Phys Rev E Stat Nonlin Soft Matter Phys 82: 041918, 2010. doi: 10.1103/PhysRevE.82.041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308: L344–L357, 2015. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J 79: 144–152, 2000. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.MacKay JL, Hammer DA. Stiff substrates enhance monocytic cell capture through E-selectin but not P-selectin. Integr Biol 8: 62–72, 2016. doi: 10.1039/C5IB00199D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Macpherson I, Montagnier L. Agar Suspension Culture for the Selective Assay of Cells Transformed by Polyoma Virus. Virology 23: 291–294, 1964. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- 146.Mandal K, Aroush DR, Graber ZT, Wu B, Park CY, Fredberg JJ, Guo W, Baumgart T, Janmey PA. Soft Hyaluronic Gels Promote Cell Spreading, Stress Fibers, Focal Adhesion, and Membrane Tension by Phosphoinositide Signaling, not Traction Force. ACS Nano 13: 203–214, 2019. doi: 10.1021/acsnano.8b05286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maroudas NG. Chemical and mechanical requirements for fibroblast adhesion. Nature 244: 353–354, 1973. doi: 10.1038/244353a0. [DOI] [PubMed] [Google Scholar]
- 148.Migliorini E, Ban J, Grenci G, Andolfi L, Pozzato A, Tormen M, Torre V, Lazzarino M. Nanomechanics controls neuronal precursors adhesion and differentiation. Biotechnol Bioeng 110: 2301–2310, 2013. doi: 10.1002/bit.24880. [DOI] [PubMed] [Google Scholar]
- 149.Mollaeian K, Liu Y, Bi S, Wang Y, Ren J, Lu M. Nonlinear Cellular Mechanical Behavior Adaptation to Substrate Mechanics Identified by Atomic Force Microscope. Int J Mol Sci 19: 3461, 2018. doi: 10.3390/ijms19113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Monzack EL, Gu X, Masters KS. Efficacy of simvastatin treatment of valvular interstitial cells varies with the extracellular environment. Arterioscler Thromb Vasc Biol 29: 246–253, 2009. doi: 10.1161/ATVBAHA.108.179218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morris BA, Burkel B, Ponik SM, Fan J, Condeelis JS, Aguirre-Ghiso JA, Castracane J, Denu JM, Keely PJ. Collagen Matrix Density Drives the Metabolic Shift in Breast Cancer Cells. EBioMedicine 13: 146–156, 2016. doi: 10.1016/j.ebiom.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moshayedi P, Costa LF, Christ A, Lacour SP, Fawcett J, Guck J, Franze K. Mechanosensitivity of astrocytes on optimized polyacrylamide gels analyzed by quantitative morphometry. J Phys Condens Matter 22: 194114, 2010. doi: 10.1088/0953-8984/22/19/194114. [DOI] [PubMed] [Google Scholar]
- 153.Mosley MC, Lim HJ, Chen J, Yang YH, Li S, Liu Y, Smith Callahan LA. Neurite extension and neuronal differentiation of human induced pluripotent stem cell derived neural stem cells on polyethylene glycol hydrogels containing a continuous Young’s Modulus gradient. J Biomed Mater Res A 105: 824–833, 2017. doi: 10.1002/jbm.a.35955. [DOI] [PubMed] [Google Scholar]
- 154.Munevar S, Wang Y, Dembo M. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys J 80: 1744–1757, 2001. doi: 10.1016/S0006-3495(01)76145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Murikipudi S, Methe H, Edelman ER. The effect of substrate modulus on the growth and function of matrix-embedded endothelial cells. Biomaterials 34: 677–684, 2013. doi: 10.1016/j.biomaterials.2012.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Murrell M, Kamm R, Matsudaira P. Substrate viscosity enhances correlation in epithelial sheet movement. Biophys J 101: 297–306, 2011. doi: 10.1016/j.bpj.2011.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Murrell MP, Voituriez R, Joanny JF, Nassoy P, Sykes C, Gardel ML. Liposome adhesion generates traction stress. Nat Phys 10: 163–169, 2014. doi: 10.1038/nphys2855. [DOI] [Google Scholar]
- 158.Nasrollahi S, Walter C, Loza AJ, Schimizzi GV, Longmore GD, Pathak A. Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory. Biomaterials 146: 146–155, 2017. doi: 10.1016/j.biomaterials.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nicolas A, Besser A, Safran SA. Is the Mechanics of Cell-Matrix Adhesion Amenable to Physical Modeling? J Adhes Sci Technol 24: 2203–2214, 2010. doi: 10.1163/016942410X507984. [DOI] [Google Scholar]
- 160.Nobis M, Herrmann D, Warren SC, Kadir S, Leung W, Killen M, Magenau A, Stevenson D, Lucas MC, Reischmann N, Vennin C, Conway JRW, Boulghourjian A, Zaratzian A, Law AM, Gallego-Ortega D, Ormandy CJ, Walters SN, Grey ST, Bailey J, Chtanova T, Quinn JMW, Baldock PA, Croucher PI, Schwarz JP, Mrowinska A, Zhang L, Herzog H, Masedunskas A, Hardeman EC, Gunning PW, Del Monte-Nieto G, Harvey RP, Samuel MS, Pajic M, McGhee EJ, Johnsson AE, Sansom OJ, Welch HCE, Morton JP, Strathdee D, Anderson KI, Timpson P. A RhoA-FRET Biosensor Mouse for Intravital Imaging in Normal Tissue Homeostasis and Disease Contexts. Cell Rep 21: 274–288, 2017. doi: 10.1016/j.celrep.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 161.Nöding H, Schön M, Reinermann C, Dörrer N, Kürschner A, Geil B, Mey I, Heussinger C, Janshoff A, Steinem C. Rheology of Membrane-Attached Minimal Actin Cortices. J Phys Chem B 122: 4537–4545, 2018. doi: 10.1021/acs.jpcb.7b11491. [DOI] [PubMed] [Google Scholar]
- 162.Noguchi S, Saito A, Mikami Y, Urushiyama H, Horie M, Matsuzaki H, Takeshima H, Makita K, Miyashita N, Mitani A, Jo T, Yamauchi Y, Terasaki Y, Nagase T. TAZ contributes to pulmonary fibrosis by activating profibrotic functions of lung fibroblasts. Sci Rep 7: 42595, 2017. doi: 10.1038/srep42595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Oakes PW, Patel DC, Morin NA, Zitterbart DP, Fabry B, Reichner JS, Tang JX. Neutrophil morphology and migration are affected by substrate elasticity. Blood 114: 1387–1395, 2009. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Okamoto T, Takagi Y, Kawamoto E, Park EJ, Usuda H, Wada K, Shimaoka M. Reduced substrate stiffness promotes M2-like macrophage activation and enhances peroxisome proliferator-activated receptor γ expression. Exp Cell Res 367: 264–273, 2018. doi: 10.1016/j.yexcr.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 166.Olsen AL, Bloomer SA, Chan EP, Gaça MDA, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol 301: G110–G118, 2011. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254, 2005. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 168.Patel NR, Bole M, Chen C, Hardin CC, Kho AT, Mih J, Deng L, Butler J, Tschumperlin D, Fredberg JJ, Krishnan R, Koziel H. Cell elasticity determines macrophage function. PLoS One 7: e41024, 2012. doi: 10.1371/journal.pone.0041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pelham RJ Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94: 13661–13665, 1997. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Petronis S, Gold J, Kasemo B. Microfabricated force-sensitive elastic substrates for investigation of mechanical cell-substrate interactions. J Micromech Microeng 13: 900–913, 2003. doi: 10.1088/0960-1317/13/6/313. [DOI] [Google Scholar]
- 171.Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol 204: 198–209, 2005. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- 172.Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 27: 4881–4893, 2006. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 173.Pogoda K, Bucki R, Byfield FJ, Cruz K, Lee T, Marcinkiewicz C, Janmey PA. Soft Substrates Containing Hyaluronan Mimic the Effects of Increased Stiffness on Morphology, Motility, and Proliferation of Glioma Cells. Biomacromolecules 18: 3040–3051, 2017. doi: 10.1021/acs.biomac.7b00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pogoda K, Chin L, Georges PC, Byfield FJ, Bucki R, Kim R, Weaver M, Wells RG, Marcinkiewicz C, Janmey PA. Compression stiffening of brain and its effect on mechanosensing by glioma cells. New J Phys 16: 075002, 2014. doi: 10.1088/1367-2630/16/7/075002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pogoda K, Janmey PA. Glial Tissue Mechanics and Mechanosensing by Glial Cells. Front Cell Neurosci 12: 25, 2018. doi: 10.3389/fncel.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Poh YC, Chowdhury F, Tanaka TS, Wang N. Embryonic stem cells do not stiffen on rigid substrates. Biophys J 99: L19–L21, 2010. doi: 10.1016/j.bpj.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pontes B, Monzo P, Gole L, Le Roux AL, Kosmalska AJ, Tam ZY, Luo W, Kan S, Viasnoff V, Roca-Cusachs P, Tucker-Kellogg L, Gauthier NC. Membrane tension controls adhesion positioning at the leading edge of cells. J Cell Biol 216: 2959–2977, 2017. doi: 10.1083/jcb.201611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Qiu Y, Brown AC, Myers DR, Sakurai Y, Mannino RG, Tran R, Ahn B, Hardy ET, Kee MF, Kumar S, Bao G, Barker TH, Lam WA. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc Natl Acad Sci USA 111: 14430–14435, 2014. doi: 10.1073/pnas.1322917111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Reimer M, Petrova Zustiak S, Sheth S, Martin Schober J. Intrinsic Response Towards Physiologic Stiffness is Cell-Type Dependent. Cell Biochem Biophys 76: 197–208, 2018. doi: 10.1007/s12013-017-0834-1. [DOI] [PubMed] [Google Scholar]
- 180.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J 95: 6044–6051, 2008. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Reiter R, Wetzel M, Hamesch K, Strnad P, Asbach P, Haas M, Siegmund B, Trautwein C, Hamm B, Klatt D, Braun J, Sack I, Tzschätzsch H. Comparison of non-invasive assessment of liver fibrosis in patients with alpha1-antitrypsin deficiency using magnetic resonance elastography (MRE), acoustic radiation force impulse (ARFI) Quantification, and 2D-shear wave elastography (2D-SWE). PLoS One 13: e0196486, 2018. doi: 10.1371/journal.pone.0196486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rens R, Vahabi M, Licup AJ, MacKintosh FC, Sharma A. Nonlinear Mechanics of Athermal Branched Biopolymer Networks. J Phys Chem B 120: 5831–5841, 2016. doi: 10.1021/acs.jpcb.6b00259. [DOI] [PubMed] [Google Scholar]
- 183.Ricca BL, Venugopalan G, Furuta S, Tanner K, Orellana WA, Reber CD, Brownfield DG, Bissell MJ, Fletcher DA. Transient external force induces phenotypic reversion of malignant epithelial structures via nitric oxide signaling. eLife 7: e26161, 2018. doi: 10.7554/eLife.26161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rosenbluth MJ, Lam WA, Fletcher DA. Force microscopy of nonadherent cells: a comparison of leukemia cell deformability. Biophys J 90: 2994–3003, 2006. doi: 10.1529/biophysj.105.067496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sackmann E, Bruinsma RF. Cell adhesion as wetting transition? ChemPhysChem 3: 262–269, 2002. doi:. [DOI] [PubMed] [Google Scholar]
- 186.Sadtler K, Wolf MT, Ganguly S, Moad CA, Chung L, Majumdar S, Housseau F, Pardoll DM, Elisseeff JH. Divergent immune responses to synthetic and biological scaffolds. Biomaterials 192: 405–415, 2019. doi: 10.1016/j.biomaterials.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 187.Sander EA, Barocas VH, Tranquillo RT. Initial fiber alignment pattern alters extracellular matrix synthesis in fibroblast-populated fibrin gel cruciforms and correlates with predicted tension. Ann Biomed Eng 39: 714–729, 2011. doi: 10.1007/s10439-010-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol 3: a003228, 2011. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schuh E, Hofmann S, Stok KS, Notbohm H, Müller R, Rotter N. The influence of matrix elasticity on chondrocyte behavior in 3D. J Tissue Eng Regen Med 6: e31–e42, 2012. doi: 10.1002/term.501. [DOI] [PubMed] [Google Scholar]
- 190.Schwarz US. Mechanobiology by the numbers: a close relationship between biology and physics. Nat Rev Mol Cell Biol 18: 711–712, 2017. doi: 10.1038/nrm.2017.109. [DOI] [PubMed] [Google Scholar]
- 191.Scott HA, Quach B, Yang X, Ardekani S, Cabrera AP, Wilson R, Messaoudi-Powers I, Ghosh K. Matrix stiffness exerts biphasic control over monocyte-endothelial adhesion via Rho-mediated ICAM-1 clustering. Integr Biol 8: 869–878, 2016. doi: 10.1039/C6IB00084C. [DOI] [PubMed] [Google Scholar]
- 192.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation - a mini-review. Gerontology 58: 227–237, 2012. doi: 10.1159/000334668. [DOI] [PubMed] [Google Scholar]
- 193.Seo E, Kim WY, Hur J, Kim H, Nam SA, Choi A, Kim YM, Park SH, Chung C, Kim J, Min S, Myung SJ, Lim DS, Kim YK. The Hippo-Salvador signaling pathway regulates renal tubulointerstitial fibrosis. Sci Rep 6: 31931, 2016. doi: 10.1038/srep31931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shebanova O, Hammer DA. Biochemical and mechanical extracellular matrix properties dictate mammary epithelial cell motility and assembly. Biotechnol J 7: 397–408, 2012. doi: 10.1002/biot.201100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shi Z, Graber ZT, Baumgart T, Stone HA, Cohen AE. Cell Membranes Resist Flow. Cell 175: 1769–1779.e13, 2018. doi: 10.1016/j.cell.2018.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shin JW, Mooney DJ. Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proc Natl Acad Sci USA 113: 12126–12131, 2016. doi: 10.1073/pnas.1611338113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shin SI, Freedman VH, Risser R, Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci USA 72: 4435–4439, 1975. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, Kamath PS, Talwalkar JA. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 11: 1573–84.e2, 2013. doi: 10.1016/j.cgh.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Smith ML, Smith MJ, Lawrence MB, Ley K. Viscosity-independent velocity of neutrophils rolling on p-selectin in vitro or in vivo. Microcirculation 9: 523–536, 2002. doi: 10.1080/713774098. [DOI] [PubMed] [Google Scholar]
- 200.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J 93: 4453–4461, 2007. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Southern BD, Grove LM, Rahaman SO, Abraham S, Scheraga RG, Niese KA, Sun H, Herzog EL, Liu F, Tschumperlin DJ, Egelhoff TT, Rosenfeld SS, Olman MA. Matrix-driven Myosin II Mediates the Pro-fibrotic Fibroblast Phenotype. J Biol Chem 291: 6083–6095, 2016. doi: 10.1074/jbc.M115.712380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Stroka KM, Aranda-Espinoza H. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood 118: 1632–1640, 2011. doi: 10.1182/blood-2010-11-321125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Syed S, Schober J, Blanco A, Zustiak SP. Morphological adaptations in breast cancer cells as a function of prolonged passaging on compliant substrates. PLoS One 12: e0187853, 2017. doi: 10.1371/journal.pone.0187853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci USA 100: 1484–1489, 2003. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tang X, Kuhlenschmidt TB, Zhou J, Bell P, Wang F, Kuhlenschmidt MS, Saif TA. Mechanical force affects expression of an in vitro metastasis-like phenotype in HCT-8 cells. Biophys J 99: 2460–2469, 2010. doi: 10.1016/j.bpj.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tao J, Sun SX. Active Biochemical Regulation of Cell Volume and a Simple Model of Cell Tension Response. Biophys J 109: 1541–1550, 2015. doi: 10.1016/j.bpj.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tee SY, Fu J, Chen CS, Janmey PA. Cell shape and substrate rigidity both regulate cell stiffness. Biophys J 100: L25–L27, 2011. doi: 10.1016/j.bpj.2010.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tilghman RW, Cowan CR, Mih JD, Koryakina Y, Gioeli D, Slack-Davis JK, Blackman BR, Tschumperlin DJ, Parsons JT. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS One 5: e12905, 2010. doi: 10.1371/journal.pone.0012905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tissot O, Pierres A, Foa C, Delaage M, Bongrand P. Motion of cells sedimenting on a solid surface in a laminar shear flow. Biophys J 61: 204–215, 1992. doi: 10.1016/S0006-3495(92)81827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tzvetkova-Chevolleau T, Stéphanou A, Fuard D, Ohayon J, Schiavone P, Tracqui P. The motility of normal and cancer cells in response to the combined influence of the substrate rigidity and anisotropic microstructure. Biomaterials 29: 1541–1551, 2008. doi: 10.1016/j.biomaterials.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 211.Ulrich TA, de Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res 69: 4167–4174, 2009. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ulrich TA, Jain A, Tanner K, MacKay JL, Kumar S. Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials 31: 1875–1884, 2010. doi: 10.1016/j.biomaterials.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 213.Urbano RL, Furia C, Basehore S, Clyne AM. Stiff Substrates Increase Inflammation-Induced Endothelial Monolayer Tension and Permeability. Biophys J 113: 645–655, 2017. doi: 10.1016/j.bpj.2017.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Van Helvert S, Storm C, Friedl P. Mechanoreciprocity in cell migration. Nat Cell Biol 20: 8–20, 2018. doi: 10.1038/s41556-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vichare S, Sen S, Inamdar MM. Cellular mechanoadaptation to substrate mechanical properties: contributions of substrate stiffness and thickness to cell stiffness measurements using AFM. Soft Matter 10: 1174–1181, 2014. doi: 10.1039/c3sm51786a. [DOI] [PubMed] [Google Scholar]
- 216.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 18: 728–742, 2017. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS One 7: e39969, 2012. doi: 10.1371/journal.pone.0039969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol 279: C1345–C1350, 2000. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 220.Wang JH, Jia F, Gilbert TW, Woo SL. Cell orientation determines the alignment of cell-produced collagenous matrix. J Biomech 36: 97–102, 2003. doi: 10.1016/S0021-9290(02)00233-6. [DOI] [PubMed] [Google Scholar]
- 221.Wang M, Chai N, Sha B, Guo M, Zhuang J, Xu F, Li F. The effect of substrate stiffness on cancer cell volume homeostasis. J Cell Physiol 233: 1414–1423, 2018. doi: 10.1002/jcp.26026. [DOI] [PubMed] [Google Scholar]
- 222.Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J 66: 2181–2189, 1994. doi: 10.1016/S0006-3495(94)81014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang S, Nie D, Rubin JP, Kokai L. Lymphatic Endothelial Cells under Mechanical Stress: Altered Expression of Inflammatory Cytokines and Fibrosis. Lymphat Res Biol 15: 130–135, 2017. doi: 10.1089/lrb.2016.0042. [DOI] [PubMed] [Google Scholar]
- 224.Wang W, Lollis EM, Bordeleau F, Reinhart-King CA. Matrix stiffness regulates vascular integrity through focal adhesion kinase activity. FASEB J 33: 1199–1208, 2019. doi: 10.1096/fj.201800841R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang X, Zheng Z, Caviglia JM, Corey KE, Herfel TM, Cai B, Masia R, Chung RT, Lefkowitch JH, Schwabe RF, Tabas I. Hepatocyte TAZ/WWTR1 Promotes Inflammation and Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab 24: 848–862, 2016. doi: 10.1016/j.cmet.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Webster KD, Ng WP, Fletcher DA. Tensional homeostasis in single fibroblasts. Biophys J 107: 146–155, 2014. doi: 10.1016/j.bpj.2014.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Weigel PH, Schmell E, Lee YC, Roseman S. Specific adhesion of rat hepatocytes to beta-galactosides linked to polyacrylamide gels. J Biol Chem 253: 330–333, 1978. [PubMed] [Google Scholar]
- 228.Weigel PH, Schnaar RL, Roseman S, Lee YC. Preparation of polyacrylamide gels containing copolymerized omega-acrylamidoalkyl glycosides. Methods Enzymol 83: 294–299, 1982. doi: 10.1016/0076-6879(82)83023-1. [DOI] [PubMed] [Google Scholar]
- 229.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 62: 1105–1110, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Weiss P, Katzberg AA. Attraction fields between growing tissue cultures. Science 115: 293–296, 1952. doi: 10.1126/science.115.2985.293. [DOI] [PubMed] [Google Scholar]
- 231.Weiss P. Cellular dynamics. Rev Mod Phys 31: 11–20, 1959. doi: 10.1103/RevModPhys.31.11. [DOI] [Google Scholar]
- 232.Willits RK, Skornia SL. Effect of collagen gel stiffness on neurite extension. J Biomater Sci Polym Ed 15: 1521–1531, 2004. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- 233.Winer JP, Oake S, Janmey PA. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLoS One 4: e6382, 2009. doi: 10.1371/journal.pone.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 105: 18782–18787, 2008. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 35: 427–433, 2010. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wolfenson H, Meacci G, Liu S, Stachowiak MR, Iskratsch T, Ghassemi S, Roca-Cusachs P, O’Shaughnessy B, Hone J, Sheetz MP. Tropomyosin controls sarcomere-like contractions for rigidity sensing and suppressing growth on soft matrices. Nat Cell Biol 18: 33–42, 2016. doi: 10.1038/ncb3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, Ulrich P, Cerami A, Lévy BI. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci USA 95: 4630–4634, 1998. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wong S, Guo WH, Wang YL. Fibroblasts probe substrate rigidity with filopodia extensions before occupying an area. Proc Natl Acad Sci USA 111: 17176–17181, 2014. doi: 10.1073/pnas.1412285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wu P-H, Aroush DR-B, Asnacios A, Chen W-C, Dokukin ME, Doss BL, Durand-Smet P, Ekpenyong A, Guck J, Guz NV, Janmey PA, Lee JSH, Moore NM, Ott A, Poh Y-C, Ros R, Sander M, Sokolov I, Staunton JR, Wang N, Whyte G, Wirtz D. A comparison of methods to assess cell mechanical properties. Nat Methods 15: 491–498, 2018. doi: 10.1038/s41592-018-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wullkopf L, West AV, Leijnse N, Cox TR, Madsen CD, Oddershede LB, Erler JT. Cancer cells’ ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol Biol Cell 29: 2378–2385, 2018. doi: 10.1091/mbc.E18-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xie K, Yang Y, Jiang H. Controlling Cellular Volume via Mechanical and Physical Properties of Substrate. Biophys J 114: 675–687, 2018. doi: 10.1016/j.bpj.2017.11.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. eLife 4: e07455, 2015. doi: 10.7554/eLife.07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yang C, DelRio FW, Ma H, Killaars AR, Basta LP, Kyburz KA, Anseth KS. Spatially patterned matrix elasticity directs stem cell fate. Proc Natl Acad Sci USA 113: E4439–E4445, 2016. doi: 10.1073/pnas.1609731113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yang X, Scott HA, Monickaraj F, Xu J, Ardekani S, Nitta CF, Cabrera A, McGuire PG, Mohideen U, Das A, Ghosh K. Basement membrane stiffening promotes retinal endothelial activation associated with diabetes. FASEB J 30: 601–611, 2016. doi: 10.1096/fj.15-277962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60: 24–34, 2005. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 246.Yusko EC, Asbury CL. Force is a signal that cells cannot ignore. Mol Biol Cell 25: 3717–3725, 2014. doi: 10.1091/mbc.e13-12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zanotelli MR, Goldblatt ZE, Miller JP, Bordeleau F, Li J, VanderBurgh JA, Lampi MC, King MR, Reinhart-King CA. Regulation of ATP utilization during metastatic cell migration by collagen architecture. Mol Biol Cell 29: 1–9, 2018. doi: 10.1091/mbc.E17-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zarrintaj P, Manouchehri S, Ahmadi Z, Saeb MR, Urbanska AM, Kaplan DL, Mozafari M. Agarose-based biomaterials for tissue engineering. Carbohydr Polym 187: 66–84, 2018. doi: 10.1016/j.carbpol.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 249.Zhang J, Hochwald SN. The role of FAK in tumor metabolism and therapy. Pharmacol Ther 142: 154–163, 2014. doi: 10.1016/j.pharmthera.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhong A, Mirzaei Z, Simmons CA. The Roles of Matrix Stiffness and β-Catenin Signaling in Endothelial-to-Mesenchymal Transition of Aortic Valve Endothelial Cells. Cardiovasc Eng Technol 9: 158–167, 2018. doi: 10.1007/s13239-018-0363-0. [DOI] [PubMed] [Google Scholar]
- 251.Zieman SJ, Melenovsky V, Clattenburg L, Corretti MC, Capriotti A, Gerstenblith G, Kass DA. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens 25: 577–583, 2007. doi: 10.1097/HJH.0b013e328013e7dd. [DOI] [PubMed] [Google Scholar]