Abstract
(1) Background: Cardio-metabolic diseases (CMD), including cardiovascular disease, stroke, and diabetes, have numerous common individual and environmental risk factors. Yet, few studies to date have considered how these multiple risk factors together affect CMD disparities between Blacks and Whites. (2) Methods: We linked daily fine particulate matter (PM2.5) measures with survey responses of participants in the Southern Community Cohort Study (SCCS). Generalized linear mixed modeling (GLMM) was used to estimate the relationship between CMD risk and social-demographic characteristics, behavioral and personal risk factors, and exposure levels of PM2.5. (3) Results: The study resulted in four key findings: (1) PM2.5 concentration level was significantly associated with reported CMD, with risk rising by 2.6% for each µg/m3 increase in PM2.5; (2) race did not predict CMD risk when clinical, lifestyle, and environmental risk factors were accounted for; (3) a significant variation of CMD risk was found among participants across states; and (4) multiple personal, clinical, and social-demographic and environmental risk factors played a role in predicting CMD occurrence. (4) Conclusions: Disparities in CMD risk among low social status populations reflect the complex interactions of exposures and cumulative risks for CMD contributed by different personal and environmental factors from natural, built, and social environments.
Keywords: cardio-metabolic disease, PM2.5, cardiovascular disease, diabetes, stroke, personal, clinical and environmental risk factors, health disparities
1. Introduction
Cardiovascular disease (CVD), stroke, and diabetes comprise a spectrum of related cardio-metabolic disease (CMD) conditions: CVD (e.g., coronary heart disease), congestive heart failure, myocardial infarction, atrial fibrillation, vascular endothelial dysfunction, stroke and atherosclerosis, and diabetes (type 1, type 2, and metabolic syndrome) [1,2,3]. Together, they are the first, fifth, and seventh leading causes of death, respectively, for both men and women in the United States [4].
These diseases taken together often are referred to as CMD due to common individual risk factors (behaviors, personal traits, cardio-metabolic characteristics) as well as shared, external environmental (natural, built, and social) exposures [5]. Common individual-level CMD risk factors include some that are fixed or non-modifiable, and unique to individuals (e.g., age, race, gender) [6], some that are modifiable (diet, exercise, and smoking), and others that are metabolic. Metabolic risk factors for CMD include hypertension [7], allostatic load [8,9,10], dyslipidemia [11], decreased high density lipoprotein (HDL) and increased low density lipoprotein (LDL) cholesterol [12,13], triglycerides [14], fasting insulin [15], serum creatinine [16], serum uric acid [17] serum high-sensitivity C-reactive protein (hsCRP) [18], inflammation [19], hypertriglyceridemia [20], thrombosis [21,22], insulin resistance [23], serum lipids [24] and blood glucose [25,26], fibrinogen [27], and homocysteine [28] (see Table 1 for an extensive list of individual risk factors for CMD).
Table 1.
Environmental risk factors for cardio-metabolic diseases.
| Domain | Subdomain | Environmental Stressor | Cardiovascular Disease (CVD) | Stroke | Diabetes |
|---|---|---|---|---|---|
| Natural | Metals | Lead | Cosselman [58] | Navas [59] | Orioli [60] |
| Arsenic | Smith [61] | Smith [61] | Smith [61] | ||
| Cosselman [58] | |||||
| Cadmium | Cosselman [58] | Peters [62] | Edwards [63] | ||
| Solvents and pesticides | Solvents | Bulka [64] | Rinsky [65] | Montgomery [66] | |
| Pesticides | Wilcosky [67] | ||||
| Air pollution | PM10, PM2.5, ultrafine PM | Mohammadi, [68] | Kowalska [69] | ||
| Gases | Carbon monoxide | Lee [70] | Hampson [71] | Huang [72] | |
| Ozone | Goodman [73] | Srebot [74] | Jerrett [75] | ||
| Nitrogen dioxide Sulfur dioxide | Kopp [76] | Amancio [77] | Coogan [40] | ||
| Built | Neighborhood conditions | Walkability | Gaglioti [78] | Kwon [79] | Sundquist [80] |
| Perceived/actual safety | Pham [81] | ||||
| Evonson [82] | |||||
| Access to healthy foods | Availability of healthy or unhealthy stores/restaurants | Lindberg [83] | Christine [84] | ||
| Gaglioti [78] | |||||
| Peolman [85] | |||||
| Social | Demographic | Population density | Rodriguez [86] | ||
| Socioeconomic status (SES) | Gebreab [87] | ||||
| Social supports | Brown [88] | Zhang [50] | |||
| Access to health care | Access to insurance and health care services | Li [89] | Medford-Davis [90] | Stark [91] | |
| Social stressors | Community stressors Residential segregation | Ford [91] | Booth [92] | Grigsby-Toussaint [93] | |
| Kershaw [94] | Patel [95] | ||||
| Cultural influences | Lack of trust in health care providers | Schoenthaler [96] | Heisler [97] | ||
| Socio-cultural beliefs and norms | |||||
| Policy | Dietary policy | Pearson [98] | Jilcott Pitts [99] | ||
| Physical activity policy | Jilcott Pitts [99] | ||||
| Endocrine-disrupting chemicals policies | Shaikh [100] | ||||
| diabetes care and prevention policy | Ackermann [101] |
Compared with individuals without diabetes, patients with type 2 diabetes mellitus (TTDM) are one and a half times more likely to have a stroke and two to four times more likely to die from heart disease. In contrast to CVD and stroke, which have declined in recent years, the incidence and prevalence of diabetes doubled nationally between 1980 and 2008, before plateauing between 2008 and 2012. Between 2012 and 2015, the incidence of diagnosed diabetes among adults aged 18 and over decreased, while the prevalence has continued to increase [29]. Increases in both the prevalence and incidence of diabetes among subgroups, however, have continued, including for non-Hispanic Black and Hispanic subpopulations and those with a high school education or less [30].
Environmental disparities in CMD outcomes have been found to be associated with exposures to chemical and non-chemical stressors found in the natural, built, and social environments. Exposures to toxicants in the natural environment linked to CMD outcomes include heavy metals (lead, mercury, cadmium, and arsenic), solvents, pesticides, indoor pollution (secondhand smoke, biomass fuels), outdoor air pollution comprised of complex mixtures of gases that include particulate matter (PM), which includes PM10 (course), PM2.5 (fine) and ultrafine PM, carbon monoxide (CO), ozone (O3), nitrogen dioxide (NO2), sulfur dioxide (SO2), diesel and other sources (see Table 1). PM is a mixture of solid and liquid droplets present in the air that vary in mass, number, size, shape, surface area, chemical composition as well as reactivity, acidity, solubility, and origin [31].
Numerous epidemiological studies have found a strong association between ambient PM (PM10, PM2.5, and ultrafine particles) and increased CMD, including myocardial infarction (MI) [32,33], cardiac arrhythmias [34,35], vascular dysfunction [36,37], hypertension [38,39], diabetes [40,41], ischemic stroke [42,43], and atherosclerosis [44,45], even at relatively low concentrations. Risk factors in the built environment that have been found to be related to CMD include interaction with nature (green space, walkability, activity supportive built environment, and lack of places to exercise) [46,47] and access to a healthy food environment (distance, affordability, and availability of healthy food and high-density of fast-food restaurants) [48] (see Table 1). Social factors associated with CMD outcomes include race/ethnicity [49], neighborhood deprivation [50], safety (neighborhood violent crime and unemployment) [51], social capital [52], and rural vs. urban [53]. Other social risk factors that have been found to be associated with CMD include population density, community stressors, residential segregation, health insurance, access to health care services, lack of trust in health care providers, socio-cultural beliefs and norms (car ownership, cultural influences), and availability of social supports (see Table 1). Public policies that have been identified as risk and protective factors for CMD disparities are local laws and regulations that have a direct or indirect outcome on CMD, including increased access to healthcare through Medicaid expansion under the Affordable Care Act, restrictions on cigarette smoking, zoning ordinances regarding parks, walking and biking paths and policies that encourage use of public transit (see Table 1).
Several studies found Black:White racial disparities in the association between exposure to PM2.5 and cardio-metabolic outcomes. The Multi-Ethnic Study of Arherosclerosis (MESA) study [54] found that Blacks compared to Whites, showed a stronger adjusted association between air pollution and left-ventricular mass index (LVMI) and left-ventricular ejection fraction (LVEF). The MESA study also found that higher exposure to multiple chemical constituents of air pollution may be a novel contributor to diabetes disparities [55]. Data from the HeartSCORE study found significant Black:White racial disparities between exposure to PM2.5 and higher blood glucose, worse arterial endothelial function, and incident CVD events [56].
The present study examined the effects of PM2.5 exposure on Black:White disparities in CMD by linking daily measures of PM2.5 with survey responses of individual and environmental risk factors and CMD history taken at enrollment of participants of the Southern Community Cohort Study (SCCS) [57].
2. Materials and Methods
2.1. Aim
The overall aim of this study is to assess self-reported clinical, personal, and environmental risk factors, measures of PM2.5, and risk for Black:White CMD disparities among participants of the SCCS.
2.2. Design
The cross-sectional study design combined individual SCCS participant survey responses at enrollment with an annualized daily measure of PM2.5 for the 12-month period prior to study enrollment.
2.3. Sample
The initial SCCS cohort consisted of 84,513 individuals who were recruited from March 2002–September 2009 in 12 southeastern states (Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, Virginia, West Virginia) [55,56]. Individuals under treatment for cancer within a year of enrollment were excluded from the original study. Participants were recruited primarily (86%) from Community Health Centers (CHCs) [57] where trained interviewers collected survey information on illness history, lifestyle, social-demographic, and environmental factors. A smaller percentage of cohort participants were enrolled using a mailed questionnaire sent to a stratified random sample of residents in the same states.
For the current study, participants were limited to those for whom 12 months of daily PM2.5 data at date of enrollment were available (n = 72,215). Only persons who identified as either White or Black and who had complete data for variables included in the model were included (n = 53,617). Persons who were not recruited through community health centers were excluded from the study. The final sample thus was comprised of those persons for whom we had 12 months of daily PM2.5 data, were either Black or White, had no missing data of those variables that were used in the model and were recruited through community health centers (n = 48,799). The current research protocol was approved by the Meharry Medical College Institutional Review Board (IRB) and the EPA Human Subjects Research Review Official, while permission for data access was provided by the SCCS governing board. The Meharry and Vanderbilt University IRBs approved and oversaw SCCS recruitment. All SCCS participants provided written informed consent.
Ethics Approval and Consent to Participate: De-identified secondary data on individual health records were used. There was no intervention or direct interaction with human subjects. The end points of the research are to identify and model the mechanisms and exposure pathways associated with CVD and other chronic diseases. The study protocol was approved by the Meharry Medical College (IRB Protocol # 17-11-783, Juarez) and by the EPA Human Subjects Research Review Official (HSR-000867).
2.4. Procedures
The following definitions were used.
Cardio-metabolic disease (CMD): The presence of CMD was defined for SCCS participants who responded to the survey administered at the time of the enrollment by a statement that a “doctor has told you that you have” one or more of the following cardio-metabolic diseases: diabetes, heart attack or coronary artery bypass surgery, or stroke.
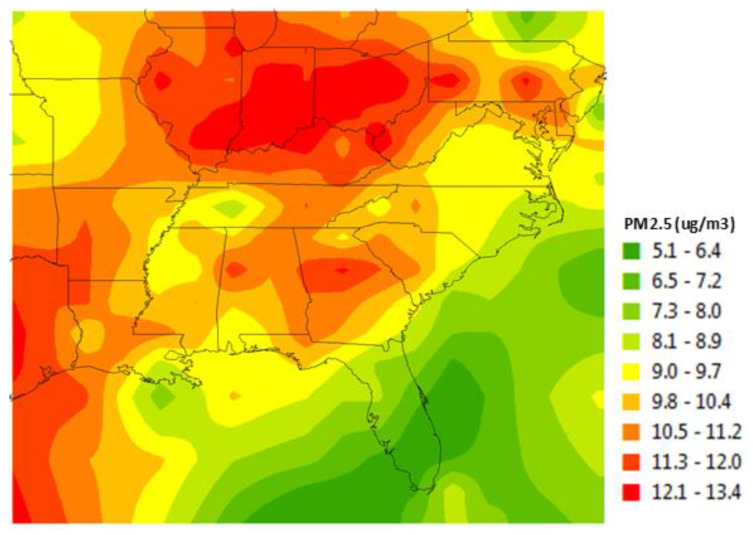
Fine particulate matter (PM2.5): PM2.5 exposure was defined as the average annual concentration (µg/m3) calculated using a continuous, spatial surface model created by Al-Hamdan et al. [102] that merged ground level ambient air measures with satellite-derived daily measures of PM2.5. Satellite measures were derived from regression models of aerosol optical depth (e.g., the measure of the degree to which sunlight is scattered and absorbed by aerosols of various sizes through the entire atmospheric column) collected by the Moderate Resolution Imaging Spectro-radiometer instrument onboard the National Aeronautics and Space Administration Aqua satellite (see [102] for more detailed information). An example of a map of annualized data is presented in Figure 1. A B-spline smoothing algorithm was used to calculate daily concentrations of PM2.5 for each 3-km grid cell for the 12 months prior to enrollment. Satellite data were used to fill the temporal and spatial gaps inherent to ground-level monitoring station data which mostly are collected from urban areas.
Figure 1.
Mean annual 3-km fine particulate matter (PM2.5) for 2009.
Geographic identifiers and residential address proxy: Geocoded individual residential addresses at time of enrollment were assigned to PM2.5, 3-km grid cells. These residential address proxy grid cells were used to link survey and environmental data in order to provide a firewall between study and SCCS data and ensure personal identification and health information remained anonymous.
Clinical risk factors: Participants were asked about the presence of clinical risk factors for CMD at enrollment, by responding yes or no to the following questions: “Has a doctor told you that you have had” high blood pressure (hypertension)? or high cholesterol? Other clinical risk factors were assessed by enrollment interviewers including height, weight, and body mass index (BMI) defined as weight (kg)/height (m)2, the latter of which is a commonly used metric to assess obesity (with categories of <18.5 = 1, 18.5–24 = 2, 25–29 = 3, 30–34 = 4, 35–39 = 5, 40 and higher = 6, respectively designated as underweight, normal weight, overweight, obesity I, obesity II, and obesity III).
Personal risk factors Self-reported personal risk factors obtained from survey responses at enrollment included: age (45–64 = 1, 65 and older = 2; age at enrollment in years was modeled); history of tobacco use/smoking status (non-smoker = 1, former smoker = 2, smoker = 3); air quality outdoors (1 = poor, 2 = fair, 3 = good, 4 = excellent); air quality indoors (1 = poor, 2 = fair, 3 = good, 4 = excellent); educational level (1 = <9 years, 2 = 9–11 years, 3 = high school or GED, 4 = vocational training, 5 = some College, 6 = college, 7 = Masters, 8 = Doctorate); household income (1 = <$15,000, 2 = $15,000–$24,999, 3 = $25,000–$49,999, 4 = $50,000–$99,999, 5 = >$100,000); marital status (married or with a partner = 1, divorced = 2, widowed = 3, single = 4); employment status (employed = 1, otherwise 0); gender (male = 1, female = 0); residence location (rural/farm = 1, urban = 0); and race (White = 0, Black = 1).
2.5. Statistical Analysis
Frequency distributions of participant characteristics were tabulated for the analytic sample. Cross-tabulations of categorical variables associated with CMD were evaluated using Chi-squared tests. Sample characteristics and percentages reporting CMD for each clinical and personal risk factor were characterized. IBM SPSS 26 (IBM Corp., Released 2019. Armonk, NY: IBM Corp.) was used to perform statistical analysis and Mathematica software was used to draw graphs.
Generalized linear mixed modeling (GLMM) was used to estimate the relationship between CMD and social-demographic characteristics, behavioral and environmental risk factors, and exposure levels of PM2.5 [103,104]. GLMM used fixed effects (age, sex, race, etc.) and a random intercept model with these data to account for clustering of observations by state, a design feature of the SCCS. A random intercept was used to take into consideration state-dependent CMD variation. State-level factors often affect measurements similarly for any given participant in a particular state. For example, each state pursues different environmental protection laws and local zoning ordinances.
To account for state-dependent CMD variation, we modeled the random effects for the intercept using state of residence with fixed effects for individual risk factor characteristics (age, sex, smoking, etc.) and PM2.5 exposure of the participants. To account for the variety of possible variance-covariance structures in the relationships among SCCS participants, we used variance components structure. This is a natural way to represent participants within a state cluster. If the true correlation structure is compound symmetry, then using a random intercept for each state will remove the correlation among the participants.
A logit link function was used to model fixed effects of the presence of CMD as a binary outcome variable. The fixed effects for a GLMM are interpreted in the same way as a regression analysis depending on the nature of the outcome variable. In this case, we interpreted the model as we would a logistic regression model. The parameter estimates given in Table 1 were estimates of the mean parameters. Estimates of covariance parameters were used to identify the variance parameter, the random intercept for each state. We used a variance component structure for this parameter with a variance of zero, such that the null hypothesis would indicate that a random effect was not needed. This was tested using the Wald Z statistic.
3. Results
Frequencies and percentages of the participants reporting CMD by personal, health, social-demographic, and environmental characteristics are presented in Table 2. The SCCS CHC cohort sample was comprised of 60.4% females and 39.6% males. The majority of respondents were Black (66.1%) and the remaining (33.9%) were non-Hispanic White. The average age at enrollment was 52 years old. Most participants at baseline were under 65 years of age (89.7%).
Table 2.
Characteristics of the sub-cohort of Southern Community Cohort Study (n = 48,799).
| Characteristics | % of Sample | % with CMD | Sig. |
|---|---|---|---|
| All Participants | 100.0 | 29.2 | |
| Gender | <0.001 | ||
| Male | 39.6 | 27.6 | |
| Female | 60.4 | 30.3 | |
| Race | 0.072 | ||
| Black | 66.1 | 28.9 | |
| Male (n = 13,292) | 27.2 | 25.9 | |
| Female (n = 18,986) | 38.9 | 31.0 | |
| White | 33.9 | 29.7 | |
| Male (n = 6046) | 12.4 | 31.1 | |
| Female (n = 10,475) | 21.5 | 28.9 | |
| Education (years completed) | 0.001 | ||
| Less than 9 years | 7.5 | 41.7 | |
| 9–11 years | 21.0 | 32.4 | |
| 12 years (or GED) | 34.3 | 27.8 | |
| Vocational/technical | 5.0 | 29.9 | |
| Some college | 20.1 | 27.0 | |
| College graduate | 7.7 | 24.7 | |
| Graduate school | 3.2 | 21.4 | |
| Doctorate | 1.3 | 19.5 | |
| Marital Status | 0.001 | ||
| Married or with partner | 34.3 | 29.6 | |
| Divorced | 34.4 | 29.1 | |
| Widowed | 9.4 | 41.6 | |
| Single | 21.9 | 23.5 | |
| Household Income | 0.001 | ||
| <$15,000 | 56.4 | 32.1 | |
| $15,000–$24,999 | 21.1 | 27.6 | |
| $25,000–$49,999 | 13.9 | 25.7 | |
| $50,000–$99,999 | 6.6 | 21.1 | |
| >$100,000 | 2.0 | 14.5 | |
| Residence | 0.001 | ||
| Urban | 54.2 | 26.4 | |
| Rural | 45.8 | 32.5 | |
| Air Quality Inside | 0.105 | ||
| Poor | 5.8 | 28.3 | |
| Fair | 28.5 | 28.7 | |
| Good | 52.7 | 29.4 | |
| Excellent | 12.9 | 30.1 | |
| Air Quality Outside | 0.001 | ||
| Poor | 7.2 | 31.5 | |
| Fair | 34.4 | 28.3 | |
| Good | 46.3 | 29.6 | |
| Excellent | 12.1 | 28.8 | |
| Body Mass Index (BMI) | 0.001 | ||
| Less than or equal 18.5 | 1.3 | 16.8 | |
| 18.5–25 | 24.0 | 17.2 | |
| 25–30 | 29.5 | 25.3 | |
| 30–35 | 21.9 | 34.0 | |
| 35–40 | 12.0 | 40.5 | |
| 40 or higher | 11.2 | 45.3 | |
| Employment Status | 0.001 | ||
| Employed | 38.5 | 19.9 | |
| Not employed | 61.5 | 35.0 | |
| Age | 0.001 | ||
| Senior 65 years and older | 10.3 | 45.2 | |
| 40–64 years old | 89.7 | 27.3 | |
| Smoking Status | 0.001 | ||
| Current | 42.0 | 23.9 | |
| Former | 22.6 | 38.0 | |
| Never | 35.4 | 29.8 | |
| Hypercholesterolemia | 0.001 | ||
| No | 65.3 | 19.4 | |
| Yes | 34.7 | 47.6 | |
| Hypertension | 0.001 | ||
| No | 44.4 | 14.3 | |
| Yes | 55.6 | 41.1 |
Overall, 29.2% of participants responded that their primary care doctor had previously told them they had a cardio-metabolic disease. The CMD prevalence was higher among seniors (45.2%) than among those younger than 65 (27.3%) but did not differ greatly between Blacks (28.9%) and Whites (29.7%) (p = 0.072). Black women reported more CMD than White women, but Black men reported less CMD than White men.
CMD prevalence was inversely related to education and family income levels. Participants with lower levels of education reported higher rates of CMD, ranging from 41.7% for all subjects with less than a high school education to 19.5% among subjects with a doctorate level of education (p < 0.001). Similarly, respondents who reported lower family incomes reported higher rates of CMD, ranging from 32.1% for those earning less than $15,000, to 14.5% for those with family incomes of $100,000 or more (p < 0.001).
Participants with clinical risk factors (i.e., hypercholesterolemia, hypertension) reported higher than average levels of CMD. In addition, CMD prevalence monotonically increased with rising BMI, with 17% of those of normal weight vs. 45% of those in obesity class III reporting CMD. In these univariate data, a low, rather than high, prevalence of CMD was seen among current smokers compared with former or never smokers.
SCCS respondents who reported poorer outdoor (p = 0.001) and indoor (p = 0.105) air quality also reported higher rates of CMD. Respondents who lived in rural areas reported higher prevalence (32.5%) of CMD than those who did not (26.4%) (p < 0.001). CMD prevalence was 29.6%, 29.0%, 29.3%, 31.2%, 28.1%, and 25.5% within the 10th, 25th, 50th, 75th, and 90th percentiles of PM2.5 concentrations. The average annual PM2.5 concentration for individuals in the 12-state sample was 13.5 μg/m3 for respondents in the 12-month period prior to enrollment, with 10th, 25th, 50th, 75th, and 90th percentile values of 11.3 μg/m3, 12.4 μg/m3, 13.5 μg/m3, 15.0 μg/m3, and 15.8 μg/m3, respectively. The annual mean PM2.5 was above the 2012 EPA regulation which defined a three-year, average annual mean exposure above 12.0 μg/m3 as harmful to public health and the environment. Among respondents, 84.1% were exposed to PM2.5 above 12.0 μg/m3 in the 12-month period prior to study enrollment.
Table 3 shows results from GLMM multivariate modeling of the CMD in relation to the risk factor data shown in Table 2. The univariate associations seen in Table 2 tended to persist after simultaneous adjustment for other factors, with strong increases in the odds of having CMD associated with lower levels of education and income, hypertension, hypercholesterolemia, and an increasing rate of BMI. However, the significantly lower risk among current vs. never smokers and higher risk in rural vs. urban areas seen in Table 2, no longer held in the adjusted analyses.
Table 3.
Generalized linear mixed model fixed effects estimates for presence of cardio-metabolic disease in FQHC cohort participants of the Southern Community Cohort Study (n = 48,799).
| Model Term | Coefficient | Std. Error | T-Value | p-Value | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval for Exp (Coefficient) | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| Intercept | −3.511 | 0.1482 | −23.692 | 0 | −3.802 | −3.221 | 0.030 | 0.022 | 0.04 |
| Enrollment Age | 0.025 | 0.001 | 24.005 | 0 | 0.023 | 0.027 | 1.025 | 1.023 | 1.027 |
| Education | |||||||||
| Doctorate | −0.2 | 0.1407 | −1.419 | 0.156 | −0.475 | 0.076 | 0.819 | 0.622 | 1.079 |
| Masters | −0.282 | 0.0579 | −4.867 | 0 | −0.395 | −0.168 | 0.754 | 0.673 | 0.845 |
| College | −0.142 | 0.0638 | −2.232 | 0.026 | −0.267 | −0.017 | 0.867 | 0.765 | 0.983 |
| Some College | −0.128 | 0.0355 | −3.607 | 0 | −0.198 | −0.059 | 0.880 | 0.820 | 0.943 |
| Vocational | −0.034 | 0.0353 | −0.953 | 0.341 | −0.103 | 0.036 | 0.967 | 0.902 | 1.036 |
| High School | −0.162 | 0.0286 | −5.673 | 0 | −0.218 | −0.106 | 0.850 | 0.804 | 0.899 |
| Some High School | −0.064 | 0.0488 | −1.311 | 0.19 | −0.16 | 0.032 | 0.938 | 0.852 | 1.032 |
| Less 9 years education | REF | - | - | - | - | - | - | - | - |
| Marital Status | |||||||||
| Single | −0.109 | 0.038 | −2.878 | 0.004 | −0.184 | −0.035 | 0.896 | 0.832 | 0.966 |
| Widowed | 0.068 | 0.037 | 1.835 | 0.067 | −0.005 | 0.14 | 1.070 | 0.995 | 1.151 |
| Divorced | −0.021 | 0.0287 | −0.727 | 0.467 | −0.077 | 0.035 | 0.979 | 0.926 | 1.036 |
| Married | REF | - | - | - | - | - | - | - | - |
| Income | |||||||||
| $100,000 plus | −0.713 | 0.0677 | −10.521 | 0 | −0.845 | −0.58 | 0.490 | 0.429 | 0.56 |
| $50,000–$99,000 | −0.381 | 0.06 | −6.356 | 0 | −0.499 | −0.264 | 0.683 | 0.607 | 0.768 |
| $25,000–$49,000 | −0.199 | 0.0429 | −4.632 | 0 | −0.283 | −0.115 | 0.820 | 0.754 | 0.892 |
| $15,000–$24,000 | −0.124 | 0.0233 | −5.337 | 0 | −0.170 | −0.079 | 0.883 | 0.844 | 0.924 |
| Less than $15,000 | REF | - | - | - | - | - | - | - | - |
| Rural or Farm | |||||||||
| Rural or Farm | 0.036 | 0.025 | 1.435 | 0.151 | −0.013 | 0.085 | 1.037 | 0.987 | 1.089 |
| Urban | REF | - | - | - | - | - | - | - | - |
| Air Quality Outside | |||||||||
| Excellent | −0.113 | 0.0384 | −2.951 | 0.003 | −0.189 | −0.038 | 0.893 | 0.828 | 0.963 |
| Good | −0.033 | 0.036 | −0.921 | 0.357 | −0.104 | 0.037 | 0.967 | 0.902 | 1.038 |
| Fair | −0.061 | 0.0342 | −1.777 | 0.076 | −0.128 | 0.006 | 0.941 | 0.880 | 1.006 |
| Poor | REF | - | - | - | - | - | - | - | - |
| Air Quality Inside | |||||||||
| Excellent | 0.15 | 0.0515 | 2.913 | 0.004 | 0.049 | 0.251 | 1.162 | 1.050 | 1.285 |
| Good | 0.01 | 0.0458 | 0.218 | 0.827 | −0.080 | 0.1 | 1.010 | 0.923 | 1.105 |
| Fair | 0.038 | 0.0424 | 0.888 | 0.374 | −0.045 | 0.121 | 1.038 | 0.956 | 1.128 |
| Poor | REF | - | - | - | - | - | - | - | - |
| BMI | |||||||||
| 40 or higher | 1.061 | 0.1134 | 9.351 | 0 | 0.838 | 1.283 | 2.888 | 2.313 | 3.607 |
| 35–39 | 0.824 | 0.1051 | 7.846 | 0 | 0.618 | 1.03 | 2.280 | 1.856 | 2.801 |
| 30–34 | 0.597 | 0.0937 | 6.374 | 0 | 0.413 | 0.781 | 1.817 | 1.512 | 2.183 |
| 25–29 | 0.252 | 0.1101 | 2.291 | 0.022 | 0.036 | 0.468 | 1.287 | 1.037 | 1.597 |
| 18.5–24 | −0.015 | 0.1042 | −0.143 | 0.886 | −0.219 | 0.189 | 0.985 | 0.803 | 1.208 |
| Less than 18.5 | REF | - | - | - | - | - | - | - | - |
| Hypertension | |||||||||
| Yes | 0.907 | 0.0284 | 31.983 | 0 | 0.852 | 0.963 | 2.478 | 2.344 | 2.620 |
| No | REF | - | - | - | - | - | - | - | - |
| Hypercholesterol | |||||||||
| Yes | 0.959 | 0.0187 | 51.155 | 0 | 0.922 | 0.996 | 2.609 | 2.515 | 2.706 |
| No | REF | - | - | - | - | - | - | - | - |
| Employment | |||||||||
| Yes | −0.504 | 0.0222 | −22.681 | 0 | −0.548 | −0.461 | 0.604 | 0.578 | 0.631 |
| No | REF | - | - | - | - | - | - | - | - |
| Race | |||||||||
| Black | 0.069 | 0.0478 | 1.435 | 0.151 | −0.025 | 0.162 | 1.071 | 0.975 | 1.176 |
| White | REF | - | - | - | - | - | - | - | - |
| Smoking History | |||||||||
| Never Smoked | −0.017 | 0.0344 | −0.487 | 0.626 | −0.084 | 0.051 | 0.983 | 0.919 | 1.052 |
| Former Smoker | 0.19 | 0.0296 | 6.404 | 0 | 0.132 | 0.248 | 1.209 | 1.141 | 1.281 |
| Current Smoker | REF | - | - | - | - | - | - | - | - |
| PM2.5 | 0.026 | 0.0093 | 2.751 | 0.006 | 0.007 | 0.044 | 1.026 | 1.007 | 1.045 |
| Gender | |||||||||
| Female | −0.197 | 0.022 | −8.93 | 0 | −0.24 | −0.154 | 0.821 | 0.787 | 0.858 |
| Male | REF | - | - | - | - | - | - | - | - |
When PM2.5 was modeled as a linear variable, we found higher occurrences of CMD among participants with a higher level of exposure to PM2.5. Exposure to increasing levels of PM2.5 (F = 7.569; p = 0.006) as measured from the algorithms developed by Al-Hamdan et al. [104] was associated with increasing prevalence of CMD for participants in the SCCS. Our findings indicate that each unit increase in PM2.5 is associated with an approximately 2.6% increase in the log odds of CMD for the study sample of SCCS participants.
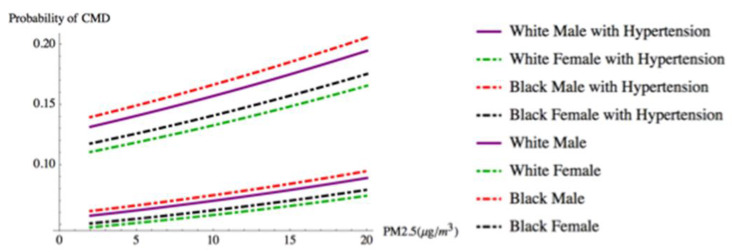
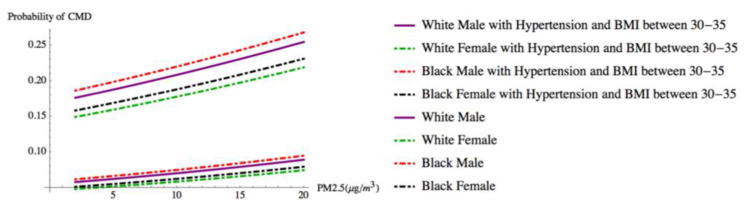
We illustrate the findings from our model by presenting the predicted probability of CMD for four “typical” types of participants as PM2.5 concentrations rise. Figure 2 shows how the presence of hypertension, alone, affects the probability of CMD by race and gender at different levels of PM2.5 exposure. This race × gender exemplar controls for other common CMD risk factors. It assumes an individual is 52 years old, married, employed, never smoked, lives in an urban area, possesses a high school diploma, has an average weight (BMI 25–30), and lives in a home where they indicate indoor and outdoor air quality as good (i.e., a person with low CMD risk factors). In contrast, Figure 3 illustrates how having two clinical factors, hypertension and being obese (BMI 30–35), while being exposed to rising levels of PM2.5, affects the predicted probability of CMD. In both cases, the presence of clinical risk factors (i.e., hypertension alone, and hypertension and obesity (high BMI)) increases the predicted probability of CMD as PM2.5 concentrations increase.
Figure 2.
Individuals by race and gender with and without hypertension.
Figure 3.
Individuals by race and gender with hypertension and BMI between 30 and 35 vs. individuals by race and gender without hypertension and BMI below 30.
We tested the GLMM using a classification table and confirmed that our model correctly predicted the presence of CMD 74.8% of the time (see Table 3). The intercept variance = 0.017 (Wald Z = 1.98, p = 0.047) (see Table 4). The null hypothesis for this parameter is a variance of zero, which would indicate that a random effect is not needed. Thus, we rejected the null hypotheses concluding a random intercept for each state is needed. These results further suggest that there are important unmeasured explanatory variables for our participants at the state level that affect CMD in a way that appears random because we do not know the value(s) of the missing explanatory variable(s).
Table 4.
Random effect.
| Random Effect Covariance | Estimate | Std. Error | Z | p-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Var (Intercept) | 0.017 | 0.008 | 1.984 | 0.047 | 0.006 | 0.045 |
Covariance structure: variance components; subject specification: state.
4. Discussion
Our study results in four key findings:
-
(1)
Residents of communities with exposure to higher levels of PM2.5 annual concentrations are more likely to have reported a CMD.
-
(2)
Race, a social risk factor for disparities in health, is not predictive of CMD when behavioral, clinical, and environmental risk factors are accounted for in the model. Similarly, residence in an urban or rural setting is not associated with CMD after PM2.5 and other risk factor information are taken into consideration.
-
(3)
A significant residual variation in the presence of CMD among participants across states was found, perhaps reflecting differences in environmental exposures, social policies, and other place-based factors. These differences will be explored further in future analyses.
-
(4)
Multiple individual and environmental risk factors are associated with self-reported CMD, consistent with a multifactorial etiology of these conditions. Our results are generally consistent with previously published literature. We found statistically significant positive associations between CMD and marital status, BMI, education, gender, age, employment, and higher concentrations of PM2.5.
However, when we controlled for other clinical and social characteristics within our study population of low socioeconomic status (SES) individuals, there were no statistically significant associations between the presence of CMD and race or residence in urban vs. rural settings. In some previous studies, race has been identified as both a biological [105] and social [106] risk factor in health disparity studies; our finding demonstrates that when clinical, behavioral, and environmental factors are included in a model ascertaining the presence of CMD, race, as a social construct, is not a statistically significant predictor of CMD risk among Black and White populations compared within the same social strata. This confirms results reported by LaVeist et al. [107] who similarly found that when comparing Blacks and Whites who live in neighborhoods with similar levels of social and economic resources/exposures, the influence of race in health outcomes largely disappeared.
Despite the completion of the human genome in 2003, and the increased attention and resources given by academicians and the NIH in support of research to identify genetic differences underlying racial health disparities, there is considerable evidence that some CMD risk factors are substantially influenced by environmental factors [108]. Our findings suggest that a more holistic, exposomics approach that incorporates a broad range of exogenous and endogenous environmental risk factors experienced over the course of life may be needed to explain CMD racial disparities.
Our findings support the notion that low-income persons, regardless of race, are exposed to social disadvantage and adverse environmental exposures resulting in intransigent inequities [109]. The underlying causes of CMD are likely a result of pathophysiological insults that occur in response to biological and personal risk factors and adverse exposures associated with the natural, built, and social environments. Extrapolating our regional findings within the SCCS, it also seems likely that racial disparities in CMD observed nationally are mainly due to differences in these multiple risk factors, rather than intrinsic to race per se. This finding has implications both for CMD research, the science of health disparities, and for the conceptualization of interventions in the clinical, public health, and policy arenas [110].
5. Conclusions
The nature of the SCCS cohort, which is overwhelmingly of low socio-economic status (SES) and within the southeastern region of the US, where CVD, stroke, and diabetes prevalence are high, allows us to compare Black and White subgroups whose disease “riskscapes” are more similar than different. Our results support the finding that race, as a risk factor for disease, as well as a way to elaborate on the patterning of CMD, should not be interpreted as “biological pre-programming”. Rather, our results indicate that CMD disparities reflect the complex interactions of personal risk factors and exposures that emerge from the social, built, and natural environments among low social status populations. Our results echo those of Geronimus and colleagues [111], who demonstrated similar mortality risks among high-poverty Black and White rural populations. Our findings underscore that position within social strata indeed matters with regards to health outcomes and the need for additional studies that critically examine racial health disparities within SES subgroups or social strata, particularly those in different geographic areas.
Modeling the contributions of multiple environmental exposures on CMD health and health disparities, as experienced in the real world, is in its formative stage. The “exposome framework” presents a new approach for considering the complex ways in which biological and personal factors interact with environmental factors to affect risk for CMD in the real world and across the life course [112]. While we only examined one environmental factor herein (PM2.5 exposure) and only across a limited time window, future studies accounting for the totality of exogenous (external) and endogenous (internal) exposures from conception onwards, and across generations, offer promise to simultaneously distinguish, characterize and quantify etiologic, mediating, moderating, and co-occurring risk and protective factors and their relationship to the onset, progression, and outcomes of personal health and population-level disparities [112]. We plan to expand our analysis in future research that incorporates over 20,000 environmental exposures and daily measures of heat over 15 years linked to SCCS participant data which include five survey waves, Medicaid and Medicare (Parts A, B, C, and D) claims data, national death index files, state cancer registries for persons with cancer, and biological samples. This will allow us to apply an exposome-wide approach and examine complete exposure pathways from source of exposure, biomarkers of exposure, effect and disease susceptibility, to population-level disparities.
Limitations
Study limitations include CMD was self-reported in response to a set of questions that asked the participant if s/he was ever told by their doctor that they had CVD, diabetes, etc. Self-reported health outcomes have been found to be affected by measurement error as the result of recall bias and social desirability [113]. However, some studies have found good concordance between self-report and more objective measures, such as medical records to identify disease history [113]. In addition, while the 3 km gridded measure of PM2.5 is a used as a proxy for exposure at the level of residential address, it is superior to either ambient or satellite measures used alone, which is a typical course of action.
Abbreviations
| CMD | Cardio-metabolic disease |
| SCCS | Southern Community Cohort Study |
| PM2.5 | Particulate matter smaller than 2.5 micrometers in diameter |
| BMI | Body mass index |
| GLMM | Generalized linear mixed model |
Author Contributions
The individual roles in the paper are described below. In addition all authors approved the submitted version and agreed to be both personally accountable for the authors’ own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the authors were not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. P.D.J. was responsible for acquisition of financial support for the project leading to this publication and for the drafting and final approval of the article; M.T. was responsible for final data analysis; R.B.V. contributed to data analysis and drafting the article; D.B.H. contributed to the writing and editing; W.I. curated data; C.M. contributed to the discussion section; C.C. and A.R. contributed to the writing of the background section; M.Z.A.-H. contributed to the writing of the methods section and developing the PM2.5 dataset; P.M.-J. and W.B. contributed to the final editing of the paper; M.Y.L. and M.M.D. contributed to writing the conclusions; D.S., M.A.L., G.L.R., and C.A.P. contributed to the data analysis section; J.F.R. contributed to the discussion section. All authors have read and agreed to the published version of the manuscript.
Funding
The research presented in this paper was supported in part by funding from: Study Title: Using an Exposome Approach to Assess the Effects of PM2.5 on CVD outcomes; Principal Investigator: Paul D. Juarez; Application/Grant/Award Number: Environmental Protection Agency. Grant #: G17D112354237. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Su J.B. Vascular endothelial dysfunction and pharmacological treatment. World J. Cardiol. 2015;7:719–741. doi: 10.4330/wjc.v7.i11.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang D., Tang X., Shen P., Si Y., Liu X., Xu Z., Wu J., Zhang J., Lu P., Lin H., et al. Multimorbidity of cardiometabolic diseases: Prevalence and risk for mortality from one million chinese adults in a longitudinal cohort study. BMJ Open. 2019;9:e024476. doi: 10.1136/bmjopen-2018-024476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Emerging Risk Factors Collaboration. Di Angelantonio E., Kaptoge S., Wormser D., Willeit P., Butterworth A.S., Bansal N., O’Keeffe L.M., Gao P., Wood A.M., et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J., Murphy S.L., Kochanek K.D., Bastian B., Arias E. Final Data for 2016. National Vital Statistics Reports. National Center for Health Statistics; Hyattsville, MD, USA: 2018. [PubMed] [Google Scholar]
- 5.Juarez P.D., Matthews-Juarez P., Hood D.B., Im W., Levine R.S., Kilbourne B.J., Langston M.A., Al-Hamdan M.Z., Crosson W.L., Estes M.G., et al. The public health exposome: A population-based, exposure science approach to health disparities research. Int. J. Environ. Res. Public Health. 2014;11:12866–12895. doi: 10.3390/ijerph111212866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahraki M., Shahraki T., Shidfar F., Ansari H. Which modifiable, non-modifiable, and socioeconomic factors have more effect on cardiovascular risk factors in overweight and obese women? J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2012;17:676–680. [PMC free article] [PubMed] [Google Scholar]
- 7.Balfour P.C., Rodriguez C.J., Ferdinand K.C. The role of hypertension in race-ethnic disparities in cardiovascular disease. Curr. Cardiovasc. Risk Rep. 2015;9:18. doi: 10.1007/s12170-015-0446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logan J., Barksdale D.J. Allostasis and allostatic load: Expanding the discourse on stress and cardiovascular disease. J. Clin. Nurs. 2008;17:201–208. doi: 10.1111/j.1365-2702.2008.02347.x. [DOI] [PubMed] [Google Scholar]
- 9.Mattei J., Demissie S., Falcon L.M., Ordovas J.M., Tucker K.L. Allostatic load is associated with chronic conditions in the boston puerto rican health study. Soc. Sci. Med. 2010;70:1988–1996. doi: 10.1016/j.socscimed.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCaffery J.M., Marsland A.L., Strohacker K., Muldoon M.F., Manuck S.B. Factor structure underlying components of allostatic load. PLoS ONE. 2012;7:e47246. doi: 10.1371/journal.pone.0047246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musunuru K. Atherogenic dyslipidemia: Cardiovascular risk and dietary intervention. Lipids. 2010;45:907–914. doi: 10.1007/s11745-010-3408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holven K.B., Retterstøl K., Ueland T., Ulven S.M., Nenseter M.S., Sandvik M., Narverud I., Berge K.E., Ose L., Aukrust P., et al. Subjects with low plasma hdl cholesterol levels are characterized by an inflammatory and oxidative phenotype. PLoS ONE. 2013;8:e78241. doi: 10.1371/journal.pone.0078241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartlett J., Predazzi I.M., Williams S.M., Bush W.S., Kim Y., Havas S., Toth P.P., Fazio S., Miller M. Is isolated low high-density lipoprotein cholesterol a cardiovascular disease risk factor? New insights from the framingham offspring study. Circ. Cardiovasc. Qual. Outcomes. 2016;9:206–212. doi: 10.1161/CIRCOUTCOMES.115.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulsegge G., Spijkerman A.M.W., van der Schouw Y.T., Bakker S.J.L., Gansevoort R.T., Smit H.A., Verschuren W.M.M. Trajectories of metabolic risk factors and biochemical markers prior to the onset of cardiovascular disease - the doetinchem cohort study. PLoS ONE. 2016;11:e0155978. doi: 10.1371/journal.pone.0155978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stout R. The impact of insulin upon atherosclerosis. Horm. Metab. Res. 1994;26:125–128. doi: 10.1055/s-2007-1000791. [DOI] [PubMed] [Google Scholar]
- 16.Tonelli M., Wiebe N., Culleton B., House A., Rabbat C., Fok M., McAlister F., Garg A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006;17:2034. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 17.Fang J., Alderman M.H. Serum uric acid and cardiovascular mortality: The nhanes i epidemiologic follow-up study, 1971–1992. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 18.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 19.Koenig W., Sund M., Fröhlich M., Fischer H.G., Löwel H., Döring A., Hutchinson W.L., Pepys M.B. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men - results from the monica (monitoring trends and determinants in cardiovascular disease) augsburg cohort study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 20.Peng J., Luo F., Ruan G., Peng R., Li X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017;16:233. doi: 10.1186/s12944-017-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tracy R.P. Thrombin, inflammation, and cardiovascular disease: An epidemiologic perspective. Chest. 2003;124:49S–57S. doi: 10.1378/chest.124.3_suppl.49S. [DOI] [PubMed] [Google Scholar]
- 22.Hoek G., Brunekreef B., Fischer P., van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001;12:355–357. doi: 10.1097/00001648-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katzke V.A., Sookthai D., Johnson T., Kühn T., Kaaks R. Blood lipids and lipoproteins in relation to incidence and mortality risks for cvd and cancer in the prospective epic-heidelberg cohort. BMC Med. 2017;15:218. doi: 10.1186/s12916-017-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulsen P., Vaag A., Kyvik K., Beck-Nielsen H. Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia. 2001;44:537–543. doi: 10.1007/s001250051659. [DOI] [PubMed] [Google Scholar]
- 26.Elder S.J., Lichtenstein A.H., Pittas A.G., Roberts S.B., Fuss P.J., Greenberg A.S., McCrory M.A., Bouchard T.J., Saltzman E., Neale M.C. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J. Lipid Res. 2009;50:1917–1926. doi: 10.1194/jlr.P900033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danesh J., Collins R., Appleby P., Peto R. Association of fibrinogen, c-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 28.Graham I.M., Daly L.E., Refsum H.M., Robinson K., Brattström L.E., Ueland P.M., Palma-Reis R.J., Boers G.H., Sheahan R.G., Israelsson B. Plasma homocysteine as a risk factor for vascular disease: The european concerted action project. JAMA. 1997;277:1775–1781. doi: 10.1001/jama.1997.03540460039030. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention . Diabetes Report Card 2017. US DHHS; Atlanta, GA, USA: 2018. [Google Scholar]
- 30.Geiss L.S., Wang J., Cheng Y.J., Thompson T.J., Barker L., Li Y., Albright A.L., Gregg E.W. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, united states, 1980-2012prevalence and incidence trends for diabetes among adultsprevalence and incidence trends for diabetes among adults. JAMA. 2014;312:1218–1226. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- 31.Kelly F.J., Fussell J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012;60:504–526. doi: 10.1016/j.atmosenv.2012.06.039. [DOI] [Google Scholar]
- 32.Hartiala J., Breton C.V., Tang W.H.W., Lurmann F., Hazen S.L., Gilliland F.D., Allayee H. Ambient air pollution is associated with the severity of coronary atherosclerosis and incident myocardial infarction in patients undergoing elective cardiac evaluation. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016;5:e003947. doi: 10.1161/JAHA.116.003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mustafić H., Jabre P., Caussin C., Murad M.H., Escolano S., Tafflet M., Périer M.C., Marijon E., Vernerey D., Empana J.P., et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA. 2012;307:713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 34.Pope Iii C.A., Renlund D.G., Kfoury A.G., May H.T., Horne B.D. Relation of heart failure hospitalization to exposure to fine particulate air pollution. Am. J. Cardiol. 2008;102:1230–1234. doi: 10.1016/j.amjcard.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 35.Link M.S., Luttmann-Gibson H., Schwartz J., Mittleman M.A., Wessler B., Gold D.R., Dockery D.W., Laden F. Acute exposure to air pollution triggers atrial fibrillation. J. Am. Coll. Cardiol. 2013;62:816–825. doi: 10.1016/j.jacc.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kampfrath T., Maiseyeu A., Ying Z., Shah Z., Deiuliis J.A., Xu X., Kherada N., Brook R.D., Reddy K.M., Padture N.P., et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via nadph oxidase and tlr4 pathways. Circ. Res. 2011;108:716–726. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelin T.D., Joseph A.M., Gorr M.W., Wold L.E. Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol. Lett. 2012;208:293–299. doi: 10.1016/j.toxlet.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brook R.D., Rajagopalan S. Particulate matter, air pollution, and blood pressure. J. Am. Soc. Hypertens. 2009;3:332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Yang B.-Y., Qian Z., Howard S.W., Vaughn M.G., Fan S.-J., Liu K.-K., Dong G.-H. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ. Pollut. 2018;235:576–588. doi: 10.1016/j.envpol.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Coogan P.F., White L.F., Yu J., Burnett R.T., Seto E., Brook R.D., Palmer J.R., Rosenberg L., Jerrett M. Pm(2.5) and diabetes and hypertension incidence in the black women’s health study. Epidemiology. 2016;27:202–210. doi: 10.1097/EDE.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dzhambov A.M., Dimitrova D.D. Exposures to road traffic, noise, and air pollution as risk factors for type 2 diabetes: A feasibility study in bulgaria. Noise Health. 2016;18:133–142. doi: 10.4103/1463-1741.181996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu H., Sun S., Tsang H., Wong C.-M., Lee R.S.-y., Schooling C.M., Tian L. Fine particulate matter exposure and incidence of stroke. Neurology. 2017;88:1709. doi: 10.1212/WNL.0000000000003903. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Eliot M.N., Wellenius G.A. Short-term changes in ambient particulate matter and risk of stroke: A systematic review and meta-analysis. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2014;3:e000983. doi: 10.1161/JAHA.114.000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araujo J.A. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual. Atmos. Health. 2011;4:79–93. doi: 10.1007/s11869-010-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai Y., Sun Q. Fine particulate matter air pollution and atherosclerosis: Mechanistic insights. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016;1860:2863–2868. doi: 10.1016/j.bbagen.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 46.Seo S., Choi S., Kim K., Kim S.M., Park S.M. Association between urban green space and the risk of cardiovascular disease: A longitudinal study in seven korean metropolitan areas. Environ. Int. 2019;125:51–57. doi: 10.1016/j.envint.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 47.Dendup T., Feng X., Clingan S., Astell-Burt T. Environmental risk factors for developing type 2 diabetes mellitus: A systematic review. Int. J. Environ. Res. Public Health. 2018;15:78. doi: 10.3390/ijerph15010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giskes K., van Lenthe F., Avendano-Pabon M., Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: Are we getting closer to understanding obesogenic environments? Obes. Rev. 2011;12:e95–e106. doi: 10.1111/j.1467-789X.2010.00769.x. [DOI] [PubMed] [Google Scholar]
- 49.Pool L.R., Ning H., Lloyd-Jones D.M., Allen N.B. Trends in racial/ethnic disparities in cardiovascular health among us adults from 1999–2012. J. Am. Heart Assoc. 2017;6:e006027. doi: 10.1161/JAHA.117.006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X., Chen X., Gong W. Type 2 diabetes mellitus and neighborhood deprivation index: A spatial analysis in zhejiang, china. J. Diabetes Investig. 2019;10:272–282. doi: 10.1111/jdi.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundquist K., Theobald H., Yang M., Li X., Johansson S.-E., Sundquist J. Neighborhood violent crime and unemployment increase the risk of coronary heart disease: A multilevel study in an urban setting. Soc. Sci. Med. 2006;62:2061–2071. doi: 10.1016/j.socscimed.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 52.Sundquist J., Johansson S.E., Yang M., Sundquist K. Low linking social capital as a predictor of coronary heart disease in sweden: A cohort study of 2.8 million people. Soc. Sci. Med. 2006;62:954–963. doi: 10.1016/j.socscimed.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention Rural Americans at Higher Risk of Death from Five Leading Causes. [(accessed on 28 July 2019)]; Available online: https://www.cdc.gov/media/releases/2017/p0112-rural-death-risk.html.
- 54.Hicken M.T., Adar S.D., Hajat A., Kershaw K.N., Do D.P., Barr R.G., Kaufman J.D., Diez Roux A.V. Air pollution, cardiovascular outcomes, and social disadvantage: The multi-ethnic study of atherosclerosis. Epidemiology. 2016;27:42–50. doi: 10.1097/EDE.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz D., Becerra M., Jagai J.S., Ard K., Sargis R.M. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care. 2018;41:193. doi: 10.2337/dc16-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erqou S., Clougherty Jane E., Olafiranye O., Magnani Jared W., Aiyer A., Tripathy S., Kinnee E., Kip Kevin E., Reis Steven E. Particulate matter air pollution and racial differences in cardiovascular disease risk. Arterioscler. Thromb. Vasc. Biol. 2018;38:935–942. doi: 10.1161/ATVBAHA.117.310305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Signorello L.B., Hargreaves M.K., Blot W.J. The southern community cohort study: Investigating health disparities. J. Health Care Poor Underserved. 2010;21:26–37. doi: 10.1353/hpu.0.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cosselman K.E., Navas-Acien A., Kaufman J.D. Environmental factors in cardiovascular disease. Nat. Rev. Cardio.l. 2015;12:627–642. doi: 10.1038/nrcardio.2015.152. [DOI] [PubMed] [Google Scholar]
- 59.Navas-Acien A., Guallar E., Silbergeld E.K., Rothenberg S.J. Lead exposure and cardiovascular disease—A systematic review. Environ. Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orioli R., Cremona G., Ciancarella L., Solimini A.G. Association between pm10, pm2.5, no2, o3 and self-reported diabetes in italy: A cross-sectional, ecological study. PLoS ONE. 2018;13:e0191112. doi: 10.1371/journal.pone.0191112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith A.H., O Lingas E., Rahman M. Contamination of Drinking Water by Arsenic in Bangladesh: A Public Health Emergency. Bull. World Health Organ. 2000;78:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 62.Peters J.L., Perlstein T.S., Perry M.J., McNeely E., Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ. Res. 2010;110:199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edwards J.R., Prozialeck W.C. Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 2009;238:289–293. doi: 10.1016/j.taap.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bulka C.M., Daviglus M.L., Persky V.W., Durazo-Arvizu R.A., Lash J.P., Elfassy T., Lee D.J., Ramos A.R., Tarraf W., Argos M. Association of occupational exposures with cardiovascular disease among us hispanics/latinos. Heart. 2019;105:439. doi: 10.1136/heartjnl-2018-313463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rinsky J.L., Hoppin J.A., Blair A., He K., Beane Freeman L.E., Chen H. Agricultural exposures and stroke mortality in the agricultural health study. J. Toxicol. Environ. Health. Part A. 2013;76 doi: 10.1080/15287394.2013.819308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandler D.P., Kamel F., Montgomery M.P., Saldana T.M., Alavanja M.C.R. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural health study, 1993–2003. Am. J. Epidemiol. 2008;167:1235–1246. doi: 10.1093/aje/kwn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilcosky T.C., Simonsen N.R. Solvent exposure and cardiovascular disease. Am. J. Ind. Med. 1991;19:569–586. doi: 10.1002/ajim.4700190503. [DOI] [PubMed] [Google Scholar]
- 68.Mohammadi A., Azhdarpoor A., Shahsavani A., Tabatabaee H. Health impacts of exposure to pm10 on inhabitants of shiraz, iran. Health Scope. 2015;4 doi: 10.17795/jhealthscope-31015. [DOI] [Google Scholar]
- 69.Kowalska M., Kocot K. Short-term exposure to ambient fine particulate matter (pm2,5 and pm10) and the risk of heart rhythm abnormalities and stroke. Postepy Higieny i Medycyny Doswiadczalnej. 2016;70:1017–1025. doi: 10.5604/17322693.1220389. [DOI] [PubMed] [Google Scholar]
- 70.Lee F.-Y., Chen W.-K., Lin C.-L., Kao C.-H. Carbon monoxide poisoning and subsequent cardiovascular disease risk: A nationwide population-based cohort study. Medicine. 2015;94:e624. doi: 10.1097/MD.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hampson N.B., Weaver L.K. Carbon monoxide poisoning and risk for ischemic stroke. Eur. J. Intern. Med. 2016;31:e7. doi: 10.1016/j.ejim.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Huang C.-C., Ho C.-H., Chen Y.-C., Lin H.-J., Hsu C.-C., Wang J.-J., Su S.-B., Guo H.-R. Increased risk for diabetes mellitus in patients with carbon monoxide poisoning. Oncotarget. 2017;8:63680–63690. doi: 10.18632/oncotarget.18887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goodman J.E., Prueitt R.L., Sax S.N., Pizzurro D.M., Lynch H.N., Zu K., Venditti F.J. Ozone exposure and systemic biomarkers: Evaluation of evidence for adverse cardiovascular health impacts. Crit. Rev. Toxicol. 2015;45:412–452. doi: 10.3109/10408444.2015.1031371. [DOI] [PubMed] [Google Scholar]
- 74.Srebot V., Gianicolo E.A.L., Rainaldi G., Trivella M.G., Sicari R. Ozone and cardiovascular injury. Cardiovasc. Ultrasound. 2009;7:30. doi: 10.1186/1476-7120-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jerrett M., Brook R., White L.F., Burnett R.T., Yu J., Su J., Seto E., Marshall J., Palmer J.R., Rosenberg L., et al. Ambient ozone and incident diabetes: A prospective analysis in a large cohort of african american women. Environ. Int. 2017;102:42–47. doi: 10.1016/j.envint.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kopp A., Lu H., Bai L., Kwong J.C., Chen H., Van Ryswyk K., Weichenthal S., Burnett R.T., Hatzopoulou M., Jerrett M., et al. Associations of long-term exposure to ultrafine particles and nitrogen dioxide with increased incidence of congestive heart failure and acute myocardial infarction. Am. J. Epidemiol. 2018;188:151–159. doi: 10.1093/aje/kwy194. [DOI] [PubMed] [Google Scholar]
- 77.Amancio C.T., Nascimento L.F.C. Association of sulfur dioxide exposure with circulatory system deaths in a medium-sized city in brazil. Braz. J. Med. Biol. Res. 2012;45:1080–1085. doi: 10.1590/S0100-879X2012007500131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gaglioti A.H., Xu J., Rollins L., Baltrus P., O’Connell L.K., Cooper D.L., Hopkins J., Botchwey N.D., Akintobi T.H. Neighborhood environmental health and premature death from cardiovascular disease. Prev. Chronic Dis. 2018;15:E17. doi: 10.5888/pcd15.170220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwon I., Choi S., Mittman B., Bharmal N., Liu H., Vickrey B., Song S., Araiza D., McCreath H., Seeman T., et al. Study protocol of “worth the walk”: A randomized controlled trial of a stroke risk reduction walking intervention among racial/ethnic minority older adults with hypertension in community senior centers. BMC Neurol. 2015;15:91. doi: 10.1186/s12883-015-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sundquist K., Eriksson U., Mezuk B., Ohlsson H. Neighborhood walkability, deprivation and incidence of type 2 diabetes: A population-based study on 512,061 swedish adults. Health Place. 2015;31:24–30. doi: 10.1016/j.healthplace.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pham D.Q., Ommerborn M.J., Hickson D.A., Taylor H.A., Clark C.R. Neighborhood safety and adipose tissue distribution in african americans: The jackson heart study. PLoS ONE. 2014;9:e105251. doi: 10.1371/journal.pone.0105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evenson K.R., Block R., Diez Roux A.V., McGinn A.P., Wen F., Rodríguez D.A. Associations of adult physical activity with perceived safety and police-recorded crime: The multi-ethnic study of atherosclerosis. Int. J. Behav. Nutr. Phys. Act. 2012;9:146. doi: 10.1186/1479-5868-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lindberg R., Sidebottom A.C., McCool B., Pereira R.F., Sillah A., Boucher J.L. Changing the restaurant food environment to improve cardiovascular health in a rural community: Implementation and evaluation of the heart of new ulm restaurant programme. Public Health Nutr. 2018;21:992–1001. doi: 10.1017/S1368980017003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Christine P.J., Auchincloss A.H., Bertoni A.G., Carnethon M.R., Sánchez B.N., Moore K., Adar S.D., Horwich T.B., Watson K.E., Diez Roux A.V. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: The multi-ethnic study of atherosclerosis (mesa) JAMA Intern. Med. 2015;175:1311–1320. doi: 10.1001/jamainternmed.2015.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poelman M., Strak M., Schmitz O., Hoek G., Karssenberg D., Helbich M., Ntarladima A.-M., Bots M., Brunekreef B., Grobbee R., et al. Relations between the residential fast-food environment and the individual risk of cardiovascular diseases in the netherlands: A nationwide follow-up study. Eur. J. Prev. Cardiol. 2018;25:1397–1405. doi: 10.1177/2047487318769458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodriguez F., Hu J., Kershaw K., Hastings K.G., López L., Cullen M.R., Harrington R.A., Palaniappan L.P. County-level hispanic ethnic density and cardiovascular disease mortality. J. Am. Heart Assoc. 2018;7:e009107. doi: 10.1161/JAHA.118.009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gebreab S.Y., Diez Roux A.V., Brenner A.B., Hickson D.A., Sims M., Subramanyam M., Griswold M.E., Wyatt S.B., James S.A. The impact of lifecourse socioeconomic position on cardiovascular disease events in african americans: The jackson heart study. J. Am. Heart Assoc. 2015;4:e001553. doi: 10.1161/JAHA.114.001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown A.F., Liang L.-J., Vassar S.D., Stein-Merkin S., Longstreth W.T., Jr., Ovbiagele B., Yan T., Escarce J.J. Neighborhood disadvantage and ischemic stroke: The cardiovascular health study (chs) Stroke. 2011;42:3363–3368. doi: 10.1161/STROKEAHA.111.622134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li S., Bruen B.K., Lantz P.M., Mendez D. Impact of health insurance expansions on nonelderly adults with hypertension. Prev. Chronic Dis. 2015;12:E105. doi: 10.5888/pcd12.150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Medford-Davis L.N., Fonarow G.C., Bhatt D.L., Xu H., Smith E.E., Suter R., Peterson E.D., Xian Y., Matsouaka R.A., Schwamm L.H. Impact of insurance status on outcomes and use of rehabilitation services in acute ischemic stroke: Findings from get with the guidelines-stroke. J. Am. Heart Assoc. 2016;5:e004282. doi: 10.1161/JAHA.116.004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stark Casagrande S., Cowie C.C. Health insurance coverage among people with and without diabetes in the u.S. Adult population. Diabetes Care. 2012;35:2243–2249. doi: 10.2337/dc12-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Booth J., Connelly L., Lawrence M., Chalmers C., Joice S., Becker C., Dougall N. Evidence of perceived psychosocial stress as a risk factor for stroke in adults: A meta-analysis. BMC Neurol. 2015;15:233. doi: 10.1186/s12883-015-0456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grigsby-Toussaint D.S., Jones A., Kubo J., Bradford N. Residential segregation and diabetes risk among latinos. Ethn. Dis. 2015;25:451–458. doi: 10.18865/ed.25.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kershaw K.N., Osypuk T.L., Do D.P., Chavez P.J., Diez Roux A.V. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: The multi-ethnic study of atherosclerosis. Circulation. 2015;131:141–148. doi: 10.1161/CIRCULATIONAHA.114.011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patel R.C., Baek J., Smith M.A., Morgenstern L.B., Lisabeth L.D. Residential ethnic segregation and stroke risk in mexican americans: The brain attack surveillance in corpus christi project. Ethn. Dis. 2015;25:11–18. [PMC free article] [PubMed] [Google Scholar]
- 96.Schoenthaler A., Montague E., Baier Manwell L., Brown R., Schwartz M.D., Linzer M. Patient-physician racial/ethnic concordance and blood pressure control: The role of trust and medication adherence. Ethn. Health. 2014;19:565–578. doi: 10.1080/13557858.2013.857764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heisler M., Bouknight R.R., Hayward R.A., Smith D.M., Kerr E.A. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J. Gen. Intern. Med. 2002;17:243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pearson-Stuttard J., Bandosz P., Rehm C.D., Penalvo J., Whitsel L., Gaziano T., Conrad Z., Wilde P., Micha R., Lloyd-Williams F., et al. Reducing us cardiovascular disease burden and disparities through national and targeted dietary policies: A modelling study. PLoS Med. 2017;14:e1002311. doi: 10.1371/journal.pmed.1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jilcott Pitts S.B., Smith T.W., Thayer L.M., Drobka S., Miller C., Keyserling T.C., Ammerman A.S. Addressing rural health disparities through policy change in the stroke belt. J. Public Health Manag. Pract. JPHMP. 2013;19:503–510. doi: 10.1097/PHH.0b013e3182893bbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shaikh S., Jagai J.S., Ashley C., Zhou S., Sargis R.M. Underutilized and under threat: Environmental policy as a tool to address diabetes risk. Curr. Diabetes Rep. 2018;18:25. doi: 10.1007/s11892-018-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ackermann R.T., Kenrik Duru O., Albu J.B., Schmittdiel J.A., Soumerai S.B., Wharam J.F., Ali M.K., Mangione C.M., Gregg E.W., Group N.-D.S. Evaluating diabetes health policies using natural experiments: The natural experiments for translation in diabetes study. Am. J. Prev. Med. 2015;48:747–754. doi: 10.1016/j.amepre.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Al-Hamdan M.Z., Crosson W.L., Economou S.A., Estes M.G., Estes S.M., Hemmings S.N., Kent S.T., Puckett M., Quattrochi D.A., Rickman D.L., et al. Environmental public health applications using remotely sensed data. Geocarto Int. 2014;29:85–98. doi: 10.1080/10106049.2012.715209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bolker B.M., Brooks M.E., Clark C.J., Geange S.W., Poulsen J.R., Stevens M.H.H., White J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 104.Snijders T.A., Bosker R.J. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. 2nd ed. Sage Publications; London, UK: 2012. [Google Scholar]
- 105.Taylor A.L., Ziesche S., Yancy C., Carson P., D’Agostino R., Ferdinand K., Taylor M., Adams K., Sabolinski M., Worcel M., et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N. Engl. J. Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 106.Braveman P.A., Egerter S.A., Cubbin C., Marchi K.S. An approach to studying social disparities in health and health care. Am. J. Public Health. 2004;94:2139–2148. doi: 10.2105/AJPH.94.12.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.LaVeist T., Pollack K., Thorpe R., Fesahazion R., Gaskin D. Place, not race: Disparities dissipate in southwest baltimore when blacks and whites live under similar conditions. Health Aff. 2011;30:1880–1887. doi: 10.1377/hlthaff.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jermendy G., Horváth T., Littvay L., Steinbach R., Jermendy Á.L., Tárnoki Á.D., Tárnoki D.L., Métneki J., Osztovits J. Effect of genetic and environmental influences on cardiometabolic risk factors: A twin study. Cardiovasc. Diabetol. 2011;10:96. doi: 10.1186/1475-2840-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Braveman P.A., Cubbin C., Egerter S., Williams D.R., Pamuk E. Socioeconomic disparities in health in the united states: What the patterns tell us. Am. J. Public Health. 2010;100(Suppl. 1):S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barouki R., Gluckman P.D., Grandjean P., Hanson M., Heindel J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health. 2012;11:42. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Geronimus A.T., Bound J., Colen C.G. Geronimus et al. Respond. Am. J. Public Health. 2011;101:1541. doi: 10.2105/AJPH.2011.300327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wild C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 113.Tisnado D.M., Adams J.L., Liu H., Damberg C.L., Hu F.A., Chen W.-P., Carlisle D.M., Mangione C.M., Kahn K.L. Does the concordance between medical records and patient self-report vary with patient characteristics? Health Serv. Outcomes Res. Methodol. 2006;6:157–175. doi: 10.1007/s10742-006-0012-1. [DOI] [Google Scholar]