Abstract
While many drugs and metabolites contain nitrogen, harnessing their diagnostic 15N NMR signature for their characterization is underutilized because of inherent detection difficulties. Here, we demonstrate how precise ultralow field signal amplification by reversible exchange (±0.2 mG) in conjunction parahydrogen and an iridium precatalyst of the form IrCl(COD)(NHC) with the coligand d9-benzylamine allows the naturally abundant 15N NMR signatures of pyridine, pyrazine, metronidazole, and acetonitrile to be readily detected at 9.4 T in single NMR observations through >50% 15N polarization levels. These signals allow for rapid and precise reagent quantification via a response that varies linearly over the 2–70 mM concentration range.
Introduction
Hyperpolarization methods have been shown to dramatically improve the sensitivity of nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI)1,2 in a process that involves increasing the purity of the magnetic states they detect. Signal amplification by reversible exchange (SABRE) reflects one such method. It harnesses the nuclear spin order of parahydrogen (p-H2)3−5 and is a consequence of the pioneering work of Weitekamp6 and Eisenberg.7 For SABRE to operate, the symmetry of p-H2 is first broken by temporarily placing it in a metal complex so that the new hydride ligands couple distinctly to NMR active spins within the ligand sphere of the product. A process of reversible binding then allows a suitable substrate to become hyperpolarized through a catalytic process that transfers nuclear spin order within the complex rather than achieving a change in the chemical identity.3,5 Typically, this process takes place in a specified magnetic field that is often called the polarization transfer field (PTF) and can be selected to optimize efficiency.8,9 The selection of this field is made according to the chemical shift difference that exists between the interacting nuclear spins and their spin–spin couplings10,11 in a process that has been accurately modeled.12 As the active SABRE catalyst may break the symmetry of the two protons that were initially located in p-H2 through chemical or magnetic inequivalence effects, the process of catalysis can be complex.5,13 This is because for the spin order transfer from the p-H2 derived hydride ligands to take place, the receiving ligand nuclei must exhibit different spin–spin couplings to these two protons.
Knowledge of this behavior has influenced the SABRE catalyst design,14 and the resulting sensitization process has enabled the easy NMR detection of low-abundance inorganic species.13 Other studies have used deuterated coligands to improve the spin-order yields in SABRE by reducing waste through the focusing of polarization transfer into fewer receptor sites.15 When this is achieved in conjunction with 2H labeling, the associated extension of the nuclear spin-order lifetime has proven to be particularly beneficial as decoherence within the SABRE catalyst reflects one route to reduce the overall processes efficiency.16 These two effects combine to extend the duration, over which signals remain visible to NMR. As in classical terms, one T1 period is associated with 63% destruction of the hard-won polarization level. Not surprisingly, the extended lifetimes associated with molecular singlet states17−21 and their derivatives feature extensively in hyperpolarization research as one goal is often to study in vivo reactivity.22 In further developments, Tessari et al. have shown how 1H-SABRE can achieve precise analyte quantification at low substrate loadings by the involvement of a slow exchanging coligand.23,24 Furthermore, Iali et al. extended SABRE to the hyperpolarization of primary amines through catalysts of the form [Ir(H)2(IMes)(amine)3]Cl,25 and it was noted that sterically hindered amines, which failed to bind efficiently, benefited by the addition of smaller NCMe, which enables the formation of [Ir(H)2(IMes)(aniline)2(NCMe)]Cl.26 The successful use of amines reflects an important boost to SABRE because the hyperpolarized NH response can be used to sensitize other molecules through proton exchange.25 More recently developments of this ligand design route have enabled the hyperpolarization of pyruvate and acetate.27,28
Normally, although the detection of 15N by NMR is even more challenging than that of 1H because of its 0.36% natural abundance and low magnetogyric ratio, 15N detection is, however, needed for the characterization of important nucleobases, nucleosides, nucleotides, peptides, proteins, and transition metal complexes. In addition, as the T1 of 15N can exceed many minutes, magnetic state lifetimes can approach those of positron emission tomography.29−33 It is not, therefore, surprising that 15N hyperpolarization reflected an early target of both spontaneous3 and radio frequency-driven SABRE.34 Warren et al. refined these methods through SABRE SHEATH35,36 to deliver 20% 15N polarization in metronidazole.37 Several alternative radio frequency strategies have also been exemplified38−40 and given the goal of in vivo SABRE, water soluble SABRE catalysts have also been described41,42 with the MRI detection of a 15N response illustrated.42 Here, we seek to demonstrate how amines as coligands can enable the highly efficient 15N polarization of a range of target substrates (sub) via SABRE catalysis through [Ir(H)2(1)(sub)2(BnNH2)]Cl (a) or [Ir(H)2(1)(sub)(BnNH2)2]Cl (b) of Scheme 1 in order to improve the potential of the SABRE approach to achieve in vivo MRI detection.
Scheme 1. Chemical Structures of Complexes, Substrates, and Ligands.
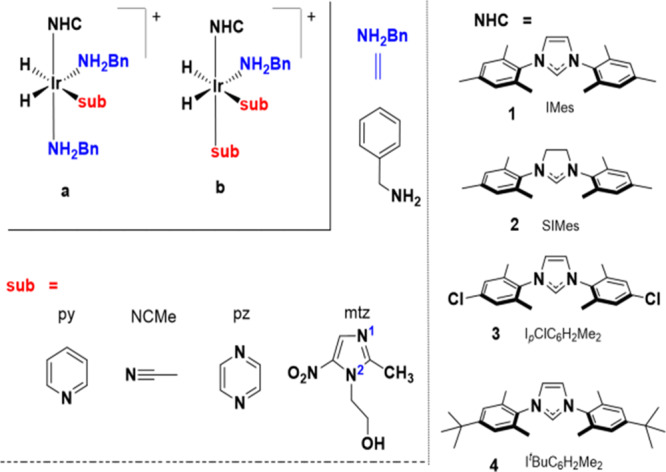
Results
Hyperpolarization of the 15N NMR Signal of Pyridine
We start by considering nonlabeled pyridine at 35 mM concentration because of its wide use in early SABRE research3,4,13 in conjunction with the precatalyst [IrCl(COD)(h22-1)]43 (5 mM) of Scheme 1. Our experimental measurements involved examining an NMR tube containing methanol-d4 solutions of these reagents under 3 bar (absolute) pressure of p-H2 at 99% purity. p-H2 gas is first dissolved by shaking the NMR tube while it is located in a preset magnetic field that lies between ±1 mG and ±70 G for ∼10 s (relative to the main NMR magnetic field orientation). Subsequently, the sample is placed in a 9.4 T magnet where the final NMR signal detection step occurs.
Under these conditions, the SABRE catalyst [Ir(H)2(h22-1)(py)3]Cl forms and a 1H NMR signal gain of 1452-fold can be seen for the ortho proton resonance of free pyridine that is present in the solution after being transferred from a 60 G field. This polarization transfer step takes 10 s to complete and the resulting polarization level (PH) is 4.65% (Px reflects the percentage polarization associated with nuclei x). In this case, the catalyst breaks the symmetry of the two p-H2-derived protons through magnetic inequivalence effects, and hence, the spin order transfer flows optimally within the equatorial plane that contains the hydride ligands into bound pyridine.44 For 15N, however, the large trans two bond 1H–15N coupling of ∼19 Hz9,35,45,46 that connects these hydride ligands to nitrogen in [Ir(H)2(h22-1)(py)3]Cl enables the efficient transfer of polarization at an approximate −1 mG field that is of the same sense to the main 9.4 T observation field. The consequence of this process is a 39200-fold (±2%) 15N NMR signal gain, which means the corresponding P15N value is 12.9% (±2%). Hence, this unlabeled 35 mM sample of pyridine can be detected by 15N NMR spectroscopy in a single scan NMR measurement at a magnetic field of 9.4 T with a signal to noise ratio of 11 using a routine inverse detection probe.
Establishment of Coligand Benzylamine As Beneficial to the Hyperpolarization of the 15N NMR Signal of Pyridine
When the coligand d7-benzylamine (d7-BnNH2) was added to such a sample, at an initial concentration of 17.5 mM, it proved to rapidly convert into its d9-benzylamine isotopologue. Consequently, we refer to d9-BnND2 throughout this article even though d7-BnNH2 is actually added to the samples. The resulting 1H NMR spectra reveal that in addition to this labeling change, two new inorganic species are formed, which yield pairs of hydride ligand signals at δ −22.14 and −22.58, and δ −23.34 and −23.73, respectively. These hydride ligand signals arise from [Ir(H)2(h22-1)(d9-BnND2)(py)2]Cl and [Ir(H)2(h22-1)(d9-BnND2)2(py)]Cl, respectively, that are present in the solution in the ratio 2.6:1. The two complexes contain inequivalent hydride ligands that differ from one another according to the identity of the axial ligands in the complex, as detailed in Scheme 1 and the Supporting Information. Furthermore, as their proportions match the value seen when a similar sample is created by the initial addition of benzylamine and H2 to [IrCl(COD)(h22-1)], but before pyridine addition takes place, it can be concluded that these two complexes are in equilibrium. Hence, the separation of their roles in the underlying SABRE process is impractical, but we note it would be expected that both will contribute to this process. In addition, it is important to recognize that both of these complexes contain chemically and magnetically distinct hydride ligands. The result of this change is that spin-order transfer can now proceed into ligands that lie trans and cis to hydride, which means that spin dilution, associated with polarization of the axial ligands, is expected and this will reduce the SABRE signal gains that are seen for the free substrate.44 Hence, the involvement of polarization transfer protecting d9-BnND2, which limits spin-order wastage should be of significant benefit to the SABRE outcome.
When the resulting d9-BnND2 solutions were examined for SABRE, the 1H NMR response resulting from this mixture of catalysts proved to contain a free pyridine ortho proton resonance that was 880-fold (±50) larger than expected after being transferred from a 60 G field. As this gain is smaller than the value achieved by [Ir(H)2(h22-1)(py)3]Cl we can conclude that under these conditions the [Ir(H)2(h22-1)(d9-BnND2)(py)2]Cl/[Ir(H)2(h22-1)(d9-BnND2)2(py)]Cl mixture is actually less efficient at hyperpolarizing the 1H NMR signals of pyridine than [Ir(H)2(h22-1)(py)3]Cl. More notable is the fact that the corresponding 15N NMR spectrum contains a signal that is indicative of a P15N value of 18% (53,300 ± 6000-fold) in conjunction with a PTF of approximately −1 mG (Figure 1a). This reflects a 27% improvement in the SABRE efficiency when compared to that achieved by [Ir(H)2(h22-1)(py)3]Cl and confirms that there is a benefit to using the coligand d9-benzylamine when seeking 15N polarization.
Figure 1.
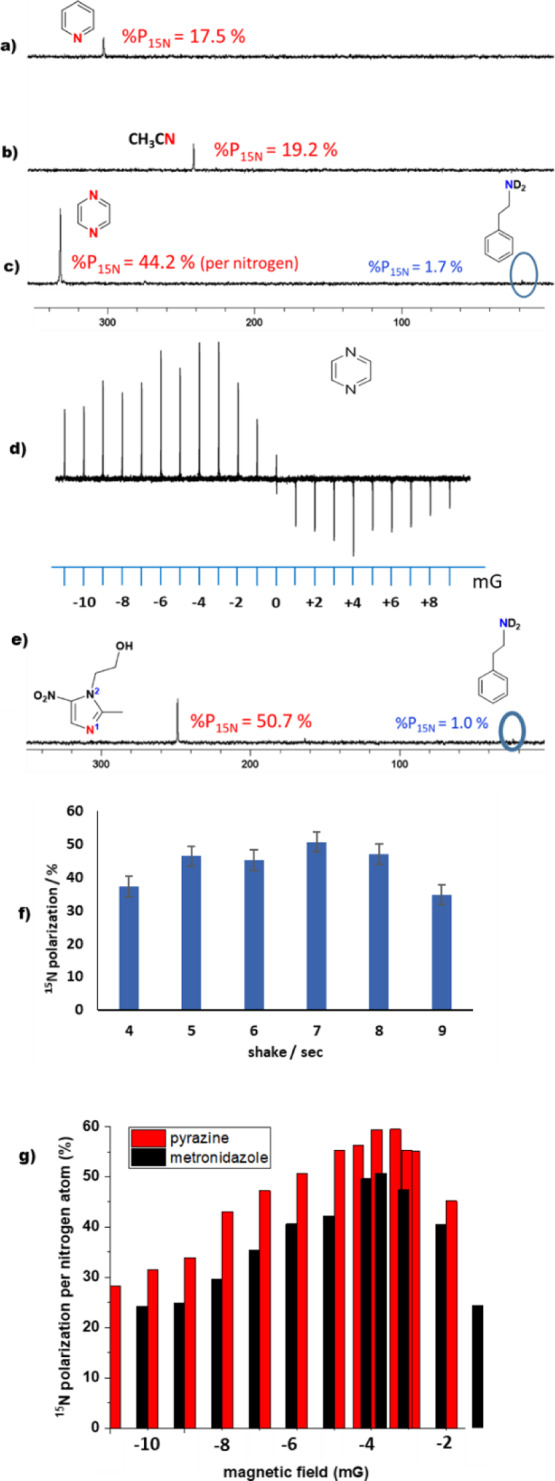
Polarized 15N NMR signals of (a) pyridine, (b) acetonitrile, (c, d) pyrazine, and (e) metronidazole. Levels indicted in figure alongside agent. In (d), the series of 15N NMR signals for pyrazine vary in intensity according to the magnitude of the PTF. (f) Shake time dependence of the P15N level in metronidazole with [IrCl(d34-4)(COD)]. (g) P15N level for metronidazole (black) and pyrazine (red) in a 10 mm sample tube as a function of PTF magnitude.
Upon changing to [IrCl(d22-1)(COD)], and completing a similar series of d9-BnND2 promoted measurements, the levels of signal gain seen in the pyridine ortho proton 1H NMR signal rises to 1324-fold, although the 15N polarization level proved to be unaffected. Hence, while catalyst deuteration is not successful at improving SABRE 15N activity, it is able to improve the level of 1H signal gain because of reduced spin order wastage and improved 1H relaxation.16 This suggests that low-field 15N-relaxation within the catalyst is not improved.
While it is well known that the optimum SABRE catalyst changes with the identity of the substrate, it has been clearly demonstrated here that there is also a further dependence on the efficiency of SABRE transfer within a given substrate according to whether 1H or 15N is the target. The optimum rate of ligand exchange for 1H transfer has been proposed by Barskiy to be 4.5 s–1 in complexes of the type [Ir(H)2(h22-1)(py)3]Cl. Consequently, the rate of pyridine substrate dissociation in [Ir(H)2(h22-1)(py)2(d9-BnND2)]Cl in methanol-d4 solution was determined using the EXSY method and found to be 0.06 s–1 at 268 K. This value increases to 1.04 s–1 upon warming at 298 K, and 2.1 s–1 at 308 K. Our associated SABRE measurements reveal that the corresponding 1H NMR signal gains change from 600-fold, through 4530-fold to 3550-fold at the 308 K setting. Hence, it appears that a rate closer to 1.04 s–1 is optimal for 1H transfer into pyridine using this catalyst. Our experiments also reveal that there is a 30% growth in efficiency of 15N polarization for pyridine on moving from 268 to 298 K, and a further 22% improvement on moving to 308 from 298 K. Consequently, we can confirm that the two different nuclei are best served with different rates of ligand exchange.
Hyperpolarization of the 15N NMR Signal of Acetonitrile
In order to develop this method further, acetonitrile was tested at a similar 35 mM concentration in conjunction with the SABRE catalyst [Ir(H)2(h22-1)(NCMe)3]Cl. This catalyst also relies on magnetic inequivalence to break the symmetry of the hydride ligands and it yields a 1H NMR signal gain of just 83-fold per methyl proton in the unbound acetonitrile present in the solution after transfer at 298 K from a 70 G field. The SABRE-derived 15N NMR signal gain for CH3CN was found to be far more substantial, at 41,800 ± 6000-fold (14% polarization) after transfer from an approximate −1 mG field.
Acetonitrile hyperpolarization was then studied in conjunction with 3.6 equivalents of the coligand d9-benzylamine relative to 5.2 mM iridium concentration. Both [Ir(H)2(d9-BnND2)2(NCMe)(h22-1)]Cl and [Ir(H)2(d9-BnND2)3(h22-1)]Cl form in these experiments, in a 2:1 ratio. They both possess chemically distinct hydride ligands. The resulting 1H NMR response after SABRE showed an improved 1H NMR signal gain of 160-fold per proton for CH3CN while its 15N polarization level rose to 19% (Figure 1b).
For the corresponding 2H labeled precatalyst [IrCl(d22-1)(COD)], the 1H NMR signal again improves further to 367-fold per proton in accordance with reduced spin dilution that arises as a consequence of hydride ligand chemical inequivalence in [Ir(H)2(d9-BnND2)2(NCMe)(h22-1)]Cl and [Ir(H)2(d9-BnND2)3(h22-1)]Cl, but now the achieved P15N level fell to 10%. Hence, 2H-catalyst labeling of the NHC ligand is now detrimental to the 15N polarization level. In this case, the appreciable concentration of [Ir(H)2(d9-BnNH2)2(NCMe)(d22-1)]Cl, where there will be coupling between the 2H labels of the NHC and the 15N of NCMe, could result in a reduction in 15N-SABRE efficiency. Barskiy’s observations that in micro-tesla transfer fields, scaler relaxation of the second kind47 associated with the quadrupolar 14N–13C interaction limits the level of 13C polarization under SABRE support this view.48 The gain in the 1H signal intensity relative to the situation with h22-1 is, however, consistent with a reduction in the polarization transfer into this ligand through deuteration and an extension of the hydride ligand relaxation times.14
Hyperpolarization of the 15N NMR Signal of Pyrazine
We next consider pyrazine (pz). This substrate was tested by taking 5.2 mM methanol-d4 solutions of [IrCl(COD)(h22-1)] that contained a sevenfold excess of pz under 3 bar of p-H2. The resulting 1H NMR signal gain for pz was now 900-fold per proton (2.9% polarization) and a P15N value of 16% (±2, per nitrogen used throughout) was observed after the transfer from −3 mG.
Studies with added h7-BnND2 resulted in a 1H NMR signal gain of 566-fold (0.8%) and a 15N signal gain of 12% due to the associated spin dilution effects. However, when d9-BnND2 and h22-1 were used with a PTF of 60 G, radiation damping resulted with 1H signal detection. In order to aid the analysis, this artifact could be suppressed if a less efficient PTF of 120 G was used. Analysis under these conditions was used to deduce that the corresponding PH level is 13.5% (±0.6) per proton for a 60 G measurement while for 15N it was 38% (per nitrogen). The 1H NMR signal gain grew further to 30.9% (±0.7) when [IrCl(d22-1)(COD)] was used, but the corresponding 15N signal response fell in intensity meaning that the scaler relaxation of the second kind is again important. We also tested the related SIMes containing precatalyst [IrCl(COD)(2)]49 with pyrazine and discovered that a P15N value of 15.8% could be achieved without a coligand. Samples containing both the d7-benzylamine and pyrazine yield [Ir(H)2(pz)2(d9-BnND2)(h22-2)]Cl and [Ir(H)2(d9-BnND2)2(pz)(h22-2)]Cl in the ratio 2:1 and a P15N value of 44.2% via PTF from an approximate −1.9 mG field (Figure 1c). This falls to 31.8% with d22-SIMes in agreement with a role for 2H-drive relation in the SABRE catalyst at low field. Figure 1d shows that the sign of the PTF, relative to that of the main observation field, affects the measured 15N pz signal gains. This is because upon moving the sample slowly between the points of polarization transfer and measurement, if it experiences a zero-field point, there is a loss in the spin order due to relaxation at this point.
The rate of pyrazine dissociation from [Ir(H)2(pz)2(d9-BnND2)(h22-2)]Cl was determined using the EXSY method to be 0.33 s–1 at 268 K when the 1H NMR signal gain is 660-fold. This rate increases to 1.8 s–1 at 298 K where the 1H signal gain is 2200-fold. Our experiments reveal that the 20% growth in the efficiency of 15N polarization on moving from 268 to 298 K for pyrazine is a consequence of this rate increase, which is faster than that of pyridine loss in the related complex [Ir(H)2(h22-1)(py)2(d9-BnND2)]Cl. This kinetic difference is consistent with the relative 15N polarization efficiencies of 44.2 and 18%, respectively.
Hyperpolarization of the 15N NMR Signal of Metronidazole
Biologically significant metronidazole50,51 has been well-studied by Chekmenev et al.(52−55) We conducted control measurements for 5.2 mM methanol-d4 solutions of [IrCl(COD)(h22-1)] and [IrCl(COD)(h22-2)] with a sevenfold excess of metronidazole relative to iridium and a 3 bar pressure of p-H2 but failed to see significant polarization in either samples. However, once a 3.6-fold excess of d7-benzylamine was added, polarization transfer to proton and 15N was readily seen with both precursors. For [IrCl(COD)(h22-1)], the P15N value was 22% while for [IrCl(COD)(h22-2)], it was 24% (transfer at −2 mG and 2% P15N seen for d7-benzylamine itself). When the 2H labeled versions of these catalysts, [IrCl(COD)(d22-1)] or [IrCl(COD)(d22-2)], were used, these P15N values rose to 27%. In all cases, the reaction with d9-benzylamine and metronidazole formed [Ir(H)2(mtz)2(d9-BnND2)(NHC)]Cl and [Ir(H)2(d9-BnND2)2(mtz)(NHC)]Cl with the ratio being 1.4:1 for d22-2.
Data were now collected on the d22-2 system to demonstrate that the PTF value can be used to control which of the two substrates present in solution receives polarization. This effect serves to illustrate how selectivity can be introduced into the analysis of mixtures if peak overlap is an issue (see the Supporting Information). Furthermore, a catalyst change to [IrCl(COD)(d34-4)] increased the N1 value to 51% for metronidazole with 4% polarization being achieved on N2 and 1% on d9-benzylamine (Table 1).
Table 1. Absolute Value of 1H (Total Proton) and 15N NMR (per Site) Signal Enhancement Levels for Pyrazine and Metronidazole at the Specified PTF for Samples with d9-Benzylamine as a Coligand.
| signal
gain (P) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nucleus (PTF) | 1 | d22-1 | 2 | d22-2 | 3 | d16-3 | 4 | d34-4 | error, %, ± | |
| pyrazine | 1H (120 G)/fold | 1372 | 3151 | 2220 | 6028 | 2533 | 558 | 556 | 673 | 4 |
| 15N (%, as indicated PTF mG) | 38 (−3) | 35 (−3) | 44 (−1.9) | 37 (−5) | 26 (−2) | 31 (−4) | 32 (−5) | 28 (−3) | 2 | |
| metronidazole-N1 | 1H (60 G)/fold | 326 | 474 | 560 | 446 | 814 | 1038 | 676 | 856 | 5 |
| 15N (%, at PTF of −2 mG) | 22 | 27 | 24 | 27 | 23 | 23 | 32 | 51 | 3 | |
The rates of metronidazole dissociation from the resulting complex [Ir(H)2(mtz)2(d9-BnND2)(d34-4)]Cl were determined in methanol-d4 solution at 268, 298, and 308 K by the EXSY method as being 0.80, 2.37, and 5.5 s–1, respectively. For the 1H signal gain, 298 K proved to be the best, yielding an enhancement of 856-fold. We now see an 80% growth in the efficiency of 15N polarization on moving from 268 to 298 K, but the P15N values falls to just 18% at 308 K. Hence, increasing the ligand exchange rate beyond 2.4 s–1 seems detrimental.
Usage of Higher Proportions of p-H2 to Improve the NMR Signal Gain
A series of measurements were then completed on metronidazole using a 10 mm NMR tube to deploy a larger excess of p-H2 in conjunction with [IrCl(COD)(d34-4)] and d9-benzylamine. A slight increase in the 15N polarization level to 54% results alongside a reduction in response variability to 2%. Consequently, as shown in Figure 1g, a −3.6 mG PTF can be deduced as being optimal. Similar 10 mm measurements were then made for pyridine with [IrCl(COD)(h22-1), acetonitrile with [IrCl(COD)(h22-1), and pyrazine with [IrCl(COD)(h22-2) in the presence of d9-benzylamine. These studies saw the P15N level for pyridine increase to 48% at 4 bar p-H2 pressure. When acetonitrile was examined, a 30.7% P15N level was reached, but for pyrazine it became 59.4% per nitrogen. Further increases in the pyrazine % P15N level can be achieved through reagent dilution such that when an initial 5 mM solution of [IrCl(COD)(h22-2)] with a 3.6-fold excess of d9-benzylamine and sevenfold excess of pyrazine based on iridium is diluted 10 fold, the P15N value increases to 79%; the S/N ratio in this case is 11.3. In this case, the effect is directly analogous to increasing the volume of p-H2 available.
Quantification of Reagent Concentrations at the mM Level through a SABRE-Enhanced 15N Signal
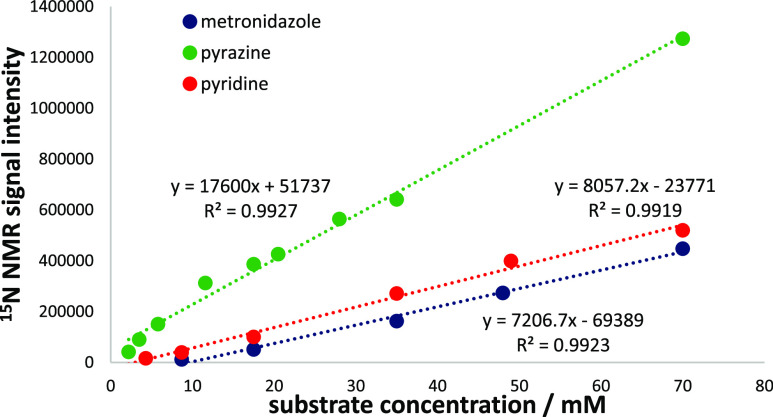
Once we had ascertained how to achieve these polarization levels, we tested how the magnitude of the pyridine, pyrazine, and metronidazole response varied as a function of substrate concentrations between 2.2 and 70 mM. These solutions were made up by simply diluting a stock solution with an initial catalyst, d7-benzylamine, and substrate concentration of 10, 36, and 70 mM, respectively. We discovered that there was a linear variation in the signal response in each case, as shown in Figure 2.
Figure 2.
Raw signal intensity resulting from a series of hyperpolarized 15N NMR spectra of pyridine, metronidazole, and pyrazine as a function of their concentration. The PTF was optimized for each substrate. The stock solution of the sample ([Ir] = 6.5 mM, substrate = 70 mM, and 22.7 mM d9-BnND2) was diluted during these measurements, from 70 mM substrate to 2.2 mM substrate concentration. The straight lines result from linear regression analysis and the square of the sample correlation coefficient—R2-confirms linear behavior.
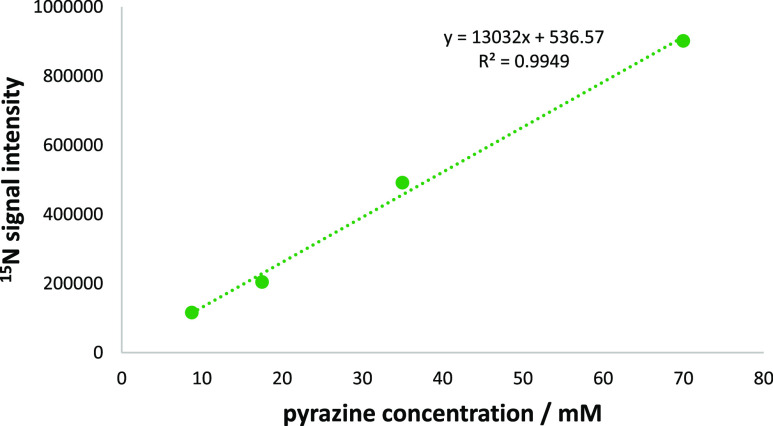
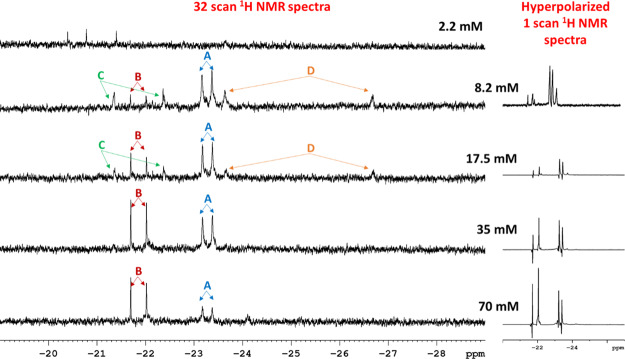
In the second series of studies, we maintained a constant iridium and coligand concentration while changing the pyrazine concentration. A linear change in the 15N signal intensity was again observed (Figure 3) despite, in this case, observing some changes in the catalyst form. The hydride region of the polarized NMR spectra confirm that both [Ir(H)2(pz)(d9-BnND2)2(h22-2)]Cl (A) and [Ir(H)2(pz)2(d9-BnND2)(h22-2)]Cl (B) of Figure 4 form, with the former being favored at low pyrazine loadings. As the concentration of pyrazine decreases, the amount of the formed complex B decreases, and as a result of it, the 15N polarization of pyrazine linearly decreases as well. This suggests that the main SABRE—15N catalyst is the type B complex. We are currently exploring this behavior in more detail. These data, therefore, confirm that substrate detection and quantification is feasible via a 15N SABRE signal (see the Supporting Information).
Figure 3.
Raw signal intensity resulting from a series of hyperpolarized 15N NMR spectra of pyrazine as a function of its concentration. The PTF used was −1.9 mG. The concentration of the [Ir]-precatalyst ([IrCl(COD)(h22-2)]) was kept constant at 6.5 mM. 3.6 equivalents of d9-BnND2 were added relative to the metal. Subsequently, the concentration of added pyrazine was varied from 8.2 to 70 mM. Straight line behavior results thereby confirmed that the absolution concentration of pyrazine can be estimated from such data.
Figure 4.
Effect of pyrazine loading on catalyst speciation when methanol-d4 solutions of [IrCl(COD)(h22-2)] are examine with d9-BnND2 and pyrazine in the presence of p-H2 seen through the hydride region of the corresponding 1H NMR spectra. Left, thermally equilibrated NMR spectra and right, initial SABRE-enhanced NMR spectra. Species A and B are defined in the text while C is Ir(H)2(Cl)(d9-BnND2)(pz)(h22-2) and D [Ir(H)2(d9-BnND2)(methanol-d4)(h22-2)]Cl.
Conclusions
We have described here how the addition of the coligand d9-benzylamine to a precatalyst based on [IrCl(NHC)(COD)] under p-H2 results in very high levels of 15N polarization in a range of substrates. The high field measurements were made in conjunction with the simple shake and drop approach, and it takes approximately 17 s to complete a measurement. In the case of the substrates pyridine and acetonitrile, [IrCl(h22-1)(COD)] led to P15N values of 48 and 30.9%, respectively, after being transferred from an appropriate mG field. In contrast, a 59.4 P15N value for pyrazine was achieved using the precatalyst [IrCl(h22-2)(COD)]. These reactions involve the formation of a range of SABRE catalysts of the form [Ir(H)2(sub)2(d9-BnND2)(NHC)]Cl and [Ir(H)2(sub)(d9-BnND2)2(NHC)]Cl, which are in equilibrium.
Previous studies have established that using deuterated NHC ligands (d22-1 and d22-2) improve SABRE hyperpolarization transfer efficiency into methylnicotinate. This improvement is based on an extension of the hydride ligand relaxation times.14 Studies here confirm that higher P1H values result in all cases in support of this benefit. However, deuteration is not beneficial for the 15N transfer in pyridine, pyrazine, and acetonitrile. Barskiy’s observations that in micro-tesla transfer fields, scaler relaxation of the second kind47 associated with the quadrupolar 14N–13C interaction limits the level of 13C polarization under SABRE offer a route to explain this view.48 For metronidazole, however, an improved value of 54% on N1 results with d9-benzylamine and [IrCl(COD)(d34-4)], compared to that seen with precatalyst [IrCl(COD)(h34-4)]. Hence, 2H labeling of the catalyst can also be of significant benefit to P15N.
The rates of ligand exchange were also assessed alongside the collection of variable temperature SABRE data. It was found that the rate of optimum ligand exchange could slower than that found for 1H transfer, despite the larger 1H–15N transfer coupling. We are currently exploring this behavior in more detail.
Data were also presented that was collected from larger 10 mm NMR tubes using a 4 bar pressure of p-H2. This acted to increase the relative excess of the hyperpolarization fuel p-H2 relative to the substrate and proved to result in greatly improved response reproducibility. Consequently, results demonstrated that a PTF precision of ±0.2 mG is needed for optimal 15N transfer. In addition, ∼50% 15N polarization levels could now be achieved in pyrazine, pyridine, or metronidazole, which makes them all highly detectable even at low concentrations.
In order to demonstrate an analytical use for these 15N signals, results were presented to demonstrate that the magnitude of the resulting NMR response scales linearly with concentration over the range 2.2–70 mM. This means that such SABRE-derived data can be used to quantify their amount in the solution when set against a suitable reference trace. Tessari have completed a growing range of studies, which demonstrate that 1H detection levels can be linked to both speciation and quantity,23,24 while we have described how 13C signals in glucose can be linked to amount.56 These studies employed a methylated triazol coligand to simplify the exchange kinetics in order to produce the necessary linear response. We were unable to benchmark our data with that of the triazol coligand as it is not commercially available. We did, however, test d6-DMSO, which is finding widespread use as a coligand for the sensitization of weakly binding substrates as an alternative. As detailed in the Supporting Information, the corresponding SABRE performance was degraded.
It is therefore clear that SABRE offers a simple and yet efficient route to analyte quantification by 15N NMR spectroscopy. Not surprisingly, we predict these results will, therefore, be of benefit if you wish to use 15N NMR as a characterization tool, or simply to quantify precise, and yet low, levels of nitrogen-containing drugs that are present in solution or to collect 15N-MRI data.
Acknowledgments
We thank Peter Rayner for providing some of the complexes used in this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpcb.0c02583.
Experimental details, NMR data, and hyperpolarization details (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Financial support from the Wellcome Trust (Grants 092506 and 098335), the MRC (MR/M008991/1) and the University of York is gratefully acknowledged.
The authors declare no competing financial interest.
Supplementary Material
References
- Dumez J.-N. Perspectives on hyperpolarised solution-state magnetic resonance in chemistry. Magn. Reson. Chem. 2017, 55, 38–46. 10.1002/mrc.4496. [DOI] [PubMed] [Google Scholar]
- Kovtunov K. V.; Pokochueva E. V.; Salnikov O. G.; Cousin S. F.; Kurzbach D.; Vuichoud B.; Jannin S.; Chekmenev E. Y.; Goodson B. M.; Barskiy D. A.; Koptyug I. V. Hyperpolarized NMR Spectroscopy:d-DNP, PHIP, and SABRE Techniques. Chem.—Asian J. 2018, 13, 1857–1871. 10.1002/asia.201800551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R. W.; Aguilar J. A.; Atkinson K. D.; Cowley M. J.; Elliott P. I. P.; Duckett S. B.; Green G. G. R.; Khazal I. G.; Lopez-Serrano J.; Williamson D. C. Reversible Interactions with para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323, 1708–1711. 10.1126/science.1168877. [DOI] [PubMed] [Google Scholar]
- Atkinson K. D.; Cowley M. J.; Elliott P. I. P.; Duckett S. B.; Green G. G. R.; López-Serrano J.; Whitwood A. C. Spontaneous Transfer of Parahydrogen Derived Spin Order to Pyridine at Low Magnetic Field. J. Am. Chem. Soc. 2009, 131, 13362–13368. 10.1021/ja903601p. [DOI] [PubMed] [Google Scholar]
- Adams R. W.; Duckett S. B.; Green R. A.; Williamson D. C.; Green G. G. R. A theoretical basis for spontaneous polarization transfer in non-hydrogenative parahydrogen-induced polarization. J. Chem. Phys. 2009, 131, 194505. 10.1063/1.3254386. [DOI] [PubMed] [Google Scholar]
- Bowers C. R.; Weitekamp D. P. Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance. Phys. Rev. Lett. 1986, 57, 2645–2648. 10.1103/physrevlett.57.2645. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. Parahydrogen-induced polarization: a new spin on reactions with molecular hydrogen. Acc. Chem. Res. 1991, 24, 110–116. 10.1021/ar00004a004. [DOI] [Google Scholar]
- Dücker E. B.; Kuhn L. T.; Münnemann K.; Griesinger C. Similarity of SABRE field dependence in chemically different substrates. J. Magn. Reson. 2012, 214, 159–165. 10.1016/j.jmr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Cowley M. J.; Adams R. W.; Atkinson K. D.; Cockett M. C. R.; Duckett S. B.; Green G. G. R.; Lohman J. A. B.; Kerssebaum R.; Kilgour D.; Mewis R. E. Iridium N-Heterocyclic Carbene Complexes as Efficient Catalysts for Magnetization Transfer from para-Hydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. 10.1021/ja200299u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. A.; Adams R. W.; Duckett S. B.; Mewis R. E.; Williamson D. C.; Green G. G. R. The theory and practice of hyperpolarization in magnetic resonance using parahydrogen. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 67, 1–48. 10.1016/j.pnmrs.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Korchak S. E.; Ivanov K. L.; Yurkovskaya A. V.; Vieth H.-M. Para-hydrogen induced polarization in multi-spin systems studied at variable magnetic field. Phys. Chem. Chem. Phys. 2009, 11, 11146–11156. 10.1039/b914188j. [DOI] [PubMed] [Google Scholar]
- Barskiy D. A.; Knecht S.; Yurkovskaya A. V.; Ivanov K. L. SABRE: Chemical kinetics and spin dynamics of the formation of hyperpolarization. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 114–115, 33–70. 10.1016/j.pnmrs.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Fekete M.; Bayfield O.; Duckett S. B.; Hart S.; Mewis R. E.; Pridmore N.; Rayner P. J.; Whitwood A. Iridium(III) Hydrido N-Heterocyclic Carbene-Phosphine Complexes as Catalysts in Magnetization Transfer Reactions. Inorg. Chem. 2013, 52, 13453–13461. 10.1021/ic401783c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner P. J.; Norcott P.; Appleby K. M.; Iali W.; John R. O.; Hart S. J.; Whitwood A. C.; Duckett S. B. Fine-tuning the efficiency of para-hydrogen-induced hyperpolarization by rational N-heterocyclic carbene design. Nat. Commun. 2018, 9, 4251. 10.1038/s41467-018-06766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete M.; Rayner P. J.; Green G. G. R.; Duckett S. B. Harnessing polarisation transfer to indazole and imidazole through signal amplification by reversible exchange to improve their NMR detectability. Magn. Reson. Chem. 2017, 55, 944–957. 10.1002/mrc.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner P. J.; Burns M. J.; Olaru A. M.; Norcott P.; Fekete M.; Green G. G. R.; Highton L. A. R.; Mewis R. E.; Duckett S. B. Delivering strong 1H nuclear hyperpolarization levels and long magnetic lifetimes through signal amplification by reversible exchange. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, E3188–E3194. 10.1073/pnas.1620457114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. S.; Norcott P.; Rayner P. J.; Green G. G. R.; Duckett S. B. A Hyperpolarizable1H Magnetic Resonance Probe for Signal Detection 15 Minutes after Spin Polarization Storage. Angew. Chem., Int. Ed. 2016, 55, 15642–15645. 10.1002/anie.201609186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. S.; Norcott P.; Rayner P. J.; Green G. G. R.; Duckett S. B. A Simple Route to Strong Carbon-13 NMR Signals Detectable for Several Minutes. Chem.—Eur. J. 2017, 23, 10496–10500. 10.1002/chem.201702767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis T.; Ortiz G. X.; Logan A. W. J.; Claytor K. E.; Feng Y.; Huhn W. P.; Blum V.; Malcolmson S. J.; Chekmenev E. Y.; Wang Q.; Warren W. S. Direct and cost-efficient hyperpolarization of long-lived nuclear spin states on universal N-15(2)-diazirine molecular tags. Sci. Adv. 2016, 2, e1501438 10.1126/sciadv.1501438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanato G.; Hill-Cousins J. T.; Håkansson P.; Roy S. S.; Brown L. J.; Brown R. C. D.; Pileio G.; Levitt M. H. A Nuclear Singlet Lifetime of More than One Hour in Room-Temperature Solution. Angew. Chem., Int. Ed. 2015, 54, 3740–3743. 10.1002/anie.201411978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumez J.-N. Perspective on long-lived nuclear spin states. Mol. Phys. 2020, 118, e1644382 10.1080/00268976.2019.1644382. [DOI] [Google Scholar]
- Levitt M. H. Long live the singlet state!. J. Magn. Reson. 2019, 306, 69–74. 10.1016/j.jmr.2019.07.029. [DOI] [PubMed] [Google Scholar]
- Sellies L.; Reile I.; Aspers R. L. E. G.; Feiters M. C.; Rutjes F. P. J. T.; Tessari M. Parahydrogen induced hyperpolarization provides a tool for NMR metabolomics at nanomolar concentrations. Chem. Commun. 2019, 55, 7235–7238. 10.1039/c9cc02186h. [DOI] [PubMed] [Google Scholar]
- Eshuis N.; Hermkens N.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Wijmenga S. S.; Tessari M. Toward Nanomolar Detection by NMR Through SABRE Hyperpolarization. J. Am. Chem. Soc. 2014, 136, 2695–2698. 10.1021/ja412994k. [DOI] [PubMed] [Google Scholar]
- Iali W.; Rayner P. J.; Duckett S. B. Using parahydrogen to hyperpolarize amines, amides, carboxylic acids, alcohols, phosphates, and carbonates. Sci. Adv. 2018, 4, eaao6250 10.1126/sciadv.aao6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iali W.; Rayner P. J.; Alshehri A.; Holmes A. J.; Ruddlesden A. J.; Duckett S. B. Direct and indirect hyperpolarisation of amines using parahydrogen. Chem. Sci. 2018, 9, 3677–3684. 10.1039/c8sc00526e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemeinhardt M. E.; Limbach M. N.; Gebhardt T. R.; Eriksson C. W.; Eriksson S. L.; Lindale J. R.; Goodson E. A.; Warren W. S.; Chekmenev E. Y.; Goodson B. M. ″Direct″ (13) C Hyperpolarization of (13) C-Acetate by MicroTesla NMR Signal Amplification by Reversible Exchange (SABRE). Angew. Chem., Int. Ed. Engl. 2020, 59, 418–423. 10.1002/anie.201910506. [DOI] [PubMed] [Google Scholar]
- Iali W.; Roy S. S.; Tickner B. J.; Ahwal F.; Kennerley A. J.; Duckett S. B. Hyperpolarising Pyruvate through Signal Amplification by Reversible Exchange (SABRE). Angew. Chem., Int. Ed. 2019, 58, 10271–10275. 10.1002/anie.201905483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst M.; Chiavazza E.; Haase A.; Aime S.; Schwaiger M.; Schulte R. F. α-trideuteromethyl[15N]glutamine: A long-lived hyperpolarized perfusion marker. Magn. Reson. Med. 2016, 76, 1900–1904. 10.1002/mrm.26104. [DOI] [PubMed] [Google Scholar]
- Cudalbu C.; Comment A.; Kurdzesau F.; van Heeswijk R. B.; Uffmann K.; Jannin S.; Denisov V.; Kirik D.; Gruetter R. Feasibility of in vivo 15N MRS detection of hyperpolarized 15N labeled choline in rats. Phys. Chem. Chem. Phys. 2010, 12, 5818–5823. 10.1039/c002309b. [DOI] [PubMed] [Google Scholar]
- Gabellieri C.; Reynolds S.; Lavie A.; Payne G. S.; Leach M. O.; Eykyn T. R. Therapeutic Target Metabolism Observed Using Hyperpolarized 15N Choline. J. Am. Chem. Soc. 2008, 130, 4598–4599. 10.1021/ja8001293. [DOI] [PubMed] [Google Scholar]
- Jagtap A. P.; Kaltschnee L.; Glöggler S. Hyperpolarization of 15N-pyridinium and 15N-aniline derivatives by using parahydrogen: new opportunities to store nuclear spin polarization in aqueous media. Chem. Sci. 2019, 10, 8577–8582. 10.1039/c9sc02970b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.; Gamliel A.; Uppala S.; Nardi-Schreiber A.; Sosna J.; Gomori J. M.; Katz-Brull R. Long-lived 15 N Hyperpolarization and Rapid Relaxation as a Potential Basis for Repeated First Pass Perfusion Imaging - Marked Effects of Deuteration and Temperature. ChemPhysChem 2018, 19, 2148–2152. 10.1002/cphc.201800261. [DOI] [PubMed] [Google Scholar]
- Atkinson K. D.; Cowley M. J.; Duckett S. B.; Elliott P. I. P.; Green G. G. R.; López-Serrano J.; Khazal I. G.; Whitwood A. C. Para-Hydrogen Induced Polarization without Incorporation of Para-Hydrogen into the Analyte. Inorg. Chem. 2009, 48, 663–670. 10.1021/ic8020029. [DOI] [PubMed] [Google Scholar]
- Theis T.; Truong M. L.; Coffey A. M.; Shchepin R. V.; Waddell K. W.; Shi F.; Goodson B. M.; Warren W. S.; Chekmenev E. Y. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. 2015, 137, 1404–1407. 10.1021/ja512242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong M. L.; Theis T.; Coffey A. M.; Shchepin R. V.; Waddell K. W.; Shi F.; Goodson B. M.; Warren W. S.; Chekmenev E. Y. 15N Hyperpolarization by Reversible Exchange Using SABRE-SHEATH. J. Phys. Chem. C 2015, 119, 8786–8797. 10.1021/acs.jpcc.5b01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colell J. F. P.; Logan A. W. J.; Zhou Z.; Shchepin R. V.; Barskiy D. A.; Ortiz G. X.; Wang Q.; Malcolmson S. J.; Chekmenev E. Y.; Warren W. S.; Theis T. Generalizing, Extending, and Maximizing Nitrogen-15 Hyperpolarization Induced by Parahydrogen in Reversible Exchange. J. Phys. Chem. C 2017, 121, 6626–6634. 10.1021/acs.jpcc.6b12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis T.; Truong M.; Coffey A. M.; Chekmenev E. Y.; Warren W. S. LIGHT-SABRE enables efficient in-magnet catalytic hyperpolarization. J. Magn. Reson. 2014, 248, 23–26. 10.1016/j.jmr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svyatova A.; Skovpin I. V.; Chukanov N. V.; Kovtunov K. V.; Chekmenev E. Y.; Pravdivtsev A. N.; Hövener J. B.; Koptyug I. V. 15 N MRI of SLIC-SABRE Hyperpolarized 15 N-Labelled Pyridine and Nicotinamide. Chem.—Eur. J. 2019, 25, 8465–8470. 10.1002/chem.201900430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Yurkovskaya A. V.; Zimmermann H.; Vieth H.-M.; Ivanov K. L. Enhancing NMR of insensitive nuclei by transfer of SABRE spin hyperpolarization. Chem. Phys. Lett. 2016, 661, 77–82. 10.1016/j.cplett.2016.08.037. [DOI] [Google Scholar]
- Kidd B. E.; Gesiorski J. L.; Gemeinhardt M. E.; Shchepin R. V.; Kovtunov K. V.; Koptyug I. V.; Chekmenev E. Y.; Goodson B. M. Facile Removal of Homogeneous SABRE Catalysts for Purifying Hyperpolarized Metronidazole, a Potential Hypoxia Sensor. J. Phys. Chem. C 2018, 122, 16848–16852. 10.1021/acs.jpcc.8b05758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovpin I. V.; Svyatova A.; Chukanov N.; Chekmenev E. Y.; Kovtunov K. V.; Koptyug I. V. 15N Hyperpolarization of Dalfampridine at Natural Abundance for Magnetic Resonance Imaging. Chem.—Eur. J. 2019, 25, 12694–12697. 10.1002/chem.201902724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres O.; Martín M.; Sola E. Labile N-Heterocyclic Carbene Complexes of Iridium. Organometallics 2009, 28, 863–870. 10.1021/om800965y. [DOI] [Google Scholar]
- Fekete M.; Bayfield O.; Duckett S. B.; Hart S.; Mewis R. E.; Pridmore N.; Rayner P. J.; Whitwood A. Iridium(III) Hydrido N-Heterocyclic Carbene-Phosphine Complexes as Catalysts in Magnetization Transfer Reactions. Inorg. Chem. 2013, 52, 13453–13461. 10.1021/ic401783c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis R. E.; Atkinson K. D.; Cowley M. J.; Duckett S. B.; Green G. G. R.; Green R. A.; Highton L. A. R.; Kilgour D.; Lloyd L. S.; Lohman J. A. B.; Williamson D. C. Probing signal amplification by reversible exchange using an NMR flow system. Magn. Reson. Chem. 2014, 52, 358–369. 10.1002/mrc.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W.; Lumata L.; Chen W.; Zhang S.; Kovacs Z.; Sherry A. D.; Khemtong C. Hyperpolarized 15N-pyridine Derivatives as pH-Sensitive MRI Agents. Sci. Rep. 2015, 5, 9104. 10.1038/srep09104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper N. C. Theory of scalar relaxation of the second kind. II. Mol. Phys. 1971, 21, 961–976. 10.1080/00268977100102131. [DOI] [Google Scholar]
- Barskiy D. A.; Shchepin R. V.; Tanner C. P. N.; Colell J. F. P.; Goodson B. M.; Theis T.; Warren W. S.; Chekmenev E. Y. The Absence of Quadrupolar Nuclei Facilitates Efficient 13C Hyperpolarization via Reversible Exchange with Parahydrogen. ChemPhysChem 2017, 18, 1493–1498. 10.1002/cphc.201700416. [DOI] [PubMed] [Google Scholar]
- Kelly R. A. III; Clavier H.; Giudice S.; Scott N. M.; Stevens E. D.; Bordner J.; Samardjiev I.; Hoff C. D.; Cavallo L.; Nolan S. P. Determination of N-Heterocyclic Carbene (NHC) Steric and Electronic Parameters using the [(NHC)Ir(CO)2Cl] System. Organometallics 2008, 27, 202–210. 10.1021/om701001g. [DOI] [Google Scholar]
- Kizaka-Kondoh S.; Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci. 2009, 100, 1366–1373. 10.1111/j.1349-7006.2009.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procissi D.; Claus F.; Burgman P.; Koziorowski J.; Chapman J. D.; Thakur S. B.; Matei C.; Ling C. C.; Koutcher J. A. In vivo 19F Magnetic Resonance Spectroscopy and Chemical Shift Imaging of Tri-Fluoro-Nitroimidazole as a Potential Hypoxia Reporter in Solid Tumors. Clin. Cancer Res. 2007, 13, 3738–3747. 10.1158/1078-0432.ccr-06-1563. [DOI] [PubMed] [Google Scholar]
- Barskiy D. A.; Shchepin R. V.; Coffey A. M.; Theis T.; Warren W. S.; Goodson B. M.; Chekmenev E. Y. Over 20% 15N Hyperpolarization in Under One Minute for Metronidazole, an Antibiotic and Hypoxia Probe. J. Am. Chem. Soc. 2016, 138, 8080–8083. 10.1021/jacs.6b04784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchepin R. V.; Jaigirdar L.; Theis T.; Warren W. S.; Goodson B. M.; Chekmenev E. Y. Spin Relays Enable Efficient Long-Range Heteronuclear Signal Amplification by Reversible Exchange. J. Phys. Chem. C 2017, 121, 28425–28434. 10.1021/acs.jpcc.7b11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchepin R. V.; Jaigirdar L.; Chekmenev E. Y. Spin-Lattice Relaxation of Hyperpolarized Metronidazole in Signal Amplification by Reversible Exchange in Micro-Tesla Fields. J. Phys. Chem. C 2018, 122, 4984–4996. 10.1021/acs.jpcc.8b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd B. E.; Gesiorski J. L.; Gemeinhardt M. E.; Shchepin R. V.; Kovtunov K. V.; Koptyug I. V.; Chekmenev E. Y.; Goodson B. M. Facile Removal of Homogeneous SABRE Catalysts for Purifying Hyperpolarized Metronidazole, a Potential Hypoxia Sensor. J. Phys. Chem. C 2018, 122, 16848–16852. 10.1021/acs.jpcc.8b05758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P. M.; Iali W.; Roy S. S.; Rayner P. J.; Halse M. E.; Duckett S. B. Rapid 13C NMR hyperpolarization delivered from para-hydrogen enables the low concentration detection and quantification of sugars. Chem. Sci. 2019, 10, 10607–10619. 10.1039/c9sc03450a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.