Abstract
Progressive muscle injury and weakness are hallmarks of Duchenne muscular dystrophy. We showed previously that quercetin (Q) partially protected dystrophic limb muscles from disease-related injury. As quercetin activates PGC-1α through Sirtuin-1, an NAD+-dependent deacetylase, the depleted NAD+ in dystrophic skeletal muscle may limit quercetin efficacy; hence, supplementation with the NAD+ donor, nicotinamide riboside (NR), may facilitate quercetin efficacy. Lisinopril (Lis) protects skeletal muscle and improves cardiac function in dystrophin-deficient mice; therefore, it was included in this study to evaluate the effects of lisinopril used with quercetin and NR. Our purpose was to determine the extent to which Q, NR, and Lis decreased dystrophic injury. We hypothesized that Q, NR, or Lis alone would improve muscle function and decrease histological injury and when used in combination would have additive effects. Muscle function of 11-mo-old DBA (healthy), D2-mdx (dystrophin-deficient), and D2-mdx mice was assessed after treatment with Q, NR, and/or Lis for 7 mo. To mimic typical pharmacology of patients with Duchenne muscular dystrophy, a group was treated with prednisolone (Pred) in combination with Q, NR, and Lis. At 11 mo of age, dystrophin deficiency decreased specific tension and tetanic force in the soleus and extensor digitorum longus muscles and was not corrected by any treatment. Dystrophic muscle was more sensitive to contraction-induced injury, which was partially offset in the QNRLisPred group, whereas fatigue was similar between all groups. Treatments did not decrease histological damage. These data suggest that treatment with Q, NR, Lis, and Pred failed to adequately maintain dystrophic limb muscle function or decrease histological damage.
NEW & NOTEWORTHY Despite a compelling rationale and previous evidence to the contrary in short-term investigations, quercetin, nicotinamide riboside, or Lisinopril, alone or in combination, failed to restore muscle function or decrease histological injury in dystrophic limb muscle from D2-mdx mice after long-term administration. Importantly, we also found that in the D2-mdx model, an emerging and relatively understudied model of Duchenne muscular dystrophy dystrophin deficiency caused profound muscle dysfunction and histopathology in skeletal muscle.
Keywords: ACE inhibitor, DMD, glucocorticoid, prednisolone, quercetin
INTRODUCTION
Duchenne muscular dystrophy (DMD) is a progressive muscle-wasting disease caused by a mutation in the dystrophin gene, resulting in dystrophin protein deficiency. By the age of 12, children with DMD are usually nonambulatory and will typically succumb to the disease in their third decade of life due to respiratory or cardiac failure (13). In addition to muscle fragility, dystrophin deficiency results in a host of cellular dysfunctions including, but not limited to, impaired autophagy, calcium mishandling, oxidative stress, and mitochondrial dysfunction (4, 20, 29). Collectively, this structural injury and cellular dysfunction results in histopathologic changes, including immune cell infiltration, fibrosis, increased necrotic area, increased fatty infiltration, and a reduction in contractile tissue (5, 17).
Transgenic or vector-mediated overexpression of PGC-1α reduced histological damage and preserved muscle function in dystrophic skeletal muscle (9, 16, 17, 27). We have pursued the capacity of quercetin, a natural flavonoid with antioxidant and anti-inflammatory properties (1, 6, 11), to attenuate disease severity as it drives PGC-1α activity through Sirtuin 1 (SIRT1), an NAD+-dependent deacetylase (12, 22). We discovered previously that 6 mo of oral quercetin delivery decreased histopathological injury in dystrophic diaphragms and hearts of mdx mice (3, 18); however, long-term administration of quercetin transiently maintained respiratory function and modestly protected limb muscles, while in corresponding hearts indices of inflammation were reduced and markers of mitochondrial biogenesis were increased (2, 26, 28).
Because quercetin relies on SIRT1 activity to drive signaling, we proposed that limited NAD+ in dystrophic muscle (8) may hinder the efficacy of quercetin (28) and that supplementation with nicotinamide riboside (NR), an NAD+ precursor, would maintain quercetin-mediated elevations in SIRT1 activity, PGC-1α activity, and muscle function. Indeed, it was recently demonstrated that muscle function was improved after 12 wk of NR supplementation (25). Independent of quercetin, early intervention with angiotensin-converting enzyme inhibitor lisinopril (Lis) improved histological injury (21) and, in combination with aldosterone receptor antagonist spironolactone, improved function and reduced histological damage of dystrophic cardiac and skeletal muscle (19, 23). Conversely, it appears that activation of aldosterone receptors on muscle fibers can potentiate muscle injury (7). Quercetin and lisinopril appear to operate through independent mechanisms, which may provide an opportunity for additive or even synergistic therapeutic benefits. The goal of this investigation was to determine the extent to which NR and/or Lis would augment therapeutic effects of quercetin on disease severity in dystrophic limb muscles. We hypothesized that the oral administration of quercetin in combination with NR and/or Lis would improve muscle function and decrease histological injury in dystrophic muscle compared with untreated dystrophic muscle.
METHODS
Animal treatments.
All animal procedures were approved by the Institutional Animal Care and Use Committees at the University of Montana (047-16JQHHP-072616) and the University of Florida (201508822). In this investigation we utilized an emerging model of DMD, the D2-mdx mouse (D2.B10-Dmdmdx/J), which was produced by crossing the traditional BL10-mdx mouse onto the DBA background such that dystrophin deficiency is caused by the identical nonsense mutation in exon 23 of the dystrophin gene. The DBA background has a 12-amino acid deletion in the latent TGF-β-binding protein gene 4 (Ltbp4). Similar mutations have been linked to accelerated losses of ambulation in DMD patients (10, 14). Additionally, the DBA background has a mutation in the Anxa6 gene that leads to the truncation of annexin A6 protein, which is involved in satellite cell self-renewal and membrane repair (30). The DBA background provides a more severe phenotype, resulting in increased fibrosis, increased muscle damage, and decreased regenerative capacity in the D2-mdx mouse (10, 15, 24). We chose this model precisely because disease severity more closely recapitulates disease severity in DMD patients than the conventional mdx model, with the hope that results using D2-mdx mice would be more predictive of outcomes (positive or negative) in DMD patients. Ten male DBA2/J (DBA; control) mice and 80 male D2.B10-Dmdmdx/J (D2-mdx; dystrophin deficient) mice were obtained from Jackson Laboratory (Bar Harbor, ME). After an acclimation period of at least 1 wk, 4-mo-old mice were assigned to 9 groups (n = 10/group): DBA, untreated D2-mdx, and D2-mdx treated with quercetin (Q; 0.2% by diet, 275 mg·kg−1·day−1), nicotinamide riboside (NR; 0.04% by diet, 55 mg·kg−1·day−1), Q and NR (QNR), lisinopril (Lis; 33 mg/L in water, 9.15 mg·kg−1·day−1), Q and Lis (QLis), QNRLis, or QNRLis + prednisolone (QNRLisPred; 10 mg/L by water, 2.77 mg·kg−1·day−1). The QNRLisPred group was included to test the most complete cocktail (QNRLis) in the context of common pharmacology used to treat DMD patients. Treatments were initiated at 4 mo of age to demonstrate the efficacy of these treatments using a rescue paradigm, which more closely replicates the onset of treatment for DMD patients. All animals were given access to food and water ad libitum regardless of treatment. Caregivers and technicians were blinded to animal treatment, and treatment groups were assigned numbers to ensure blinded conditions throughout data collection. After 7 mo of treatment, soleus and extensor digitorum longus (EDL) were collected.
Muscle function.
In vitro muscle function was assessed in the soleus and EDL at the University of Florida Myology Institute Physiological Assessment Core as previously described (28, 31). Briefly, soleus and EDL muscles were removed after surgical sedation with ketamine and xylazine and placed in a bath of oxygenated Ringers solution (120 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM HEPES, and 5.5 mM glucose). Isometric tetanic force was measured at 120 Hz and 100 Hz for EDL and soleus, respectively. To determine fatigue resistance, the soleus was stimulated with 1 contraction/sec at 100 Hz for 330 ms, with 200-μs pulses for 10 min. Fatigue resistance was reported as percentage of remaining initial force. Finally, to assess resistance to contraction-induced injury, five lengthening contractions of 500-ms duration at 80 Hz followed by a final 10% stretch beyond optimal length for 200 ms were applied to EDL muscles. Resistance to contraction-induced injury was reported as percentage of initial force production.
Histology.
Muscle damage and fibrotic area were assessed using hematoxylin and eosin (H&E) and Masson’s Trichrome (cat. no. KTMTRPT; American MasterTech, Lodi, CA) staining as previously described (26, 28). Soleus and EDL muscles were coated in Optimal Cutting Temperature media and frozen for histology in liquid nitrogen-chilled isopentane. Briefly, 10-μm sections were stained with H&E or Masson’s Trichrome and imaged at ×10 and ×40 magnification by a blinded, trained technician on an inverted Lecia DMI3000 B microscope and QICAM MicroPublisher 5.0 (model no. MP5.0-RTV-CLR-10; QIMAGING) camera with QCapture software (Surrey, BC, Canada). Contractile area was quantified using the H&E images by alternative density slicing for the hypercontracted cells and necrotic areas, then subtracting that sum from the total area using Open Laboratory Software (Improvision). The density slice function uses prescribed pixel parameters to select the dark-red, hypercontracted cells and the light-pink necrotic areas. Similarly, fibrotic area was quantified in the entire muscle cross section by using the density slice function of the Open Laboratory Software to quantify the blue area of the trichrome images, which is divided by the total area to identify the percentage of the total area that was fibrotic.
Fiber area distribution was assessed using immunohistochemistry. Sections were incubated with antilaminin (anti-rabbit; cat. no. RB-082-A; Thermo Scientific) using our standard immunohistochemistry protocol as previously described (29). Briefly, after being washed [phosphate buffered saline (PBS)] and blocked (5% goat serum, 5% bovine serum albumin, 1% DMSO in 2× PBS), slides were incubated in primary antibody (laminin, 1:100 in blocking solution) overnight at 4°C, then washed and incubated in secondary antibody (anti-rabbit Alexa Fluor 488, 1:500; cat. no. 4412S; Cell Signaling Technology) for 1 h at room temperature. Slide covers were mounted with SlowFade Gold Antifade Mountant with DAPI (cat. no. S36938; Thermo Fisher Scientific) and sealed with nail polish. Slides were imaged at ×10 magnification on the same inverted microscope. For each sample, two independent images were taken and quantified (100–400 fibers/image). Fiber areas were objectively measured using OpenLab Software.
Statistics.
To determine significant sources of variation in single time point measures, such as tetanic force, specific force, fiber area distribution, contractile area, and fibrotic area, one-way ANOVAs were performed using GraphPad Prism with a Newman-Keuls post hoc test. Data from soleus fatigue experiments were analyzed utilizing a repeated measure model in which a spatial power variance structure was used. EDL contraction-induced injury data were analyzed using a repeated measures model that implemented an autoregressive variance structure. A mixed model method was used to determine significant sources of variation (Proc Mixed, SAS V9.0, SAS Inst., Cary, NC) that included fixed effects for treatment, time, and treatment × time interaction with pretreatment value (20 min baseline measurement at 4 mo of age) as a linear covariate. When fixed effects were significant sources of variation, the PDIFF statement of SAS was used at the fixed-effect level of least squares means to separate pairwise differences.
RESULTS
Animal characteristics.
Mouse body mass was decreased by 13–38% in untreated and treated D2-mdx mice compared with healthy (DBA) mice (P < 0.05) (Table 1). Additionally, the QNRLisPred group was decreased 38% and 28% compared with DBA and D2-mdx (P < 0.05), respectively, and was similar to the remaining treatment groups. Soleus, EDL, gastrocnemii, and tibialis anterior (TA) muscles were weighed. EDL, gastrocnemius, and TA masses were decreased 29–47%, 58–71%, and 42–56%, respectively, in untreated and treated D2-mdx mice compared with muscles from DBA mice (P < 0.05) (Table 1). Interestingly, the soleus was similar between all groups with the exception of D2-mdx mice treated with QNRLisPred, which was decreased ~13% compared with all groups (P < 0.05). Similarly, the EDLs and gastrocnemius muscles from QNRLisPred were significantly decreased compared with untreated D2-mdx (P < 0.05). Lastly, EDLs from Q-treated D2-mdx mice were 7% greater than untreated D2-mdx (P < 0.05). When tissue masses were normalized to body mass, gastrocnemius and TA masses were decreased in all D2-mdx groups compared with DBA by 45–53% and 22–35%, respectively (P < 0.05) (Table 1). Similarly, D2-mdx EDL relative mass was decreased by 26% compared with DBA (P < 0.05), and treatment with Q or QNRLis increased muscle mass by 23–29% compared with EDLs from untreated D2-mdx mice (P < 0.05). Finally, soleus relative mass was increased by 16–32% in treated and untreated D2-mdx mice compared with DBA (P < 0.05) and were similar to each other.
Table 1.
Animal and muscle masses
| DBA | D2-mdx | Q | NR | QNR | Lis | QLis | QNRLis | QNRLisPred | |
|---|---|---|---|---|---|---|---|---|---|
| Body mass, g | 39.53 ± 1.81 | 34.36 ± 1.13* | 28.36 ± 1.25*# | 29.63 ± 1.14*† | 31.18 ± 1.49*† | 32.28 ± 1.71*† | 31.38 ± 0.76*† | 28.92 ± 0.84* | 24.57 ± 0.98*# |
| Soleus, mg | 7.5 ± 0.2 | 7.6 ± 0.2 | 7.1 ± 0.3† | 6.9 ± 0.3† | 7.1 ± 0.2† | 7.2 ± 0.2† | 7.5 ± 0.2† | 6.9 ± 0.2† | 6.0 ± 0.2*# |
| Rel Soleus, mg/g | 0.19 ± 0.01 | 0.22 ± 0.01* | 0.25 ± 0.01* | 0.24 ± 0.01* | 0.23 ± 0.01* | 0.23 ± 0.01* | 0.24 ± 0.01* | 0.24 ± 0.01* | 0.25 ± 0.01* |
| EDL, mg | 8.9 ± 0.1 | 5.9 ± 0.1* | 6.3 ± 0.2*#† | 5.7 ± 0.2† | 5.9 ± 0.1† | 6.0 ± 0.2† | 5.9 ± 0.1† | 5.9 ± 0.2† | 4.7 ± 0.1*# |
| Rel EDL, mg/g | 0.23 ± 0.01 | 0.17 ± 0.00* | 0.22 ± 0.01# | 0.19 ± 0.01* | 0.19 ± 0.01* | 0.19 ± 0.01* | 0.20 ± 0.00* | 0.21 ± 0.01# | 0.20 ± 0.01* |
| Gast, mg | 123.30 ± 2.2 | 51.30 ± 1.6* | 49.43 ± 2.14*† | 47.93 ± 1.49*† | 46.99 ± 1.36*† | 48.26 ± 1.12*† | 48.85 ± 1.58*† | 46.03 ± 2.03*† | 36.29 ± 1.09*# |
| Rel gast, mg/g | 3.17 ± 0.09 | 1.50 ± 0.05* | 1.74 ± 0.06* | 1.63 ± 0.06* | 1.52 ± 0.06* | 1.52 ± 0.07* | 1.56 ± 0.05* | 1.60 ± 0.08* | 1.49 ± 0.05* |
| TA, mg | 50.00 ± 3.5 | 28.40 ± 0.9* | 28.24 ± 0.73* | 25.83 ± 0.75* | 26.73 ± 0.86* | 27.33 ± 0.82* | 28.87 ± 0.74*† | 28.03 ± 0.71* | 22.01 ± 0.95*# |
| Rel TA, mg/g | 1.28 ± 0.08 | 0.83 ± 0.03* | 1.00 ± 0.03* | 0.88 ± 0.04* | 0.87 ± 0.03* | 0.85 ± 0.03* | 0.92 ± 0.02* | 0.97 ± 0.03* | 0.91 ± 0.05* |
Masses and relative (rel) masses are represented as means ± SE; n = no. of mice. Body mass was decreased in dystrophic (D2-mdx) compared with healthy (DBA) mice, and treatment with QNRLis + prednisolone (QNRLisPred) further decreased body mass. Absolute muscle masses were reduced in dystrophic mice compared with healthy mice, except the soleus was similar between groups. Treatment with QNRLisPred further decreased absolute muscle masses. Similarly, relative muscle masses were decreased in dystrophic mice, regardless of tissue or treatment, DBA, n = 10; D2-mdx, n = 8; quercetin (Q), n = 9; nicotinamide riboside (NR), n = 8; QNR, n = 8; lisinopril (Lis), n = 7; QLis, n = 10; QNRLis, n = 8; and QNRLisPred, n = 9. EDL, extensor digitorum longus; Gast, gastrocnemius; TA, tibialis anterior.
Significantly different from DBA;
significantly different from D2-mdx; and
significantly different from QNRLisPred, P < 0.05.
In vitro muscle function.
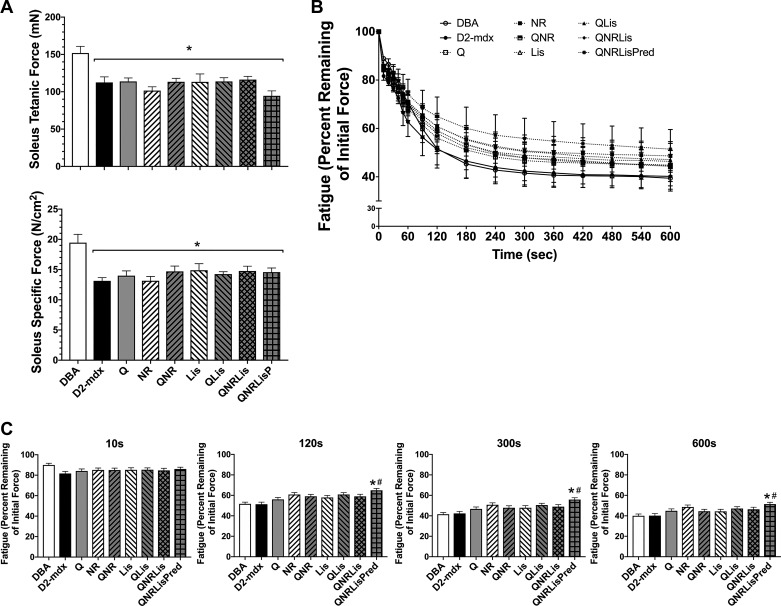
Muscle function was assessed in the soleus and EDL after 7 mo of treatment (Figs. 1 and 2). Soleus tetanic force and specific tension were decreased by 32% in D2-mdx compared with DBA (P < 0.05) with no effect of treatments (Fig. 1A). After 10 s of a fatigue protocol, force production was similar between groups; however, after 120 s, QNRLisPred produced 25% greater force than DBA (P < 0.05) mice whereas DBA and D2-mdx were similar. Additionally, QNRLisPred produced 28% more force than D2-mdx and DBA after 300 s of a fatigue protocol that was maintained through 600 s of a 1 contraction/sec fatigue protocol (P < 0.05; Fig. 1C).
Fig. 1.
Soleus muscle function. A: tetanic force and specific force were decreased in D2-mdx mice, regardless of treatment, compared with DBA. B: resistance to fatigue was measured over 10 min. C: despite similar fatigue resistance between DBA and D2-mdx at these time points, QNRLis + prednisolone (QNRLisPred) increased fatigue resistance at 120, 300, and 600 s compared with D2-mdx. DBA, n = 10 mice; D2-mdx, n = 7–8 mice; quercetin (Q), n = 8 mice; nicotinamide riboside (NR), n = 8 mice; QNR, n = 8 mice; lisinopril (Lis), n = 7 mice; QLis, n = 9–10 mice; QNRLis, n = 7 mice; and QNRLisPred, n = 9 mice. Statistical significance was established at P < 0.05; *significantly different from DBA; #significantly different from D2-mdx.
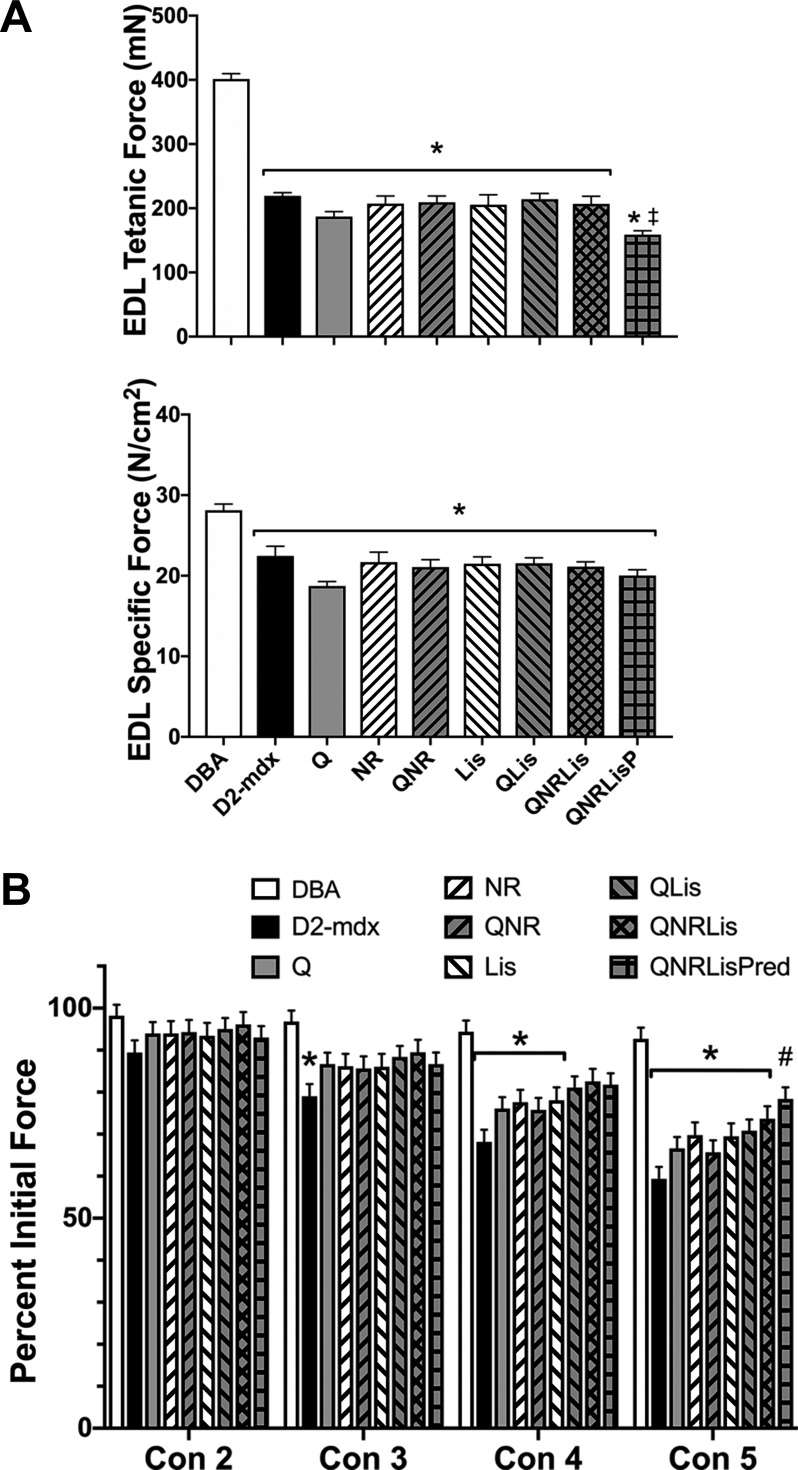
Fig. 2.
Extensor digitorum longus muscle (EDL) function. A: tetanic force and specific force were decreased in D2-mdx mice, regardless of treatment, compared with DBA. QNRLis + prednisolone (QNRLisPred) further decreased tetanic force compared with all groups but had specific tension similar to D2-mdx groups. B: resistance to contraction-induced injury was decreased in D2-mdx mice. Treatment with QNRLisPred improved resistance to contraction-induced injury after contraction 5. DBA, n = 9 mice; D2-mdx, n = 7–8 mice; quercetin (Q), n = 8–9 mice; nicotinamide riboside (NR), n = 8 mice; QNR, n = 8 mice; lisinopril (Lis), n = 7 mice; QLis, n = 9–10 mice; QNRLis, n = 7–8 mice; and QNRLisPred, n = 9 mice. Statistical significance was established at P < 0.05; *significantly different from DBA; #significantly different from D2-mdx; ‡significantly different from all other D2-mdx groups.
In the EDL, tetanic and specific tension were significantly decreased by 50% and 20%, respectively, in D2-mdx groups compared with DBA (Fig. 2, A and B; P < 0.05). Additionally, EDL tetanic tension was further decreased by 25% in QNRLisPred-treated mice compared with remaining D2-mdx groups (P < 0.05). Resistance to contraction-induced injury was assessed by measuring peak force during five eccentric contractions, in which the muscles were stretched 10% past optimal length. After contractions 3, 4, and 5, D2-mdx produced 18%, 27%, and 36% less force compared with DBA (P < 0.05), respectively. After contraction 4, Q, NR, QNR, and Lis produced 17–19% less force than DBA (P < 0.05). Similarly, after contraction 5, Q-, NR-, Lis-, QLis-, and QNRLis-treated groups produced 20–29% less force than DBA (P < 0.05). We noted that Q-, NR-, Lis-, QLis-, and QNRLis-treated mice appeared to produce greater force than untreated D2-mdx mice particularly after contractions 3–5; however, these numerical changes did not reach statistical significance. QNRLisPred-treated mice produced 32% more force during the fifth contraction than D2-mdx did and were statistically similar to DBA, suggesting protection from contraction-induced injury (Fig. 2B).
Histology.
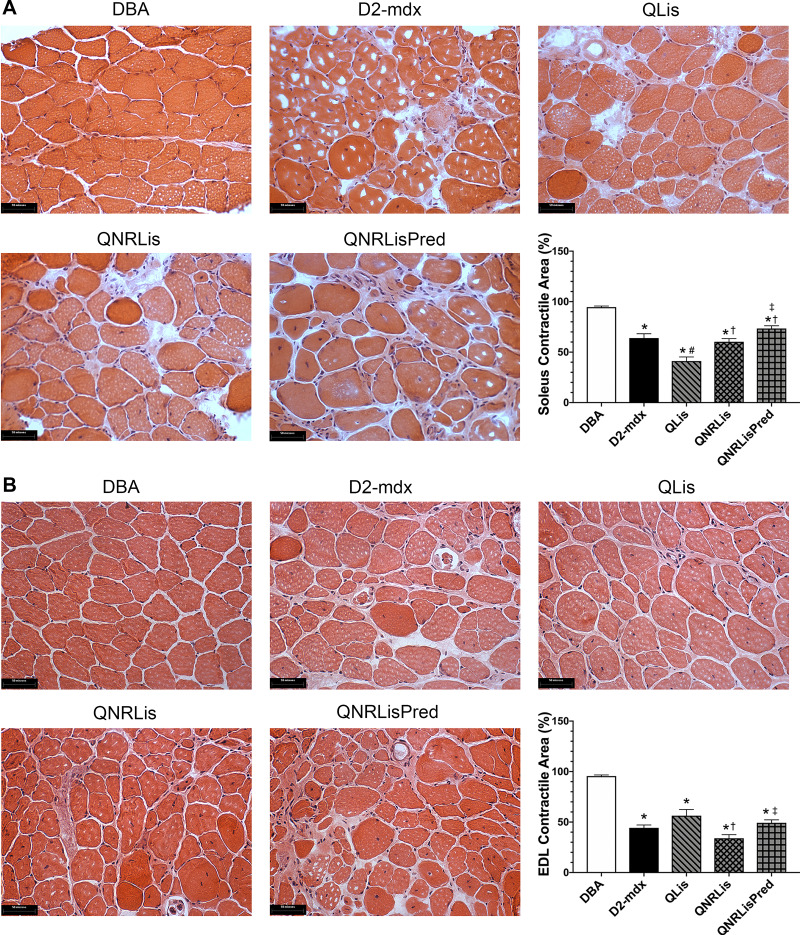
Given that muscle function was largely similar between untreated and treated D2-mdx mice, we performed histopathological examination on groups that showed therapeutic promise (numerical changes suggestive of therapeutic potential) (Fig. 3). Hence, we assessed histological muscle damage in the DBA, D2-mdx, QLis, QNRLis, and QNRLisPred groups. Soleus contractile area decreased from 95% in DBA mice to ~65–73% in D2-mdx, QNRLis, and QNRLisPred (P < 0.05). Surprisingly, contractile area was only 41% in QLis. In the EDL, contractile area was decreased by 41–64% in D2-mdx groups compared with DBA mice (Fig. 3; P < 0.05). Treatment with QNRLis further decreased contractile area compared with QLis and QNRLisPred.
Fig. 3.
Hematoxylin and eosin (H&E) staining of soleus and extensor digitorum longus (EDL). A: representative H&E images (×40) of soleus muscle and quantification of contractile area. B: representative H&E images (×40) of EDL and quantification of contractile area. Contractile area was significantly decreased in D2-mdx mice, which was not corrected by these treatments. DBA, n = 8–9 mice; D2-mdx, n = 8 mice; quercetin and lisinopril (QLis), n = 10 mice; Q, nicotinamide riboside, and Lis (QNRLis), n = 7–9 mice; and QNRLis + prednisolone (QNRLisPred), n = 8 mice. Statistical significance was established at P < 0.05; *significantly different from DBA; #significantly different from D2-mdx; †significantly different from QLis; ‡significantly different from QNRLis.
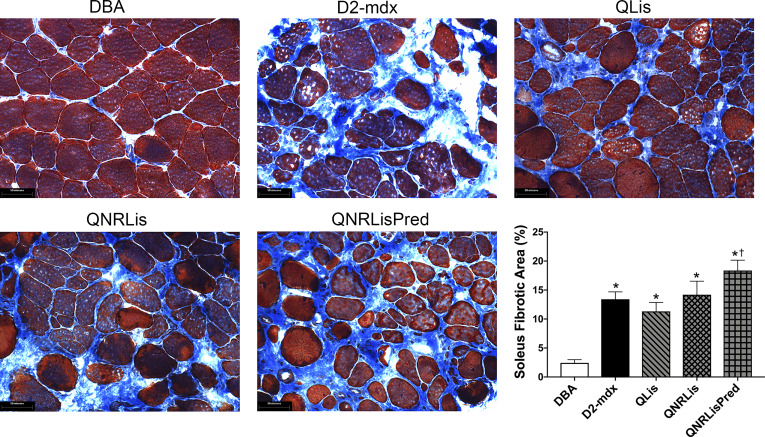
Given the above findings, secondary analyses were performed on the soleus to gain further insights. Fibrotic area increased from less than 5% in DBA to 11–18% in D2-mdx groups (P < 0.05). Treatments did not significantly decrease fibrotic area compared with D2-mdx, but treatment with QNRLisPred significantly increased fibrotic area compared with QLis (Fig. 4; P < 0.05).
Fig. 4.
Trichrome staining of the soleus. Representative trichrome images (×40) of soleus muscle and quantification of fibrotic area. Fibrotic area was increased in dystrophic muscle. DBA, n = 9 mice; D2-mdx, n = 8 mice; quercetin and lisinopril (QLis), n = 10 mice; Q, nicotinamide riboside, and Lis (QNRLis), n = 8 mice; and QNRLis + prednisolone (QNRLisPred), n = 8 mice. Statistical significance was established at P < 0.05; *significantly different from DBA; †significantly different from QLis.
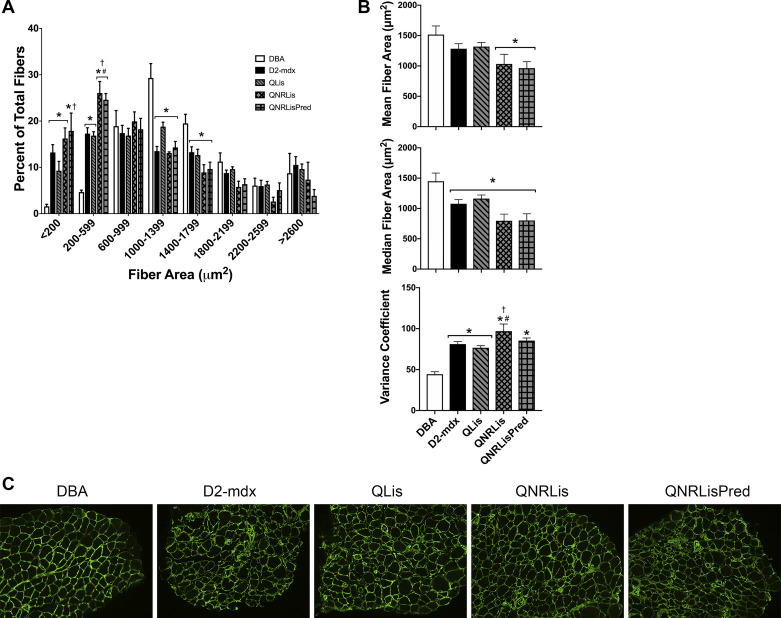
Distribution of fiber cross-sectional area was shifted toward an overabundance of smaller fibers and a corresponding underrepresentation of larger fibers in D2-mdx groups compared with DBA (Fig. 5A). The mean fiber area was similar between DBA, D2-mdx, and QLis but was decreased by 32% in QNRLis and QNRLisPred compared with DBA (Fig. 5B; P < 0.05). Median fiber cross-sectional area was decreased by 20–45% in all D2-mdx groups compared with DBA (Fig. 5B; P < 0.05). Additionally, the variance coefficient was at least doubled in all D2-mdx groups compared with DBA and increased 19% in QNRLis compared with D2-mdx (Fig. 5B; P < 0.05).
Fig. 5.
Fiber area distribution of the soleus. A: dystrophic fiber area distribution was shifted toward an abundance of small fibers. B: mean fiber area was similar between healthy and dystrophic muscle, but median was decreased in all D2-mdx groups. Additionally, the variance coefficient was elevated in dystrophic muscle, regardless of treatment. C: representative immunohistochemistry images (×10) of laminin of soleus muscle. DBA, n = 8 mice; D2-mdx, n = 7 mice; quercetin and lisinopril (QLis), n = 7 mice; Q, nicotinamide riboside, and Lis (QNRLis), n = 4 mice; and QNRLis + prednisolone (QNRLisPred), n = 5 mice. Groups were compared within each fiber area bin using a one-way ANOVA. Significance was established at P < 0.05; *significantly different from DBA; #significantly different from D2-mdx; †significantly different from QLis.
DISCUSSION
DMD is caused by a mutation in the dystrophin gene, resulting in the absence of functional dystrophin protein and a complicated sequela. Researchers and clinicians continue to pursue a variety of strategies intended to counter these cellular dysfunctions and slow or prevent disease progression. Although transformative therapies are currently in various phases of Food and Drug Administration approval to treat tomorrow’s patients, pragmatic solutions are urgently needed to treat today’s patients. In this study, our purpose was to determine the extent to which treatment of D2-mdx mice with quercetin, nicotinamide riboside (NR), and/or lisinopril (Lis) improved muscle function and decreased disease-related muscle damage. Counter to our hypotheses, we failed to demonstrate that treatment with quercetin, NR, or lisinopril independently or in combination meaningfully improved muscle function or decreased histopathological injury. Only a cocktail containing Pred (QNRLisPred) provided some protection against fatigue and contraction-induced injury, but it did not decrease histological injury.
Consistent with our earlier work using long-term quercetin treatment of mdx mice, a relatively less-severe rodent model of DMD (28), application of quercetin over 7 mo to D2-mdx mice did not correspond with improved limb muscle function. Previously, we demonstrated that quercetin improved soleus specific force but did not potentiate other parameters of muscle function. We then surmised that the transient nature of quercetin-mediated protection (28) may be due to depleted NAD+ and successfully treated with NR (28). Early support for this rationale was established by prior observations that NAD+ depletion in mdx mice was corrected after 12 wk of NR supplementation (25). This promising outcome was accompanied by an improved exercise capacity despite elevated energetic stress due to decreased mitochondrial oxidative phosphorylation rate and increased mitochondrial ATP production load (25). Despite dosing differences in this investigation, it is likely that the advanced disease progression in the present investigation (older mice, more-severe model) and/or the long-term use of NR limited its efficacy. Given that 1) the D2-mdx model more accurately recapitulates injury in humans than the mdx mouse model and 2) long-term treatment should be expected with DMD patients, the applicability of NR to DMD seems tenuous based on the current findings.
In the present investigation, lisinopril did not improve measures of muscle function or decrease histological damage. Similarly, short-term treatment with lisinopril did not improve muscle function in an mdx/Utrn+/− model, although lisinopril decreased histological injury (21). When used in combination with spironolactone, an aldosterone receptor antagonist, lisinopril increased EDL and diaphragm function and decreased histological injury in the quadriceps when treatment was started at 4 wk but not at 8 wk in mdx/Utrn+/− mice (23). Interestingly, in the mdx model, treatment with lisinopril and spironolactone did not improve muscle function or decrease histological damage (19). Although differences in animal model, duration of study, dose (33 mg/L or 66 mg/L), and use of drug in combination likely account for some differences between these investigations, collectively they provide underwhelming support for continued use of lisinopril alone as a skeletal muscle therapy for DMD. Notably, the combination of lisinopril and metoprolol (β-blocker) mitigate some aspects of disease severity in cardiac muscle in a clinical population (32).
Within the context of preclinical murine experiments to date, DMD has been most commonly modeled by the C57BL/10-mdx (mdx) mouse. Although this mouse model has been useful for studies related to disease mechanism, its mild phenotype may contribute to translational limitations of therapeutic findings to human populations. In this study we used an emerging model of DMD, the DBA/2J-mdx (D2-mdx) mouse, which has a relatively severe, progressive pathology and early-onset cardiac dysfunction (10). Consistent with our data from 48-wk-old D2-mdx mice, EDL tetanic force and specific force were decreased at 7, 28, and 52 wk (10). Moreover, similar to our current findings in soleus, increased fibrosis was previously reported in tibialis anterior, gastrocnemius, and quadriceps in 6- and 8-mo-old D2-mdx mice compared with control (15). Interestingly, fat accumulation was significantly increased at 6 mo in the gastrocnemius and at 6 and 8 mo in the quadriceps (15), but in this investigation fatty infiltration was not visually apparent in the 11-mo-old soleus or EDL. Ostensibly, increased fatty infiltration decreased contractile cross-sectional area in this prior work (15) and supports functional observations in the current investigation. Moreover, as in this investigation, Coley et al. (10) discovered that the distribution of fiber area was shifted toward a greater frequency of small fibers. These data, in combination with current findings and when paired with decreased muscle mass and findings from H&E and trichrome analyses, support a reduction in fiber size as well as functional cross-sectional area of the whole muscle, which translate to dramatic reductions in muscle function.
In summary, and counter to our study hypotheses, quercetin, nicotinamide riboside, and lisinopril (alone and in combination) were not associated with resistance to dystrophic disease progression in limb muscle. Treatment with QNRLisPred provided partial improvements in muscle function but did not mitigate other indices of disease progression. Furthermore, some indices of disease were exacerbated in the QNRLisPred group, raising the possibility of negative interactions of these compounds. Collectively, these data indicate that quercetin and these quercetin-based cocktails have limited value in the treatment of dystrophin-deficient limb muscles, and alternative approaches should be considered.
GRANTS
Project Parent Muscular Dystrophy (01297), Ryan’s Quest, and Michael’s Cause supported this work. University of Florida Myology Institute Physiological Assessment Core collected the muscle function data (U54 AR052646).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.R.S., T.Q., J.C.Q., and J.T.S. conceived and designed research; H.R.S., T.Q., K.H., J.C.Q., and J.T.S. performed experiments; H.R.S., T.Q., K.H., J.C.Q., and J.T.S. analyzed data; H.R.S., T.Q., K.H., J.C.Q., and J.T.S. interpreted results of experiments; H.R.S., K.H., and J.T.S. prepared figures; H.R.S. and J.T.S. drafted manuscript; H.R.S., T.Q., J.C.Q., and J.T.S. edited and revised manuscript; H.R.S., T.Q., K.H., J.C.Q., and J.T.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge ChromaDex for the kind donation of quercetin and nicotinamide riboside.
REFERENCES
- 1.Anhê GF, Okamoto MM, Kinote A, Sollon C, Lellis-Santos C, Anhê FF, Lima GA, Hirabara SM, Velloso LA, Bordin S, Machado UF. Quercetin decreases inflammatory response and increases insulin action in skeletal muscle of ob/ob mice and in L6 myotubes. Eur J Pharmacol 689: 285–293, 2012. doi: 10.1016/j.ejphar.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Ballmann C, Denney T, Beyers RJ, Quindry T, Romero M, Selsby JT, Quindry JC. Long-term dietary quercetin enrichment as a cardioprotective countermeasure in mdx mice. Exp Physiol 102: 635–649, 2017. doi: 10.1113/EP086091. [DOI] [PubMed] [Google Scholar]
- 3.Ballmann C, Hollinger K, Selsby JT, Amin R, Quindry JC. Histological and biochemical outcomes of cardiac pathology in mdx mice with dietary quercetin enrichment. Exp Physiol 100: 12–22, 2015. doi: 10.1113/expphysiol.2014.083360. [DOI] [PubMed] [Google Scholar]
- 4.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15: 325–330, 2009. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82: 291–329, 2002. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 6.Boots AW, Drent M, de Boer VC, Bast A, Haenen GR. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr 30: 506–512, 2011. doi: 10.1016/j.clnu.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Chadwick JA, Hauck JS, Lowe J, Shaw JJ, Guttridge DC, Gomez-Sanchez CE, Gomez-Sanchez EP, Rafael-Fortney JA. Mineralocorticoid receptors are present in skeletal muscle and represent a potential therapeutic target. FASEB J 29: 4544–4554, 2015. doi: 10.1096/fj.15-276782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalkiadaki A, Igarashi M, Nasamu AS, Knezevic J, Guarente L. Muscle-specific SIRT1 gain-of-function increases slow-twitch fibers and ameliorates pathophysiology in a mouse model of duchenne muscular dystrophy. PLoS Genet 10: e1004490, 2014. doi: 10.1371/journal.pgen.1004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan MC, Rowe GC, Raghuram S, Patten IS, Farrell C, Arany Z. Post-natal induction of PGC-1α protects against severe muscle dystrophy independently of utrophin. Skelet Muscle 4: 2, 2014. doi: 10.1186/2044-5040-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coley WD, Bogdanik L, Vila MC, Yu Q, Van Der Meulen JH, Rayavarapu S, Novak JS, Nearing M, Quinn JL, Saunders A, Dolan C, Andrews W, Lammert C, Austin A, Partridge TA, Cox GA, Lutz C, Nagaraju K. Effect of genetic background on the dystrophic phenotype in mdx mice. Hum Mol Genet 25: 130–145, 2016. doi: 10.1093/hmg/ddv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, González-Gallego J. Quercetin decreases oxidative stress, NF-κB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr 135: 2299–2304, 2005. doi: 10.1093/jn/135.10.2299. [DOI] [PubMed] [Google Scholar]
- 12.Dong J, Zhang X, Zhang L, Bian HX, Xu N, Bao B, Liu J. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKα1/SIRT1. J Lipid Res 55: 363–374, 2014. doi: 10.1194/jlr.M038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord 12: 926–929, 2002. doi: 10.1016/S0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 14.Flanigan KM, Ceco E, Lamar KM, Kaminoh Y, Dunn DM, Mendell JR, King WM, Pestronk A, Florence JM, Mathews KD, Finkel RS, Swoboda KJ, Gappmaier E, Howard MT, Day JW, McDonald C, McNally EM, Weiss RB; United Dystrophinopathy Project . LTBP4 genotype predicts age of ambulatory loss in Duchenne muscular dystrophy. Ann Neurol 73: 481–488, 2013. doi: 10.1002/ana.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukada S, Morikawa D, Yamamoto Y, Yoshida T, Sumie N, Yamaguchi M, Ito T, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Genetic background affects properties of satellite cells and mdx phenotypes. Am J Pathol 176: 2414–2424, 2010. doi: 10.2353/ajpath.2010.090887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1α regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev 21: 770–783, 2007. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollinger K, Gardan-Salmon D, Santana C, Rice D, Snella E, Selsby JT. Rescue of dystrophic skeletal muscle by PGC-1α involves restored expression of dystrophin-associated protein complex components and satellite cell signaling. Am J Physiol Regul Integr Comp Physiol 305: R13–R23, 2013. doi: 10.1152/ajpregu.00221.2012. [DOI] [PubMed] [Google Scholar]
- 18.Hollinger K, Shanely RA, Quindry JC, Selsby JT. Long-term quercetin dietary enrichment decreases muscle injury in mdx mice. Clin Nutr 34: 515–522, 2015. doi: 10.1016/j.clnu.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Janssen PM, Murray JD, Schill KE, Rastogi N, Schultz EJ, Tran T, Raman SV, Rafael-Fortney JA. Prednisolone attenuates improvement of cardiac and skeletal contractile function and histopathology by lisinopril and spironolactone in the mdx mouse model of Duchenne muscular dystrophy. PLoS One 9: e88360, 2014. doi: 10.1371/journal.pone.0088360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuznetsov AV, Winkler K, Wiedemann FR, von Bossanyi P, Dietzmann K, Kunz WS. Impaired mitochondrial oxidative phosphorylation in skeletal muscle of the dystrophin-deficient mdx mouse. Mol Cell Biochem 183: 87–96, 1998. doi: 10.1023/A:1006868130002. [DOI] [PubMed] [Google Scholar]
- 21.Lowe J, Wodarcyk AJ, Floyd KT, Rastogi N, Schultz EJ, Swager SA, Chadwick JA, Tran T, Raman SV, Janssen PM, Rafael-Fortney JA. The angiotensin converting enzyme inhibitor lisinopril improves muscle histopathology but not contractile function in a mouse model of duchenne muscular dystrophy. J Neuromuscul Dis 2: 257–268, 2015. doi: 10.3233/JND-150099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280: 16456–16460, 2005. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 23.Rafael-Fortney JA, Chimanji NS, Schill KE, Martin CD, Murray JD, Ganguly R, Stangland JE, Tran T, Xu Y, Canan BD, Mays TA, Delfín DA, Janssen PM, Raman SV. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in Duchenne muscular dystrophy mice. Circulation 124: 582–588, 2011. doi: 10.1161/CIRCULATIONAHA.111.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues M, Echigoya Y, Maruyama R, Lim KRQ, Fukada SI, Yokota T. Impaired regenerative capacity and lower revertant fibre expansion in dystrophin-deficient mdx muscles on DBA/2 background. Sci Rep 6: 38371, 2016. doi: 10.1038/srep38371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mázala DA, Mouchiroud L, Marshall PL, Campbell MD, Ali AS, Knowels GM, Bellemin S, Iyer SR, Wang X, Gariani K, Sauve AA, Cantó C, Conley KE, Walter L, Lovering RM, Chin ER, Jasmin BJ, Marcinek DJ, Menzies KJ, Auwerx J. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med 8: 361ra139, 2016. doi: 10.1126/scitranslmed.aaf5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selsby JT, Ballmann CG, Spaulding HR, Ross JW, Quindry JC. Oral quercetin administration transiently protects respiratory function in dystrophin-deficient mice. J Physiol 594: 6037–6053, 2016. doi: 10.1113/JP272057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selsby JT, Morine KJ, Pendrak K, Barton ER, Sweeney HL. Rescue of dystrophic skeletal muscle by PGC-1α involves a fast to slow fiber type shift in the mdx mouse. PLoS One 7: e30063, 2012. doi: 10.1371/journal.pone.0030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spaulding HR, Ballmann CG, Quindry JC, Selsby JT. Long-term quercetin dietary enrichment partially protects dystrophic skeletal muscle. PLoS One 11: e0168293, 2016. doi: 10.1371/journal.pone.0168293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaulding HR, Kelly EM, Quindry JC, Sheffield JB, Hudson MB, Selsby JT. Autophagic dysfunction and autophagosome escape in the mdx mus musculus model of Duchenne muscular dystrophy. Acta Physiol (Oxf) 222: e12944, 2018. doi: 10.1111/apha.12944. [DOI] [PubMed] [Google Scholar]
- 30.Swaggart KA, Demonbreun AR, Vo AH, Swanson KE, Kim EY, Fahrenbach JP, Holley-Cuthrell J, Eskin A, Chen Z, Squire K, Heydemann A, Palmer AA, Nelson SF, McNally EM. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proc Natl Acad Sci USA 111: 6004–6009, 2014. doi: 10.1073/pnas.1324242111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vassilakos G, Lei H, Yang Y, Puglise J, Matheny M, Durzynska J, Ozery M, Bennett K, Spradlin R, Bonanno H, Park S, Ahima RS, Barton ER. Deletion of muscle IGF-I transiently impairs growth and progressively disrupts glucose homeostasis in male mice. FASEB J 33: 181–194, 2019. doi: 10.1096/fj.201800459R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viollet L, Thrush PT, Flanigan KM, Mendell JR, Allen HD. Effects of angiotensin-converting enzyme inhibitors and/or beta blockers on the cardiomyopathy in Duchenne muscular dystrophy. Am J Cardiol 110: 98–102, 2012. doi: 10.1016/j.amjcard.2012.02.064. [DOI] [PubMed] [Google Scholar]