Abstract
Background and Aims
Accessing the pancreatobiliary region in patients with a history of Roux-en-Y gastric bypass (RYGB) can be challenging. Traditionally, techniques such as percutaneous biliary drainage, enteroscopy-assisted ERCP, and laparoscopy-assisted ERCP have been used. However, each technique has its limitations. EUS–directed transgastric ERCP (EDGE) using a lumen-apposing metal stent (LAMS) has emerged as a novel endoscopic technique for ERCP in patients who have undergone RYGB. The aim of this case series was to highlight LAMS-related shortcomings and adverse events during the periprocedural period.
Methods
This was a retrospective review of 4 patients with RYGB anatomy who underwent EDGE for the management of pancreaticobiliary disease and experienced LAMS-related adverse events. Techniques for managing and avoiding these events are discussed.
Results
Four patients underwent EDGE with both technical and clinical success. Slight LAMS migration with partial mucosal overgrowth was encountered in 1 case and was managed by LAMS removal. A large, bleeding, distal marginal ulcer after the EDGE procedure was encountered in the second case and was managed with proton pump inhibitor and removal of the LAMS, with fistula treatment with argon plasma coagulation used to enhance closure. The third case was complicated by moderate intraprocedural bleeding after LAMS dilation, which was managed by applying balloon tamponade and placing a through-the-scope esophageal stent across the LAMS. Last, preferential food passage to the excluded stomach was noted in the fourth case and resulted in symptomatic distention. The symptomatic distention was managed by another de novo jejunogastrostomy using a LAMS for drainage.
Conclusions
Despite its feasibility and acceptable safety profile, the use of LAMSs during EDGE could be associated with several procedure-specific adverse events, which can be avoided or managed endoscopically with no further consequence.
Abbreviations: EDGE, EUS-directed transgastric ERCP; LAMS, lumen-apposing metal stent; RYGB, Roux-en-Y gastric bypass
Accessing the biliary tree in patients with surgically altered GI anatomy can be very challenging. Patients who have undergone Roux-en-Y gastric bypass (RYGB) pose a unique challenge because of multiple possible anatomic, technical, and logistical issues that tend to increase failure rates and prolong hospital stay.1, 2, 3, 4 Traditionally, techniques for managing pancreatobiliary disease in these patients involve enteroscopy-assisted and laparoscopy-assisted ERCP.3, 4, 5 Other techniques include percutaneous biliary drainage and EUS-guided biliary drainage. However, owing to the limitations6,7 of each technique, there is currently no well-defined algorithmic approach for performing ERCP in patients who have undergone RYGB.
Methods
EUS-directed transgastric ERCP (EDGE) has emerged as a novel technique for accessing the pancreatobiliary region in patients with RYGB anatomy. It involves deployment of a transgastric (or transjejunal) lumen-apposing metal stent (LAMS) under EUS guidance, with the stent then acting as a gateway to the excluded stomach.8 Once access to the excluded stomach is obtained, ERCP may be performed with a duodenoscope and standard ERCP instruments. Because the technique has a high success rate and acceptable safety profile,1,9 its use is growing among interventional endoscopists. However, as with other devices in interventional endoscopy, LAMS placement for gastrogastrostomy or jejunogastrostomy may have shortcomings or lead to adverse events. In this report, we will highlight 4 instructive cases of such adverse events.
Video description
Patient 1: Embedded Lumen-Apposing Metal Stent
A 50-year-old woman with a history of RYGB and cholecystectomy presented with right upper quadrant abdominal pain and elevated liver function test results. The patient underwent a successful EGDE with a jejunogastrostomy approach and use of a 20- × 10-mm LAMS. There were no acute adverse events, and liver function test results had a downward trend postprocedure. Interval history was noncontributory, and the patient returned for upper endoscopy follow-up 1 month postprocedure.
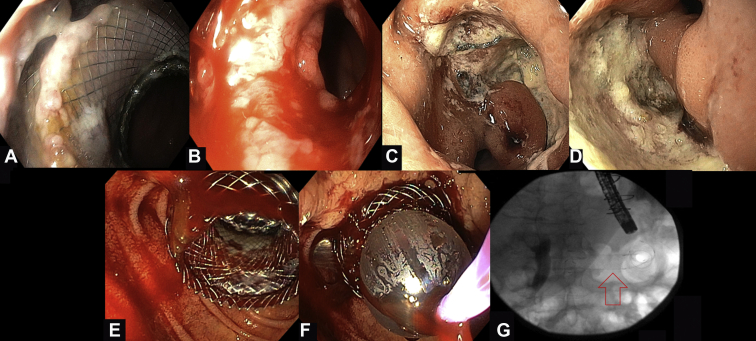
The previously deployed LAMS had migrated slightly into the excluded stomach, and it was partially embedded in the mucosa (Fig. 1A). Given the absence of any filling defects on cholangiography, the decision was made to remove the LAMS because there was no anticipated need for reintervention. Removal of the LAMS with a Raptor forceps (Rescue™ Retrieval Devices, Boston Scientific, Natick, Massachusetts) was difficult because of tissue growth over the stent. However, with application of moderate traction the stent was removed with no adverse events except for minor, self-limited bleeding (Fig. 1B; Video 1, available online at www.VideoGIE.org).
Figure 1.
Intraprocedural and postprocedural lumen-apposing metal stent (LAMS)-related adverse events during EUS–directed transgastric ERCP. A, Endoscopic view from the proximal end of the jejunogastrostomy 1 month after the procedure revealing the embedded LAMS. B, Endoscopic view right after the removal of the LAMS. Minor bleeding can be noted. C, D, Endoscopic images 9 months after the procedure. Two large, nonbleeding ulcers at the surgical gastrojejunostomy site. E, Moderate bleeding that occurred after dilation of the stent. F, Reinsertion of the balloon catheter through the stent and application of tamponade with 18-mm inflated balloon for 1.5 minutes. G, Fluoroscopic image showing the fully covered esophageal stent across the LAMS.
Patient 2: Large Marginal Ulcer Distal to the LAMS
A 51-year-old woman with a history of RYGB underwent cholecystectomy, which was complicated by iatrogenic biliary injury. The patient underwent single-session EGDE with a gastrogastric approach and use of a 20- × 10-mm LAMS; a plastic biliary stent (10F × 9 cm) was placed.
The patient was lost to follow-up; however, outside hospital records revealed that she was hospitalized twice for upper GI bleed from an ulcer at the surgical gastrojejunostomy site, requiring blood transfusion and endoscopic management. After the second hospitalization, the patient was referred back to our center. The LAMS was in situ across the gastrogastrostomy; however, 2 large, nonbleeding marginal ulcers were noted at the surgical gastrojejunostomy (Fig. 1C and D). The duodenoscope was advanced through the LAMS toward the ampulla, where cholangiography revealed no leak, and the biliary stent was removed. The LAMS was removed, and the fistula was treated with argon plasma coagulation to enhance spontaneous closure (Video 1, available online at www.VideoGIE.org).
Patient 3: Moderate Bleeding after LAMS Dilatation
A 69-year-old woman with a history of RYGB and choledocholithiasis underwent successful creation of a jejunogastrostomy with a 20- × 10-mm LAMS; however, upon stent dilation with an 18-19-20–mm balloon, moderate bleeding was noted, necessitating the reintroduction of the balloon across the LAMS to tamponade the bleeding (Fig. 1E and F). After 1.5 minutes of tamponade, the bleeding ceased and ERCP was carried out successfully. On withdrawal of the duodenoscope after ERCP, active bleeding was noted again at the site of the LAMS. This was treated with placement of an 18- × 80-mm fully covered through-the-scope esophageal stent across the LAMS to further tamponade the site (Fig. 1G).
Two days postprocedure, the patient reported abdominal pain and had 1 episode of melenic stool. CT imaging showed a large hematoma in the stomach and proximal jejunum with no active bleeding. Because her hemoglobin level was stable, the patient was managed conservatively. One month later, both stents were removed, and the tract was treated with argon plasma coagulation (Video 2, available online at www.VideoGIE.org).
Patient 4: Preferential Food Passage to the Excluded Stomach after EDGE
A 72-year-old woman with a history of RYGB presented with painless obstructive jaundice. Initial imaging suggested a diagnosis of pancreatic cancer. The patient underwent successful creation of a gastrogastrostomy with a 20- × 10-mm LAMS, which was followed by EUS-guided fine-needle biopsy and ERCP during the same session. No procedural adverse events occurred.
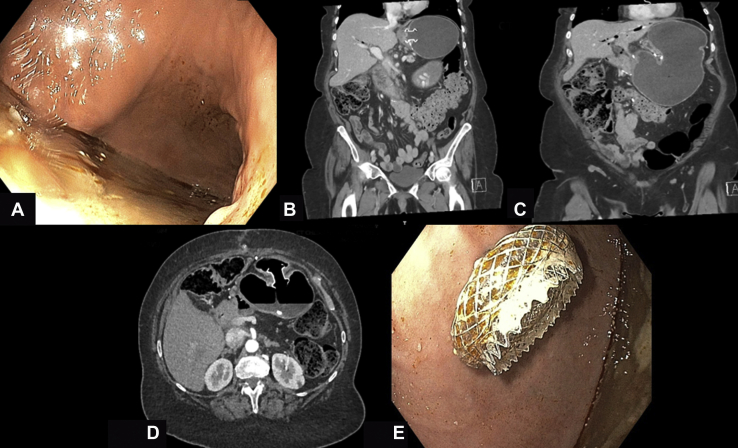
A diagnosis of pancreatic adenocarcinoma was confirmed. A month later, the patient underwent a successful Whipple procedure. The surgeon elected to leave the bypass anatomy intact because of the presence of the gastrogastrostomy. Shortly after surgery, the patient experienced persistent reflux, nausea, and vomiting. On CT, progressive dilatation of the excluded stomach was noted, whereas the gastric pouch and efferent limb remained decompressed (Fig. 2B and C).
Figure 2.
Decompression of the excluded stomach after a Whipple procedure in the setting of a previously created gastrogastrostomy. A, Endoscopic view of the excluded stomach after the advancement of the endoscope through the gastrogastric fistula. A copious amount of food material can be noted. B, C, Computed tomography (CT) imaging of the abdomen showing significant dilation of the excluded stomach. D, Abdominal CT scan showing the lumen-apposing metal stent (LAMS) in the de novo–created jejunogastric fistula. E, Endoscopic view of the distal side of the newly deployed LAMS as seen from the excluded stomach.
On endoscopy, the LAMS was noted to be just distal to the gastroesophageal junction, resulting in preferential and direct passage of food into the excluded stomach. Copious amounts of liquid and solid contents were noted in the excluded stomach (Fig. 2C). Hence, a jejunogastrostomy was created using a 20- × 10-mm LAMS under fluoroscopic and EUS guidance (Fig. 2D and E). This resulted in a rapid symptom resolution. Successively, 1 week later, the proximal gastrogastrostomy was closed by the removal of the proximal LAMS, and closure of the fistula was accomplished with endoscopic suturing (Video 2, available online at www.VideoGIE.org).
Conclusion
Mucosal overgrowth over LAMSs has been reported in the literature,10,11 and its removal can be technically challenging and result in bleeding. We hypothesize that a LAMS deployed in the antrum as opposed to the proximal stomach is more prone to embedment or migration. This may contribute to the more pronounced wall contractions in the antrum. Consider creating gastrogastrostomy or gastrostomy with access to the proximal rather than distal excluded stomach.
A marginal ulcer can develop at the gastrojejunal anastomosis distal to the LAMS as the result of increased exposure to acidic secretions from the excluded stomach. Our current standard of care is to advise all patients to continue use of proton pump inhibitors until documentation of fistula closure. This merits further studies to consider the benefits of proton pump inhibitor treatment in patients after EDGE.
Significant bleeding after LAMS placement can be managed with tamponading (using a balloon or via placement of a second stent across the LAMS).
The proximity of the LAMS to the gastroesophageal junction might lead to preferential passage of food to the excluded stomach, leading to distention. This might be problematic in the rare instances in which a patient has undergone multiple reconstructive surgeries, and drainage of the excluded stomach is not optimal. In general, a LAMS used for the creation of a de novo fistula in EDGE should be distant enough from the gastroesophageal junction to prevent preferential passage of food to the excluded stomach.
Disclosure
M.A. Khashab is a consultant for Boston Scientific, Olympus, and Medtronic. All other authors disclosed no financial relationships.
Supplementary data
Partial lumen-apposing metal stent (LAMS) embedment and migration after endoscopic ultrasonography (EUS)-guided jejunogastrostomy (Case 1). Large marginal ulcer distal to LAMS after EUS-guided gastrogastrostomy (Case 1).
Moderate bleeding after lumen-apposing metal stent dilatation after endoscopic ultrasonography (EUS)-guided jejunogastrostomy (Case 3). Preferential food passage to the excluded stomach after EUS-guided gastrogastrostomy (Case 4).
References
- 1.James T.W., Baron T.H. Endoscopic ultrasound-directed transgastric ERCP (EDGE): a single-center US experience with follow-up data on fistula closure. Obes Surg. 2019;29:451–456. doi: 10.1007/s11695-018-3531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner M., Popa D., Neumann H. ERCP with the overtube-assisted enteroscopy technique: a systematic review. Endoscopy. 2014;46:560–572. doi: 10.1055/s-0034-1365698. [DOI] [PubMed] [Google Scholar]
- 3.Schreiner M.A., Chang L., Gluck M. Laparoscopy–assisted versus balloon enteroscopy–assisted ERCP in bariatric post–Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75:748–756. doi: 10.1016/j.gie.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Abbas A.M., Strong A.T., Diehl D.L. Multicenter evaluation of the clinical utility of laparoscopy-assisted ERCP in patients with Roux-en-Y gastric bypass. Gastrointest Endosc. 2018;87:1031–1039. doi: 10.1016/j.gie.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee N., Parepally M., Byrne T.K. Systematic review of transgastric ERCP in Roux-en-Y gastric bypass patients. Surg Obes Relat Dis. 2017;13:1236–1242. doi: 10.1016/j.soard.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Inamdar S., Slattery E., Sejpal D.V. Systematic review and meta-analysis of single-balloon enteroscopy–assisted ERCP in patients with surgically altered GI anatomy. Gastrointest Endosc. 2015;82:9–19. doi: 10.1016/j.gie.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Jirapinyo P., Lee L.S. Endoscopic ultrasound-guided pancreatobiliary endoscopy in surgically altered anatomy. Clin Endosc. 2016;49:515–529. doi: 10.5946/ce.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kedia P., Sharaiha R.Z., Kumta N.A. Internal EUS-directed transgastric ERCP (EDGE): game over. Gastroenterology. 2014;147:566–568. doi: 10.1053/j.gastro.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 9.Runge T.M., Thomas E., Baron T.H. Living on the edge-success, long-term complications, and implications following EUS-directed transgastric ERCP: a multicenter study [abstract] Gastrointest Endosc. 2019;89:AB131. [Google Scholar]
- 10.Bang J.Y., Hasan M., Navaneethan U. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut. 2017;66:2054–2056. doi: 10.1136/gutjnl-2016-312812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabbri C., Luigiano C., Marsico M. A rare adverse event resulting from the use of a lumen-apposing metal stent for drainage of a pancreatic fluid collection: “the buried stent.”. Gastrointest Endosc. 2015;82:585–587. doi: 10.1016/j.gie.2015.04.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Partial lumen-apposing metal stent (LAMS) embedment and migration after endoscopic ultrasonography (EUS)-guided jejunogastrostomy (Case 1). Large marginal ulcer distal to LAMS after EUS-guided gastrogastrostomy (Case 1).
Moderate bleeding after lumen-apposing metal stent dilatation after endoscopic ultrasonography (EUS)-guided jejunogastrostomy (Case 3). Preferential food passage to the excluded stomach after EUS-guided gastrogastrostomy (Case 4).