Abstract
There is marked sexual dimorphism in the current coronavirus disease 2019 (COVID-19) pandemic. Here we report that estrogen can regulate the expression of angiotensin-converting enzyme 2 (ACE2), a key component for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cell entry, in differentiated airway epithelial cells. Further studies are required to elucidate the mechanisms by which sex steroids regulate SARS-CoV-2 infectivity.
Keywords: coronavirus, COVID-19, epithelial cells, estrogen, SARS-CoV-2
INTRODUCTION
There are pronounced sex-related differences in the ongoing coronavirus disease 2019 (COVID-19) pandemic, with higher intensive care unit admissions and deaths in males compared with females (3–5). This is consistent with epidemiological reports from prior coronavirus outbreaks, such as the 2002–2003 severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome epidemics (1, 8). Murine studies have confirmed the increased susceptibility of males to pathogenic coronaviruses (CoVs). Interestingly, SARS-CoV-infected female mice treated with an estrogen receptor antagonist have a higher mortality rate compared with their vehicle-treated female counterparts. Overall, this suggests a protective role for estrogen signaling in the setting of a SARS-CoV infection (2).
The novel SARS-CoV-2 depends on angiotensin-converting enzyme 2 (ACE2) for cell entry and engages the serine protease transmembrane protease serine 2 (TMPRSS2) for priming of the viral spike protein (6). Therefore, both ACE2 and TMPRSS2 are crucial for the ability of SARS-CoV-2 to cause infection. Here, we sought to determine whether 17β-estradiol (E2), a primarily female sex steroid, can regulate the gene expression of ACE2 and TMPRSS2 in differentiated normal human bronchial epithelial (NHBE) cells.
MATERIALS AND METHODS
NHBE cells from a female donor were purchased from a commercial source (Lonza) and grown at the air-liquid interface (ALI) on collagen-coated porous cell culture inserts. During the 3-wk differentiation process, the NHBE cells were treated with either 100 nM E2 (Sigma-Aldrich) or vehicle (ethanol). Total cellular RNA was extracted with the RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. Complementary DNA was synthetized with the Transcriptor Reverse Transcriptase Kit (Roche). Quantitative RT-PCR was performed with the LightCycler Green 480 SYBR Green I Master on the Roche LC480 Light Cycler (ABI). The following primers were used: 1) human ACE2 forward 5′-GGACCCAGGAAATGTTCAGA-3′ and reverse 5′-GGCTGCAGAAAGTGACATGA-3′, 2) human TMPRSS2 forward 5′-CACTGTGCATCACCTTGACC-3′ and reverse 5′-ACACACCGATTCTCGTCCTC-3′, and 3) human ribosomal protein S16 forward 5′-GCTTTCCTTTTCCGGTTGCG-3′ and reverse 5′-ACACGGATGTCTACACCAGC-3′. Gene expression was calculated relative to the expression of ribosomal protein S16 (housekeeping gene) and is reported as copies of the gene of interest per 104 copies of ribosomal protein S16, as previously published (10).
RESULTS
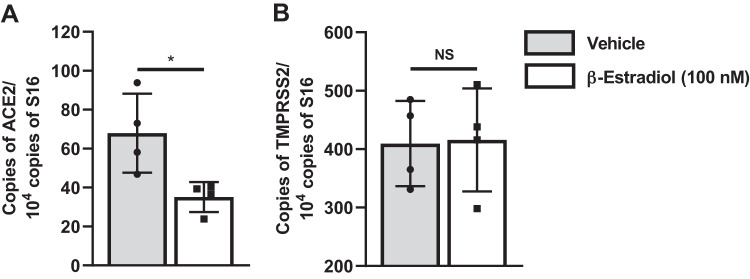
Here we demonstrate that E2-treated NHBE cells expressed lower levels of ACE2 mRNA compared with the vehicle-treated controls (Fig. 1A). This E2-driven downregulation of ACE2 expression is particularly relevant, as the efficiency of ACE2 usage by SARS-CoV has been shown to be an important determinant of viral replication and disease severity (6, 9). Furthermore, we also show that the levels of TMPRSS2 mRNA were not affected by E2 treatment (Fig. 1B). Finally, we confirmed prior reports that undifferentiated NHBE cells express very low levels of ACE2 (data not shown) (7). Therefore, it is critical to use fully differentiated NHBE cells grown at the ALI to accurately study ACE2 gene expression.
Fig. 1.
Angiotensin-converting enzyme 2 (ACE2; A) and transmembrane protease serine 2 (TMPRSS2; B) mRNA expression in normal human bronchial epithelial cells grown at the air-liquid interface and treated with vehicle (gray bars) or β-estradiol (white bars). The quantities of ACE2 and TMPRSS2 mRNA were normalized against that of ribosomal protein S16 mRNA. n = 4 experimental replicates. *P < 0.05 by 2-tailed Student’s t test. NS, not significant.
DISCUSSION
Limitations of our study include the use of a single female donor of NHBE cells. Further studies with NHBE cells from multiple male and female donors are warranted. In addition, it is important to note that the E2 concentration used for our experiments is only seen under physiological conditions during pregnancy and not in nonpregnant women (11). Finally, we should emphasize that the observed E2-induced reduction of ACE2 mRNA might not necessarily translate into a reduction of ACE2 protein at the cell surface.
In conclusion, we have shown that E2 can regulate the expression of ACE2 in differentiated NHBE cells. Given the striking sexual dimorphism in the COVID-19 pandemic, it is important to further elucidate the mechanisms by which sex hormones regulate the cellular components required for SARS-CoV-2 infectivity and ability to cause life-threatening disease.
GRANTS
This work was supported by National Institute of Allergy and Infectious Diseases Grant K08 AI-141765.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.C.-E. and S.E.C. conceived and designed research; K.E.S. and S.E.C. performed experiments; K.E.S. and S.E.C. analyzed data; K.E.S., F.C.-E., M.S., S.B., Y.S.P., and S.E.C. interpreted results of experiments; S.E.C. prepared figures; F.C.-E., M.S., S.B., and S.E.C. drafted manuscript; K.E.S., F.C.-E., M.S., S.B., Y.S.P., and S.E.C. edited and revised manuscript; K.E.S., F.C.-E., M.S., S.B., Y.S.P., and S.E.C. approved final version of manuscript.
REFERENCES
- 1.Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, Alghamdi MM, El-Sheemy MA. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med 7: 417–423, 2014. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol 198: 4046–4053, 2017. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507–513, 2020. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, Nailescu A, Corona A, Zangrillo A, Protti A, Albertin A, Forastieri Molinari A, Lombardo A, Pezzi A, Benini A, Scandroglio AM, Malara A, Castelli A, Coluccello A, Micucci A, Pesenti A, Sala A, Alborghetti A, Antonini B, Capra C, Troiano C, Roscitano C, Radrizzani D, Chiumello D, Coppini D, Guzzon D, Costantini E, Malpetti E, Zoia E, Catena E, Agosteo E, Barbara E, Beretta E, Boselli E, Storti E, Harizay F, Della Mura F, Lorini FL, Donato Sigurtà F, Marino F, Mojoli F, Rasulo F, Grasselli G, Casella G, De Filippi G, Castelli G, Aldegheri G, Gallioli G, Lotti G, Albano G, Landoni G, Marino G, Vitale G, Battista Perego G, Evasi G, Citerio G, Foti G, Natalini G, Merli G, Sforzini I, Bianciardi L, Carnevale L, Grazioli L, Cabrini L, Guatteri L, Salvi L, Dei Poli M, Galletti M, Gemma M, Ranucci M, Riccio M, Borelli M, Zambon M, Subert M, Cecconi M, Mazzoni MG, Raimondi M, Panigada M, Belliato M, Bronzini N, Latronico N, Petrucci N, Belgiorno N, Tagliabue P, Cortellazzi P, Gnesin P, Grosso P, Gritti P, Perazzo P, Severgnini P, Ruggeri P, Sebastiano P, Covello RD, Fernandez-Olmos R, Fumagalli R, Keim R, Rona R, Valsecchi R, Cattaneo S, Colombo S, Cirri S, Bonazzi S, Greco S, Muttini S, Langer T, Alaimo V, Viola U; COVID-19 Lombardy ICU Network . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 323: 1574–1581, 2020. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79: 14614–14621, 2005. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol 159: 229–231, 2004. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Zhang C, Sui J, Kuhn JH, Moore MJ, Luo S, Wong SK, Huang IC, Xu K, Vasilieva N, Murakami A, He Y, Marasco WA, Guan Y, Choe H, Farzan M. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J 24: 1634–1643, 2005. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 33: 1–47, 2012. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]