Abstract
Depression is a major contributor to the overall global burden of disease, with high prevalence and relapse rate. Several factors have been considered in order to reduce the depression burden. Among them, physical activity (PA) showed a potential protective role. However, evidence is contrasting probably because of the differences in PA measurement. The aim of this systematic review with meta-analysis is to assess the association between objectively measured PA and incident and prevalent depression. The systematic review was conducted according to methods recommended by the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Relevant papers published through 31 August 2019 were identified searching through the electronic databases PubMed/MEDLINE, Excerpta Medica dataBASE (Embase), PsycINFO, Scopus, Web of Science (WoS), and the Cochrane Library. All analyses were conducted using ProMeta3. Finally, 42 studies met inclusion criteria. The overall Effect size (ES) of depression for the highest vs. the lowest level of PA was −1.16 [(95% CI = −1.41; −0.91), p-value < 0.001] based on 37,408 participants. The results of the meta-analysis showed a potential protective effect of PA on prevalent and incident depression.
Keywords: depressive symptoms, depression, physical activity, accelerometer, objectively measure, meta-analysis
1. Introduction
Depression is one of the major leading causes of disability worldwide, affecting approximately 400 million people [1], with 9% of men and 17% of women experiencing depressive symptoms at least once in their life. Mainly due to social prejudices, depression continues to be frequently under-diagnosed and inadequately treated [2]. Depression can have several negative consequences, being characterized by sad mood and/or loss of interest, affecting thoughts, feelings, behaviors, physical health and impairing social and occupational functioning [3,4]. Furthermore, over 80% of depressed patients have more than one depressive episode during their lifespan [5,6]. In this context, innovative and effective preventive and therapeutic strategies are required.
Current studies are focusing on the important role played by lifestyles and in particular physical activity (PA), in both preventing and treating depression [7]. Several biological mechanisms are potentially involved in the association between PA and depression, such as the noradrenergic and serotoninergic effects of physical activity [8], the hypothalamic-pituitary-adrenal axis regulation [9], the production of neurotrophic factor [10], and lastly the improvement in vascular function and oxygenation [11,12]. However, despite the high number of potential explanations, evidence is not concordant in proven positive association between PA and depression, for both prevention and treatment. One of the main reasons behind these contrasting results could be the different methods used to measure physical activity.
Two recent meta-analyses focusing on prevalent depression and incident depression found an inverse association between prevalent depression and PA [13], while this association was not significant when incident depression has been considered [14]. However, the study conducted by Schuch et al. retrieved only one paper using the objectively measured PA [13]. The meta-analysis conducted by Krogh et al. included trials that prescribed different types of exercise sessions without objectively measuring PA [14]. On the other hand, growing evidence is focusing on objectively measured physical activity, using for instance accelerometer and pedometer, showing how objectively measured PA is more precise than self-reported one. This was particularly true in estimating duration, total amount and intensity [15].
We performed a systematic review with meta-analysis of the evidence from the literature to assess the relation between physical activity objectively measured and incident and prevalent depression.
2. Materials and Methods
We conducted this systematic review according to the methods recommended by the Cochrane Collaboration [16] and to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [17] and documented the process and results in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. The review protocol has been registered on PROSPERO [19], the International Prospective Register of Systematic Reviews funded by the National Institute of Health Research (https://www.crd.york.ac.uk/prospero/).
2.1. Information Sources and Search Strategy
Studies were identified searching through the electronic databases PubMed/MEDLINE, Embase, Scopus, Web of Science (WoS), PsycINFO and the Cochrane Library. We combined the search strategy of free text terms and exploded MESH headings for the topics of depression, physical activity, objective measurements, and type of study. The strategy was firstly developed in PubMed/MEDLINE and then adapted for use in the other databases (Supplementary Table S1). Studies conducted on human subjects and published in English through 31 August 2019 were included.
2.2. Inclusion and Exclusion Criteria
We considered studies that investigated the relation between physical activity objectively measured and depression, both as a continuous and as a binary variable. Adult participants of both sexes were considered. As done before [20,21], both population-based and hospital-based studies were included. Among hospital-based studies, inpatients, day-hospital, and outpatient subjects were included while emergency care records were excluded as considered non-representative. All experimental and observational study designs were included apart from case reports. Narrative and systematic reviews, letters to the editor and book chapters were excluded. Table 1 shows a detailed description of inclusion/exclusion criteria according to the Population, Exposure, Outcomes and Study design (PEOS) [22], adjusted for observational studies extended with time and language filters, as recommended by the Cochrane Collaboration [16].
Table 1.
Detailed description of inclusion/exclusion criteria according to a Population, Exposure, Outcomes and Study design (PEOS).
| Search Strategy | Details |
|---|---|
| Inclusion criteria | P: adults (men and women) E: physical activity objectively measured O: Depressive disorder S: Trials, cohort studies, case-control, cross-sectional |
| Exclusion criteria | P: people < 18 years old E: physical activity not objectively measured (self-reported) O: other psychological disorders S: not original papers (opinion paper, review article, commentary, letter, protocols, article without quantitative data) |
| Language filter | English |
| Time filter | No filter (from inception) |
| Database | PubMed/Medline; EMBASE, Web of Science; Scopus, PsycoInfo, Cochrane |
2.3. Study Selection and Data Extraction
Identified studies were independently reviewed for eligibility by two couples of authors (VG, LB, MM, SC) in a two-step process: a first screening was performed based on title and abstract, while full texts were retrieved for the second screening. At both stages disagreements by reviewers were resolved by consensus. Data were independently extracted by three authors (LB, MM, SC) and supervised by a senior author (VG) using an ad-hoc developed data extraction spreadsheet. The data extraction spreadsheet was piloted on 10 randomly selected papers and modified accordingly. As done before [23,24,25], both qualitative and quantitative data was extracted from the original studies. Qualitative data recorded included the following items: name of first author and year of publication, country where the study was conducted and period during which the study was performed, device used to measure PA and tool used for depression diagnosis. Moreover, characteristics of the subjects were recorded (e.g., age, gender, comorbidities). Quantitative data extracted includes: sample size, number of participants lost (attrition), duration of PA measurement, distribution of depressed participants in the sample, level of PA performed and the results estimating the association between PA objectively measured and depression.
2.4. Quality Evaluation
The quality evaluation of the included publications were independently assessed by two authors using the New-Ottawa Scale [26] for observational studies and Cochrane Collaboration tool for trials [27].
2.5. Meta-Analysis
We pooled individual studies data using ProMeta3® (Internovi, Milano, Italy) software. Due to heterogeneity, a random effects meta-analysis was employed. In order to reduce the heterogeneity, two sensitivity analyses were conducted, considering the following items: (i) study design, (ii) participants’ comorbidities. Moreover, a subgroup analysis by gender was conducted in order to estimate potential different effects among the two groups. We assessed publication bias with the visual inspection of a funnel plot [27] and the Begg [28] and Egger [29] tests.
3. Results
3.1. Literature Search
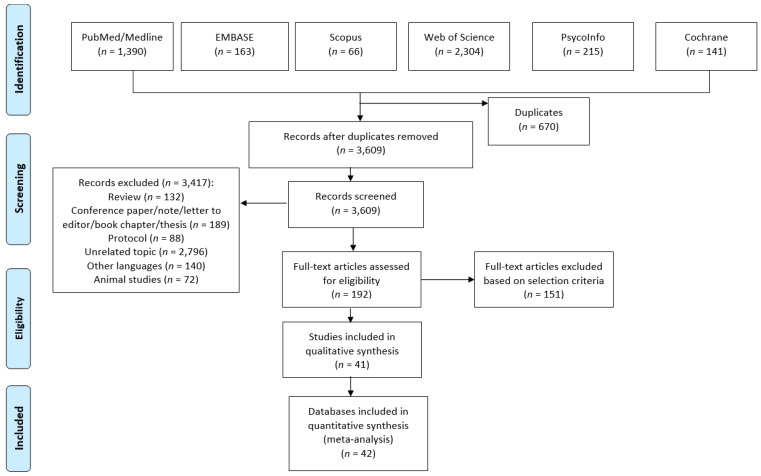
A total of 4279 articles were retrieved. After a preliminary screening 670 articles were excluded because of duplicates, 409 not original papers (reviews, letters to the editor, editorials, protocols, etc.), and 2796 covering a different topic. After title and abstract screening, a total of 192 full-text articles were consulted, while at the end of the screening process only 41 were included in the systematic review [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]. As it was not possible to extrapolate data from one study, it was not included in the quantitative evaluation [67]. Figure 1 shows the selection process. Two studies reported separate data for men and women [49,54] and for this reason they were considered separately, resulting in 42 datasets being included in the meta-analysis.
Figure 1.
Flow diagram of the selection process.
The characteristics of the included studies are reported in Table 2. The majority of the studies were conducted in Europe (n = 18, 43%) and North America (n = 12, 29%). The first study assessing objectively measure PA and depression was published in 2004 [68]. The smallest sample size included in a study was of 23 participants [70], whereas the largest sample size was of 16,415 participants [62]. Twenty-six of the 42 datasets were cross-sectional (62%), eight trials (19%), six cohort studies (14%), and one case-control study (2%). The quality assessment of trials is reported in Supplementary Table S2. Thirty-two datasets (76%) used an accelerometer as the measurement device, while nine datasets (21%) used a pedometer. In almost all studies participants were asked to wear the device for 7 days, and even in cohort studies PA was measured only at baseline. With regard to depression, heterogeneous tools were used to make diagnosis, such as the Hospital Anxiety and Depression Scales (HADS), the Patient Health Questionnaire-9 (PHQ-9), the Beck Depression Inventory (BDI-II) and the Center for Epidemiologic Studies Depression Scale (CESD). Most of the time HADS was used (n = 11), followed by PHQ-9 questionnaire (n = 9); however almost all studies used a validated tool. At the same time, the results were expressed using different measures, as for instance Odd Ratio (OR), Relative Risk (RR), β coefficient (β) and Spearman’s Rho (r).
Table 2.
Descriptive characteristics of the included studies stratified by study design and listed in alphabetical order.
| Author Year [Reference] |
Country | Characteristics | Study Period | Age and Gender | Sample Size and Gender | Depressed Subjects | Attrition + | Device Used | Duration of Measurement | Tool Used for Depression Diagnosis | PA | Results | QS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional studies | |||||||||||||
| Al-Eisa, 2014 [31] |
Saudi Arabia | Female students | 2014 | Mean: 20.9 ± 1.4 y, F | 76 | 52% | 29 | Pedometer | 3 weeks | BDI-II | PA = 8715 steps/day | R = −0.78 p ≤ 0.01; |
4 |
| Alosco, 2012 [32] |
USA | Persons with heart failure | n.a. | Mean: 68.81 ± 8.8 y, M, F | 96 (M = 63, 5%, F = 36, 5%) |
n.a. | 27 | GT1M+ accelerometer (ActiGraph, Pensacola, Florida) | 7 days at baseline, 3 months, 12 months | BDI-II | MVPA = 3.24 ± 9.0 min/day | β = −64.35 p < 0.05 t = −2.32 |
6 |
| Altenburg, 2013 [33] |
The Netherlands | Patients with stable chronic obstructive pulmonary disease (COPD) | n.a. | Mean: 62 (54–69) y M, F |
155 (M = 102, F = 53) | n.a. | 0 | Yamax-Digiwalker pedometer (SW-200) | 2 weeks | HADS | PA = 4206 (2387–6284) steps/day | R = −0.191 p < 0.05 |
4 |
| Arrieta, 2018 [34] |
Spain | Partecipants from nursing home | October 2016–June 2017 | 84.9 ± 6.9 years | 114 (81 F, 33 M) | 25% (at risk of depression | 0 | Actigraph GT3X model | 7 days | GDS | MVPA = 0.9 ± 1.2 min/day | β = 1.142 p = 0.028 |
7 |
| Bade, 2018 [35] |
USA | Lung Cancer Patients | 2014–2015 | Mean: 66 ± 7.75(SD) y (51–80) M, F |
30 (M = 20, F = 10) |
n.a. | 43 | Accelerometer (Fitbit Zip) | 7 days | PHQ-9 | PA = 4877 ± 305 | R = −0.40 | 5 |
| Barriga, 2014 [36] |
Portugal | COPD patients | n.a. | Mean: 67 ± 9.6 y, M | 55 (sex n.s.) | n.a. | 0 | Pedometer | Number of steps per day, on three consecutive days | HADS | PA = 4972.4 ± 2242.3 | R = −0.424 p < 0.01 |
3 |
| Di Marco, 2014 [37] |
Italy | COPD patients | n.a. | Mean: 71 ± 6 y M, F |
70 (M = 52, F = 18); No Depression = 51 (18% F) |
19 (47% F) | 0 | Accelerometer (SenseWear Pro Armband, BodyMedia) | 5 days | HADS | No Depression PA = 6950 ± 2431 Depresed PA = 5055 ± 2576 | β = 0.106 p = 0.84 |
6 |
| Dillon, 2017 [38] |
Ireland | Patients in the 50–69 year age group. | 2011 | Mean: 59.6 ± 5.5 y M, F |
397 (M = 182, F = 214) |
18.2% | 78 | Accelerometer (ActivInsights Ltd.) | 7 days | CESD-20 | Mean Light PA No Depression = 103 min/day Depressed = 105 min/day |
β = −0.34 (95% CI = −0.64 to −0.04) |
7 |
| Drieling, 2014 [39] |
USA | Obese latino immigrants | July 2009–September 2010 | n.a. | 207 (48 M, 159 F) | 36.7% | 0 | Pedometer | 7 days | CESD | 6.3 ± 3.1 steps/day in thousands | β = −0.02 SE 0.01 p = 0.03 |
6 |
| Elbelt, 2015 [41] |
Germany | High grade obesity | 2008–2010 | Mean: 42 ± 12 y | 50 (10 M, 40 F) | 36% | 0 | Accelerometer | 3 days | PHQ-9 | No depressed: 6023 ± 2459 steps/day Depressed: 6532 ± 3085 steps/day |
r = 0.023 | 7 |
| Fenton, 2017 [42] |
England | Rheumatoid Arthritis patients | n.a. | Mean: 54.92 ± 12.39 y | 61 (F = 67.2%) | n.a. | 36 | Actigraph GT3X+, accelerometer (Pensacola, FL) | 7 days | HADS | LPA = 269.35 ± 69.35 min/day | β = −0.30 p < 0.05 |
10 |
| Gaskin, 2016 [44] |
Australia | Prostate cancer survivors | n.a. | 65.6 ± 8.5 y | 98 (M) | n.a. | n.a. | ActiGraph GT1 M (Pensacola, FL) | 7 days | CESD | MVPA = 38 min/day | β = ·0.00 p = 0.97 |
10 |
| Howie, 2018 [49] |
Australia | Subsample of the 22th follow-up measurement of the Raine cohort Study. | 2011 | n.a. | 475 (256 F, 219 M) | 1.3% | 299 | Actigraph GT3X+, accelerometer Pensacola, FL | 7 days | DASS-21 | MVPA F = 27 min/day M = 34.1 min/day |
F: RR = 0.99 (95% CI = 0.98–1.00), p = 0.078 M: RR= 1.01 (95% CI = 0.99–1.02), p = 0.300 |
10 |
| Huong, 2013 [58] |
USA | COPD patients | n.a. | Mean: 66.5 ± 8.8 y M, F |
148 (M = 115, F = 33) |
29% | 0 | Accelerometer Stepwatch 3 Activity Monitor (OrthoCare Innovations LLC) | 7 days | HADS | Mean = 6.079 ± 3718 | β = −0.19 p = 0.02) |
7 |
| Jung, 2018 [50] |
Japan | Community-dwelling older Japanese adults. | 2013 | Mean: >75 y M, F |
3054 (M = 1491, F = 1563) |
598 | 2.203 | Accelerometer (GT40-020) | 7–40 days | GDS | No Depression = 5059.6 ± 53.7 steps/day Depressed = 5003.0 ± 112.1 steps/day |
Coehns = 0.03 p = 0.359 |
7 |
| Kangasniemi, 2014 [51] |
Finland | Adults, general population | 2011 | Mean 43 ± 5.2 y, | 108 (58 F, 50 M) | n.a. | 109 | ActiGraph-GT1M, accelerometer LLC, Pensacola, Florida | 7 days | BDI- II | Less Active: 24.3 ± 12.4 min/day More active: 62.7 ± 24.7 min/day |
r = −0.24, (95% CI 0.38, 0.08) | 7 |
| King, 2014 [52] |
USA | Adults with ≥class 2 obesity. | 2009 | Mean 45 (18–78) y | 850 (673 F, 177 M) | 31.8% | 3626 | StepWatch™ 3 Activity Monitor (OrthoCare Innovations, Washington, D.C.) | 7 days | BDI- II | PA ≥ 1000 steps/day Mean: 7321.0 steps/day |
OR = 1.03 (95% CI 0.97–1.09)c | 7 |
| Loprinzi, 2012 [54] |
USA | non-institutionalized U.S. civilians | 2005–2006 | 48.4 ± 0.8 y | 1146 (611 M) | 9.5% | n.a. | ActiGraph AM-7164, accelerometer Walton, Beach, FL. | 7 days | PHQ-9 | MVPA = 2020–5998 steps/min | M: OR 0.71 (95% CI 0.53–0.95) F:OR = 0.74 (95% CI 0.57–0.96) |
10 |
| Loprinzi 2013 (A) [55] |
USA | non-institutionalized USA civilians | 2006 | Mean: 73.5 ± 0.2 y | 708 (57.2% M) | 14.9% | n.a. | ActiGraph AM-7164, accelerometer Walton, Beach, FL. | 7 days | PHQ-9 | MVPA = 10.0 ± 0.9 min/day | OR = 0.78 (95% CI 0.64–0.94) | 9 |
| Loprinzi, 2013 (B) [56] |
USA | Diabetic non-institutionalized USA civilians | 2006 | Mean: 59.6 ± 1.2 y | 372 (51.4% F) | 3.1% | n.a. | ActiGraph AM-7164, accelerometer Walton, Beach, FL. | 7 days | PHQ-9 | MVPA = 12.2 ± 1.3 min/day | β = −0.03 (95% CI −0.05—−0.006) p < 0.05 |
10 |
| Ludwig, 2018 [57] |
UK | UK residents | 2013–2015 | 69 ± 4.1 y | 1720 (M = 85.5%) | 4% | 20 | ActiGraph GT3X accelerometer (ActiGraph, Florida, USA) | 7 days | PHQ-9 | PA = 6151 steps/day | β = −0.170 p < 0.001 |
7 |
| Park, 2017 [60] |
UK | Subjects living facilities across England | n.a. | 77.5 ± 8.2 y | 85 (M = 31.8%) | n.a. | 0 | GT3X+, WGT3X-BT; ActiGraph, Pensacola, FL, USA | n.a. | HADS | MVPA = 9.74 min/day | Χ2 = 8.45 p = 0.004 |
5 |
| Song, 2011 [64] |
USA | community residents older than 20 years | 2006 | ≥20 y | 4058 (51.32% F) | 19.5% | 6290 | ActiGraph® AM-7164, accelerometer Walton, Beach, FL. | 7 days | PHQ-9 | MPA = 30 min daily and more than 3 days a week | OR = 0.72 (95% CI 0.54–0.97) p < 0.05 |
7 |
| Vallance JK, 2011 [66] |
USA | non-institutionalized civilian US citizens | 2005–2006 | 45.7 ± 13.7 y | 2862 (1417 M) | 195 | n.a. | ActiGraph AM-7164, accelerometer Walton, Beach, FL. | 7 days | PHQ-9 | MVPA = 20.2 ± 0.2 min/day | OR = 0.37, (95% CI, 0.20 to 0.70) p < 0.01 |
9 |
| Vallance J.K, 2015 [67] |
Canada | Colon cancer survivors | n.a. | Mean: 64.3 ± 10.3 y M, F | 180 (M = 99, F = 81) |
8.5% | 17 | Actigraph GT3X+ accelerometer | 7 days | PHQ-9 | non-extrapolatable | non-extrapolatable | 8 |
| Whitaker, 2014 [69] |
USA | Overweight and obese women | n.a. | Mean: 38.3 ± 7.6 y | 196 (F) | n.a. | 34 | ActiGraph-GT1M, accelerometer LLC, Pensacola, Florida | 7 days | CESD-10 | MVPA ≥ 2400 steps/min | t = 0.30 p = 0.77 |
9 |
| Case-control studies | |||||||||||||
| O’Brien JT, 2016 [59] |
UK | adults > 60yo | 2015 | 74 ± 6 y | 58 (43 F) | 29 | 0 | Accelerometer | 7 days | Montgomery–Åsberg Depression Rating Scale (MADRS); GDS-15 | 0.17 acceleration/min/day | r = −0.37 p ≤ 0.05 |
7 |
| Cohort studies | |||||||||||||
| Duenas-Espin, 2016 [40] |
Europe (Athens, Leuven, London, Groningen). | COPD patients | July–November 2011 | M, F Mean: 67 ± 8y |
220 (149 M, 71 F) | 5% | n.a. | Accelerometer Dynaport MoveMonitor (McRoberts BV, The Hague, the Netherlands). | 7 days at baseline, 6 and 12 months | HADS hospital anxiety and depression scale) (depression>11 points) |
4812 ± 3147 steps/day | β = 0.6 (95% CI 0.5 to 0.8) p = 0.01 |
5 |
| Follow-up = 1 y | |||||||||||||
| Po-Wen, 2017 [53] |
Taiwan | community-dwelling older adults | 2012–2014 | Mean: 74.5 y M, F |
285 (M = 125, F = 149) |
n.a. | 11 | ActiGraph GT3X-BT (ActiGraph, Pensacola, FL) | 7 day at baseline | 15-item Geriatric Depression Scale | MVPA>1951 steps/min | RR: 0.88 95% CI (0.79–0.98) p = 0.021 |
8 |
| Follow-up = 2 y | |||||||||||||
| Raudsepp, 2017 [61] |
Estonia | generally healthy community-dwelling individuals aged 67–74 years | 2011–2013 | 67–74 y M, F |
195 (M = 85, F = 110) | n.a. | 23 | Yamax-Digiwalker pedometer (SW-200-024) | 1 week each year, per 3 years | 15-Item Geriatric Depression Scale | 6394.5 daily walking steps | β = −0.17 Χ2 = 83.27 |
6 |
| Follow-up = 3 y | |||||||||||||
| Rethorst, 2017 [62] |
USA | Hispanic/Latino men and women, age 18 to 74 years at time | 2008–2011 | Mean: 41.06 ± 0.25 y M, F |
16,415 (52.13% F) | n.a. | n.a. | Actical B-1 version accelerometer | 7 days at baseline | Center for Epidemiological Studies Depression Scale 10 | VPA≥3962 steps/min | β = −0.936 | 4 |
| Follow-up = 7 days | |||||||||||||
| Sylvester, 2017 [64] |
Canada | Breast cancer women over 1 year post-treatment | n.a. | 55.01 ± 10.96 y | 201 F | n.a. | 0 | ActiGraph GT3X-BT (ActiGraph, Pensacola, FL) | 7 days every 3 months | 10-item Center for Epidemiologic Studies Depression Scale | MPA = 14.73 ± 11.6 min/day | β = −0.73; p = 0.03 | 8 |
| Follow-up = 1 y | |||||||||||||
| Trinh, 2015 [65] |
Canada | Patients with breast cancer in stage I–III without metastatic disease | 2010–2012 | Mean: 55 ± 11 y F |
199 (F) | n.a. | 4 | ActiGraph GT3X-BT (ActiGraph, Pensacola, FL) | 7 days at baseline | CES-D10 | MVPA mean 107.1 ± 81.3 min/week) | β = −0.10 p = 0.19 |
4 |
| Follow-up = 7 days | |||||||||||||
| Trial studies | |||||||||||||
|
Author
Year |
Country | Characteristics | Study Period | Age and Gender | Sample Size | Depressed Subjects | Attrition + | Device Used | Duration of Measurement | Tool Used for Depression Diagnosis | PA | Results | Follow-up |
| Abedi, 2015 [30] |
Iran | Post-menopausal women | n.a. | n.a. | 106 F | n.a. | n.a. | Pedometer | 12 weeks | BDI-II | Before 76,377 steps/months; after: 106398/month | Intervention vs. control group 13.7 ± 5 vs. 19.6 ± 4.79 p < 0.001 |
12 weeks |
| Freitas, 2018 [43] |
Brazil | Obese adults with asthma | n.a. | 30–60 y | 51 F | 58.8% | n.a | ActiGraph GT3X-BT (ActiGraph, Pensacola, FL) | 7 days | HADS | Training group (after): 10,000 steps/day Control group(after): ~8000 steps/day |
r = 0.52 p < 0.01 |
3 months |
| Golsteijn, 2018 [45] |
Holland | prostate and colorectal cancer patients survivors | 2015–2016 | 66.5 ± 7.1 y | 427 (M, F) | n.a. | na | ActiGraph GT3X-BT (ActiGraph, Pensacola, FL) | 7 days | HADS | MVPA > 3 MET MVPA = 271 ± 211 min/week |
β = −0.64 p = 0.005 |
6 months |
| Hallam, 2018 [46] |
India, Australia, and 21 other countries | General Population, of Stepathlon corporate challenge | 2015/16 | 16–74 y | 1963 (1458 M, 505 F) | n.a. | na | own personal pedometer, or activity monitoring device | 100 days | DASS | n.a. | r = − 0.026 p = 0.254 |
100 days |
| Hartescu I, 2015 [47] |
UK | Inactive people with insomnia | 2014 | 59.8 ± 9.46 yo | 41 (30 F, 11 M) | n.a. | n.a. | ActiGraph GT3X-BT (ActiGraph, Pensacola, FL) | 6 months | BDI-II | Intervention group 66.50 ± 30.37 (min per week) |
Cohen: 0.87 (0.19–1.56) | 6 months |
| Hospes G, 2009 [48] |
Netherlands | COPD patients | 2008 | 63.1 ± 8.3 y | 35 (21 M) | n.a. | n.a. | Pedometer Digiwalker SW-2000 (Yamax; Tokyo, Japan) | 12 weeks | BDI-II | Intervention group Before 7087 ± 4058 After 7872 ± 3962 |
β = 0.93 p = 0.01 |
12 weeks |
| van den Berg-Emons, 2004 [68] |
Netherlands | Patients with stable chronic heart failure | n.a. | 58.6 ± 12.1 | 34 (25 M e 9 F) | n.a. | n.a. | Accelerometer (AM, Temec Instruments, Kerkrade | 48 h | HADS | Intervention group: 9.9% (of 24 h) Control group: 7.4% |
Intervention group: 3.4(±4.0); Control group: 4.8 ± (3.1) |
3 months |
| Vetrovsky T, 2017 [70] |
Czech Republic | inactive people from general population in primary care setting | 2015 | 41 ± 10 y | 23 (12 M, 11 F) | 0 at baseline | 0 | tri- axial pedometer (eVito 3D Step Counter SL; HMM Diagnostics GmbH, Dossenheim, Germany) | 7 days | HADS | After = +1676 | Mean difference = −2.4 [95% CI −3.7, −1.2] p = 0.001 |
3 months |
+ Number of subjects lost or incomplete data; n.a. not available; n.s. not specified; QS = quality score; COPD Chronic obstructive pulmonary disease; UK United Kingdom; USA United States of America; MVPA moderate-to-vigorous physical activity; M male; F female; BDI-II Beck Depression Inventory-II; HADS Hospital Anxiety and Depression Scale; GDS Goldberg Depression Scale; Center for Epidemiologic Studies for Depression Scale CESD-10; PHQ-9 Patient Health Questionnaire-9; DASS-21 Depression Anxiety Stress Scales.
3.2. Results of Meta-Analysis
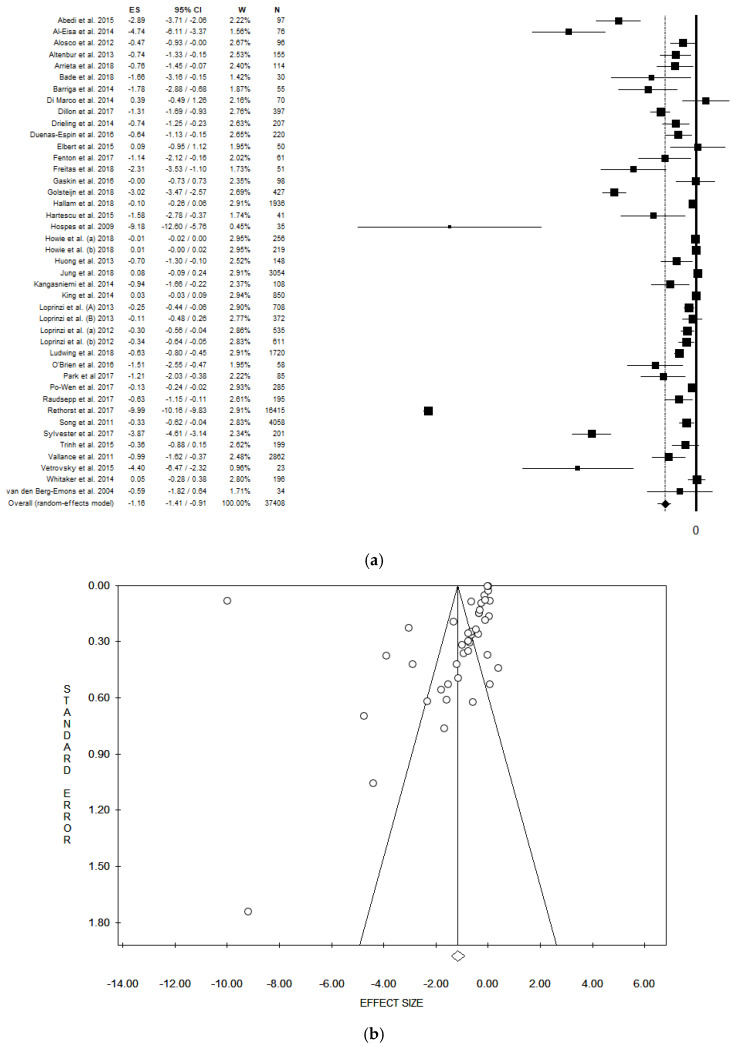
The pooled ES was −1.16 [(95% CI = −1.41; −0.91), p-value < 0.001] based on 37,408 participants (Figure 2a), with high statistical heterogeneity (Chi2 = 15,090.18, df = 41, I2 = 99.73, p-value < 0.001). A potential publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept −5.85, t = −1.91, p-value = 0.063). However, the ES estimated did not change after the trim and fill method (Figure 2b).
Figure 2.
(a) Forest plot, (b) funnel plot and of the meta-analysis assessing the association between physical activity and depression. ES, effect size; CI, confidence interval.
3.3. Sensitivity Analysis by Participants’ Comorbidities
The sub-group analysis considering only the general population (without diseases), included 21 datasets, and the pooled ES was −1.32 [(95% CI = −1.67; −0.97), p-value < 0.001] based on 33,812 subjects. High statistical heterogeneity was found (Chi2 = 14,715.47, df = 20, I2 = 99.86, p-value < 0.001). However, no publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept −9.46, t = −1.50, p-value = 0.150). The sub-group analysis considering patients with chronic obstructive pulmonary disease (COPD), included 6 datasets, and the pooled ES was −1.08 [(95% CI = −1.91; −0.24), p-value = 0.012] based on 683 subjects. High statistical heterogeneity was found (Chi2 = 33.35, df = 5, I2 = 85.01, p-value < 0.001). However, no publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept −4.12, t = −2.06, p-value = 0.109). The sub-group analysis considering obese participants, included 5 datasets, and the pooled ES was −0.35 [(95% CI = −0.80; 0.10), p-value = 0.128] based on 1354 participants. High statistical heterogeneity was found (Chi2 = 22.86, df = 4, I2 = 82.50, p-value < 0.001). However, no publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept −1.86, t = −1.65, p-value = 0.197). The sub-group analysis considering participants with (any type of) cancer, included 5 datasets, and the pooled ES was −1.79 [(95% CI = −3.35; −0.22), p-value = 0.025] based on 955 participants. High statistical heterogeneity was found (Chi2= 112.21, df = 4, I2 = 96.44, p-value < 0.001). However, no publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept 1.27, t = 0.16, p-value = 0.885).
3.4. Sensitivity Analysis by Study Design
The sub-group analysis considering only observational studies (cross-sectional, cohort and case-control studies), included 34 datasets, and the pooled ES was −0.99 [(95% CI = −1.26; −0.72), p-value < 0.001] based on 34,764 participants. High statistical heterogeneity was found (Chi2 = 14,809.58, df = 33, I2 = 99.78, p-value < 0.001). However, no publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept −6.13, t = −1.61, p-value = 0.118). The sub-group analysis considering only cross-sectional analysis, included 27 datasets, and the pooled ES was −0.23 [(95% CI = −0.30; −0.16), p-value < 0.001] based on 17,191 participants. A high statistical heterogeneity was found (Chi2 = 240.33, df = 26, I2 = 89.18, p-value < 0.001). A publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept −2.25, t = −4.89, p-value < 0.001). The sub-group analysis considering only cohort datasets, included 6 datasets, and the pooled ES was −2.61 [(95% CI = −7.41; 2.21), p-value < 0.289] based on 17,515 participants. High statistical heterogeneity was found (Chi2 = 10105.57, df = 5, I2 = 99.95, p-value < 0.001). However, no publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept −4.06, t = −0.12, p-value = 0.909). The sub-group analysis considering only interventional studies (trials), included 8 datasets, and the pooled ES was −2.63 [(95% CI = −4.06; −1.20), p-value < 0.001] based on 2644 participants. High statistical heterogeneity was found (Chi2 = 224.80, df = 7, I2 = 96.89, p-value < 0.001). Potential publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept −5.12, t = −2.56, p-value = 0.043).
3.5. Subgroup Analysis by Gender
Considering The sub-group analysis considering only women, included seven datasets, and the pooled ES was −1.91 [(95% CI = −2.77; −1.04), p-value < 0.001] based on 1415 participants. High statistical heterogeneity was found (Chi2 = 217.37, df = 6, I2 = 97.24, p-value < 0.001). Potential publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept −5.29, t = −3.82, p-value = 0.012). The sub-group analysis considering only men, included three datasets, and the pooled ES was −0.11 [(95% CI = −0.38; 0.16), p-value = 0.430] based on 928 participants. A high statistical heterogeneity was found (Chi2 = 240.33, df = 26, I2 = 89.18, p-value < 0.001). However, no publication bias was found by the visual assessment of the funnel plot and confirmed by the Egger’s linear regression test (Intercept −1.20, t = −0.99, p-value = 0.503).
4. Discussion
The current systematic review with meta-analysis—which included 43 studies in qualitative evaluation and 42 studies in the quantitative analysis—provided data on the association between objectively measured PA and the risk of depression. Since some studies expressed data separated for gender, a total of 42 datasets have been considered. The pooled ES based on 37,408 subjects indicated a significantly protective effect of PA on depression [−1.16 (95% CI = −1.41; −0.91), p-value < 0.001] while, in the subgroup analysis including only cross-sectional datasets, the risk of prevalent depression was estimated on 17,191 participants and the ES was −0.23 [(95% CI = −0.30; −0.16)]. In subgroup analysis including only longitudinal datasets, the risk of incident depression, estimated on 17,515 participants, was lower −2.61 [(95% CI = −7.41; 2.21).
With the purpose of deeply understanding the strength of the association between objectively measured PA and depression, a sub-group analysis by participants’ comorbidity has been conducted. When studies assessing the association among participants with comorbidities were considered, the ES were not statistically significant (apart for COPD participants). However, prescription of adapted PA among participants affected by co-morbidities should be considered [71]. To the contrary, when only studies with general population (otherwise healthy people) were considered, the pooled ES was statistically significant, indicating an inverse association between PA objectively measured and depression (more PA was associated with lower risk of depression). A subgroup analysis by gender was conducted as well, showing a protective effect of PA only for women. However, this result should be considered carefully, since only three studies assessed PA and depression only in men, reducing the sample size.
These results are extremely important considering that depression is one of the leading causes of disabilities worldwide [1]. In the last fifty years a great concern was casted on physical health of depressed individuals. This could be due because physical exercise seems to improve several biomarkers implicated in depression (e.g., impaired neuroplasticity, autonomic and immune imbalances) [9]. In in-vivo models, physical activity showed a serotoninergic effect as some antidepressant medications [8]. Moreover, PA has demonstrated an effect on inflammatory processes, through the hypothalamic-pituitary-adrenal axis regulation involved in the development of depression [9]. Additionally, higher levels of brain derived neurotrophic factor have been found after physical exercise [10]. Lastly, the level of PA directly affects the upper limit of oxygen uptake which depends on the capacity of the cardiorespiratory system to transport oxygen to the organs, including the brain. A lower oxygenation of the brain may result in a chronic cerebral ischemia and, if the affected areas are involved in a mood regulation, this may increase the risk of depression [12].
In the last decades, several studies have shown that a healthy lifestyle, in particular the intensity and length of physical activity [72,73], are important in the prevention and treatment of depression [7]. In our analysis we could not assess the relation between severity of depression and intensity of PA, as in most of the primary studies included, severity of depression was not reported and PA intensity was expressed using different methods. The results from our review confirm the beneficial effect of PA on depression, especially for participants without comorbidities. In this regard, health education campaigns aimed to promote PA should be fostered [74,75,76], especially because approximately 40% of the adult population worldwide is insufficiently physical active [77]. However, in order to better interpret our results, another important aspect should be considered: indeed, even if several sub-group analyses have been conducted, the value of heterogeneity remained stably high. Although a sensitivity analysis including only datasets with otherwise healthy people has been conducted, the I2 remained extremely high. However, a I2 value higher than 90% means that heterogeneity is directly due to heterogeneity among studies, instead of sampling error [78]. Moreover, primary papers expressed the level of PA using different types of unit of measures and also the results were reported using different modalities. Even if the pooled ES has been estimated by log OR, allowing comparability, this underlying heterogeneity might have affected the assessment of the I2 [79]. Another potential explanation of heterogeneity could be the different type of duration of measurement, the device used and the questionnaire adopted to diagnose depression. Furthermore, a variety of confounding variables were selected in original studies and, in order to control the results, we pooled the models with the highest level of adjustment.
Limitantions and Strengths
The main limitation of this systematic review is the high I2 value that might reduce the generalizability of our results. Most studies are observational and based on cross-sectional analysis. Nevertheless, we performed sensitivity analyses only including trials and longitudinal studies, increasing the robustness of our results. Due to the high heterogeneity in reporting the level of PA performed by participants in original studies, it was not possible to identify a recommended level of PA. The inability to estimate an association between severity of depression and PA is another important limitation. The main strengths of this review are being systematic in nature and its comprehensive way to include the entire scientific evidence published so far on the main medical-scientific databases. Furthermore, the pooled ES was significantly large, based on 37,408 participants, and sub-group analyses have been conducted based on participants’ comorbidity and study design. In the primary studies, diagnosis of depression was consistently based on the DSM criteria and was established by trained investigators using validated assessment scales mainly with interrater reliability.
5. Conclusions
To conclude, the results of this systematic review and meta-analysis clearly show a statistically significant protective effect of objectively measured PA on prevalent and incident depression. An increased PA is associated with lower risk of depression. The advantages of our study are several. Firstly, this study offers a systematic overview of previous studies assessing objectively measured PA and depression. Secondly, this study highlights the usefulness of objectively measured PA compared to self-reported one. Objectively measured PA is not only more precise in estimating duration, total amount, and intensity of PA, but indirectly it can also better strengths the association with some diseases, as depression. Thirdly, this study shows the importance to promote physical activity forasmuch it can help to reduce the high burden of depression in our society. Lastly, our findings are relevant for both policy makers and clinicians as physical activity is one of the cheapest, non-pharmacological treatment that might be prescribed to the general population with potentially major public health impact. Physical activity is important across ages and should be integrated into daily life.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/10/3738/s1, F, Table S1: Search strategy in PubMed/MEDLINE, Table S2: Assessment of risk of bias for trials, using The Cochrane Collaboration’s.
Author Contributions
V.G. conceptualized and designed the study, analyzed and interpreted data, and write manuscript. L.B., S.C. and M.M. contributed to data collection, and managed the database. C.S., A.A. and A.O. provided important intellectual supports in various steps of the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization . The Global Burden of Disease: 2004 Update. Health Statistics and Information Systems; Geneva, Switzerland: 2008. [Google Scholar]
- 2.Peveler R., Carson A., Rodin G. Depression in medical patients. BMJ. 2002;325:149–152. doi: 10.1136/bmj.325.7356.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; Arlington, VA, USA: 2013. [Google Scholar]
- 4.Odone A., Landriscina T., Amerio A., Costa G. The impact of the current economic crisis on mental health in Italy: Evidence from two representative national surveys. Eur. J. Public Health. 2018;28:490–495. doi: 10.1093/eurpub/ckx220. [DOI] [PubMed] [Google Scholar]
- 5.Burcusa S.L., Iacono W.G. Risk for Recurrence in Depression. Clin. Psychol. Rev. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amerio A., Odone A., Marchesi C., Ghaemi S.N. Is depression one thing or many? Br. J. Psychiatry. 2014;204:488. doi: 10.1192/bjp.204.6.488. [DOI] [PubMed] [Google Scholar]
- 7.Belvederi Murri M., Ekkekakis P., Magagnoli M., Zampogna D., Cattedra S., Capobianco L., Serafini G., Calcagno P., Zanetidou S., Amore M. Physical Exercise in Major Depression: Reducing the Mortality Gap While Improving Clinical Outcomes. Front. Psychiatry. 2018;9:762. doi: 10.3389/fpsyt.2018.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meeusen R., De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20:160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Rimmele U., Zellweger B.C., Marti B., Seiler R., Mohiyeddini C., Ehlert U., Heinrichs M. Trained men show lower cortisol, heart rate and psychological responses to psychosocial stress compared with untrained men. Psychoneuroendocrinology. 2007;32:627–635. doi: 10.1016/j.psyneuen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Szuhany K.L., Bugatti M., Otto M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Agtmaal M.J.M., Houben A., Pouwer F., Stehouwer C.D.A., Schram M.T. Association of Microvascular Dysfunction with Late-Life Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2017;74:729–739. doi: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor W.D., Aizenstein H.J., Alexopoulos G.S. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol. Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuch F., Vancampfort D., Firth J., Rosenbaum S., Ward P., Reichert T., Bagatini N.C., Bgeginski R., Stubbs B. Physical activity and sedentary behavior in people with major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2017;210:139–150. doi: 10.1016/j.jad.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 14.Krogh J., Hjorthoj C., Speyer H., Gluud C., Nordentoft M. Exercise for patients with major depression: A systematic review with meta-analysis and trial sequential analysis. BMJ Open. 2017;7:e014820. doi: 10.1136/bmjopen-2016-014820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strath S.J., Kaminsky L.A., Ainsworth B.E., Ekelund U., Freedson P.S., Gary R.A., Richardson C.R., Smith D.T., Swartz A.M., American Heart Association Physical Activity Committee of the Council on Lifestyle et al. Guide to the assessment of physical activity: Clinical and research applications: A scientific statement from the American Heart Association. Circulation. 2013;128:2259–2279. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2013. [(accessed on 1 November 2018)]. Version 5.1.0. Available online: www.training.cochrane.org/handbook. [Google Scholar]
- 17.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B., et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 19.Gianfredi V., Blandi L., Cacitti S., Minelli M. Physical Activity and Depression: A Systematic Review and Meta-Analysis on the Association between Patterns of Objectively Measured Physical Activity and Risk of Depression in Adults. [(accessed on 29 April 2020)]; CRD42020132860, Prospero 2020. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020132860.
- 20.Amerio A., Stubbs B., Odone A., Tonna M., Marchesi C., Ghaemi S.N. Bipolar I and II Disorders; A Systematic Review and Meta-Analysis on Differences in Comorbid Obsessive-Compulsive Disorder. Iran. J. Psychiatry Behav. Sci. 2016;10:e3604. doi: 10.17795/ijpbs-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amerio A., Ossola P., Scagnelli F., Odone A., Allinovi M., Cavalli A., Iacopelli J., Tonna M., Marchesi C., Ghaemi S.N. Safety and efficacy of lithium in children and adolescents: A systematic review in bipolar illness. Eur. Psychiatry. 2018;54:85–97. doi: 10.1016/j.eurpsy.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Brown P., Brunnhuber K., Chalkidou K., Chalmers I., Clarke M., Fenton M., Forbes C., Glanville J., Hicks N.J., Moody J., et al. How to formulate research recommendations. BMJ. 2006;333:804–806. doi: 10.1136/bmj.38987.492014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianfredi V., Nucci D., Abalsamo A., Acito M., Villarini M., Moretti M., Realdon S. Green Tea Consumption and Risk of Breast Cancer and Recurrence-A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2018;10:1886. doi: 10.3390/nu10121886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianfredi V., Bragazzi N.L., Nucci D., Villarini M., Moretti M. Cardiovascular diseases and hard drinking waters: Implications from a systematic review with meta-analysis of case-control studies. J. Water Health. 2017;15:31–40. doi: 10.2166/wh.2016.131. [DOI] [PubMed] [Google Scholar]
- 25.Gianfredi V., Nucci D., Fatigoni C., Salvatori T., Villarini M., Moretti M. Extent of Primary DNA Damage Measured by the Comet Assay in Health Professionals Exposed to Antineoplastic Drugs: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health. 2020;17:523. doi: 10.3390/ijerph17020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells G.A., Shea B., O’Connell D., Paterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 1 November 2018)];2014 Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 27.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 29.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abedi P., Nikkhah P., Najar S. Effect of pedometer-based walking on depression, anxiety and insomnia among postmenopausal women. Climacteric. 2015;18:841–845. doi: 10.3109/13697137.2015.1065246. [DOI] [PubMed] [Google Scholar]
- 31.Al-Eisa E., Buragadda S., Melam G.R. Association between physical activity and psychological status among Saudi female students. BMC Psychiatry. 2014;14:238. doi: 10.1186/s12888-014-0238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alosco M.L., Spitznagel M.B., Miller L., Raz N., Cohen R., Sweet L.H., Colbert L.H., Josephson R., Waechter D., Hughes J., et al. Depression is associated with reduced physical activity in persons with heart failure. Health Psychol. 2012;31:754–762. doi: 10.1037/a0028711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altenburg W.A., Bossenbroek L., De Greef M.H., Kerstjens H.A., Ten Hacken N.H., Wempe J.B. Functional and psychological variables both affect daily physical activity in COPD: A structural equations model. Respir. Med. 2013;107:1740–1747. doi: 10.1016/j.rmed.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Arrieta H., Rezola-Pardo C., Echeverria I., Iturburu M., Gil S.M., Yanguas J.J., Irazusta J., Rodriguez-Larrad A. Physical activity and fitness are associated with verbal memory, quality of life and depression among nursing home residents: Preliminary data of a randomized controlled trial. BMC Geriatr. 2018;18:80. doi: 10.1186/s12877-018-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bade B.C., Brooks M.C., Nietert S.B., Ulmer A., Thomas D.D., Nietert P.J., Scott J.B., Silvestri G.A. Assessing the Correlation between Physical Activity and Quality of Life in Advanced Lung Cancer. Integr. Cancer Ther. 2018;17:73–79. doi: 10.1177/1534735416684016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barriga S., Rodrigues F., Barbara C. Factors that influence physical activity in the daily life of male patients with chronic obstructive pulmonary disease. Rev. Port. Pneumol. 2014;20:131–137. doi: 10.1016/j.rppneu.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Di Marco F., Terraneo S., Roggi M.A., Repossi A.C., Pellegrino G.M., Veronelli A., Santus P., Pontiroli A.E., Centanni S. Physical activity impairment in depressed COPD subjects. Respir. Care. 2014;59:726–734. doi: 10.4187/respcare.02848. [DOI] [PubMed] [Google Scholar]
- 38.Dillon C.B., McMahon E., O’Regan G., Perry I.J. Associations between physical behaviour patterns and levels of depressive symptoms, anxiety and well-being in middle-aged adults: A cross-sectional study using isotemporal substitution models. BMJ Open. 2018;8:e018978. doi: 10.1136/bmjopen-2017-018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drieling R.L., Goldman Rosas L., Ma J., Stafford R.S. Community resource utilization, psychosocial health, and sociodemographic factors associated with diet and physical activity among low-income obese Latino immigrants. J. Acad. Nutr. Diet. 2014;114:257–265. doi: 10.1016/j.jand.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duenas-Espin I., Demeyer H., Gimeno-Santos E., Polkey M.I., Hopkinson N.S., Rabinovich R.A., Dobbels F., Karlsson N., Troosters T., Garcia-Aymerich J. Depression symptoms reduce physical activity in COPD patients: A prospective multicenter study. Int. J. Chron. Obstruct. Pulmon. Dis. 2016;11:1287–1295. doi: 10.2147/COPD.S101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elbelt U., Ahnis A., Riedl A., Burkert S., Schuetz T., Ordemann J., Strasburger C.J., Klapp B. F Associations of physical activity with depressiveness and coping in subjects with high-grade obesity aiming at bariatric surgery: A cross-sectional study. Biopsychosoc. Med. 2015;9:16. doi: 10.1186/s13030-015-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fenton S.A.M., Van Zanten J., Metsios G.S., Rouse P.C., Yu C.A., Kitas G.D., Dudaa J.L. Autonomy support, light physical activity and psychological well-being in Rheumatoid Arthritis: A cross-sectional study. Ment. Health Phys. Act. 2018;14:11–18. doi: 10.1016/j.mhpa.2017.12.002. [DOI] [Google Scholar]
- 43.Freitas P.D., Silva A.G., Ferreira P.G., Silva D.A., Salge J.M., Carvalho-Pinto R.M., Cukier A., Brito C.M., Mancini M.C., Carvalho C.R.F. Exercise Improves Physical Activity and Comorbidities in Obese Adults with Asthma. Med. Sci. Sports Exerc. 2018;50:1367–1376. doi: 10.1249/MSS.0000000000001574. [DOI] [PubMed] [Google Scholar]
- 44.Gaskin C.J., Craike M., Mohebbi M., Salmon J., Courneya K.S., Broadbent S., Livingston P.M. Associations of objectively measured moderate-to-vigorous physical activity and sedentary behavior with quality of life and psychological well-being in prostate cancer survivors. Cancer Causes Control. 2016;27:1093–1103. doi: 10.1007/s10552-016-0787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golsteijn R.H.J., Bolman C., Volders E., Peels D.A., De Vries H., Lechner L. Short-term efficacy of a computer-tailored physical activity intervention for prostate and colorectal cancer patients and survivors: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2018;15:106. doi: 10.1186/s12966-018-0734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallam K.T., Bilsborough S., De Courten M. “Happy feet”: Evaluating the benefits of a 100-day 10,000 step challenge on mental health and wellbeing. BMC Psychiatry. 2018;18:19. doi: 10.1186/s12888-018-1609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartescu I., Morgan K., Stevinson C.D. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: A randomized controlled trial. J. Sleep Res. 2015;24:526–534. doi: 10.1111/jsr.12297. [DOI] [PubMed] [Google Scholar]
- 48.Hospes G., Bossenbroek L., Ten Hacken N.H., Van Hengel P., De Greef M.H. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: Results of an exercise counseling program. Patient Educ. Couns. 2009;75:274–278. doi: 10.1016/j.pec.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Howie E.K., McVeigh J.A., Winkler E.A.H., Healy G.N., Bucks R.S., Eastwood P.R., Straker L.M. Correlates of physical activity and sedentary time in young adults: The Western Australian Pregnancy Cohort (Raine) Study. BMC Public Health. 2018;18:916. doi: 10.1186/s12889-018-5705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung S., Lee S., Lee S., Bae S., Imaoka M., Harada K., Shimada H. Relationship between physical activity levels and depressive symptoms in community-dwelling older Japanese adults. Geriatr. Gerontol. Int. 2018;18:421–427. doi: 10.1111/ggi.13195. [DOI] [PubMed] [Google Scholar]
- 51.Kangasniemi A., Lappalainen R., Kankaanpaa A., Tammelin T. Mindfulness skills, psychological flexibility, and psychological symptoms among physically less active and active adults. Ment. Health Phys. Act. 2014;7:121–127. doi: 10.1016/j.mhpa.2014.06.005. [DOI] [Google Scholar]
- 52.King W.C., Kalarchian M.A., Steffen K.J., Wolfe B.M., Elder K.A., Mitchell J.E. Associations between physical activity and mental health among bariatric surgical candidates. J. Psychosom. Res. 2013;74:161–169. doi: 10.1016/j.jpsychores.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Po-Wen K., Steptoe A., Liao Y., Sun W.J., Chen L.J. Prospective relationship between objectively measured light physical activity and depressive symptoms in later life. Int. J. Geriatr. Psychiatry. 2018;33:58–65. doi: 10.1002/gps.4672. [DOI] [PubMed] [Google Scholar]
- 54.Loprinzi P.D., Cardinal B.J. Interrelationships among physical activity, depression, homocysteine, and metabolic syndrome with special considerations by sex. Prev Med. 2012;54:388–392. doi: 10.1016/j.ypmed.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Loprinzi P.D., Franz C., Hager K.K. Accelerometer-assessed physical activity and depression among US adults with diabetes. Ment. Health Phys. Act. 2013;6:79–82. doi: 10.1016/j.mhpa.2013.04.003. [DOI] [Google Scholar]
- 56.Loprinzi P.D. Objectively measured light and moderate-to-vigorous physical activity is associated with lower depression levels among older US adults. Aging Ment. Health. 2013;17:801–805. doi: 10.1080/13607863.2013.801066. [DOI] [PubMed] [Google Scholar]
- 57.Ludwig V.M., Bayley A., Cook D.G., Stahl D., Treasure J.L., Asthworth M., Greenough A., Winkley K., Bornstein S.R., Ismail K. Association between depressive symptoms and objectively measured daily step count in individuals at high risk of cardiovascular disease in South London, UK: A cross-sectional study. BMJ Open. 2018;8:e020942. doi: 10.1136/bmjopen-2017-020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huong N.Q., Fan V.S., Herting J., Lee J., Fu M., Chen Z., Borson S., Kohen R., Matute-Bello G., Pagalilauan G., et al. Patients with COPD with higher levels of anxiety are more physically active. Chest. 2013;144:145–151. doi: 10.1378/chest.12-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Brien J.T., Gallagher P., Stow D., Hammerla N., Ploetz T., Firbank M., Ladha C., Ladha K., Jackson D., McNaney R., et al. A study of wrist-worn activity measurement as a potential real-world biomarker for late-life depression. Psychol. Med. 2017;47:93–102. doi: 10.1017/S0033291716002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S., Thogersen-Ntoumani C., Ntoumanis N., Stenling A., Fenton S.A., Veldhuijzen van Zanten J.J. Profiles of Physical Function, Physical Activity, and Sedentary Behavior and their Associations with Mental Health in Residents of Assisted Living Facilities. Appl. Psychol. Health Well Being. 2017;9:60–80. doi: 10.1111/aphw.12085. [DOI] [PubMed] [Google Scholar]
- 61.Raudsepp L., Riso E.M. Longitudinal Association between Objectively Measured Walking and Depressive Symptoms among Estonian Older Adults. J. Aging Phys. Act. 2017;25:639–645. doi: 10.1123/japa.2016-0303. [DOI] [PubMed] [Google Scholar]
- 62.Rethorst C.D., Moncrieft A.E., Gellman M.D., Arredondo E.M., Buelna C., Castaneda S.F., Daviglus M.L., Khan U.I., Perreira K.M., Sotres-Alvarez D., et al. Isotemporal Analysis of the Association of Objectively Measured Physical Activity with Depressive Symptoms: Results from Hispanic Community Health Study/Study of Latinos (HCHS/SOL) J. Phys. Act. Health. 2017;14:733–739. doi: 10.1123/jpah.2016-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song M.R., Lee Y.S., Baek J.D., Miller M. Physical activity status in adults with depression in the National Health and Nutrition Examination Survey, 2005–2006. Public Health Nurs. 2012;29:208–217. doi: 10.1111/j.1525-1446.2011.00986.x. [DOI] [PubMed] [Google Scholar]
- 64.Sylvester B.D., Lee Y.S., Baek J.D., Miller M. Changes in light-, moderate-, and vigorous-intensity physical activity and changes in depressive symptoms in breast cancer survivors: A prospective observational study. Support. Care Cancer. 2017;25:3305–3312. doi: 10.1007/s00520-017-3745-1. [DOI] [PubMed] [Google Scholar]
- 65.Trinh L., Amireault S., Lacombe J., Sabiston C.M. Physical and psychological health among breast cancer survivors: Interactions with sedentary behavior and physical activity. Psychooncology. 2015;24:1279–1285. doi: 10.1002/pon.3872. [DOI] [PubMed] [Google Scholar]
- 66.Vallance J.K., Winkler E.A., Gardiner P.A., Healy G.N., Lynch B.M., Owen N. Associations of objectively-assessed physical activity and sedentary time with depression: NHANES (2005–2006) Prev. Med. 2011;53:284–288. doi: 10.1016/j.ypmed.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Vallance J.K., Boyle T., Courneya K.S., Lynch B.M. Accelerometer-assessed physical activity and sedentary time among colon cancer survivors: Associations with psychological health outcomes. J. Cancer Surviv. 2015;9:404–411. doi: 10.1007/s11764-014-0409-8. [DOI] [PubMed] [Google Scholar]
- 68.Van den Berg-Emons R., Balk A., Bussmann H., Stam H. Does aerobic training lead to a more active lifestyle and improved quality of life in patients with chronic heart failure? Eur. J. Heart Fail. 2004;6:95–100. doi: 10.1016/j.ejheart.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Whitaker K.M., Sharpe P.A., Wilcox S., Hutto B.E. Depressive symptoms are associated with dietary intake but not physical activity among overweight and obese women from disadvantaged neighborhoods. Nutr. Res. 2014;34:294–301. doi: 10.1016/j.nutres.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vetrovsky T., Cupka J., Dudek M., Kuthanova B., Vetrovska K., Capek V., Bunc V. A pedometer-based walking intervention with and without email counseling in general practice: A pilot randomized controlled trial. BMC Public Health. 2018;18:635. doi: 10.1186/s12889-018-5520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romano-Spica V., Macini P., Fara G.M., Giammanco G., GSMS Working Group on Movement Sciences for Health Italian Society of Hygiene Preventive Medicine Public Health Adapted Physical Activity for the Promotion of Health and the Prevention of Multifactorial Chronic Diseases: The Erice Charter. Ann. Ig. 2015;27:406–414. doi: 10.7416/ai.2015.2028. [DOI] [PubMed] [Google Scholar]
- 72.Nebiker L., Lichtenstein E., Minghetti A., Zahner L., Gerber M., Faude O., Donath L. Moderating Effects of Exercise Duration and Intensity in Neuromuscular vs. Endurance Exercise Interventions for the Treatment of Depression: A Meta-Analytical Review. Front. Psychiatry. 2018;9:305. doi: 10.3389/fpsyt.2018.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paolucci E.M., Loukov D., Bowdish D.M.E., Heisz J.J. Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 2018;133:79–84. doi: 10.1016/j.biopsycho.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 74.Gianfredi V., Monarca S., Moretti M., Villarini M. Health education, what is the role for pharmacist? Results from a cross sectional study in Umbria, Italy. Recent. Prog. Med. 2017;108:433–441. doi: 10.1701/2802.28356. [DOI] [PubMed] [Google Scholar]
- 75.Gianfredi V., Grisci C., Nucci D., Parisi V., Moretti M. Communication in health. Recent. Prog. Med. 2018;109:374–383. doi: 10.1701/2955.29706. [DOI] [PubMed] [Google Scholar]
- 76.Gianfredi V., Balzarini F., Gola M., Mangano S., Carpagnano L.F., Colucci M.E., Gentile L., Piscitelli A., Quattrone F., Scuri S., et al. Leadership in Public Health: Opportunities for Young Generations within Scientific Associations and the Experience of the “Academy of Young Leaders”. Front. Public Health. 2019;7:378. doi: 10.3389/fpubh.2019.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.World Health Organization Physical Activity. [(accessed on 2 April 2020)];2018 Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity.
- 78.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 79.Gianfredi V., Nucci D., Salvatori T., Dallagiacoma G., Fatigoni C., Moretti M., Realdon S. Rectal Cancer: 20% Risk Reduction Thanks to Dietary Fibre Intake. Systematic Review and Meta-Analysis. Nutrients. 2019;11:1579. doi: 10.3390/nu11071579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.