Abstract
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is a new rapidly spreading infectious disease. Current guidance from the World Health Organization (WHO) highlights asthmatics as a high-risk group for severe illness from COVID-19. Viruses are common triggers of asthma exacerbations and the current SARS-CoV-2 pandemic raises several questions regarding the optimum management strategies. Here, we discuss the contentious issue of whether the mainstay therapy systemic corticosteroids should be used in the routine management of COVID-19-associated asthma exacerbations. Recent guidance from the WHO has advised against the use of corticosteroids if COVID-19 is suspected due to concerns that these agents may impair protective innate antiviral immune responses. This may not be appropriate in the unique case of asthma exacerbation, a syndrome associated with augmented type 2 inflammation, a disease feature that is known to directly inhibit antiviral immunity. Corticosteroids, through their suppressive effects on type 2 inflammation, are thus likely to restore impaired antiviral immunity in asthma and, in contrast to non-asthmatic subjects, have beneficial clinical effects in the context of SARS-CoV-2 infection.
Keywords: asthma, corticosteroid, COVID-19, exacerbation
INTRODUCTION
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is a new, rapidly spreading infectious disease. Current guidance from the World Health Organization (WHO) highlights asthmatics as a high-risk group for severe illness from COVID-19 (2) and widespread shielding of these patients has been advocated. Published case series of COVID-19 to date have not reported asthma as a common comorbidity (22) and there is currently limited evidence to inform on the optimum management of COVID-19-associated asthma exacerbations. Here, we discuss the contentious issue of whether the mainstay therapy systemic corticosteroids should be used in the routine management of COVID-19-associated asthma exacerbations.
VIRUSES AND SARS-CoV-2 AS TRIGGERS FOR ASTHMA EXACERBATIONS
Asthma is a chronic respiratory condition punctuated by the occurrence of acute symptomatic deteriorations (“exacerbations”). The link between virus infection and exacerbations is well established: rhinoviruses are the most commonly isolated pathogen at exacerbation (∼50–80% of virally triggered episodes) with a range of other viruses (including coronaviruses) less frequently identified (17, 23). Experimental human infection challenge studies have confirmed unequivocally that viruses play a causal role in precipitating asthma exacerbations (10), driving augmented airway inflammation, mucus hypersecretion, and lower respiratory tract symptoms (8, 10, 12). As with other coronaviruses, SARS-CoV-2 is anticipated to similarly exacerbate disease, although the precise immunopathological mechanisms through which this occurs are yet to be characterized.
SYSTEMIC CORTICOSTEROIDS IN ASTHMATICS WITH COVID-19: BENEFICIAL OR HARMFUL?
Current asthma guidelines advocate treatment with oral corticosteroids in acute exacerbations [typically oral prednisolone, 40–50 mg for 5–7 days (1)] and this therapy is prescribed almost ubiquitously for hospitalized episodes. Asthma exacerbations are associated with augmented airway inflammation which drives increased respiratory symptoms (10); corticosteroids are broad immunosuppressive agents that reduce these features to promote clinical recovery. Conversely, recent guidance from the WHO advises against the use of corticosteroids if COVID-19 is suspected, although with the caveat that they may be considered if there is underlying asthma or COPD (2). The recommendation of avoidance has been formulated based on previous data showing that, despite potentially beneficial anti-inflammatory effects, corticosteroids (inhaled or systemic) can inhibit production of the critical antiviral mediators type I and III interferons. This has been shown in a range of in vitro and in vivo human and animal studies for several asthma-relevant viruses including rhinovirus, influenza, and respiratory syncytial virus (RSV) (7, 18, 19). These effects precipitate increased virus replication (7, 18, 19) and augment virus-driven pathology including mucus hypersecretion and secondary bacterial infections (18). Similar detrimental effects are expected to occur with the use of corticosteroids in the context of COVID-19. Accordingly, studies in patients with other coronavirus infections (e.g., SARS-CoV-1, MERS-CoV) have shown that corticosteroids increase viremia and delay viral clearance with no evidence of clinical benefit (16).
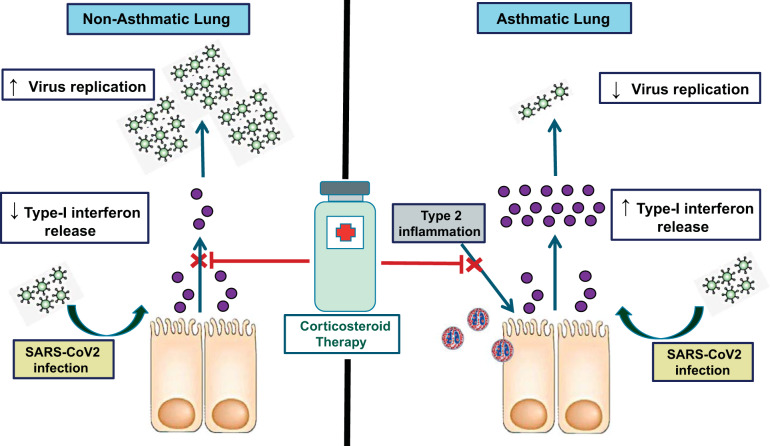
Asthma is associated with an inherent impairment of interferon responses to virus infection (4, 20), and therefore, in the context of overwhelming viral illness, further inhibition of interferon by corticosteroids in an already deficient state could have deleterious consequences. This leaves clinicians with the conundrum of whether oral corticosteroids, which are effective guideline-recommended therapies for asthma exacerbations, should be used in COVID-19-related exacerbation. However, multiple other lines of evidence in asthma indicate that certain disease mechanisms might counterbalance any potential adverse effects upon antiviral immunity. Increased T-helper cell 2 (Th2) inflammation is present in a large proportion of asthmatic subjects and is augmented upon viral infection (10, 12). Sputum eosinophilia correlates negatively with impaired IFN induction in cultured asthmatic cells (4) and Th2 mediators (IL-4, IL-13) can directly inhibit epithelial production of type I interferon (3). Interferon-α can additionally suppress Th2 cell polarization in T cell or mixed leukocyte culture models, attenuating expression of GATA3, IL-4, IL-5, and IL-13 (9, 14). Furthermore, mice deficient in the type I IFN receptor-deficient mice (Ifnar−/−) have augmented pulmonary eosinophilia and type 2 inflammation in response to influenza infection (5). As corticosteroids suppress type 2 inflammation, their use in the context of COVID-19-associated exacerbation may thus lead to the beneficial effect of secondary restoration of impaired antiviral immunity (see Fig. 1). This was suggested by a previous study showing that inhaled budesonide did not impair CD8+ T cell infiltration into the bronchial epithelium following experimental rhinovirus infection in asthmatics (6) contrary to the clear suppressive effects of corticosteroids on T cells observed in the absence of preexisting Th2 inflammation (18). Accordingly, in a recent report of histopathological autopsy findings from a nonasthmatic patient with COVID-19 treated with systemic corticosteroids, suppressed peripheral blood CD8+ T cell numbers were observed (21).
Fig. 1.
Proposed differences between effects of corticosteroid therapy in asthmatic and nonasthmatic subjects infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
In a clinical setting, objective evidence of augmented type 2 inflammation could be ascertained by the presence of bronchoconstriction (since type 2 mediators, particularly IL-13, are major drivers of airway hyperresponsiveness) or by measurement of objective biomarkers of type 2 inflammation such as blood eosinophils. Interestingly, in case series to date, SARS-CoV-2 infection appears to be associated with low blood eosinophils (15). A future hypothesis to explore is whether the presence of normal or elevated eosinophils in asthmatics infected with SARS-CoV-2 may reflect a type 2 inflammatory process that could be used as a biomarker for corticosteroid therapy. Although systemic steroids are highly effective in eosinophilic disease, exacerbations of airways disease are heterogeneous and viral exacerbations may also be characterized by neutrophilic inflammation (11) which is typically less responsive to steroids. It is currently unclear whether steroids can be safely withheld in patients with low blood eosinophil counts, although studies addressing this question are needed. Moreover, early data suggest that the primary focus of lung pathology in COVID-19 may be the parenchyma rather than the airways, with evidence of diffuse alveolar damage, pneumocyte desquamation, and interstitial mononuclear inflammatory infiltrates reported (21). It remains unclear whether more prominent bronchial involvement will be observed in asthmatic subjects, and larger case series with inclusion of such patients should shed light on this.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
We remain in the early stages of our understanding of how COVID-19 affects patients with chronic respiratory diseases such as asthma and optimum management strategies still need to be determined and refined. However, the contraindication to corticosteroids that is being advocated for individuals who acquire SARS-CoV-2 infection is predominantly based on a lack of efficacy in treating COVID-19 disease, rather than evidence of harm. This should be balanced against their proven efficacy in reducing asthma symptoms and risk of relapse in patients with asthma (13), particularly those with evidence of augmented type 2 inflammation where corticosteroid use may restore antiviral immunity and confer benefit. Therefore, the decision to treat an asthmatic infected with SARS-CoV-2 will require careful consideration on a “per-patient” basis. Future studies should focus on characterizing the immunopathology of COVID-19-related asthma exacerbation including the extent to which augmented type 2 inflammation drives pathology. This will facilitate determination of the optimum approaches to management of these patients.
GRANTS
K.K. is supported by funding from the National Institute for Health Research (NIHR) Biomedical Research Centre. T.S.C.H. is supported by grants from the Wellcome Trust (104553/z/14/z, 211050/Z/18/z) and the NIHR Oxford Biomedical Research Centre (BRC). A.S. is supported by funding from the Wellcome Trust (215275/Z/19/Z) and the Association of Physicians of Great Britain & Ireland.
DISCLOSURES
K.K. reports no conflict of interests. T.S.C.H. reports grants from The Wellcome Trust, grants from The Guardians of the Beit Fellowship, personal fees from Astra Zeneca, personal fees from TEVA, and personal fees from Peer Voice outside of the submitted work. A.S. reports personal fees from Astra Zeneca outside of the submitted work.
AUTHOR CONTRIBUTIONS
K.K. and A.S. prepared figure; A.S. drafted manuscript; K.K., T.S.H., and A.S. edited and revised manuscript; K.K., T.S.H., and A.S. approved final version of manuscript.
REFERENCES
- 1.Global Initiative for Asthma (GINA) Management and Prevention Guidelines (Online). https://www.ginasthma.org/severeasthma/. 2019.
- 2.World Health Organization Clinical management of severe acute respiratory infection when novel COVID-19 is suspected (Online). Geneva: World Health Organization, January 28, 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [20 March 2020]. [Google Scholar]
- 3.Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, Stanciu LA, Gnesini G, Pastore A, Spanevello A, Morelli P, Johnston SL, Caramori G, Papi A. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 70: 910–920, 2015. doi: 10.1111/all.12627. [DOI] [PubMed] [Google Scholar]
- 4.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 12: 1023–1026, 2006. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 5.Duerr CU, McCarthy CD, Mindt BC, Rubio M, Meli AP, Pothlichet J, Eva MM, Gauchat JF, Qureshi ST, Mazer BD, Mossman KL, Malo D, Gamero AM, Vidal SM, King IL, Sarfati M, Fritz JH. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol 17: 65–75, 2016. doi: 10.1038/ni.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grünberg K, Sharon RF, Sont JK, In ’t Veen JC, Van Schadewijk WA, De Klerk EP, Dick CR, Van Krieken JH, Sterk PJ. Rhinovirus-induced airway inflammation in asthma: effect of treatment with inhaled corticosteroids before and during experimental infection. Am J Respir Crit Care Med 164: 1816–1822, 2001. doi: 10.1164/ajrccm.164.10.2102118. [DOI] [PubMed] [Google Scholar]
- 7.Gustafson LM, Proud D, Hendley JO, Hayden FG, Gwaltney JM Jr. Oral prednisone therapy in experimental rhinovirus infections. J Allergy Clin Immunol 97: 1009–1014, 1996. doi: 10.1016/S0091-6749(96)80077-7. [DOI] [PubMed] [Google Scholar]
- 8.Hewson CA, Haas JJ, Bartlett NW, Message SD, Laza-Stanca V, Kebadze T, Caramori G, Zhu J, Edbrooke MR, Stanciu LA, Kon OM, Papi A, Jeffery PK, Edwards MR, Johnston SL. Rhinovirus induces MUC5AC in a human infection model and in vitro via NF-κB and EGFR pathways. Eur Respir J 36: 1425–1435, 2010. doi: 10.1183/09031936.00026910. [DOI] [PubMed] [Google Scholar]
- 9.Huber JP, Ramos HJ, Gill MA, Farrar JD. Cutting edge: Type I IFN reverses human Th2 commitment and stability by suppressing GATA3. J Immunol 185: 813–817, 2010. doi: 10.4049/jimmunol.1000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, Jerico Del-Rosario, Telcian AG, Nikonova A, Zhu J, Aniscenko J, Gogsadze L, Bakhsoliani E, Traub S, Dhariwal J, Porter J, Hunt D, Hunt T, Hunt T, Stanciu LA, Khaitov M, Bartlett NW, Edwards MR, Kon OM, Mallia P, Papadopoulos NG, Akdis CA, Westwick J, Edwards MJ, Cousins DJ, Walton RP, Johnston SL. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med 190: 1373–1382, 2014. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Message SD, Johnston SL. The immunology of virus infection in asthma. Eur Respir J 18: 1013–1025, 2001. doi: 10.1183/09031936.01.00228701. [DOI] [PubMed] [Google Scholar]
- 12.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, Jeffery PK, Stanciu LA, Johnston SL. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA 105: 13562–13567, 2008. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev (5): CD011801, 2016. doi: 10.1002/14651858.CD011801.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritchard AL, Carroll ML, Burel JG, White OJ, Phipps S, Upham JW. Innate IFNs and plasmacytoid dendritic cells constrain Th2 cytokine responses to rhinovirus: a regulatory mechanism with relevance to asthma. J Immunol 188: 5898–5905, 2012. doi: 10.4049/jimmunol.1103507. [DOI] [PubMed] [Google Scholar]
- 15.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. In press. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395: 473–475, 2020. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satia I, Cusack R, Greene JM, O’Byrne PM, Killian KJ, Johnston N. Prevalence and contribution of respiratory viruses in the community to rates of emergency department visits and hospitalizations with respiratory tract infections, chronic obstructive pulmonary disease and asthma. PLoS One 15: e0228544, 2020. doi: 10.1371/journal.pone.0228544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singanayagam A, Glanville N, Girkin JL, Ching YM, Marcellini A, Porter JD, Toussaint M, Walton RP, Finney LJ, Aniscenko J, Zhu J, Trujillo-Torralbo MB, Calderazzo MA, Grainge C, Loo SL, Veerati PC, Pathinayake PS, Nichol KS, Reid AT, James PL, Solari R, Wark PAB, Knight DA, Moffatt MF, Cookson WO, Edwards MR, Mallia P, Bartlett NW, Johnston SL. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat Commun 9: 2229, 2018. doi: 10.1038/s41467-018-04574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas BJ, Porritt RA, Hertzog PJ, Bardin PG, Tate MD. Glucocorticosteroids enhance replication of respiratory viruses: effect of adjuvant interferon. Sci Rep 4: 7176, 2014. doi: 10.1038/srep07176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 201: 937–947, 2005. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420–422, 2020. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy all.14238, 2020. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 23.Zheng XY, Xu YJ, Guan WJ, Lin LF. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol 163: 845–853, 2018. doi: 10.1007/s00705-017-3700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]