Abstract
DNA damage accrued in induced pluripotent stem cell (iPSC)-derived cardiomyocytes during in vitro culture practices lessens their therapeutic potential. We determined whether DNA-damage-free iPSCs (DdF-iPSCs) can be selected using stabilization of p53, a transcription factor that promotes apoptosis in DNA-damaged cells, and differentiated them into functionally competent DdF cardiomyocytes (DdF-CMs). p53 was activated using Nutlin-3a in iPSCs to selectively kill the DNA-damaged cells, and the stable DdF cells were cultured further and differentiated into CMs. Both DdF-iPSCs and DdF-CMs were then characterized. We observed a significant decrease in the expression of reactive oxygen species and DNA damage in DdF-iPSCs compared with control (Ctrl) iPSCs. Next-generation RNA sequencing and Ingenuity Pathway Analysis revealed improved molecular, cellular, and physiological functions in DdF-iPSCs. The differentiated DdF-CMs had a compact beating frequency between 40 and 60 beats/min accompanied by increased cell surface area. Additionally, DdF-CMs were able to retain the improved molecular, cellular, and physiological functions after differentiation from iPSCs, and, interestingly, cardiac development network was prominent compared with Ctrl-CMs. Enhanced expression of various ion channel transcripts in DdF-CMs implies DdF-CMs are of ventricular CMs and mature compared with their counterparts. Our results indicated that DdF-iPSCs could be selected through p53 stabilization using a small-molecule inhibitor and differentiated into ventricular DdF-CMs with fine-tuned molecular signatures. These iPSC-derived DdF-CMs show immense clinical potential in repairing injured myocardium.
NEW & NOTEWORTHY Culture-stress-induced DNA damage in stem cells lessens their performance. A robust small-molecule-based approach, by stabilizing/activating p53, to select functionally competent DNA-damage-free cells from a heterogeneous population of cells is demonstrated. This protocol can be adopted by clinics to select DNA-damage-free cells before transplanting them to the host myocardium. The intact DNA-damage-free cells exhibited with fine-tuned molecular signatures and improved cellular functions. DNA-damage-free cardiomyocytes compared with control expressed superior cardiomyocyte functional properties, including, but not limited to, enhanced ion channel signatures. These DNA-intact cells would better engraft, survive, and, importantly, improve the cardiac function of the injured myocardium.
Keywords: DNA damage, iPS-CM, iPSCs, p53
INTRODUCTION
Regenerative medicine is facing challenges due to reduced ability, survival, and engraftment of cells derived from induced pluripotent stem cells (iPSCs; Ref. 38). Continuous, rapid, and large-scale expansion of cells is employed to achieve the high numbers needed for cell transplantation. Culture stress is induced when cells are rapidly propagated on/in inappropriate substrate (e.g., tissue culture plastic wares; Ref. 9), serum (most cells experience plasma, not serum, in vivo; Ref. 40), and oxidative stress (culturing in atmospheric O2, a hyperoxidative condition; Ref. 26). Unfortunately, iPSCs and iPSC-derived cardiomyocytes (iPS-CMs) often experience culture stress as any one or all of the stresses mentioned above are involved in culturing them. Reactive oxygen species (ROS) begin to accrue over time, causing DNA damage and leading to genomic instability in stem cells (25). Consequently, such propagated cell cultures would be of a heterogeneous nature: cells with lesser potential to survive and accumulated DNA-damaged and competent DNA-damage-free (DdF) cells. Differentiating and transplanting these heterogeneous populations of cells may experience poor survival, the ability to proliferate, and eventually portend the host myocardium as well. Therefore, strategies to select the functionally competent cells (devoid of DNA damage) from the heterogeneous pool to survive at the wound site (infarctions) of the host myocardium are in immediate need.
The transcription factor p53 controls the fate of cells by regulating their survival, growth, aging, and death (3, 4). p53 is activated in response to a wide range of cellular stresses, including oxidative stress, DNA damage, aberrant growth signals, and hypoxia. Depending on the stimuli, through transactivation of the appropriate target genes, p53 can activate cell cycle arrest, cellular senescence, or apoptosis in DNA-damaged cells while promoting cell cycle processes in healthy cells (3, 4, 14). With the use of this selective nature of p53, we stabilized p53 using a small-molecule inhibitor, Nutlin-3a (N3a), in iPSCs and selected DdF-iPSCs from the heterogeneous cell pool. We then differentiated these DdF-iPSCs into DNA-damage-free cardiomyocytes (DdF-CMs) and demonstrated their engraftment potentials in a mouse myocardial ischemia model. These DdF-CMs showed an enhanced engraftment rate compared with CMs differentiated from nonselected control iPSCs (15). Here, we have provided the selection of DdF cells and the molecular characterization of the DdF-iPSCs and DdF-CMs.
MATERIALS AND METHODS
Human iPSC culture.
The human iPSCs (hiPSCs) were previously generated and characterized by Dr. Zhang (14, 15, 22, 23, 41). Briefly, iPSCs were cultured and maintained on Matrigel membrane matrix (Corning)-coated plates using mTeSR1 medium (STEMCELL Technologies).
DdF-iPSC selection.
hiPSCs were treated with a murine double minute 2 (MDM2) inhibitor, N3a (SML0580; Sigma-Aldrich), at a concentration of 4 μM for 24 h. Following the treatment, the dead cells were gently washed off of the culture using PBS, and the remaining cells were cultured as mentioned above. Without further propagation, DdF-iPSCs were subjected to further analysis or differentiated into CMs.
Reactive oxygen species and superoxide measurement.
The quantities of reactive oxygen and superoxide species were monitored in live iPSCs using Cellular ROS/Superoxide Detection Assay Kit (ab139476) following the manufacturer’s instruction. Briefly, 2 × 105 cells were seeded into each well of 96-well black bottom plates (Corning). ROS and superoxide (SO) detection reagents from the kit were added to cells, and FITC and tetramethylrhodamine isothiocyanate channel fluorescence from the wells as a measure of ROS and SO, respectively, were quantified using fluorimeter. The average fluorescences of ROS and SO were calculated by dividing by DAPI fluorescence.
DNA damage analysis.
DNA damage in iPSCs was assessed by comet assay as previously described (13, 14, 23). Briefly, 2 × 105 cells were embedded in 70 μl of low-melting-point agarose and placed on a microscope slide. Slides were treated with alkaline lysis buffer to remove proteins and form nucleoids containing supercoiled DNA. Electrophoresis was performed to induce the formation of comets. Slides were stained with Vista Green dye and analyzed by fluorescence microscopy. The distance between the center of the head and the center of the tail, i.e., the tail moment length, was measured with ImageJ using comet assay plug-in. The tail moment was then calculated by the product of the percentage of damaged DNA and the tail moment length (13, 14, 23).
iPSC immunofluorescent staining.
iPSCs were grown on Matrigel-coated coverslips/chamber slides, fixed with 4% paraformaldehyde, and blocked with 3% BSA containing 10% normal donkey serum. The cells were then immunolabeled with rabbit anti-phospho-histone H2A.X antibody (cat. no. 9718; Cell Signaling Technology), mouse anti-phospho-p53 (Ser15) antibody (cat. no. 9286; Cell Signaling Technology), phalloidin (cat. nos. A22287 and A12380; Thermo Fisher Scientific), and pluripotent markers (ab109884; Abcam), and the nuclei were counterstained with DAPI (15, 23).
p53 Knockdown with siRNA.
The transcription factor p53 was knocked down with Silencer Pre-Designed siRNA (cat. no. am51331; Thermo Fisher Scientific) following manufacturer’s instruction. Briefly, iPSCs were culture as stated above, and when they reached 50% confluence siRNA transfection was performed. One hundred nanomolar either p53 siRNA or control siRNA was transfected into iPSCs using Lipofectamine RNAiMAX reagent (cat. no. 13778075; Thermo Fisher Scientific). Ambion In Vivo Negative Control #1 siRNA (cat. no. 4457287; Thermo Fisher Scientific) was used as control siRNA. p53 Knockdown was confirmed through transcript analysis.
p53 Ubiquitination.
The transcription factor p53 was immunoprecipitated using anti-p53 monoclonal antibody (cat. no. 2527; Cell Signaling Technology) from control iPSC and DdF-IPSC protein extracts. Immunoprecipitated proteins were separated on gels, and Western blot was performed to identify ubiquitinated p53 using anti-ubiquitin antibody (cat. no. 3933S; Cell Signaling Technology).
hiPSC-CM differentiation.
The hiPSCs were cultured until 95% confluent, and then hiPSC-CM differentiation was performed as described previously (10, 22, 23, 42). Briefly, the iPSCs were treated with 6 μM CHIR99021 (a GSK-3 inhibitor) in basal medium (RPMI 1640 medium supplemented with 2% B-27 Supplement minus insulin) for 24 h. Then, 72 h later, cells were treated with 5 μM IWR-1 (a Wnt inhibitor) for 48 h. Between the treatments, cells were cultured in basal medium, and following the treatment cells were cultured in growth medium (RPMI 1640 supplemented with 2% B-27). Beating CMs appeared on days 9–12 of this differentiation protocol. iPS-CMs from days 25 to 30 of the differentiation were used for further analysis.
iPS-CM immunofluorescent staining.
iPS-CMs were grown on Matrigel-coated coverslips/chamber slides, fixed with 4% paraformaldehyde, and blocked with 3% BSA containing 10% normal donkey serum. The cells were then immunolabeled with rabbit anti-human troponin T antibody (ab91605; Abcam) and rabbit anti-connexin 43 antibody (ab11370; Abcam), and the nuclei were counterstained with DAPI. After troponin T antibody labeling was performed, cells were blocked with rabbit IgG before connexin 43 labeling (15, 23).
RNA isolation and real-time quantitative polymerase chain reaction.
Total RNA was extracted from 3.0 × 106 cells using the Qiagen RNeasy kit (cat. no. 79306). After sample purity was confirmed via NanoDrop One C spectrophotometer (Thermo Fisher Scientific), 1–2 μg of RNA was used to synthesize cDNA. A 20-μl reaction containing 25–50 ng of cDNA templates, respective primer assays, and SYBR Green (cat. no. 218075; Qiagen) was amplified according to the manufacturer’s instructions in a Roche LightCycler 480 (Roche Life Science). Relative expression was determined according to the comparative cycle threshold method using GAPDH as housekeeping gene.
Next-generation RNA sequencing.
Total RNA was extracted from induced control (Ctrl) iPSCs, DdF-iPSCs, Ctrl-CMs, and DdF-CMs using RNeasy Mini Kit (cat. no. 74106; Qiagen; Ref. 33). Thermo Fisher Scientific NanoDrop One was used to quantify the RNA concentration and its quality. oligo(dT) magnetic beads were used to purify the intact poly(A) transcripts from total RNA, and mRNA sequencing libraries were prepared using the TruSeq Stranded mRNA Library Preparation Kit (cat. nos. RS-122-2101 and RS122-2102; Illumina). Detailed protocol for next-generation RNA sequencing (NGS) was discussed in our recent reports (28, 29). The RNA sequencing data sets are available in the supplemental material, all of which is at https://doi.org/10.6084/m9.figshare.11640159.
Ingenuity Pathway Analysis.
Following RNA sequence analysis, Ingenuity Pathway Analysis (IPA) was performed. For generating networks, a data set containing gene identifiers and corresponding expression values was uploaded into IPA. Each identifier was mapped to its corresponding object in Ingenuity Knowledge Base. A fold change cut-off of ±2 and q value <0.05 were set to identify molecules for which expression was significantly differentially regulated. These molecules, called Network Eligible Molecules, were overlaid onto a global molecular network developed from information contained in Ingenuity Knowledge Base. Network Eligible Molecules were then algorithmically generated based on their connectivity. The Functional Analysis identified the biological functions and/or diseases that were most significant to the entire data set. Molecules from the data set that met the fold change cut-off of ±2 and q value <0.05 and were associated with biological functions and/or diseases in Ingenuity Knowledge Base were considered for the analysis. Right-tailed Fisher exact test was used to calculate a P value determining the probability that each biological function and/or disease assigned to that data set is due to chance alone. Details on the number of molecules analyzed per sample by IPA are provided in Supplement 4.
Statistical analysis.
Data are presented as means ± SD, and each in vitro experiment was performed at least three times. Significance (P < 0.05) was determined via the Student’s t-test for comparisons between two groups and via two-way ANOVA for comparisons between three or more groups.
RESULTS
p53 Stabilization and selection of DdF-iPSCs.
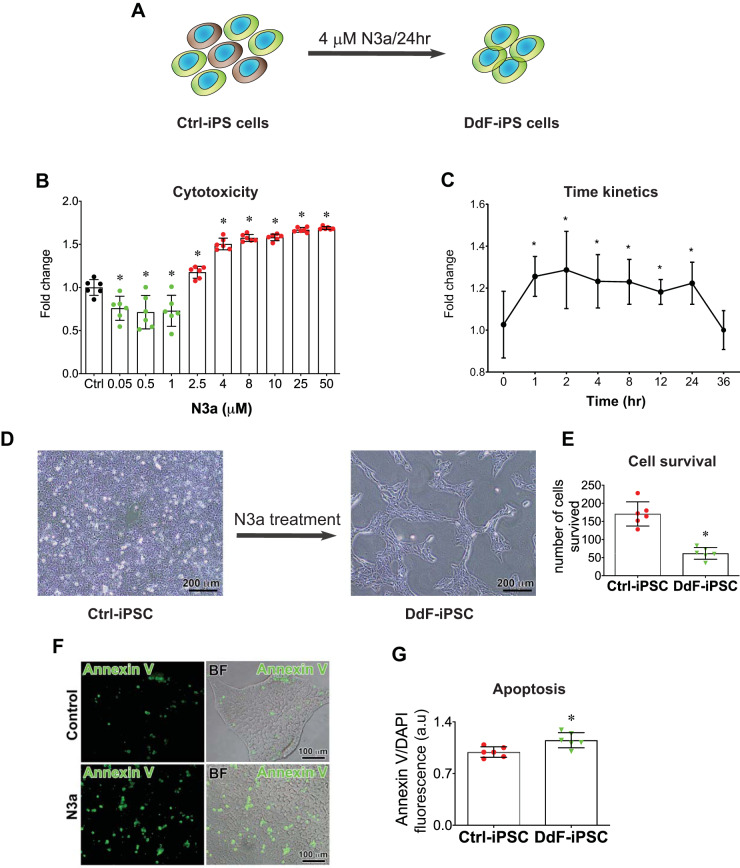
The primary cell fate regulator is the well-known tumor-suppressor protein p53. When p53 is activated in DNA-damaged cells, the cells either proceed with apoptosis or go into a state of cellular senescence (4). With the use of this discriminating nature of p53, we sought to isolate healthy cells from the pool of hiPSC cultures. hiPSCs were cultured as described and treated with N3a, an MDM2 inhibitor, to activate p53 signaling and selectively kill DNA-damaged cells. N3a binds and blocks the site on MDM2 where p53 binds. Thus p53 is released from MDM2 and becomes stabilized (Supplement 1, A–D). N3a treatment induced cell death in iPSCs in a dose- and time-dependent manner (Fig. 1, A–C). From the dose and time kinetics, we selected the dose 4 μM N3a and the treatment time 24 h (Fig. 1A) and treated iPSCs. A large number of cells were eliminated from the culture, and only a third of cells survived (Fig. 1, D and E). The elimination of the cells due to N3a treatment may be due to activation of the apoptotic pathway, as observed via annexin V staining (Fig. 1, F and G).
Fig. 1.
p53 Stabilization and selection of DNA-damage-free induced pluripotent stem cells (DdF-iPS cells). A: schematic showing the DdF-iPS cell selection. B: iPS cells were treated with different concentrations of Nutlin-3a (N3a) for 24 h, and cytotoxicity was evaluated using 3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Black, control (Ctrl); green, proliferative; red, cytotoxic; n = 6. ANOVA was performed. Data are means ± SD. *P < 0.05 vs. Ctrl. C: iPS cells were treated with 4 μM N3a at indicated time points, and cytotoxicity was evaluated using MTS assay (n = 3). ANOVA was performed. Data are means ± SD. *P < 0.05 vs. time 0. D and E: iPS cells (iPSC) were treated with 4 μM N3a for 24 h, and the number of cells that survived were counted (n = 6). t-test was performed. Data are means ± SD. *P < 0.05 vs. Ctrl-iPSC. F and G: iPS cells were treated with 4 μM N3a for 4 h, and the early apoptotic cell death was quantified by annexin V assay (n = 6). t-test was performed. Data are means ± SD. *P < 0.05 vs. Ctrl-iPSC. H: iPS cells were treated with 4 μM N3a for 4 h, and γH2A.X foci and phosphorylation of p53 at serine 15 (ser15) were identified by immunolabeling with corresponding antibodies. The area under the white box is shown in higher magnification on the right. I and J: control siRNA- and p53 siRNA-transfected iPS cells were treated with 4 μM N3a for 24 h, and γH2A.X foci were identified by immunolabeling. p53 Knockdown by siRNA was confirmed by transcript analysis using quantitative RT-PCR (J; n = 3). K: Western blot showing p53 and ubiquitination (Ub) of p53 in N3a-treated and nontreated iPS cells (control; left). Right: quantification of blots (n = 3). t-test was performed. Data are means ± SD. *P < 0.05 vs. Ctrl. R.Q., relative quantity; a.u., arbitrary units; BF, bright-field; IP, immunoprecipitation; ND, not determined.
The above-mentioned p53 stabilization by N3a, induction of apoptosis in damaged cells, and deactivation of p53 in normal cells are well documented in literature (19). Our results are also well aligned with the previous findings (19). DNA damage, identified by the presence of γH2A.X foci, and p53 phosphorylation and nuclear translocation in control iPSCs and N3a-treated iPSCs were analyzed by using immunofluorescent labeling and confocal imaging. The data indicate the presence of basal levels of γH2A.X foci and p53 Ser15 in Ctrl-iPSCs. The iPSCs were treated with N3a for 4 h to capture the initial p53 nuclear translocation. The N3a-treated cells showed no changes in γH2A.X foci and enhanced p53 Ser15 and nuclear localization (Fig. 1H). To further analyze the role of p53 in DdF cell selection, p53 was knocked down using siRNA. After p53 knockdown, DdF selection protocol was carried out and DNA damage was assessed in the control siRNA- and p53 siRNA-treated cells. Control siRNA-treated iPSCs yielded DdF cells as expected. However, p53 siRNA-treated iPSCs failed to produce DdF cells and were accompanied by γH2A.X foci (Fig. 1I). The p53 knockdown was confirmed by transcript quantification by RT-PCR (Fig. 1J). Together, these data clearly indicate that p53 plays a major role in DNA damage repair and producing DdF cells.
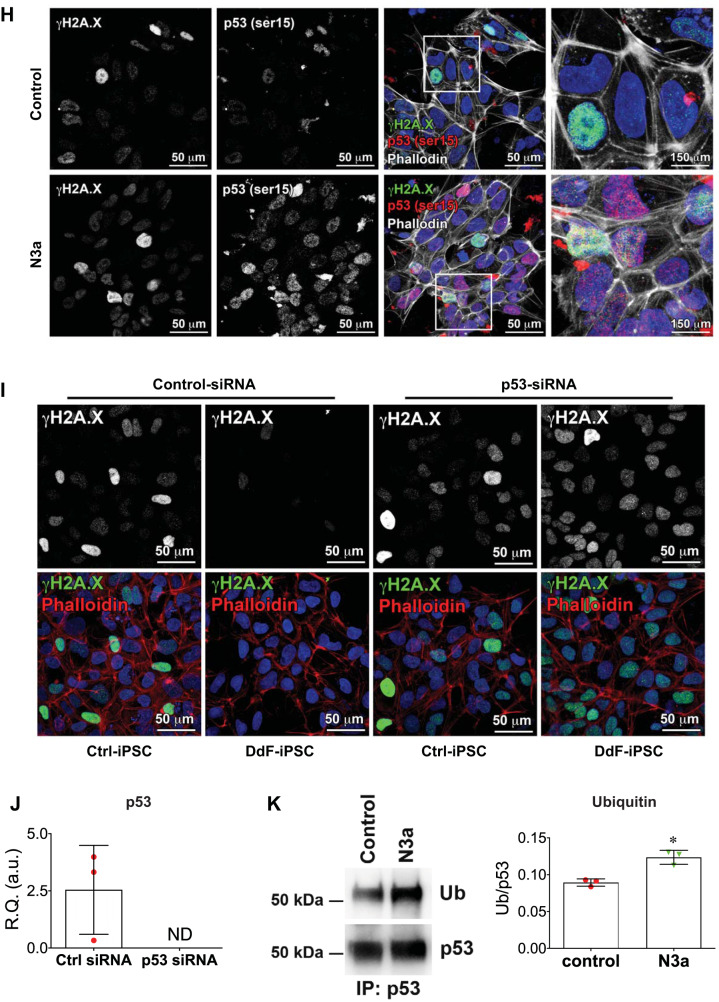
Whether N3a treatment had any effect on pluripotent marker expression in iPSCs was analyzed by immunofluorescence imaging (Fig. 2A) and transcript quantification (Fig. 2B). The results from both experiments showed that there are no significant differences between N3a-treated DdF-iPSCs and nontreated Ctrl-iPSCs. The iPSCs were then analyzed for reactive oxygen species (ROS) and superoxide (SO), a lethal form of ROS. ROS (including SO) was significantly reduced in DdF-iPSCs compared with Ctrl-iPSCs (Fig. 2, C–E). Then, the amount of DNA damage in iPSCs was quantified using comet assay, a standard method for assessing DNA fragmentation in cells (Fig. 2F). The amount and frequency of DNA damage (tail moment) were significantly reduced in DdF-iPSCs compared with their counterparts, indicating that the DdF-iPSCs have intact DNA strands. Together, these data clearly show that 1) treating iPSCs with 4 μM N3a for 24 h selectively removed the cells exhibiting accumulated oxidative stress and DNA damage and 2) the selected DdF-iPSCs retained the expression of pluripotent markers Oct4, Nanog, and Sox2.
Fig. 2.
Characterization of DNA-damage-free induced pluripotent stem cells (DdF-iPSCs). A: confocal micrographs of control (Ctrl) iPSCs and DdF-iPSCs showing expression of pluripotent markers Sox2, Oct4, and Nanog. B: DdF-iPSCs and Ctrl-iPSCs analyzed by quantitative RT-PCR for transcript expression of pluripotent markers Sox2, Oct4, and Nanog. (n = 6). t-test was performed. Data are means ± SD. C–E: reactive oxygen species (ROS; green) evaluated using fluorescence staining in Ctrl-iPSCs and DdF-iPSCs. Quantification of relative expression of ROS (D; n = 9) and superoxide (E; Ctrl, n = 8; DdF, n = 9) to number of cells is shown. t-test was performed. Data are means ± SD. *P < 0.05. F: DNA damage in nucleoids of Ctrl-iPSCs analyzed by comet assay. G: quantity of damaged DNA in nuclei of iPSCs in Ctrl-iPSCs (n = 58 comets) and DdF-iPSCs (n = 71 comets). t-test was performed. Data are means ± SD. *P < 0.05. R.Q., relative quantity; a.u., arbitrary units.
Characterization of DdF-iPSCs.
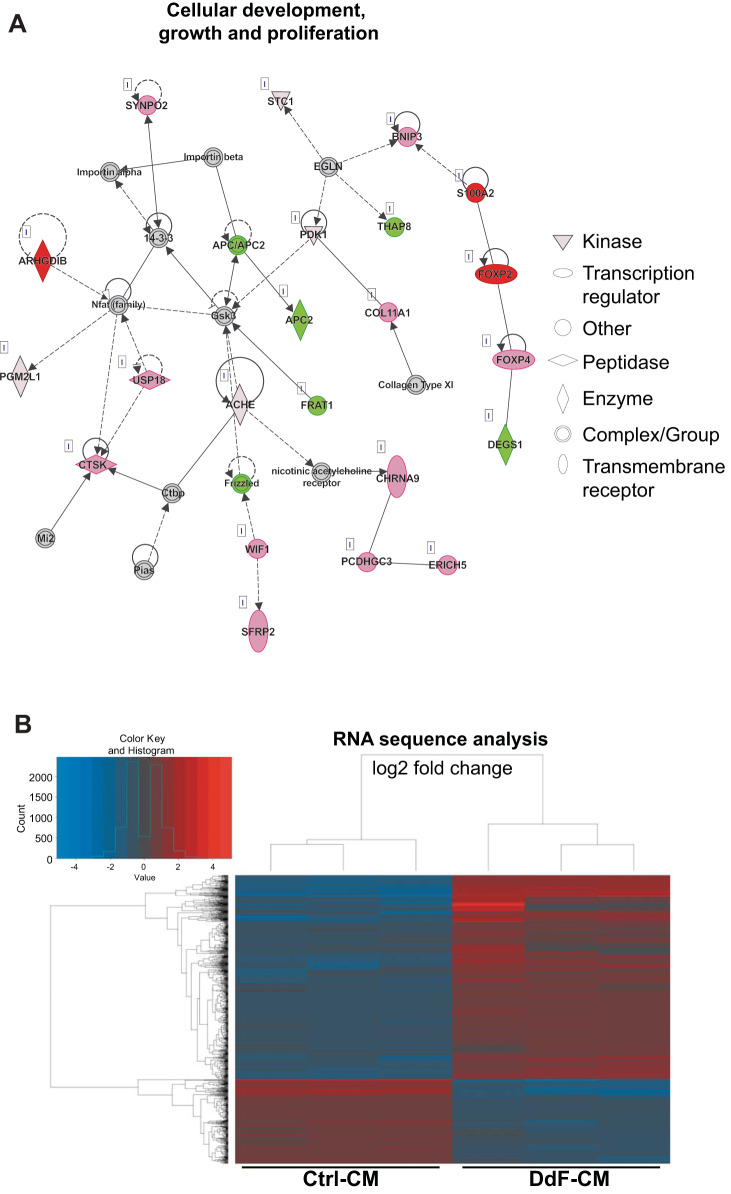
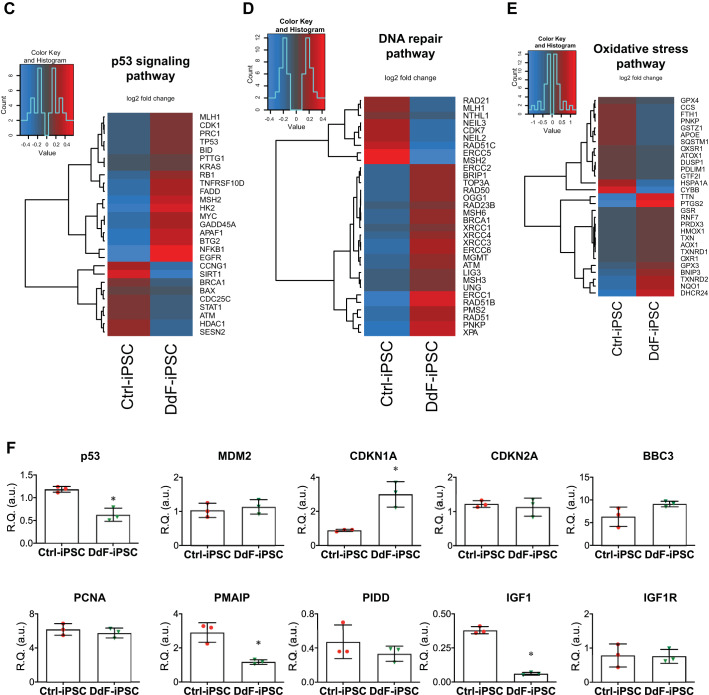
DNA damage affects the DNA integrity of cells. Accumulated DNA damage and mutations lead to loss or truncated protein expression, which would affect the regular functional dynamics of cells. To determine whether the molecular, cellular, and physiological functions of iPSCs were improved in DdF-iPSCs, NGS was performed on total RNAs isolated from Ctrl-iPSCs and DdF-iPSCs (Fig. 3, A–E; Supplement 2). Several genes were found to be differentially expressed in DdF-iPSCs compared with Ctrl-iPSCs (Fig. 3B). IPA on the RNA sequencing data revealed several genes and associated pathways that are responsible for molecular, cellular, and physiological functions were significantly upregulated in DdF-iPSCs. The top five hits of molecular and cellular functions and physiological system development are shown in Supplement 2, A and B, respectively. Most importantly, the cellular development, growth, and proliferation regulatory network in DdF-iPSCs was prominent compared with the Ctrl-iPSCs (Fig. 3A). Genes that are selectively involved in pathways such as p53 signaling (Fig. 3C), DNA repair (Fig. 3D), and oxidative stress (Fig. 3E) were selected from the NGS data, and a heat map was plotted. The gene expression levels obtained through NGS were validated by RT-PCR for p53-regulated genes such as MDM2, CDKN1A, CDKN2A, BBC3, PCNA, PMAIP, PIDD, IGF1, and IGF1R (Fig. 3F). Together, the RNA sequencing, IPA, and RT-PCR experiments show that DdF-iPSCs conceal a unique gene expression profile indicating DdF-iPSCs may have enhanced cellular functions compared with Ctrl-iPSCs.
Fig. 3.
Molecular characterization of DNA-damage-free induced pluripotent stem cells (DdF-iPSCs). RNA sequencing followed by Ingenuity Pathway Analysis was performed on control (Ctrl) iPSCs and DdF-iPSCs. A: cellular development, growth, and proliferation regulatory network was found to be well developed in DdF-iPSCs (continuous lines and arrows, direct relationship; broken lines and arrows, indirect relationship). B: a heat map of differentially expressed genes in Ctrl-iPSCs and DdF-iPSCs. The heat map visualizes 2-way hierarchical clustering of 980 differentially expressed genes in DdF-iPSCs compared with Ctrl-iPSCs (n = 3; P < 0.05, DdF-iPSC/Ctrl-iPSC log2 fold change ≥ 2). C–E: heat maps of genes that are involved in the p53 signaling pathway (C), DNA repair pathway (D), and oxidative stress signaling pathway (E). n = 3; P < 0.05, DdF-iPSC/Ctrl-iPSC fold change > 2. F: transcript expression levels of various genes quantified by RT-PCR in Ctrl-iPSCs and DdF-iPSCs. n = 3. Data are means ± SD. *P < 0.05 vs. Ctrl-iPSC. R.Q., relative quantity; a.u., arbitrary units.
DdF-iPSCs express enhanced cardiomyocyte differentiation.
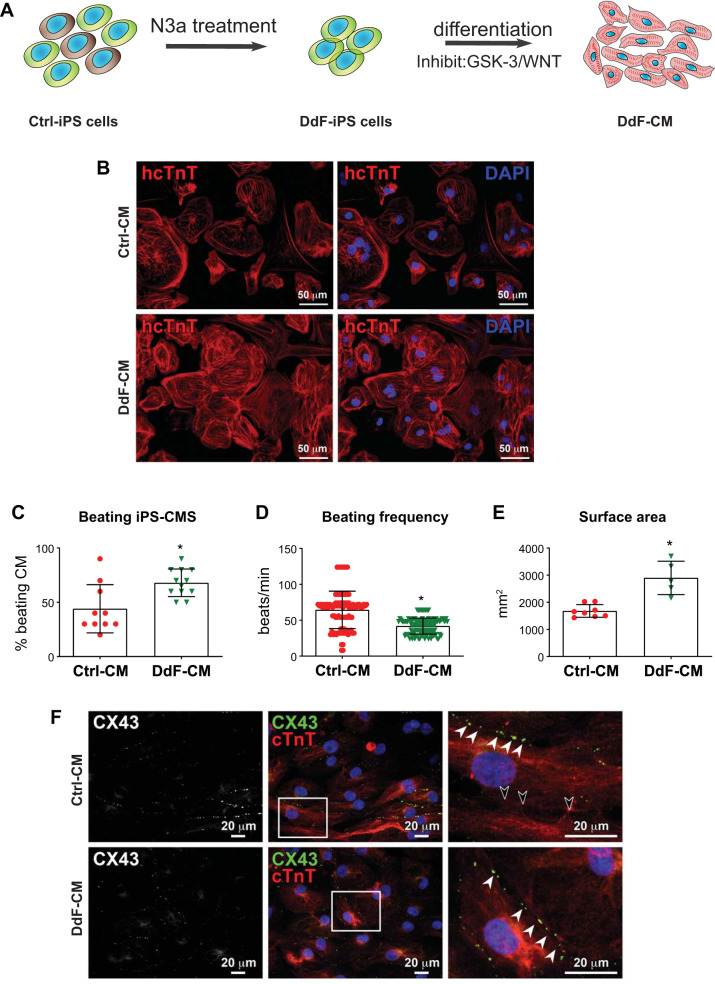
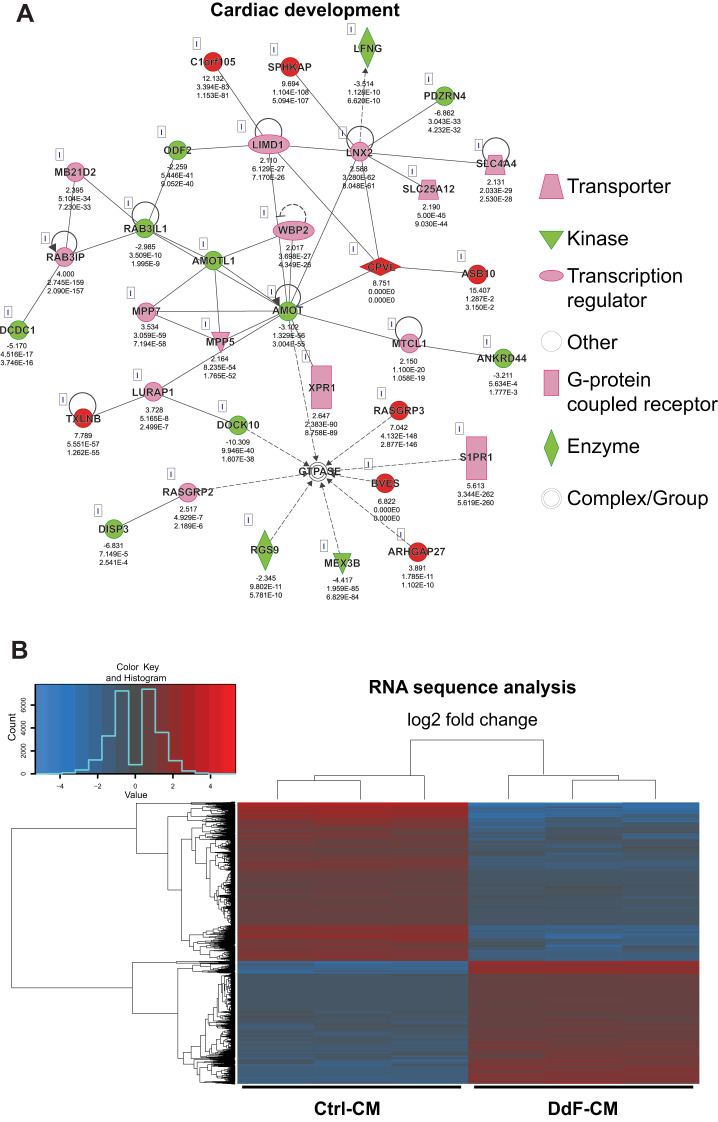
Whether the DdF-iPSCs with improved cellular functions allow for improved cardiomyocyte differentiation potential was then investigated. To address this critical question, Ctrl-iPSCs and DdF-iPSCs were differentiated into CMs (Fig. 4A) as previously described (41). Following differentiation, the cells were immunofluorescence-stained, and the micrograph is shown in Fig. 4B. Both Ctrl-CM and DdF-CM showed positivity for cardiac troponin T. The number of beating CMs following the differentiation protocol was counted manually. DdF-iPSCs differentiated into a significantly higher number of beating CMs compared with Ctrl-iPSCs (Fig. 4C). Beating frequency, i.e., the number of beats per minute, of the cells was also measured from videos obtained from the iPS-CM cultures. Whereas the beating frequency in Ctrl-CMs ranged from 16 to 124 beats/min, DdF-CM exhibited a compact, 30–64 beats/min range. This indicates, after transplanting, these homogenous beating DdF-CMs have the potential to synchronize well with endogenous myocyte beating and may minimize cell transplantation-induced arrhythmia in the host myocardium. The surface area of CMs, as measured from the fluorescent micrographs, was found to be significantly increased in DdF-CMs (Fig. 4E). Expression of the gap junction protein connexin 43 (CX43) in Ctrl-CM and DdF-CM was analyzed by immunofluorescent labeling. Both of the cell types expressed CX43 and formed gap junctions (Fig. 4F) with neighboring cells. However, CX43 expression in Ctrl-CM was not observed in all of the cells (Fig. 4F), whereas homogenous expression of CX43 is observed in DdF-CMs. To determine whether DdF-CMs continue to uphold their enhanced molecular signatures, RNA sequencing was performed on both Ctrl-CMs and DdF-CMs on day 30 of differentiation. Interestingly, several genes representing pathways involved in physiological system development and functions (Supplement 3A) as well as molecular and cellular functions (Supplement 3B) were upregulated in DdF-CMs compared with Ctrl-CMs. Most importantly, the cardiovascular development network was significantly improved in DdF-CM cells (Fig. 5A). A heat map differentially expressing genes between Ctrl-CMs and DdF-CMs is presented in Fig. 5B.
Fig. 4.
DNA-damage-free induced pluripotent stem cells (DdF-iPS cells) show enhanced cardiomyocyte (CM) differentiation. A: schematic of DdF-iPS cell selection and differentiation. B: immunolabeling of human-specific cardiac troponin T (hcTnT; red; all panels) and nuclei counterstained with DAPI (blue; right) in control (Ctrl) CM (top) and DdF-CM (bottom). C: the fraction of beating CMs generated by differentiating Ctrl-iPS and DdF-iPS cells. n = 3 (total 10–12 fields analyzed in each group) in all cases. Significance was analyzed by t-test. Data are means ± SD. *P < 0.05 vs. Ctrl-CM. D: frequency of beating by Ctrl-CM (n = 3, total 100 CMs) and DdF-CM (n = 3, total 120 CMs). t-test was performed. Data are means ± SD. *P < 0.05 vs. Ctrl-CM. E: the average surface area of Ctrl-CM and DdF-CM. n = 3 (total 5–8 fields analyzed in each group) in all cases. t-test was performed. Data are means ± SD. *P < 0.05 vs. Ctrl-CM. F: confocal imaging of the immunofluorescence-labeled gap junction protein connexin 43 (CX43) and cardiac troponin T in Ctrl-CM and DdF-CM (white arrowheads, CX43 in cell-to-cell junction; black arrowheads, absence of CX43 in cell-to-cell junction). N3a, nutlin-3a.
Fig. 5.
Characterization of DNA-damage-free cardiomyocytes (DdF-CMs). RNA sequencing followed by Ingenuity Pathway Analysis was performed on control (Ctrl) CMs and DdF-CMs. A: cardiac development regulatory network in DdF-CMs was upregulated compared with Ctrl-CMs (continuous lines and arrows, direct relationship; broken lines and arrows, indirect relationship). B: heat map of gene expression in Ctrl-CMs and DdF-CMs. The heat map visualizes 2-way hierarchical clustering of 4,245 differentially expressed genes in DdF-CMs compared with Ctrl-CMs (n = 3; P < 0.05, DdF-CM/Ctrl-CM log2 fold change ≥ 2). C and D: transcript expression levels of various differentiation and myocyte maturation markers quantified by RT-PCR in Ctrl-CMs and DdF-CMs. t-test was performed. n = 3. Data are means ± SD. *P < 0.05 vs. Ctrl-CM. R.Q., relative quantity; a.u., arbitrary units.
DdF-CMs express a mature cardiomyocyte phenotype.
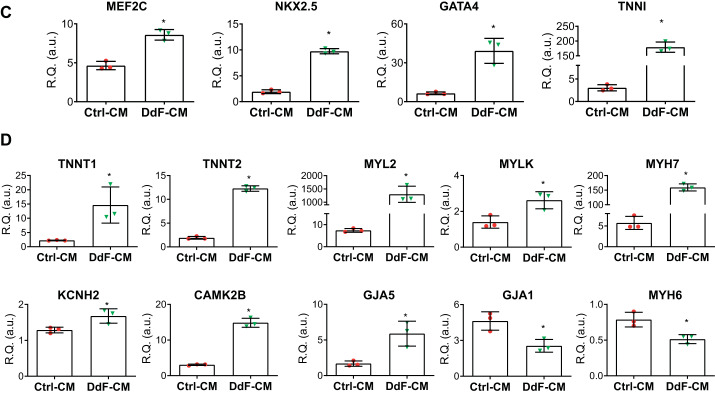
iPS-CMs in long-term culture yields mature CMs (12). To test whether continuous culture affects the CM maturation, both Ctrl-CMs and DdF-CMs were allowed to mature in culture and were analyzed on day 30. Whether an increase in surface area, as shown in Fig. 4E, is associated with differential expression of myocyte markers and to confirm the RNA sequencing data, several transcripts were assessed by RT-PCR. Myocyte markers MEF2C, NKX2.5, GATA4, and TNNI were significantly enriched in DdF-CM (Fig. 5C). Moreover, myocyte maturation markers TNNT, MYL2, MYLK, and MYH, potassium channel marker KCNH2, calcium channel marker CAMK2B, and gap junctional protein GJA5 were significantly enhanced in DdF-CM compared with Ctrl-CM (Fig. 5D).
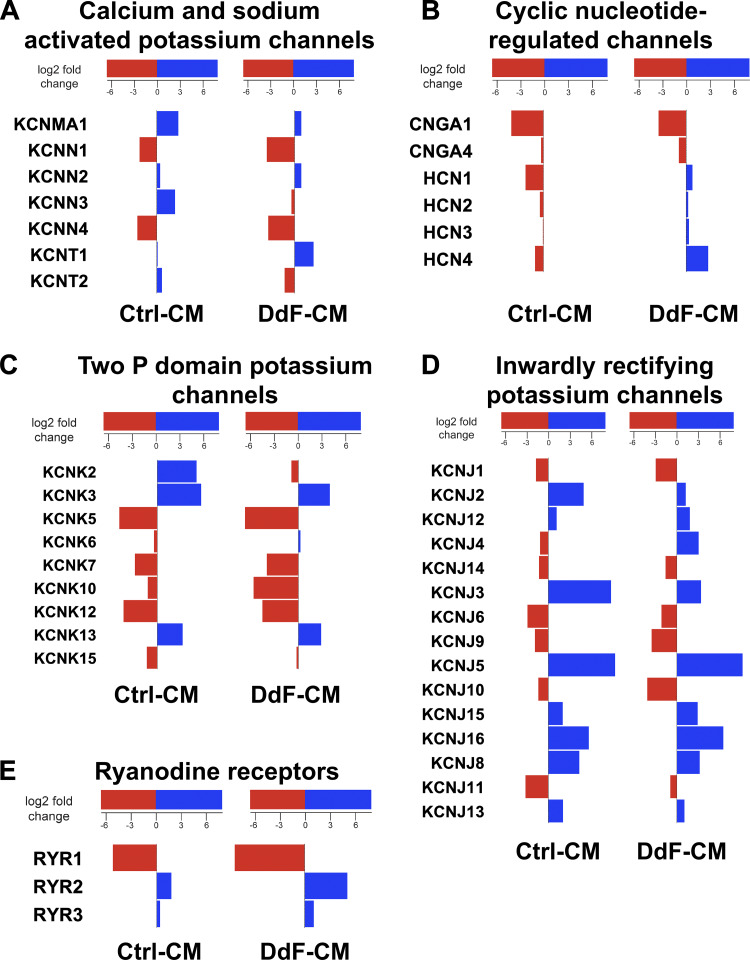
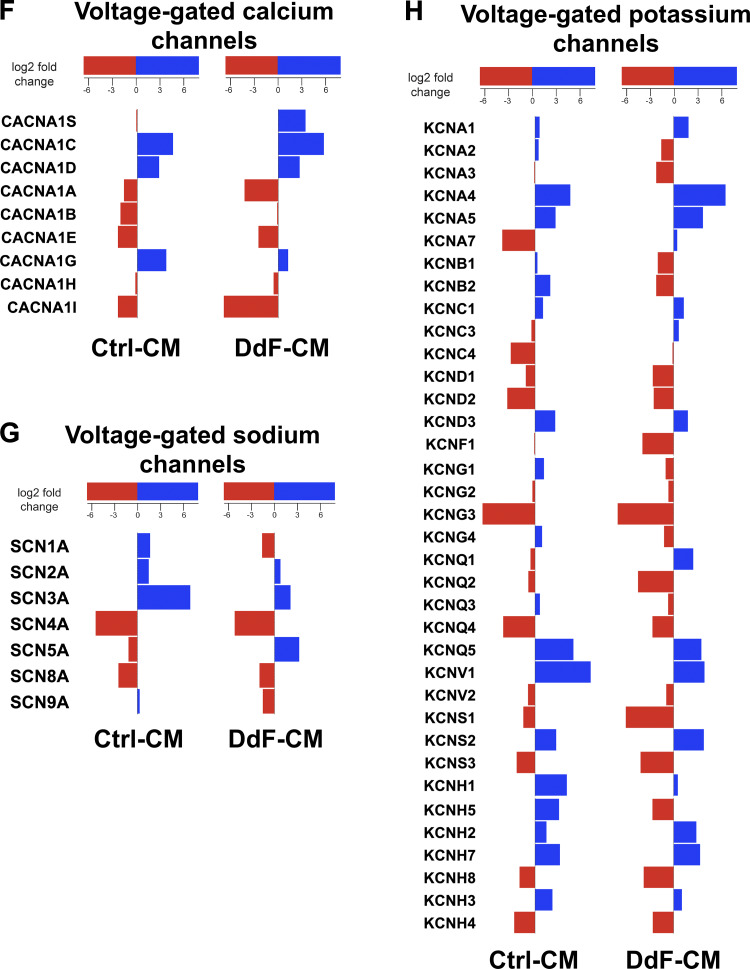
Transcript expression levels of various ion channels in Ctrl-CMs and DdF-CMs were analyzed using RNA sequencing. Notable differential gene expression was observed in specific calcium (Ca2+) and sodium (Na+) potassium-activated channels of the DdF-CMs compared with Ctrl-CMs. The most significant difference was seen in the downregulation of KCNN3 and KCNT2 as well as the upregulation of KCNT1. Small-conductance Ca2+-activated K+ (SK channels, KCa channels, KCNN) channels are widely expressed throughout the body, specifically in excitable cells where they aid in integrating changes in intracellular Ca2+ with membrane potential (1, 16, 18). Reduction in the expression of SK3 (Fig. 6A) suggests that the DdF-CMs were expressing stronger ventricular, rather than atrial, characteristics (34, 37). Increases in gene expression of the hyperpolarization-activated cyclic nucleotide-gated channels (Fig. 6B) not only support the increased rhythmicity demonstrated by the DdF-CMs, but also suggest that these cells may possess a more mature phenotype (2, 5). Most interesting to note, of the numerous shifts in two-pore-domain potassium channels (Fig. 6C), the downregulation of KCNK2 further supports the idea that the DdF-CMs are less likely to lead to arrhythmic events (7, 11). The expression of inward rectifier potassium channel genes, especially the upregulation of KCNJ4 (Fig. 6D), once again supports the fact that healthy, ventricular myocytes were generated from DdF-iPSCs (24, 27, 36).
Fig. 6.
p53 Stabilization mediates differential expression of ion channels in DNA-damage-free cardiomyocytes (DdF-CMs). RNA sequencing was performed on both control (Ctrl) CMs and DdF-CMs. Differentially expressed genes in calcium- and sodium-activated potassium channels (A), cyclic nucleotide-regulated channels (B), 2-pore (P)-domain potassium channels (which generally form “leak channels”; C), inwardly rectifying potassium channels (D), ryanodine receptors (E), voltage-gated calcium channels (F), voltage-gated sodium channels (G), and voltage-gated potassium channels (H) are shown. Red bars indicate downregulation, and blue bars indicate upregulation. n = 3. P < 0.05.
The RYR2 ryanodine receptor isoform is the primary cellular mediator of calcium-induced calcium release in mammalian cells and has been used as both an indicator of cellular function (in 2- vs. 3-dimensional culture) as well as a primary indicator of cellular maturity (17, 31, 32, 35). DdF-CMs expressed a significantly higher amount of RYR2 compared with their control counterparts (Fig. 6E). Ca2+-gated voltage channel associated with excitation-contraction coupling (Fig. 6F) was found to be significantly upregulated in DdF-CMs following in vitro culture, suggestive of the enhanced maturation of calcium handling capabilities (6, 8). The downregulation of mRNA levels of SCN1A (Fig. 6G) in the DdF-CMs can potentially also be used as an indicator of cell maturation. Especially of interest is the fact that SCN1A RNA levels have been found to increase immediately following birth but decrease in the weeks that follow (20). Furthermore, numerous changes in voltage-gated potassium channels (Fig. 6H) were observed. Of these, the upregulation of KCNQ1, for example, a potassium channel responsible for the slow delayed rectifier potassium currents, therefore advocates faster repolarization of the cell membrane, which can be linked to shorter action potential duration (30). Furthermore, an upregulation in KCNQ1 has been associated with the maturation of CMs, as its levels have been reported to be about twofold higher in adult hearts compared with human pluripotent stem cells (39).
Collectively, these data point to the fact that not only differentiating the DdF-iPSCs yields an increased number of CMs, but also these CMs possess improved physiological, molecular, and cellular functions. In addition to enhancing the overall functions of the CMs being cultured, the increase in the cellular surface area along with the enhanced ion channel transcript expression observed in DdF-CMs suggests that these cells also possess a more mature phenotype.
DISCUSSION
The cell genome can be damaged by multiple factors, including, but not limited to, their environment, oxidative metabolism, and errors in DNA replication or mitosis during the process of cell division (3). Necessarily, there are various options for the cells to undergo after this damage: they can attempt to repair this damage, become senescent, or go through apoptosis and die (3). Unfortunately, due to rapid and continuous culturing, DNA-damaged cells accumulate in the cell culture and become heterogeneous, containing both healthy and DNA-damaged cells. Transplanting these DNA-damaged and functionally incompetent cells results in poor engraftment into hosts and substantial downstream problems. Here, we demonstrate a robust method to select DdF cells from the pool of heterogeneous cells. Since this technique is based on the stabilization of p53 using a small molecule, the approach can be applied to any stem cell culture to select DdF cells for transplantation purposes. However, optimizations of N3a dose and treatment time are recommended for each cell type. Although the described method may be robust in eliminating DNA-damaged cells, whether N3a treatment will eliminate iPSCs with genetic defects and epigenetic modifications from the culture pools or even correct those defects cannot be answered now. Further experiments aiming for this direction may address these questions.
Multiple studies using gene manipulation techniques have shown enhanced engraftment, proliferation, and differentiation of transplanted cells (21, 42). For ethical reasons, cells with modified genes may never be used in the clinics. Moreover, the approaches of overexpressing or deleting one or two critical downstream genes to overcome physiological concerns are impermanent, and these approaches may fail when the alternative mechanisms are initiated by the cells and organs. Therefore, a robust approach to fine-tune upstream genes to obtain functionally competent cells with intact DNA was established in this study.
To our knowledge, this is the first study to show 1) p53 stabilization mediated elimination of DNA-damaged cells in culture, 2) DdF-iPSCs possessing improved molecular, cellular, and physiological functions, 3) DdF-iPSCs yield higher numbers of beating CMs, 4) DdF-CMs with compact beating frequencies, 5) DdF-CMs with enhanced cardiac development network supported by 4,245 genes, and 6) DdF-CMs with developed ion channel machinery. All of these novel findings make this a prominent study in the cardiac regeneration field.
Conclusion.
For the first time, we demonstrate that by stabilizing p53, a robust approach to select functionally competent cells from a heterogeneous population of cells, DNA-intact cells can be isolated. DdF-CMs expressed superior cardiomyocyte functional properties, including, but not limited to, enhanced ion channel signatures, reduced arrhythmogenic potential, as well as larger cell surface area, which could be attributed to a more mature phenotype. However, the morphological aspects of maturation, such as CX43 confinement to the intercalated disc in DdF-CM, are not encouraging. Collating all of the results from this study makes it abundantly evident that DdF-CMs have the potential to alleviate some of the issues challenged in current cardiac regeneration strategies.
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute (NHLBI) Grants R01-HL-95077, HL-114120, HL-131017, HL-138023, and U01-HL-134764 to J. Zhang, the American Heart Association Scientist Development Grant (17SDG33670677) to R. Kannappan, and NHLBI Grants 1-R01-HL-118067 and 2-R01-HL-118067 and start-up funds (Department of Pathology) to N. S. Rajasekaran.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Z. and R.K. conceived and designed research; J.M.M., N.M.M., N.S.R., and R.K. performed experiments; J.M.M., N.M.M., and R.K. analyzed data; J.M.M., N.M.M., D.P., P.K., N.S.R., J.Z., and R.K. interpreted results of experiments; R.K. prepared figures; D.P. and R.K. drafted manuscript; J.M.M., D.P., P.K., N.S.R., J.Z., and R.K. edited and revised manuscript; J.M.M., N.M.M., D.P., P.K., N.S.R., J.Z., and R.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Vasanthi Rajasekaran for excellent technical assistance.
REFERENCES
- 1.Barfod ET, Moore AL, Lidofsky SD. Cloning and functional expression of a liver isoform of the small conductance Ca2+-activated K+ channel SK3. Am J Physiol Cell Physiol 280: C836–C842, 2001. doi: 10.1152/ajpcell.2001.280.4.C836. [DOI] [PubMed] [Google Scholar]
- 2.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 83: 59–115, 2003. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 3.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol 11: S27–S31, 2001. doi: 10.1016/S0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 4.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120: 513–522, 2005. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Chan YC, Ting S, Lee YK, Ng KM, Zhang J, Chen Z, Siu CW, Oh SK, Tse HF. Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. J Cardiovasc Transl Res 6: 989–999, 2013. doi: 10.1007/s12265-013-9510-z. [DOI] [PubMed] [Google Scholar]
- 6.Chun YW, Balikov DA, Feaster TK, Williams CH, Sheng CC, Lee JB, Boire TC, Neely MD, Bellan LM, Ess KC, Bowman AB, Sung HJ, Hong CC. Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiomyocytes. Biomaterials 67: 52–64, 2015. doi: 10.1016/j.biomaterials.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decher N, Ortiz-Bonnin B, Friedrich C, Schewe M, Kiper AK, Rinné S, Seemann G, Peyronnet R, Zumhagen S, Bustos D, Kockskämper J, Kohl P, Just S, González W, Baukrowitz T, Stallmeyer B, Schulze-Bahr E. Sodium permeable and “hypersensitive” TREK-1 channels cause ventricular tachycardia. EMBO Mol Med 9: 403–414, 2017. doi: 10.15252/emmm.201606690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu JD, Rushing SN, Lieu DK, Chan CW, Kong CW, Geng L, Wilson KD, Chiamvimonvat N, Boheler KR, Wu JC, Keller G, Hajjar RJ, Li RA. Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS One 6: e27417, 2011. doi: 10.1371/journal.pone.0027417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fusenig NE, Boukamp P. Multiple stages and genetic alterations in immortalization, malignant transformation, and tumor progression of human skin keratinocytes. Mol Carcinog 23: 144–158, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y, Zhang J. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 137: 1712–1730, 2018. doi: 10.1161/CIRCULATIONAHA.117.030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein SA. Sodium leak through K2P potassium channels and cardiac arrhythmia, an emerging theme. EMBO Mol Med 9: 399–402, 2017. doi: 10.15252/emmm.201607479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, Yamanaka S, Kimura T. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J 77: 1307–1314, 2013. doi: 10.1253/circj.CJ-12-0987. [DOI] [PubMed] [Google Scholar]
- 13.Kannappan R, Matsuda A, Ferreira-Martins J, Zhang E, Palano G, Czarna A, Cabral-Da-Silva MC, Bastos-Carvalho A, Sanada F, Ide N, Rota M, Blasco MA, Serrano M, Anversa P, Leri A. p53 Modulates the fate of cardiac progenitor cells ex vivo and in the diabetic heart in vivo. EBioMedicine 16: 224–237, 2017. doi: 10.1016/j.ebiom.2017.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannappan R, Mattapally S, Wagle PA, Zhang J. Transactivation domain of p53 regulates DNA repair and integrity in human iPS cells. Am J Physiol Heart Circ Physiol 315: H512–H521, 2018. doi: 10.1152/ajpheart.00160.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannappan R, Turner JF, Miller JM, Fan C, Rushdi AG, Rajasekaran NS, Zhang J. Functionally competent DNA damage-free induced pluripotent stem cell-derived cardiomyocytes for myocardial repair. Circulation 140: 520–522, 2019. doi: 10.1161/CIRCULATIONAHA.119.040881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keating DJ, Rychkov GY, Roberts ML. Oxygen sensitivity in the sheep adrenal medulla: role of SK channels. Am J Physiol Cell Physiol 281: C1434–C1441, 2001. doi: 10.1152/ajpcell.2001.281.5.C1434. [DOI] [PubMed] [Google Scholar]
- 17.Keung W, Boheler KR, Li RA. Developmental cues for the maturation of metabolic, electrophysiological and calcium handling properties of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther 5: 17, 2014. doi: 10.1186/scrt406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273: 1709–1714, 1996. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 19.Kojima K, Konopleva M, McQueen T, O’Brien S, Plunkett W, Andreeff M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood 108: 993–1000, 2006. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause U, Alflen C, Steinmetz M, Müller MJ, Quentin T, Paul T. Characterization of maturation of neuronal voltage-gated sodium channels SCN1A and SCN8A in rat myocardium. Mol Cell Pediatr 2: 5, 2015. doi: 10.1186/s40348-015-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Li S. Effects of transplantation of hypoxia-inducible factor-1α genemodified cardiac stem cells on cardiac function of heart failure rats after myocardial infarction. Anatol J Cardiol 20: 318–329, 2018. doi: 10.14744/AnatolJCardiol.2018.91979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattapally S, Zhu W, Fast VG, Gao L, Worley C, Kannappan R, Borovjagin AV, Zhang J. Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice. Am J Physiol Heart Circ Physiol 315: H327–H339, 2018. doi: 10.1152/ajpheart.00688.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JM, Mardhekar NM, Rajasekaran V, Zhang J, Kannappan R. Assessing stem cell DNA integrity for cardiac cell therapy. J Vis Exp: 2019. doi: 10.3791/58971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morishige K, Takahashi N, Jahangir A, Yamada M, Koyama H, Zanelli JS, Kurachi Y. Molecular cloning and functional expression of a novel brain-specific inward rectifier potassium channel. FEBS Lett 346: 251–256, 1994. doi: 10.1016/0014-5793(94)00483-8. [DOI] [PubMed] [Google Scholar]
- 25.Nagaria P, Robert C, Rassool FV. DNA double-strand break response in stem cells: mechanisms to maintain genomic integrity. Biochim Biophys Acta 1830: 2345–2353, 2013. doi: 10.1016/j.bbagen.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 5: 741–747, 2003. [Erratum in Nat Cell Biol 5: 839, 2003.] doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Périer F, Radeke CM, Vandenberg CA. Primary structure and characterization of a small-conductance inwardly rectifying potassium channel from human hippocampus. Proc Natl Acad Sci USA 91: 6240–6244, 1994. doi: 10.1073/pnas.91.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quiles JM, Narasimhan M, Mosbruger T, Shanmugam G, Crossman D, Rajasekaran NS. Identification of transcriptome signature for myocardial reductive stress. Redox Biol 13: 568–580, 2017. doi: 10.1016/j.redox.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quiles JM, Narasimhan M, Shanmugam G, Milash B, Hoidal JR, Rajasekaran NS. Differential regulation of miRNA and mRNA expression in the myocardium of Nrf2 knockout mice. BMC Genomics 18: 509, 2017. doi: 10.1186/s12864-017-3875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribeiro MC, Tertoolen LG, Guadix JA, Bellin M, Kosmidis G, D’Aniello C, Monshouwer-Kloots J, Goumans MJ, Wang YL, Feinberg AW, Mummery CL, Passier R. Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro – correlation between contraction force and electrophysiology. Biomaterials 51: 138–150, 2015. doi: 10.1016/j.biomaterials.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 31.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556: 239–243, 2018. [Erratum in Nature 572: E16–E17, 2019.] doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M, Murry CE. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation 134: 1557–1567, 2016. doi: 10.1161/CIRCULATIONAHA.114.014998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanmugam G, Narasimhan M, Sakthivel R, Kumar R R, Davidson C, Palaniappan S, Claycomb WW, Hoidal JR, Darley-Usmar VM, Rajasekaran NS. A biphasic effect of TNF-α in regulation of the Keap1/Nrf2 pathway in cardiomyocytes. Redox Biol 9: 77–89, 2016. doi: 10.1016/j.redox.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vázquez AE, Young JN, Glatter KA, Chiamvimonvat N. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol 289: H2714–H2723, 2005. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 35.Veerman CC, Kosmidis G, Mummery CL, Casini S, Verkerk AO, Bellin M. Immaturity of human stem-cell-derived cardiomyocytes in culture: fatal flaw or soluble problem? Stem Cells Dev 24: 1035–1052, 2015. doi: 10.1089/scd.2014.0533. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation 98: 2422–2428, 1998. doi: 10.1161/01.CIR.98.22.2422. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vázquez AE, Yamoah EN, Chiamvimonvat N. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem 278: 49085–49094, 2003. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 38.Yanamandala M, Zhu W, Garry DJ, Kamp TJ, Hare JM, Jun HW, Yoon YS, Bursac N, Prabhu SD, Dorn GW 2nd, Bolli R, Kitsis RN, Zhang J. Overcoming the roadblocks to cardiac cell therapy using tissue engineering. J Am Coll Cardiol 70: 766–775, 2017. doi: 10.1016/j.jacc.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res 114: 511–523, 2014. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaswen P, Stampfer MR. Molecular changes accompanying senescence and immortalization of cultured human mammary epithelial cells. Int J Biochem Cell Biol 34: 1382–1394, 2002. doi: 10.1016/S1357-2725(02)00047-X. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Guo J, Zhang P, Xiong Q, Wu SC, Xia L, Roy SS, Tolar J, O’Connell TD, Kyba M, Liao K, Zhang J. Derivation and high engraftment of patient-specific cardiomyocyte sheet using induced pluripotent stem cells generated from adult cardiac fibroblast. Circ Heart Fail 8: 156–166, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu W, Zhao M, Mattapally S, Chen S, Zhang J. CCND2 overexpression enhances the regenerative potency of human induced pluripotent stem cell-derived cardiomyocytes: remuscularization of injured ventricle. Circ Res 122: 88–96, 2018. doi: 10.1161/CIRCRESAHA.117.311504. [DOI] [PMC free article] [PubMed] [Google Scholar]