Abstract
Obesity and aging reduce skeletal muscle contractile function. However, it remains unclear whether obesity additively promotes muscle contractile dysfunction in the setting of aging. In this study, we investigated skeletal muscle contractile function ex vivo and intracellular Ca2+ release in male C57BL/6J mice fed a low-fat diet (LFD) or a high-fat diet (HFD) for 4 or 20 mo. Tetanic force production in the extensor digitorum longus muscle was decreased by aging or HFD feeding, and the further reduction was observed in aged HFD mice. The 20-mo HFD-fed mice, not the 20-mo LFD-fed mice or 4-mo HFD-fed mice, showed reduced intracellular Ca2+ peak levels by high concentration of caffeine (25 mM) compared with 4-mo LFD mice. Aging and HFD feeding additively increased intramyocellular lipid (IMCL) levels and were associated with the degree of impaired muscle contractile force and peak Ca2+ level. These data suggest that impairment in the contractile force in aged muscle is aggravated by HFD, which may be due, at least in part, to dysfunction in intracellular Ca2+ release. The IMCL level may be a marker for impaired muscle contractile force caused by aging and HFD.
NEW & NOTEWORTHY The aim of this study was to examine the effect of high-fat diet (HFD)-induced obesity on contractile function and Ca2+ release capacity in aged skeletal muscle. Not only were the force production and peak Ca2+ levels decreased by aging and HFD feeding, respectively, but also, these interventions had an additive effect in aged HFD-fed mice. These data suggest that the impairment in the contractile force in aged muscle is aggravated by a HFD, which may be due to synergistic dysfunction in intracellular Ca2+ release.
Keywords: aging, calcium, high-fat diet, muscle contraction, obesity
INTRODUCTION
Developed countries are aging rapidly, causing both health and economic problems (5, 42). Physical function declines with age, and it was shown that decreased muscle strength, but not reduced muscle mass, was associated with increased mortality (33). On the other hand, obesity due to overeating and lack of exercise causes major health problems worldwide (15, 48), and thus the number of elderly obese individuals is increasing. To overcome this health problem, we need to understand the pathophysiology related to various aging-related diseases in this population. The influence of obesity on impaired muscle strength caused by aging is an important issue but one that has not yet been fully clarified.
It has been reported that muscle-force production normalized to muscle mass is substantially lower in aged (18) and obese mice (10). Skeletal muscle contractile properties are regulated by the contraction-relaxation cycle that depends on intracellular Ca2+ release and uptake into the sarcoplasmic reticulum (SR) (2, 12, 44), and previous studies showed that Ca2+ release levels were decreased in aged (3) and obese (7, 45) skeletal muscle. These results suggest that both aging and obesity may impair muscle contractile function through reduced Ca2+ release capacity, but the detailed mechanism has not been fully elucidated. It also remains unclear whether obesity further exaggerates impaired muscle contractile force by aging.
Against this background, this study investigated the effects of high-fat diet (HFD)-induced obesity and aging on contractile dysfunction and impaired Ca2+ release capacity in skeletal muscle.
MATERIALS AND METHODS
Animals.
Male C57BL/6J mice were housed in cages in a temperature-controlled room under a 12-h light-dark cycle. During a 1-wk adaptation period, all mice were fed standard chow and water ad libitum. After an initial acclimatization period, animals (2 mo of age) were randomly assigned to the following groups: 4-mo low-fat diet (LFD; n = 16), 4-mo HFD (n = 17), 20-mo LFD (n = 18), and 20-mo HFD (n = 10). The HFD mice were fed a diet containing 20% protein, 60% fat, and 20% carbohydrate (D12492; Research Diets, New Brunswick, NJ), and the LFD mice were fed standard chow containing 22.6% protein, 5.6% fat, and 53.8% carbohydrate [a pelleted Charles River Formula (CRF)-1 diet; Charles River, Japan]. After 4 mo (when mice were 6 mo old) or 20 mo (when mice were 22 mo old) of the HFD or standard chow diet, we performed blood and muscle sampling for further analysis as described below. All animal experiments in this study were approved by the Animal Experimental Committee of Juntendo University.
Glucose and insulin tolerance tests.
Intraperitoneal glucose tolerance tests (GTTs) were performed after mice had fasted overnight (16–18 h; 0.5 g/kg body weight). Intraperitoneal insulin tolerance tests (ITTs) were performed (5–6 h) with regular human insulin (0.75 U/kg body weight; Eli Lilly). Glucose and insulin were injected intraperitoneally, and blood glucose levels were assessed by tail bleeds at 0, 15, 30, 60, 90, 120, and 150 min. The fasting plasma insulin levels were measured using an ELISA kit (Morinaga Co., Kanagawa, Japan), according to the manufacturer’s protocol. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as previously described (31).
Muscle preparation.
Mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (70 mg/kg body weight). Once a surgical level of anesthesia was reached, the muscles were dissected, and the abdominal visceral fat was collected and weighed (41). The extensor digitorum longus (EDL), soleus (SOL), and flexor digitorum brevis (FDB) muscles were used.
Muscle contraction measurement.
The force-frequency relationship was assessed in intact EDL and SOL muscle samples, as previously described (13, 14). Briefly, isolated EDL and SOL muscle preparations were mounted on a force transducer (UL-100; Minebea, Tokyo, Japan), and the hock was fixed in a chamber containing Krebs solution [in mM: 120 NaCl, 5 KCl, 2 CaCl2, 1 magnesium chloride (MgCl2), 1 NaH2PO4, 25 NaHCO3, and 11 glucose], bubbled with 95% O2-5% CO2. At the end of the 5-min equilibration, the isolated muscle was stimulated with 500 ms trains of current pulses at 1, 10, 20, 30, 40, 50, 70, 100, and 150 Hz at 1-min intervals, and the contractile force was measured at an acquisition rate of 1 kHz. The optimal muscle length was determined by the maximum twitch force. After muscle length was measured, the muscles were removed from the chamber, the tendons were dissected out, and muscle weight was measured. Specific force was calculated from the absolute force, muscle weight, and muscle length, assuming a density of 1.056 g/mL. At the end of the experiments, the EDL muscles were frozen rapidly in isopentane, cooled in liquid nitrogen, and subsequently used for histochemical staining.
Intracellular Ca2+ measurement.
Intracellular Ca2+ measurement was performed in intact FDB muscle samples, as previously described (14). The excised, intact FDB muscles were digested under gentle agitation for 2 h at 37°C in a rodent Krebs solution, supplemented with 2 mg/mL collagenase type II (CLS-2; Worthington Biochemical, Lakewood, NJ). The muscles were then suspended in Ringer’s without collagenase type II and triturated repeatedly using Pasteur pipettes in which their tips had been fire polished to produce openings of a successively smaller size. Single FDB fibers were then plated on 35 mm glass-bottomed dishes and allowed to settle for >30 min before experimentation. The fibers were used within 7 h of isolation. Single FDB fibers were loaded with 5 µM Fluo-4 AM (Invitrogen/Molecular Probes, Waltham, MA) and 5 µM Fura-2 AM (Dojindo, Kumamoto, Japan) in the dark for 30 min at room temperature (22–25°C), followed by a washing with dye-free Krebs solution (in mM: 142 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 HEPES, and 10 glucose, pH 7.4). Then, single FDB fibers were washed in dye-free solution, supplemented with 25 μM N-benzyl-p-toluene sulfonamide (BTS; AAJ64910MA; Fisher Scientific) for 10 min to block contractions. Fluorescence images of the Fluo-4- and/or Fura-2-loaded fibers were captured via an objective lens [CFI Super Fluor× 20, numerical aperture (NA) 0.75; Nikon, Tokyo, Japan] and a cooled charge-coupled device (CCD) camera (ORCA-ER; Hamamatsu Photonics, Shizuoka, Japan) at an acquisition rate of 200 Hz (Fluo-4) and 1.42–10 Hz (Fura-2). The fluorescence images were processed using AQUA-COSMOS/ratio software (Hamamatsu Photonics). Ca2+ concentration ([Ca2+]) levels were assessed using Fluo-4 during electrical stimulation with individual 500 ms trains at 1 and or 100 Hz. The peak fluorescence intensity value (F) was measured during contraction, and the quantification data were plotted as changes from the precontraction level (F0).
In the pharmacological experiment in intact FDB muscle, resting [Ca2+] levels were assessed by changes in the Fura-2 signal intensity in response to caffeine (C0750; Sigma-Aldrich, St. Louis, MO) and 4-chloro-m-cresol (4-CmC; C55402; Sigma-Aldrich) (36). We superfused caffeine or 4-CmC with a Ca2+-free solution, and peak [Ca2+] levels were assessed using Fura-2 during caffeine (120 s) or 4-CmC (30 s) stimulation. Single FDB fibers were preincubated in Krebs-Henseleit buffer (KHB) solution, supplemented with 30 mM magnesium chloride (MgCl2; M1028; Sigma-Aldrich) for 10 min before 4-CmC stimulation. Adenosine triphosphate (ATP) was used from Sigma-Aldrich (A2383). The peak fluorescence intensity value (R; ratiometric images) was measured during stimulation, and the ratiometric quantification data (ΔR/R0 ratio) are expressed as changes from the prestimulation levels (R0). In each experiment, the solutions were superfused at a rate of 2 mL/min. We placed a plastic ring wall around the glass-bottom dishes to limit the perfusion volume less than ~300 μL.
Western blotting analysis.
The expression levels of skeletal muscle Ca2+ regulatory proteins were analyzed by Western blotting, as previously described (13). The following primary antibodies were used: anti-type 1 ryanodine receptor (RyR) antibody 34C (MA3-925; Thermo Fisher Scientific, Waltham, MA), anti-dihydropyridine receptor (DHPR) antibody 20A (ab2864; Abcam, Cambridge, MA), anti-calsequestrin (CSQ) antibody VIIID12 (MA3-913; Thermo Scientific), anti-sarcalumenin (SAR) antibody XIIC4 (sc-58845; Santa Cruz Biotechnology, Dallas, TX), anti-SR Ca2+ ATPase 1 (SERCA1) antibody IIH11 (MA3-911; Thermo Scientific), anti-parvalbumin (PV) antibody (ab32895; Abcam), anti-FK506 binding protein 12 (FKBP12) antibody (ab2918; Abcam), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody 14C10 (Number 2118; Cell Signaling Technology, Danvers, MA). The protein levels were normalized to the GAPDH expression level and are expressed relative to the levels in 4-mo LFD mice.
Immunofluorescence microscopy.
Histological sections of mouse muscle fibers were examined and analyzed as previously described (14). The lipid droplets in muscle sections were measured using LipiDye (Lipid Droplet Green; Funakoshi, Tokyo, Japan). In brief, sections fixed with 4% formaldehyde were incubated with 1 μM LipiDye for 30 min at 4°C. Then, the sections were washed two times with phosphate-buffered saline (PBS). Images (1,024 × 1,024 pixels) were acquired using the laser lines of an LSM780 confocal laser-scanning microscope [40×/1.4 NA oil objective lens (PlanApo; Carl Zeiss MicroImaging, Inc., Oberkochen, Germany)]. The image was analyzed using either Spectralyzer (custom-designed) or ImageJ software. The fluorescence percentage of individual fibers was calculated with an ImageJ macro application. The quantitative fluorescence analyses were performed by counting ~39 random muscle fiber sections in each group.
Electron microscopy.
Sample preparation and electron microscopy quantitative analysis were performed, as previously described (12). In brief, the EDL muscles were fixed with 2.5% glutaraldehyde (TAAB) in 0.1 M phosphate buffer (pH 7.4), followed by postfixation with 1% OsO4 in the same buffer. Fixed specimens were dehydrated with a graded series of ethanol and embedded in Epok 812 (Oken Shoji, Tokyo, Japan). Ultrathin sections were cut and stained with uranyl acetate and lead citrate. The specimens were examined with an HT7700 transmission electron microscope (Hitachi, Tokyo, Japan). Next, electron micrographs were taken at high magnification using the Soft Imaging System. Individual lipid droplets from three mice per group were manually traced in longitudinal orientations using ImageJ software. Quantitative analyses were performed using ~114 random lipid droplets in a longitudinal muscle area from mice in each group.
Statistical analysis.
Values are expressed as means ± SE. Statistical analyses were performed using Prism version 7.0 (GraphPad Software, San Diego, CA). To compare two nonpaired groups, Student’s t test was applied. For comparisons of more than two groups, a two-way ANOVA was applied, followed by Bonferroni post hoc tests. In all tests, P < 0.05 was considered statistically significant.
RESULTS
Effects of HFD on physical characteristics and metabolic parameters in aged mice.
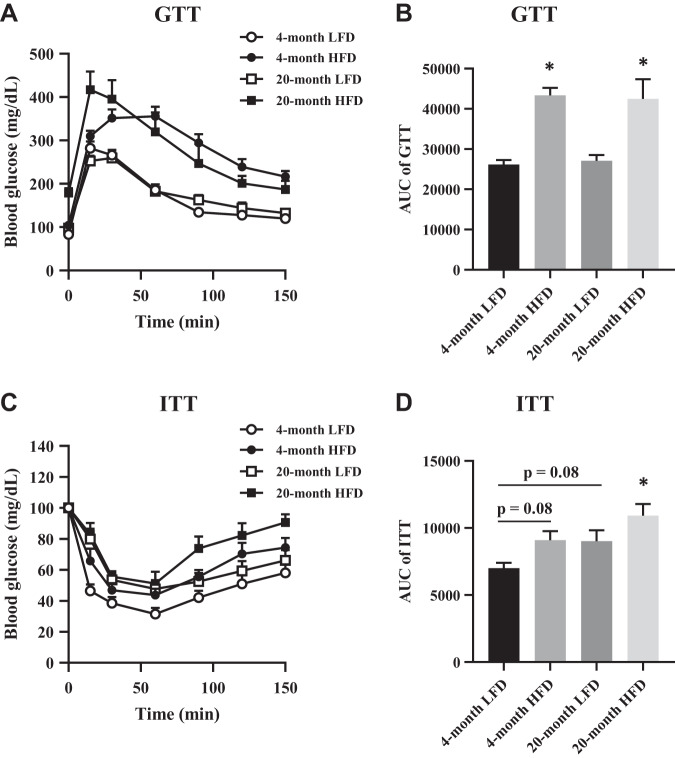
Metabolic parameters were measured after LFD or HFD administration for 4 mo (when mice were 6 mo old) or 20 mo (when mice were 22 mo old). Compared with LFD, HFD feeding resulted in a significant increase in body weight, abdominal visceral fat, fasting glucose levels, insulin level, and HOMA-IR in both the 4-mo and 20-mo groups (Table 1). Similarly, the HFD significantly deteriorated glucose tolerance at both time points (Fig. 1, A and B). In addition, ITT revealed that insulin sensitivity in the 4-mo HFD and 20-mo LFD groups was trending to be lower, whereas significantly deteriorating in the 20-mo HFD group (Fig. 1, C and D). The weights of the EDL muscles were lower in the 20-mo LFD group but unchanged in the 4-mo HFD or 20-mo HFD group compared with the 4-mo LFD group (Table 1).
Table 1.
Physiological parameters
| 4-mo LFD | 4-mo HFD | 20-mo LFD | 20-mo HFD | |
|---|---|---|---|---|
| Body weight, g | 30.8 ± 0.5 | 49.4 ± 0.7** | 34.4 ± 0.7 | 56.8 ± 4.9**#†† |
| EDL weight, mg | 13.1 ± 0.4 | 13.0 ± 0.4 | 11.3 ± 0.8* | 13.0 ± 0.2 |
| SOL weight, mg | 10.0 ± 0.3 | 11.4 ± 0.4 | 10.5 ± 0.2 | 10.8 ± 0.5 |
| Abdominal visceral fat, g | 0.48 ± 0.3 | 2.53 ± 0.2** | 0.99 ± 1.0 | 3.47 ± 0.6**#†† |
| Fasting glucose, mg/dL | 83.9 ± 2.7 | 128.1 ± 12.0** | 97.9 ± 3.1 | 178.4 ± 10.2**#†† |
| Fasting insulin, ng/mL | 0.23 ± 0.04 | 1.07 ± 0.18** | 0.28 ± 0.02 | 0.89 ± 0.08** |
| HOMA-IR, IU/L × mg/dL/405 | 1.20 ± 0.16 | 8.63 ± 1.61** | 1.76 ± 0.10 | 10.38 ± 1.36** |
Values are the means ± SE; n = 5–18 per group. EDL, extensor digitorum longus; HFD, high-fat diet; HOMA-IR, homeostasis model assessment of insulin resistance; LFD, low-fat diet; SOL, soleus.
P < 0.05 and
P < 0.01 vs. 4-mo LFD mice.
P < 0.01 vs. 4-mo HFD mice.
P < 0.01 vs. 20-mo LFD mice.
Fig. 1.
Parameters of insulin sensitivity. A: glucose tolerance test (GTT). B: area under the curve (AUC) quantification of GTT results; n = 5–10 per group. C: insulin tolerance test (ITT). D: AUC quantification of ITT results; n = 5–9 per group. HFD, high-fat diet. The data are presented as the means ± SE. *P < 0.05 vs. 4-mo low-fat diet (LFD).
Effects of HFD on muscle contractile function in aged mice.
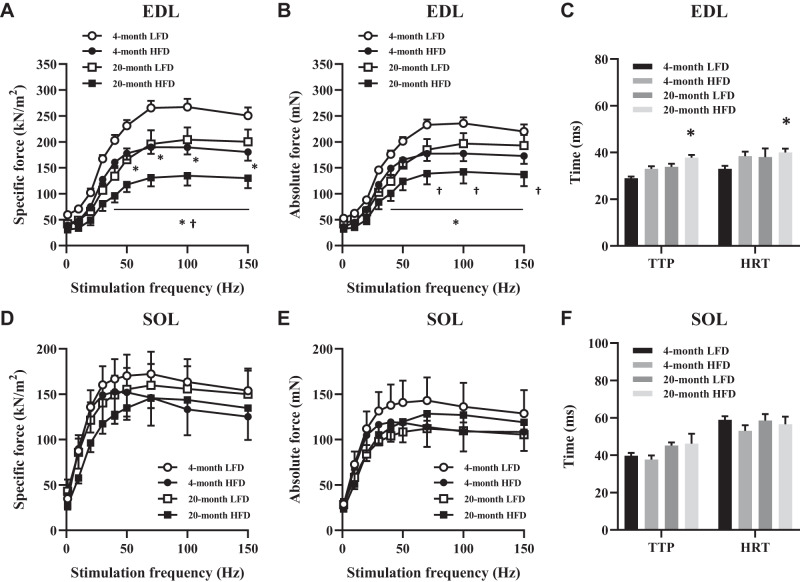
Next, we measured muscle contractile function in EDL and SOL muscles in all groups. The specific force at stimulation frequencies ranging from 50 to 150 Hz in the EDL muscles was lower in the 4-mo HFD and 20-mo LFD groups compared with the 4-mo LFD group. Furthermore, the contractile force was further reduced in the 20-mo HFD group (Fig. 2A). Similar findings were seen in absolute force data (Fig. 2B). Indeed, the time-to-peak (TTP) force and the half-relaxation time (HRT) during twitch stimulation were prolonged in EDL muscle in the 20-mo HFD group (Fig. 2C). On the other hand, the specific force, the absolute force, and TTP and HRT in SOL muscle were comparable among groups (Fig. 2, D–F). These data suggest that HFD and aging additively impair muscle contractile force in the EDL muscle but not the SOL muscle. In addition, prolonged TTP and HRT in EDL muscle samples in the 20-mo HFD group suggest the presence of impaired calcium regulation in the muscle.
Fig. 2.
Force-frequency curve in fast- and slow-twitch skeletal muscle. Specific force-frequency relationship (A) and absolute force-frequency relationship (B) in the extensor digitorum longus (EDL) muscle. C: time to peak (TTP) and half-relaxation time (HRT) of muscle twitches in the EDL. Specific force-frequency relationship (D) and absolute force-frequency relationship (E) in the soleus (SOL) muscle. F: TTP and HRT of muscle twitches in the SOL muscle; n = 5–7 per group. The data are presented as the means ± SE. *P < 0.05 vs. 4-mo low-fat diet (LFD); †P < 0.05 vs. 4-mo high-fat diet (HFD) and 20-mo LFD.
Effect of HFD on [Ca2+] release in aged mice.
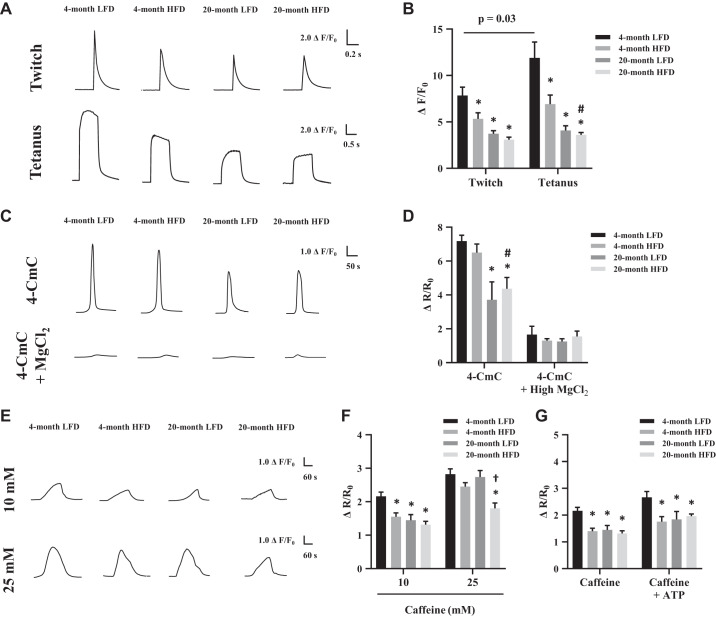
Next, we measured Ca2+ flux in intact FDB muscles during electrically stimulated contraction and pharmacological stimulation. Contraction-stimulated Ca2+ peak levels in the 4-mo HFD, 20-mo LFD, and 20-mo HFD groups were lower compared with the 4-mo LFD group (Fig. 3, A and B). In particular, a substantial reduction of Ca2+ peak levels was observed during tetanic stimulation in the 20-mo HFD group compared with the 4-mo LFD and HFD groups. For the pharmacological experiments, we first used a direct activator of RyR, 4-CmC (36). The Ca2+ peak levels stimulated by 4-CmC were reduced in the 20-mo LFD and HFD groups compared with the 4-mo LFD group (Fig. 3, C and D). In addition, 4-CmC significantly reduced the Ca2+ peak levels in the 20-mo HFD group compared with the 4-mo HFD group. Of note, the Ca2+ peak levels following 4-CmC administration were dramatically reduced in all groups by the presence of MgCl2, which suppresses RyR-dependent Ca2+ release (28) (Fig. 3, C and D). Intriguingly, with a high concentration of caffeine (25 mM), which promoted greater cytosolic calcium, Ca2+ peak levels only in the 20-mo HFD group were significantly decreased compared with other groups (Fig. 3, E and F). Next, we investigated intracellular Ca2+ levels following the administration of ATP and caffeine, which are known to stimulate the opening and activation of the RyR1 receptor (11). We found that 10 mM caffeine, with or without ATP stimulation, reduced Ca2+ peak levels in the 4-mo HFD, 20-mo LFD, and 20-mo HFD groups compared with the 4-mo LFD group (Fig. 3G). Together, these data suggest that both aging and HFD cause skeletal muscle calcium dysregulation. Whereas the degree of the impairments in each group was variable, the most severe impairment for the calcium regulation consistently occurred in the 20-mo HFD group.
Fig. 3.
Ca2+ flux during electrically stimulated contraction and pharmacological ryanodine receptor (RyR) stimulation. A: representative twitch (1 Hz) and tetanus (100 Hz) stimulation. B: average peak Ca2+ responses (Fluo-4 fluorescence) during twitch and tetanus stimulation. C: representative 1 mM 4-chloro-m-cresol (4-CmC) stimulation, with or without 30 mM MgCl2. D: average peak Ca2+ responses (Fura-2 fluorescence) during 4-CmC, with or without 30 mM MgCl2 stimulation. E: representative 10 mM or 25 mM caffeine stimulation. F: average peak Ca2+ responses (Fura-2 fluorescence) during 10 mM and 25 mM caffeine. G: average peak Ca2+ responses (Fura-2 fluorescence) during 10 mM caffeine, with or without 2 mM adenosine triphosphate (ATP). The peak fluorescence intensity (F or R, ratiometric images) during the stimulation was measured, and the quantified data are shown in a graph as changes (ΔF or ΔR) from the prestimulation level (F0 or R0). Intracellular Ca2+ concentration ([Ca2+]i) is expressed as the Δ ratio (ΔF/F0 or ΔR/R0); n = 7–20 fibers and 3–4 mice per group. The data are presented as the means ± SE. *P < 0.05 vs. 4-mo low-fat diet (LFD); #P < 0.05 vs. 4-mo high-fat diet (HFD); †P < 0.05 vs. 4-mo HFD and 20-mo LFD.
Effect of HFD on calcium-related proteins in aged mice.
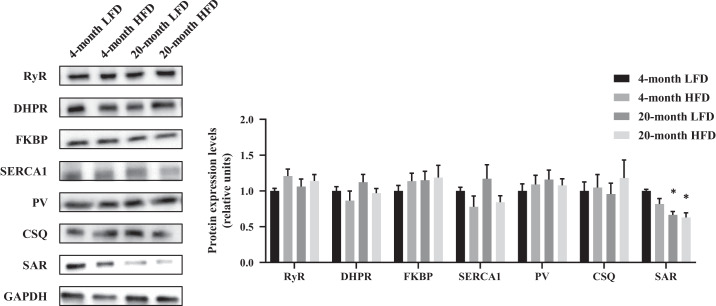
To identify the molecule mechanisms responsible for the impaired Ca2+ regulation by aging and HFD, we investigated the protein abundance of these enzymes. As shown in Fig. 4, the expressions of RyR, DHPR, FKBP, SERCA1, PV, and CSQ proteins were comparable among the groups. Instead, the SAR protein levels in the 20-mo LFD and 20-mo HFD groups were decreased compared with those in the 4-mo LFD group. These data indicate that skeletal muscle SAR protein is reduced with aging but not with HFD feeding.
Fig. 4.
Abundance of calcium-regulated proteins. Representative Western blots are shown of ryanodine receptor (RyR), dihydropyridine receptor (DHPR), FK506 binding proteins (FKBP), sarcoplasmic reticulum calcium ATPase type 1 (SERCA1), parvalbumin (PV), calsequestrin (CSQ), sarcalumenin (SAR), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression in extensor digitorum longus muscle. HFD, high-fat diet. The data are presented as the means ± SE; n = 5–7 per group. *P < 0.05 vs. 4-mo low-fat diet (LFD).
Effect of HFD on lipid accumulation in aged mice.
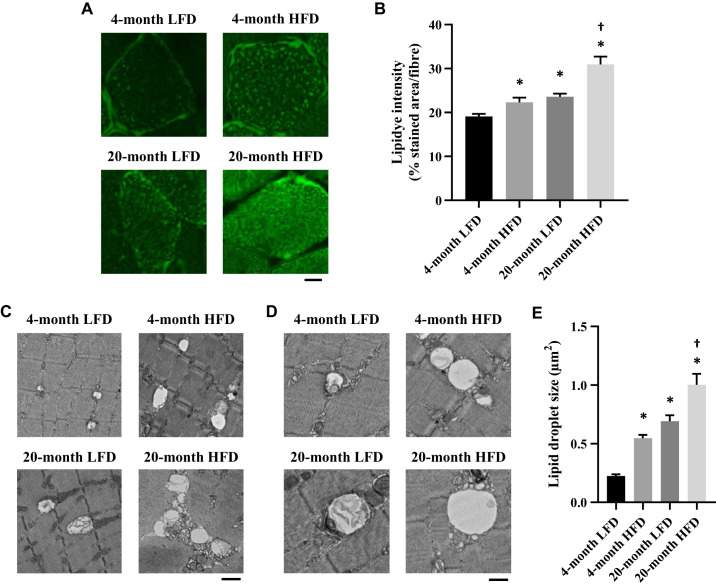
Intramyocellular lipid (IMCL) accumulation is associated with muscle weakness (4, 9, 13). Thus we assessed IMCL accumulation in EDL muscle in each group by histochemical analyses. The quantification of lipid accumulation by LipiDye intensity showed that lipid droplet volume was increased in the 4-mo HFD and 20-mo LFD groups compared with that in the 4-mo LFD group (Fig. 5, A and B). Furthermore, this effect was additive in the 20-mo HFD group (Fig. 5B). We also assessed the IMCL droplet size with electron microscopy. Similar to the LipiDye results, IMCL droplet size was also greater in the 4-mo HFD and 20-mo LFD groups compared with that in the 4-mo LFD group (Fig. 5, C–E), and this effect was additive in the 20-mo HFD group (Fig. 5E).
Fig. 5.
Quantification of intramyocellular lipid by histocytochemical analysis and electron microscopy analyses. A: transverse sections of the extensor digitorum longus (EDL) muscle stained with LipiDye. Scale bar, 10 μm. B: quantification of the fluorescence intensity per individual fiber (n = 30–39 fibers from 4 to 6 mice). Representative transmission electron microscope images at low (C; scale bar, 1 µm) and high (D; scale bar, 500 nm) magnification from EDL muscles. E: quantification of lipid droplet size to determine the intermyofibrillar area of EDL muscle samples (samples comprise 60–114 pictures/10 fibers in 3 animals). The data are presented as the means ± SE. *P < 0.05 vs. 4-mo low-fat diet (LFD); †P < 0.05 vs. 4-mo high-fat diet (HFD) and 20-mo LFD.
Next, with the use of the mean values in each group, we investigated the relationship between the IMCL droplet size and the specific forces during twitch stimulation and tetanic stimulation, as well as Ca2+ peak levels and TTP during twitch stimulation. As shown in Fig. 6, increased IMCL droplet size by aging and HFD seems to be associated with lowered specific forces (Fig. 6, A and B), calcium peak levels (Fig. 6C), and delayed TTP (Fig. 6D), respectively. These data suggest that aging and HFD additively increased IMCL level, and these IMCL levels were associated with the degree of impaired muscle contractile force in each group.
Fig. 6.
Relationship between Ca2+ release capacity and lipid droplet size. Relationship between twitch force (A) and tetanic force (B) in the extensor digitorum longus (EDL) muscle. C: average peak Ca2+ responses (Fluo-4 fluorescence) during twitch forces. D: time to peak (TTP) and lipid morphology size. Assessed by transmission electron microscopy (lipid droplet size). ΔF/F0, ratio of change in peak fluorescence intensity/prestimulation level; HFD, high-fat diet; LFD, low-fat diet.
DISCUSSION
The present study found that skeletal muscle-force production was decreased with aging and HFD feeding and that this effect was additive in the aged HFD-fed mice. Consistent with these results, electrically stimulated Ca2+ peak levels were also reduced by aging and HFD feeding, with additive effects in the aged HFD-fed mice. Experiments with a high concentration of caffeine also support this notion. Furthermore, increased IMCL droplet size was associated with impaired muscle contractile function or lowered Ca2+ release capacity. Finally, SAR, a calcium-binding protein in the SR that contributes to SR Ca2+ content, was decreased in the skeletal muscle of aged mice.
Many studies have demonstrated skeletal muscle contractile dysfunction in mice with HFD-induced obesity (1, 10, 13, 44) and in aged mice (3, 6, 30). Regarding muscle strength in aged mice with HFD-induced obesity, a previous study demonstrated that 14-mo-old mice (middle aged) that consumed a HFD for 5 mo exhibited decreased skeletal muscle strength compared with control mice (29). Similarly, Abrigo et al. (1) showed that the specific force in isolated fast-twitch skeletal muscle was lower in 50-wk-old mice that had consumed a HFD for 30 wk. In terms of the fiber type that is affected by aging and HFD, recent studies, including ours, have shown that only fast-twitch muscle, not slow-twitch muscle, exhibits a reduction in specific force in mice with HFD-induced obesity (10, 13, 19, 44). Consistent with these data, in this study, we demonstrated that force production in fast-twitch skeletal muscle was impaired by aging and HFD-induced obesity, whereas slow-twitch skeletal muscle was barely affected.
Consistent with earlier reports (3, 39), we found reduced peak Ca2+ levels during muscle contraction in aged mice (20-mo LFD group) and young mice with HFD-induced obesity (4-mo HFD group). Since Marks and colleagues (11) demonstrated that caffeine and ATP augment RyR1 opening, we investigated peak Ca2+ levels using RyR agonists (4-CmC, caffeine, and caffeine/ATP) in muscle samples from HFD mice, aged mice, and aged HFD mice compared with young LFD mice. Interestingly, only 20-mo HFD mice showed reduced Ca2+ peak levels following stimulation with high-concentration caffeine (Fig. 3F). A previous study demonstrated that the amount of releasable Ca2+ content from the SR was decreased in the skeletal muscle of elderly humans (27), and we confirmed that relative to the 4-mo LFD group, TTP (which reflects Ca2+ release capacity during muscle-twitch stimulation) was delayed only in the 20-mo HFD group (Fig. 2C). Taken together, our data suggest that aging and HFD-induced obesity additively impair intracellular Ca2+ release in muscle.
Transient increases of intracellular Ca2+ in myocytes are initiated by sarcolemmal and transverse tubule depolarization, triggering Ca2+ release from the SR via RyR. Ca2+ release from RyR is activated through charge-dependent conformational changes in the DHPR (43). CSQ is located in the SR lumen and modulates the sensitivity of RyR (47). FKBP12 is involved in both orthograde and retrograde coupling between the L-type Ca2+ channel and RyR1, affecting SR Ca2+ release (3, 46). SAR is a Ca2+-binding protein localized in the SR of the intracellular Ca2+ store (35, 49, 50). Previous studies have shown that the expression levels of these Ca2+ release proteins in skeletal muscle are similar in aged and young mice (3, 37). In contrast, SAR is reduced in aged rodent muscle (25, 35) and muscle in elderly women (20). Among the proteins that we investigated, we only found a consistent reduction in SAR proteins in the skeletal muscles of aged LFD-fed and HFD-fed mice (Fig. 4). We recently demonstrated that 6 wk of exercise training improved contractile dysfunction and the failure of Ca2+ release and increased protein expression of SAR in the muscle in type 2 diabetic mice (14). Intriguingly, a recent study showed that 9 wk of training by electrical stimulation increased SAR in old muscles (32). In addition, a previous study suggested that SAR is involved in exercise ability after endurance exercise training (23). It is expected that exercise intervention may increase SAR and improve calcium handling and contractile dysfunction in the muscle. However, previous studies demonstrated that muscle contractile force was not reduced in SAR-knockout mice (49). Thus it is still unclear whether decreased SAR protein levels contribute to the reduced muscle contraction caused by aging and HFD, and further investigations are required to clarify the mechanisms.
IMCL accumulates in obese (24) and elderly people (38) and is associated not only with insulin resistance (24, 38) but also with decreased muscle contractile force (9). We (13) and others (4) also demonstrated that a HFD increased IMCL and impaired muscle contractile force in an animal model. In the present study, we also identified a relationship between increased IMCL and Ca2+ release capacity, the latter expressed as the specific force and TTP during twitch stimulation in skeletal muscle (Fig. 6). Previous studies demonstrated that higher IMCL levels were associated with decreased muscle-specific force and impaired physical function in obese older adults (9). In skeletal muscle, muscle contraction and relaxation speeds are critically dependent on effective SR Ca2+ handling (2). A previous study demonstrated that IMCL was increased, and TTP was delayed in skeletal muscle in a streptozocin-induced type 1 diabetic mouse model (26). Here, we showed that IMCL was correlated with impairment of muscle contractile force by aging and HFD, and therefore, the IMCL level may serve as a good marker for impaired muscle contractility in this setting. Although in this study, we only measured the amount of IMCL, several studies suggested that altered IMCL composition may cause changes in skeletal muscle contractile function via calcium kinetics. One previous study showed that sphingosine blocked calcium release from the RyR and reduced the activity of RyR channels reconstituted into planar lipid bilayers (40); indeed, sphingolipids, such as ceramide, sphingosine, and sphingosine-1-phosphate, promote skeletal muscle weakness (34). In addition, studies using fatty acid synthase knockout mice (17) or choline/ethanolamine phosphotransferase 1 knockout mice (16) showed that decreased intramyocellular phosphatidylethanolamine may impair SERCA activity and cause muscle weakness. Thus changes in IMCL levels caused by aging and a HFD may be accompanied by alterations in IMCL species, which in turn, may impair Ca2+ release capacity in skeletal muscle.
Although our study demonstrated that HFD-induced obesity results in reduced contractile function in aged skeletal muscle, Hill et al. (21) showed that the short term (9 wk) of high fat diet-induced obesity did not cause muscle contractile dysfunction in aged (79-wk) female mice. On the other hand, Hurst et al. (22) suggested that 8-wk HFD, but not 4-wk HFD, reduces muscle contractile function at 20 wk in female mice. In addition, contractile properties in skeletal muscle in female mice are different from those in male mice (8). Thus the effect of HFD on contractile function might be influenced by sex, age, and duration of HFD. Since we provided the HFD to male mice for 20 mo, one should be careful when generalizing the results in the present study.
In conclusion, our study demonstrates that HFD-induced obesity results in significantly reduced contractile function and Ca2+ release capacity in aged skeletal muscle. These changes may be driven by a diet- and age-induced lipidomic milieu, which interferes with SR Ca2+ cycling, in skeletal muscle. The data confirm the idea that diet plays an important role in the maintenance of skeletal muscle contractile property and demonstrate for the first time that these diet-dependent effects are equally relevant with aging. The synergistic effects of obesity and aging on muscle function may exacerbate morbidity and mortality. Further studies will investigate the potential role of the lipid stress signal that may interfere with muscle contractile function with diet and aging.
GRANTS
This work was supported, in part, by the Strategic Research Foundation at Private Universities and KAKENHI (Grants 16J11193 and 15H06597) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Suzuken Memorial Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.E. conceived and designed research; H.E., R. Kakigi, and R.H. performed experiments; H.E. analyzed data; H.E., Y.T., S.K., R. Kakigi, R.H., K.F., R. Kawamori, and H.W. interpreted results of experiments; H.E. prepared figures; H.E. and Y.T. drafted manuscript; H.E., Y.T., S.K., R. Kakigi, R.H., K.F., R. Kawamori, and H.W. edited and revised manuscript; H.E., Y.T., S.K., R. Kakigi, R.H., K.F., R. Kawamori, and H.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Miyuki Iwagami, Naoko Daimaru, Eriko Magoshi, Hiroko Tsujimura, and Sumie Ishikawa for their excellent technical assistance.
REFERENCES
- 1.Abrigo J, Rivera JC, Aravena J, Cabrera D, Simon F, Ezquer F, Ezquer M, Cabello-Verrugio C. High fat diet-induced skeletal muscle wasting is decreased by mesenchymal stem cells administration: implications on oxidative stress, ubiquitin proteasome pathway activation, and myonuclear apoptosis. Oxid Med Cell Longev 2016: 1–13, 2016. doi: 10.1155/2016/9047821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 3.Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14: 196–207, 2011. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrich DE, Ou Y, Melbouci L, Leduc-Gaudet JP, Auclair N, Mercier J, Secco B, Tomaz LM, Gouspillou G, Danialou G, Comtois AS, St-Pierre DH. Altered lipid metabolism impairs skeletal muscle force in young rats submitted to a short-term high-fat diet. Front Physiol 9: 1327, 2018. doi: 10.3389/fphys.2018.01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, Lloyd-Sherlock P, Epping-Jordan JE, Peeters GMEEG, Mahanani WR, Thiyagarajan JA, Chatterji S. The world report on ageing and health: a policy framework for healthy ageing. Lancet 387: 2145–2154, 2016. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruton JD, Katz A, Lännergren J, Abbate F, Westerblad H. Regulation of myoplasmic Ca(2+) in genetically obese (ob/ob) mouse single skeletal muscle fibres. Pflugers Arch 444: 692–699, 2002. doi: 10.1007/s00424-002-0882-1. [DOI] [PubMed] [Google Scholar]
- 8.Chan S, Head SI. Age- and gender-related changes in contractile properties of non-atrophied EDL muscle. PLoS One 5: e12345, 2010. doi: 10.1371/journal.pone.0012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi SJ, Files DC, Zhang T, Wang ZM, Messi ML, Gregory H, Stone J, Lyles MF, Dhar S, Marsh AP, Nicklas BJ, Delbono O. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J Gerontol A Biol Sci Med Sci 71: 557–564, 2016. doi: 10.1093/gerona/glv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciapaite J, van den Berg SA, Houten SM, Nicolay K, van Dijk KW, Jeneson JA. Fiber-type-specific sensitivities and phenotypic adaptations to dietary fat overload differentially impact fast- versus slow-twitch muscle contractile function in C57BL/6J mice. J Nutr Biochem 26: 155–164, 2015. doi: 10.1016/j.jnutbio.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 11.des Georges A, Clarke OB, Zalk R, Yuan Q, Condon KJ, Grassucci RA, Hendrickson WA, Marks AR, Frank J. Structural basis for gating and activation of RyR1. Cell 167: 145–157.e17, 2016. doi: 10.1016/j.cell.2016.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eshima H, Miura S, Senoo N, Hatakeyama K, Poole DC, Kano Y. Improved skeletal muscle Ca2+ regulation in vivo following contractions in mice overexpressing PGC-1α. Am J Physiol Regul Integr Comp Physiol 312: R1017–R1028, 2017. doi: 10.1152/ajpregu.00032.2017. [DOI] [PubMed] [Google Scholar]
- 13.Eshima H, Tamura Y, Kakehi S, Kurebayashi N, Murayama T, Nakamura K, Kakigi R, Okada T, Sakurai T, Kawamori R, Watada H. Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle. Physiol Rep 5: e13250, 2017. doi: 10.14814/phy2.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshima H, Tamura Y, Kakehi S, Nakamura K, Kurebayashi N, Murayama T, Kakigi R, Sakurai T, Kawamori R, Watada H. Dysfunction of muscle contraction with impaired intracellular Ca2+ handling in skeletal muscle and the effect of exercise training in male db/db mice. J Appl Physiol (1985) 126: 170–182, 2019. doi: 10.1152/japplphysiol.00048.2018. [DOI] [PubMed] [Google Scholar]
- 15.Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA 288: 1758–1761, 2002. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- 16.Funai K, Lodhi IJ, Spears LD, Yin L, Song H, Klein S, Semenkovich CF. Skeletal muscle phospholipid metabolism regulates insulin sensitivity and contractile function. Diabetes 65: 358–370, 2016. doi: 10.2337/db15-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funai K, Song H, Yin L, Lodhi IJ, Wei X, Yoshino J, Coleman T, Semenkovich CF. Muscle lipogenesis balances insulin sensitivity and strength through calcium signaling. J Clin Invest 123: 1229–1240, 2013. doi: 10.1172/JCI65726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González E, Messi ML, Delbono O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol 178: 175–183, 2000. doi: 10.1007/s002320010025. [DOI] [PubMed] [Google Scholar]
- 19.Graber TG, Kim JH, Grange RW, McLoon LK, Thompson LV. C57BL/6 life span study: age-related declines in muscle power production and contractile velocity. Age (Dordr) 37: 9773, 2015. doi: 10.1007/s11357-015-9773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gueugneau M, Coudy-Gandilhon C, Gourbeyre O, Chambon C, Combaret L, Polge C, Taillandier D, Attaix D, Friguet B, Maier AB, Butler-Browne G, Béchet D. Proteomics of muscle chronological ageing in post-menopausal women. BMC Genomics 15: 1165, 2014. doi: 10.1186/1471-2164-15-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill C, James RS, Cox VM, Tallis J. Does dietary-induced obesity in old age impair the contractile performance of isolated mouse soleus, extensor digitorum longus and diaphragm skeletal muscles? Nutrients 11: E505, 2019. doi: 10.3390/nu11030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurst J, James RS, Cox VM, Hill C, Tallis J. Investigating a dose-response relationship between high-fat diet consumption and the contractile performance of isolated mouse soleus, EDL and diaphragm muscles. Eur J Appl Physiol 119: 213–226, 2019. doi: 10.1007/s00421-018-4017-6. [DOI] [PubMed] [Google Scholar]
- 23.Jiao Q, Bai Y, Akaike T, Takeshima H, Ishikawa Y, Minamisawa S. Sarcalumenin is essential for maintaining cardiac function during endurance exercise training. Am J Physiol Heart Circ Physiol 297: H576–H582, 2009. doi: 10.1152/ajpheart.00946.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24: 933–941, 2001. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 25.Kelley RC, McDonagh B, Ferreira LF. Advanced aging causes diaphragm functional abnormalities, global proteome remodeling, and loss of mitochondrial cysteine redox flexibility in mice. Exp Gerontol 103: 69–79, 2018. doi: 10.1016/j.exger.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause MP, Riddell MC, Gordon CS, Imam SA, Cafarelli E, Hawke TJ. Diabetic myopathy differs between Ins2Akita+/− and streptozotocin-induced type 1 diabetic models. J Appl Physiol (1985) 106: 1650–1659, 2009. doi: 10.1152/japplphysiol.91565.2008. [DOI] [PubMed] [Google Scholar]
- 27.Lamboley CR, Wyckelsma VL, Dutka TL, McKenna MJ, Murphy RM, Lamb GD. Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J Physiol 593: 2499–2514, 2015. doi: 10.1113/JP270179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laver DR, Baynes TM, Dulhunty AF. Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J Membr Biol 156: 213–229, 1997. doi: 10.1007/s002329900202. [DOI] [PubMed] [Google Scholar]
- 29.Lee SR, Khamoui AV, Jo E, Park BS, Zourdos MC, Panton LB, Ormsbee MJ, Kim JS. Effects of chronic high-fat feeding on skeletal muscle mass and function in middle-aged mice. Aging Clin Exp Res 27: 403–411, 2015. doi: 10.1007/s40520-015-0316-5. [DOI] [PubMed] [Google Scholar]
- 30.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 41: 1234–1238, 2006. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Mosole S, Zampieri S, Furlan S, Carraro U, Löefler S, Kern H, Volpe P, Nori A. Effects of electrical stimulation on skeletal muscle of old sedentary people. Gerontol Geriatr Med 4: 2333721418768998, 2018. doi: 10.1177/2333721418768998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61: 72–77, 2006. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 34.Nikolova-Karakashian MN, Reid MB. Sphingolipid metabolism, oxidant signaling, and contractile function of skeletal muscle. Antioxid Redox Signal 15: 2501–2517, 2011. doi: 10.1089/ars.2011.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell K, Gannon J, Doran P, Ohlendieck K. Reduced expression of sarcalumenin and related Ca2+-regulatory proteins in aged rat skeletal muscle. Exp Gerontol 43: 958–961, 2008. doi: 10.1016/j.exger.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Oláh T, Bodnár D, Tóth A, Vincze J, Fodor J, Reischl B, Kovács A, Ruzsnavszky O, Dienes B, Szentesi P, Friedrich O, Csernoch L. Cannabinoid signalling inhibits sarcoplasmic Ca2+ release and regulates excitation-contraction coupling in mammalian skeletal muscle. J Physiol 594: 7381–7398, 2016. doi: 10.1113/JP272449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne AM, Zheng Z, González E, Wang ZM, Messi ML, Delbono O. External Ca(2+)-dependent excitation—contraction coupling in a population of ageing mouse skeletal muscle fibres. J Physiol 560: 137–155, 2004. doi: 10.1113/jphysiol.2004.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300: 1140–1142, 2003. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russ DW, Grandy JS, Toma K, Ward CW. Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function. Acta Physiol (Oxf) 201: 391–403, 2011. doi: 10.1111/j.1748-1716.2010.02191.x. [DOI] [PubMed] [Google Scholar]
- 40.Sabbadini RA, Danieli-Betto D, Betto R. The role of sphingolipids in the control of skeletal muscle function: a review. Ital J Neurol Sci 20: 423–430, 1999. doi: 10.1007/s100720050062. [DOI] [PubMed] [Google Scholar]
- 41.Sackmann-Sala L, Berryman DE, Munn RD, Lubbers ER, Kopchick JJ. Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity (Silver Spring) 20: 101–111, 2012. doi: 10.1038/oby.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shrestha LB. Population aging in developing countries. Health Aff (Millwood) 19: 204–212, 2000. doi: 10.1377/hlthaff.19.3.204. [DOI] [PubMed] [Google Scholar]
- 43.Sorrentino V. The ryanodine receptor family of intracellular calcium release channels. Adv Pharmacol 33: 67–90, 1995. doi: 10.1016/S1054-3589(08)60666-3. [DOI] [PubMed] [Google Scholar]
- 44.Tallis J, Hill C, James RS, Cox VM, Seebacher F. The effect of obesity on the contractile performance of isolated mouse soleus, EDL, and diaphragm muscles. J Appl Physiol (1985) 122: 170–181, 2017. doi: 10.1152/japplphysiol.00836.2016. [DOI] [PubMed] [Google Scholar]
- 45.Tallis J, James RS, Seebacher F. The effects of obesity on skeletal muscle contractile function. J Exp Biol 221: jeb163840, 2018. doi: 10.1242/jeb.163840. [DOI] [PubMed] [Google Scholar]
- 46.Tang W, Ingalls CP, Durham WJ, Snider J, Reid MB, Wu G, Matzuk MM, Hamilton SL. Altered excitation-contraction coupling with skeletal muscle specific FKBP12 deficiency. FASEB J 18: 1597–1599, 2004. doi: 10.1096/fj.04-1587fje. [DOI] [PubMed] [Google Scholar]
- 47.Wei L, Hanna AD, Beard NA, Dulhunty AF. Unique isoform-specific properties of calsequestrin in the heart and skeletal muscle. Cell Calcium 45: 474–484, 2009. doi: 10.1016/j.ceca.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Wilborn C, Beckham J, Campbell B, Harvey T, Galbreath M, La Bounty P, Nassar E, Wismann J, Kreider R. Obesity: prevalence, theories, medical consequences, management, and research directions. J Int Soc Sports Nutr 2: 4–31, 2005. doi: 10.1186/1550-2783-2-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida M, Minamisawa S, Shimura M, Komazaki S, Kume H, Zhang M, Matsumura K, Nishi M, Saito M, Saeki Y, Ishikawa Y, Yanagisawa T, Takeshima H. Impaired Ca2+ store functions in skeletal and cardiac muscle cells from sarcalumenin-deficient mice. J Biol Chem 280: 3500–3506, 2005. doi: 10.1074/jbc.M406618200. [DOI] [PubMed] [Google Scholar]
- 50.Zhao X, Yoshida M, Brotto L, Takeshima H, Weisleder N, Hirata Y, Nosek TM, Ma J, Brotto M. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol Genomics 23: 72–78, 2005. doi: 10.1152/physiolgenomics.00020.2005. [DOI] [PubMed] [Google Scholar]