Abstract
Thirteen known (1–12 and 16) and three previously undescribed Amaryllidaceae alkaloids of belladine structural type, named carltonine A-C (13–15), were isolated from bulbs of Narcissus pseudonarcissus cv. Carlton (Amaryllidaceae) by standard chromatographic methods. Compounds isolated in sufficient amounts, and not tested previously, were evaluated for their in vitro acetylcholinesterase (AChE; E.C. 3.1.1.7), butyrylcholinesterase (BuChE; E.C. 3.1.1.8) and prolyl oligopeptidase (POP; E.C. 3.4.21.26) inhibition activities. Significant human BuChE (hBUChE) inhibitory activity was demonstrated by newly described alkaloids carltonine A (13) and carltonine B (14) with IC50 values of 913 ± 20 nM and 31 ± 1 nM, respectively. Both compounds displayed a selective inhibition pattern for hBuChE with an outstanding selectivity profile over AChE inhibition, higher than 100. The in vitro data were further supported by in silico studies of the active alkaloids 13 and 14 in the active site of hBuChE.
Keywords: Amaryllidaceae, Narcissus pseudonarcissus cv. Carlton, alkaloids, carltonine A–C, Alzheimer’s disease, butyrylcholinesterase, docking studies
1. Introduction
Alzheimer’s disease (AD), a progressive neurodegenerative brain disorder featuring memory loss and cognitive impairments, has been named after German psychiatrist Alois Alzheimer. AD is the most common type of dementia among the elderly, generally diagnosed in individuals over the age of 65 years [1]. Considering population aging and increased life expectancy, the number of AD patients is predicted to increase enormously. About 4.6 million new cases are diagnosed every year throughout the world, and the World Health Organization (WHO) estimates that by 2050 over 131 million patients will suffer from AD [2]. Additionally, the current cost of dementia is about a trillion US dollars per year, and it should rise to 2 trillion US dollars by 2030 [3]. Thus, AD starts to be one of the greatest public health problems with severe impact on patients, their families and care workers.
Although the exact pathogenesis of AD remains elusive, it is currently conceived as a multifactorial disease. The main pathological hallmarks of AD are the deposits of β-amyloid peptide (Aβ) in senile plaques and neurofibrillary tangles (NFTs) formed by hyperphosphorylated τ-protein [4]. In addition to them, inflammation and oxidative stress processes also extensively contribute to the loss of synaptic integrity and neurodegeneration.
The current treatment is only symptomatic and mainly involves restoring of acetylcholine (ACh) levels through AChE inhibition [5]. Three AChE inhibitors, namely donepezil, galanthamine and rivastigmine, are currently used as the main therapeutic option for AD treatment [6]. BuChE is another serine hydrolase homologous to AChE that is encoded by a different gene and can be found ubiquitously throughout the body [6]. The role of BuChE has recently gained enormous interest, and not only in the field of AD research [7]. Regarding AD, it is well-documented that AChE activity is downregulated by up to 33–45% of normal values, while the activity of BuChE is escalated by up to 40–90% [8]. This dramatic switch between the AChE/BuChE ratio highlighted the supportive role of BuChE in hydrolysing the excess of ACh. Moreover, several lines of evidence also indicate that both cholinesterases play an important role in Aβ aggregation and maturation to oligomers, fibrils and plaques [9].
POP, a cytosolic serine peptidase, has gained interest as a target for the treatment of neuropsychiatric and neurodegenerative diseases like schizophrenia, bipolar affective disorder, Parkinson’s disease and AD [10]. POP is expressed in several brain regions, and catalyses with high specificity the cleavage of short peptides containing proline at its carboxyl site [11]. POP is responsible for the degradation of neurotransmitters, such as substance P, arginine vasopressin–oxytocin, and neurotensin, which in turn reduce the possibility of generation of toxic Aβ peptides. Generally, POP can be regarded as one of the active players in the processes of learning and memory. In fact, some POP inhibitors have been experimentally found to act as antidementia drugs [10].
Natural products represent an important source of clinical drugs, especially for their structural diversity and wide range of biological activities [12]. Alkaloids are, without a doubt, the most intriguing templates of natural origin [13]. The family Amaryllidaceae comprises about 1100 species in 85 genera, which are distributed worldwide through the tropics and warm regions [14]. Some of the species are cultivated as ornamental plants for their beautiful flowers and fragrant essences. Different types of plant extracts from this family have been long used in traditional medicine for the treatment of cancer by the ancient Greeks, as well as by Africans, Asians, and Polynesian communities for the treatment of various diseases [15,16]. The medicinal value of the Amaryllidaceae species is attributed to the presence of tyrosine-derived alkaloids, typically named as Amaryllidaceae alkaloids (AAs), that are produced exclusively by this family. These alkaloids exhibit a wide range of biological activities, including AChE inhibition, antitumor, antiviral, antimalarial, analgesic antibacterial, and cytotoxic properties. One successful example of an Amaryllidaceae alkaloid is exemplified by galanthamine, a drug indicated for the treatment of mild to moderate vascular dementia and AD [17].
Narcissus L. is the most common genus of the Amaryllidaceae family comprising 80–100 wild species, mainly distributed in Southwestern Europe and North Africa, with some populations in the Balkans, Italy and France. Plants of the genus Narcissus L. have been used in traditional medicine worldwide in cancer therapy [18,19,20,21]. Most of the species can hybridize, thus, a large number of cultivars have been developed for ornamental purposes. Interestingly, some intersectional hybrids and cultivars have also been reported as potential sources of galanthamine and other Amaryllidaceae alkaloids [22,23]. The cultivar Carlton is cultivated for the commercial extraction of galanthamine, because of its relatively high concentration in the bulbs, the large bulb size, and their availability in large volumes. Galanthamine is reported as the major alkaloid of Narcissus pseudonarcissus, followed by haemanthamine. Haemanthamine also has interesting biological activities including inhibition of protein synthesis, antimalarial and antiretroviral activity, as well as cytotoxicity against various cancer cell lines [24,25,26]. Recently, some semi-synthetic derivatives of haemanthamine have been published as promising candidates for AD therapy [27].
As a part of our ongoing research on Amaryllidaceae alkaloids with implication to AD, this work reports the isolation of several such alkaloids from fresh bulbs of Narcissus pseudonarcissus cv. Carlton. The isolated alkaloids that had not been previously studied were submitted for biological evaluation to reveal their inhibition potential towards hAChE, hBuChE, and POP. In vitro data were further supported by investigating the compound’s putative binding modes in the active site of hBuChE to display crucial interaction.
2. Results and Discussion
2.1. Phytochemical Study of Narcissus pseudonarcissus cv. Carlton
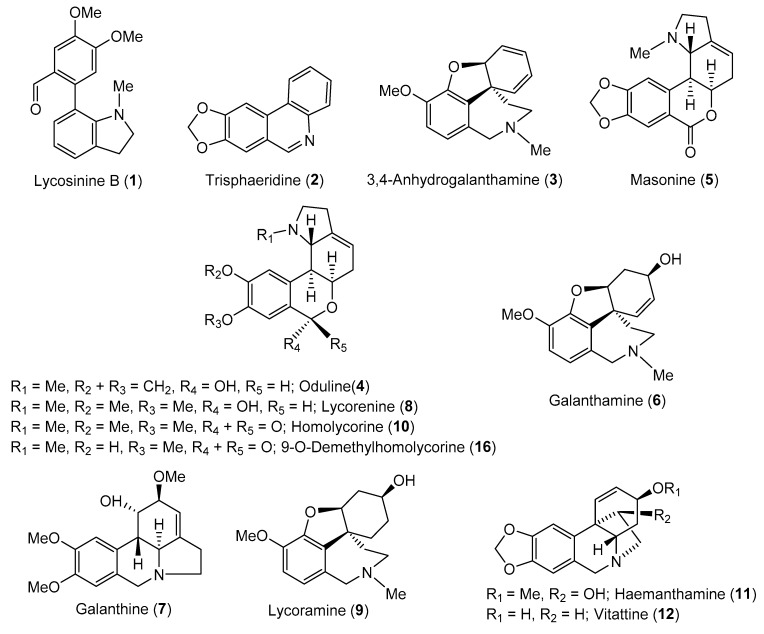
Thirteen known (1–12 and 16) and three novel AAs (13–15) were isolated from bulbs of Narcissus pseudonarcissus cv. Carlton (Amaryllidaceae) by common chromatographic methods, as described in the Experimental section. The compounds were identified by MS, ESI-HRMS, 1D and 2D NMR spectroscopic analyses and by comparison of the obtained data with the literature (Figure 1). These techniques led to the identification of lycosinine B (1) [28], trisphaeridine (2) [29], 3,4-anhydrogalanthamine (3) [30], oduline (4) [31], masonine (5) [32], galanthamine (6) [33], galanthine (7) [34], lycorenine (8) [33], lycoramine (9) [33], homolycorine (10) [31], haemanthamine (11) [20], vittatine (12) [35], and 9-O-demethylhomolycorine (16) [31]. The isolated alkaloids belong to the galanthindole (1), narciclasine (2), galanthamine (3, 6, 9), homolycorine (4, 5, 8, 10, 16), lycorine (7) and haemanthamine (11, 12) structural types; newly isolated alkaloids 13–15 belong to the beladinne-type of AAs.
Figure 1.
Structures of isolated alkaloids from Narcissus pseudonarcissus cv. Carlton.
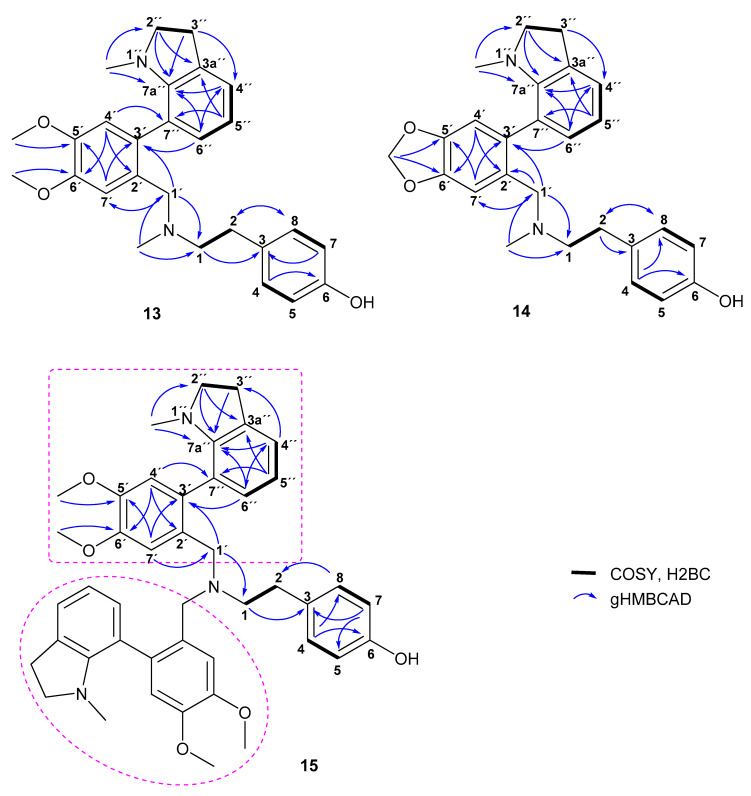
The new compound 13, named carltonine A, was obtained as a pale yellow oil. The HRMS of 13 showed a protonated molecular ion peak [M + H]+ at m/z 433.2488, corresponding to the molecular formula C27H32N2O3 (433.2486 calcd for C27H33N2O3+). The 1H NMR spectrum exhibited resonances associated with nine aromatic protons from which the presence of a p-substituted benzene ring (δH 7.00–6.96; 6.72–6.68), a 1,2,4,5-tetrasubstituted benzene ring (δH 7.10, 6.76) and a 1,2,3-trisubstituted benzene ring (δH 7.09, 6.82, 6.72) were obvious. The high-field part of the 1H NMR spectrum contained one singlet corresponding to two methoxy groups (δH 3.34), two doublets (δH 3.45, 3.29), two doublets of triplets (δH 3.32, dt, overlap, J = 17.3, 8.8 Hz; 3.20, dt, J = 17.3, 8.8 Hz), two triplets (δH 2.98, t, J = 8.8 Hz; 2.65, t, J = 7.4 Hz) and two singlets of N-methyl groups (δH 2.21, 2.20). The 13C and HSQC data revealed signals of four deshielded sp2 carbons (δC 154.0, 150.4, 148.1, 146.9), five nonprotonated sp2 carbons (δC 132.5, 132.1, 131.2, 130.1, 123.0), seven aromatic methines (δC 130.5, 129.8 (2C), 123.3, 117.9, 115.1 (2C), 113.2, 111.1), three deshielded methylenes (δC 59.2, 58.5, 57.1), two methoxy groups (δC 55.9, 55.8), two N-methyls (δC 42.3, 38.6) and two aliphatic methylene groups (δC 32.8, 28.6). The COSY spectrum, supported by H2BC correlations, showed cross-peaks for the H-1/H-2, H-2″/H-3″, H-4″/H-5″/H-6″ and AA′BB′ spin systems. Moreover, these assignments corresponded to the spin-spin splitting of signals in the 1H spectrum. HMBC correlation from H-2 to the sp2 carbons at δC 129.8 revealed the fact that the p-substituted benzene ring was attached to the aminoethyl group. The nitrogen from this substructural fragment bore a methyl at δC 42.3 and a benzylic methylene at δC 58.5. The 1,2,4,5-tetrasubstituted aromatic ring was determined by HMBC correlations from H-4′ and H-7′. This experiment also revealed the conjunction of this fragment with the methylene C-1′ by C-2′. The HMBC cross-peaks of δH 6.76 to δC 123.0 and δH 6.82 to δC 132.1 indicated the attachment of nonprotonated carbons C-3′ and C-7″. This sp2 quaternary carbon belonged to the remaining 1,2,3-trisubsituted aromatic ring which was a part of the N-methylindoline moiety. This was proven by correlation of its protons to the related carbons. Therefore, the structure of 13 was established as depicted (Figure 2). The assigned atoms are shown in Table 1.
Figure 2.
Key 2D NMR correlations of compounds 13–15.
Table 1.
1H NMR (500 MHz) and 13C NMR (125.7 MHz) data for 13–15 in CDCl3 (δ in ppm and J in Hz).
| Carltonine A (13) | Carltonine B (14) | Carltonine C (15) | ||||
|---|---|---|---|---|---|---|
| Position | δ C | δ H | δ C | δ H | δ C | δ H |
| 1 | 59.2 | 2.58–2.46, m | 59.3 | 2.56–2.45, m | 55.2; 55.4 | 2.66–2.42, m |
| 2 | 32.8 | 2.65, t (7.4) | 32.7 | 2.66–2.58, m | 32.7; 32.8 | 2.66–2.42, m |
| 3 | 132.5 | 132.2 | 132.8 | |||
| 4 | 129.8 | 7.00–6.96, m | 129.7 | 7.00–6.94, m | 129.9 | 6.93–6.86, m |
| 5 | 115.1 | 6.72–6.68, m | 115.2 | 6.75–6.72, m | 114.9 | 6.71–6.65, m |
| 6 | 154.0 | 153.9 | 153.7 | |||
| 7 | 115.1 | 6.72–6.68, m | 115.2 | 6.75–6.72, m | 114.9 | 6.71–6.65, m |
| 8 | 129.8 | 7.00–6.96, m | 129.7 | 7.00–6.94, m | 129.9 | 6.93–6.86, m |
| 1′ | 58.5 | 3.45, d (14.0) 3.29, d, overlap (14.0) |
58.3 | 3.46, d (13.2) 3.27, d (13.2) |
55.1; 55.0 | 3.43, d (14.5) 3.29, d (14.5); 3.37, s |
| 2′ | 130.1 | 130.6 | 131.2; 131.0 | |||
| 3′ | 132.1 | 133.3 | 131.9; 131.8 | |||
| 4′ | 113.2 | 6.76, s | 110.2 | 6.75–6.72, m | 113.23; 113.15 | 6.74, s |
| 5′ | 146.9 | 145.9 | 146.7 | |||
| 6′ | 148.1 | 147.0 | 147.9 | |||
| 7′ | 111.1 | 7.10, s, overlap | 108.6 | 7.07, s, overlap | 110.6; 110.5 | 7.15, s; 7.14, s |
| 2″ | 57.1 | 3.32, dt, overlap (17.3, 8.8) 3.20, dt (17.3, 8.8) |
57.1 | 3.35, dt (17.5, 8.7) 3.16, dt (17.5, 8.7) |
57.1; 57.0 | 3.27–3.17, m |
| 3″ | 28.6 | 2.98, t (8.8) | 28.6 | 3.00–2.93, m | 28.6 | 2.99–2.91, m |
| 3a″ | 131.2 | 131.2 | 131.1 | |||
| 4″ | 123.3 | 7.09, dd, overlap (7.4, 1.0) | 123.4 | 7.08, d, overlap (7.4) | 123.3 | 7.07, d (7.4) |
| 5″ | 117.9 | 6.72, t, overlap (7.4) | 118.1 | 6.70, t, overlap (7.4) | 117.9; 117.8 | 6.71–6.65, m |
| 6″ | 130.5 | 6.82, dd (7.4, 1.0) | 130.4 | 6.79, d (7.4) | 130.3; 130.4 | 6.77, d (7.4) |
| 7″ | 123.0 | 122.9 | 122.8 | |||
| 7a″ | 150.4 | 150.4 | 150.3; 150.2 | |||
| N-Me | 42.3 | 2.20, s | 41.9 | 2.18, s | - | - |
| N1″-Me | 38.6 | 2.21, s | 38.8 | 2.23, s | 38.51; 38.48 | 2.14, s; 2.12, s |
| 5′-OMe | 55.8 or 55.9 | 3.34, s | - | - | 55.9 | 3.84, s |
| 6′-OMe | 55.8 or 55.9 | 3.34, s | - | - | 55.6 | 3.82, s; 3.81, s |
| -OCH2O- | - | - | 101.0 | 5.99, s 5.98, s |
- | - |
Carltonine B (14) was obtained as a pale yellow oil. The molecular formula was determined to be C26H28N2O3 from the protonated molecular ion peak [M + H]+ found at m/z 417.2172 (417.2173 calcd for C26H29N2O3+) in the positive-ion HRMS. Due to the small quantity of sample, the 1H NMR spectrum had poor resolution, but it looked similar to that of 13. In addition, the 13C spectrum was in accordance with this observation (see Table 1). The only difference was the absence of signals assigned to two methoxy groups of the 1,2,4,5-tetrasubstituted aromatic fragment, which were replaced by signals corresponding to a strongly deshielded methylene group of a 1,3-dioxole moiety in the 1D spectra. The key correlations are presented in Figure 2.
Another new compound, carltonine C (15), was obtained as a yellowish amorphous solid. The 1D NMR spectrum, as well as the 2D NMR spectra of 15 and 13, were quite similar; however, the 13C NMR data showed doubling of most carbon resonances and the absence of the N-methyl group of the central tertiary nitrogen. Moreover, the molecular formula was determined to be C44H49N3O5 from the protonated molecular ion peak [M + H]+ found at m/z 700.3743 (700.3745 calcd for C44H50N3O5+). These prerequisites led to the identification of the structure shown in Figure 2. The framed substituents of the central tertiary nitrogen are identical; therefore, there is no reason for doubling the signals, as appeared in the 1D NMR data. Due to the steric hindrance of these two fragments, atropisomerism has been estimated as the reason for the doubling of some signals. The analysis was performed at ambient temperature and at 50 °C. The acquired results proved the suitability of this assumption. At ambient temperature, some signals were divided into two lines that broaden with temperature increase and eventually merge into a single line (see Supplementary Material). Unfortunately, the spectrum also revealed the signals of decomposition of this molecule.
2.2. Biological Activity Determination of Isolated Alkaloids
All the isolated compounds that had not been studied previously for their inhibition potential of cholinesterases, and which were obtained in sufficient amounts, were screened for their hAChE/hBuChE inhibition potency using a modified spectrophotometric method of Ellman et al. [36]. Furthermore, hAChE/hBuChE-active AAs were also studied for their ability to inhibit POP enzyme. Galanthamine and eserine were used as positive controls in the hAChE/hBuChE assays. Berberine was selected as a positive control when measuring POP inhibition. The results are summarized in Table 2. Moreover, the in vitro data are justified by docking studies proposing orientation of the top-ranked ligands within the hBuChE gorge.
Table 2.
In vitro results of hAChE, hBuChE and POP inhibition of selected AAs isolated from Narcissus pseudonarcissus cv. Carlton.
| Compound | %Inhibition hAChE ± SD a |
hAChE IC50 ± SD (µM) b | % inhibition hBuChE ± SD a |
hBuChE IC50 ± SD (µM) b | SI for hBuChE c |
POP IC50 ± SD (µM) b |
|---|---|---|---|---|---|---|
| Lycosinine B (1) | 28 ± 1 | >100 | 42 ± 1 | >100 | nc | 258 ± 14 |
| Trispheridine (2) | 6 ± 1 | >100 | 13 ± 1 | >100 | nc | nm |
| 3,4-Anhydrogalanthamine (3) | 4 ± 0 | >100 | 28 ± 1 | >100 | nc | nm |
| Carltonine A (13) | 2 ± 0 | >100 | 98 ± 1 | 0.91 ± 0.02 | >110 | 143 ± 12 |
| Carltonine B (14) | 40 ± 1 | >100 | 99 ± 1 | 0.031 ± 0.001 | >3226 | nm |
| Carltonine C (15) | 9 ± 0 | >100 | 78 ± 1 | 14.8 ± 1.1 | >7 | nm |
| Galanthamine d | nm | 1.72 ± 0.12 | nm | 42 ± 1 | 0.04 | nm |
| Eserine d | nm | 0.063 ± 0.005 | nm | 0.13 ± 0.01 | 0.48 | nm |
| Berberine d | nm | 0.72 ± 0.11 | nm | 31 ± 4 | 0.02 | 142 ± 21 |
a Tested at 100 µM compound concentration; b Compound concentration required to decrease enzyme activity by 50%; the values are the mean values ± standard deviations (SD) of three independent measurements, each performed in triplicate; c Selectivity index (SI) for hBuChE is determined as ratio hAChE IC50/hBuChE IC50; d Reference compound; nc stands for not calculated; nm stands for not measured.
The hAChE/hBuChE inhibitory activity of the AAs was initially screened at a concentration of 100 µM. Compounds displaying inhibition ability >50% against one or both cholinesterases at the screening concentration were selected for the determination of their IC50 values (Table 2).
In the hAChE assay, all studied AAs displayed marginal inhibition potency (Table 2). On the other hand, all newly isolated belladine-type alkaloids (13–15) demonstrated promising inhibition activity towards hBuChE (Table 2; Figure 3). Indeed, compounds 13 and 14 displayed IC50 values in the nanomolar range (hBuChE IC50 = 910 nM and 31 nM, for 13 and 14, respectively).
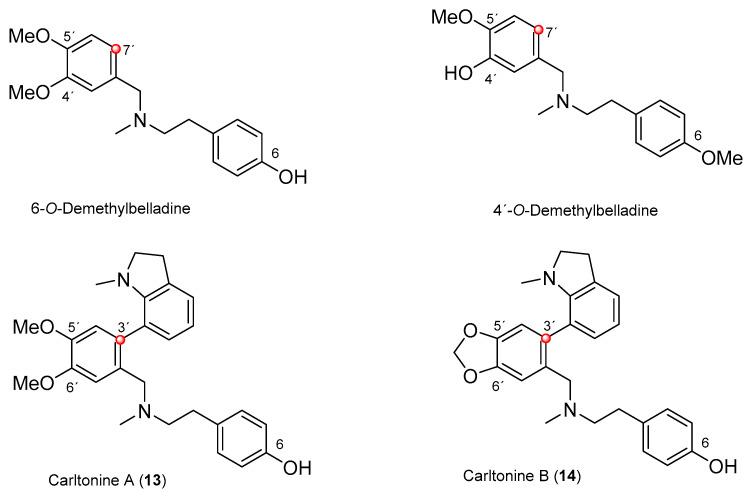
Figure 3.
Structures of newly isolated (13 and 14) and recently reported belladine-type AAs 6-O-demethylbelladine and 4′-O-demethylbelladine [37].
From the structural perspective, both AAs are endowed with the same core structure, differing only in the substitution at positions C-5′ and C-6′, respectively (Figure 2). The presence of a 1,3-dioxolane ring in 14 compared with its opened dimethoxybenzene analogue 13 is plausibly responsible for the almost 30 times drop in hBuChE inhibition activity. Both compounds showed a hBuChE selective inhibition pattern with outstanding selectivity index (SI) values higher than 100 (Table 2).
Our group has previously isolated similar compounds from fresh bulbs of Nerine bowdenii: 6-O-demethylbelladine (13) and 4′-O-demethylbelladine (14) (Figure 3) [37]. However, neither of these two alkaloids are substituted at position C-7′, and differ from each other by the absence of one methoxy group (Figure 3) [37]. 4′-O-demethylbelladine (IC50 = 30.7 ± 4.0 µM) displayed slightly better in vitro inhibition activity of hBuChE compared with galanthamine (IC50 = 42 ± 1 µM). On the other hand, the compounds isolated within this study are more than 30 to 100 more potent hBuChE inhibitors, yielding a new structural lead scaffold that may be pursued in AD research.
Since some of the alkaloids were only isolated on a small scale, only two (1 and 13) were able to be tested for POP inhibition. Alkaloid 13 demonstrated POP inhibition in the same range as berberine (Table 2) [38].
The biological profiles of the other AAs (Figure 1) have already been determined within previous studies on Amaryllidaceae plants [21,39,40,41].
2.3. Docking Study of Carltonine A (13) and Carltonine B (14)
To reveal fundamental interactions for 13 and 14 within the hBuChE active site (PDB ID: 4BDS) [42], molecular docking studies were carried out, enabling better insight into the structural requirements for inhibition. Since both 13 and 14 are tertiary amines, which are protonated at physiological pH, they behave as pseudo-enantiomers. In this study, we attempted to predict more bioactive conformers of 13 and 14 based on their energy score and their topology within hBChE. To gain a more in-depth outlook into the overall ligand-enzyme complex, we are also displaying several comparative structure overlay between (R)-13-(R)-14 (Figure S4A), (S)-13-(R)-13 (Figure S4B), (S)-13-(S)-14 (Figure S4C), and (S)-14-(R)-14 (Figure S4D) pseudo-enantiomers (see Supplementary Material).
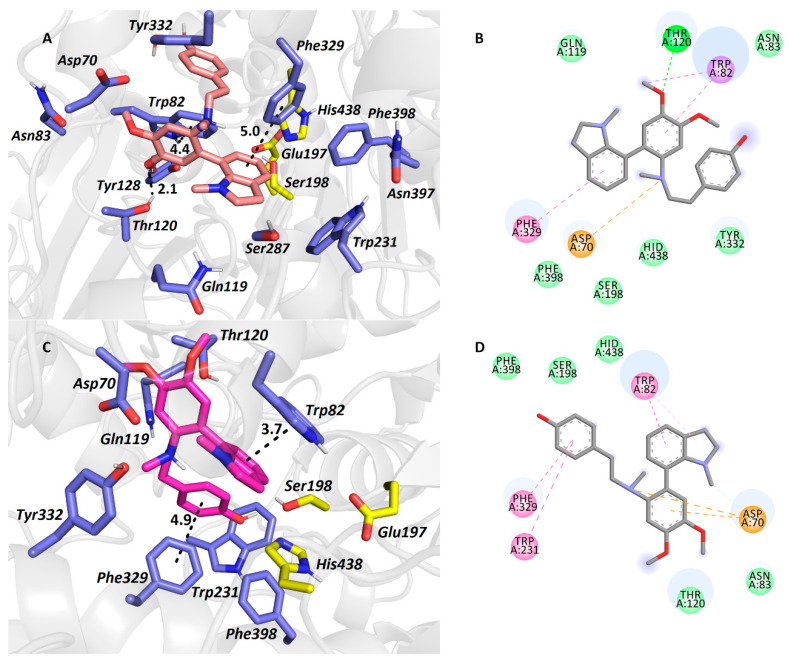
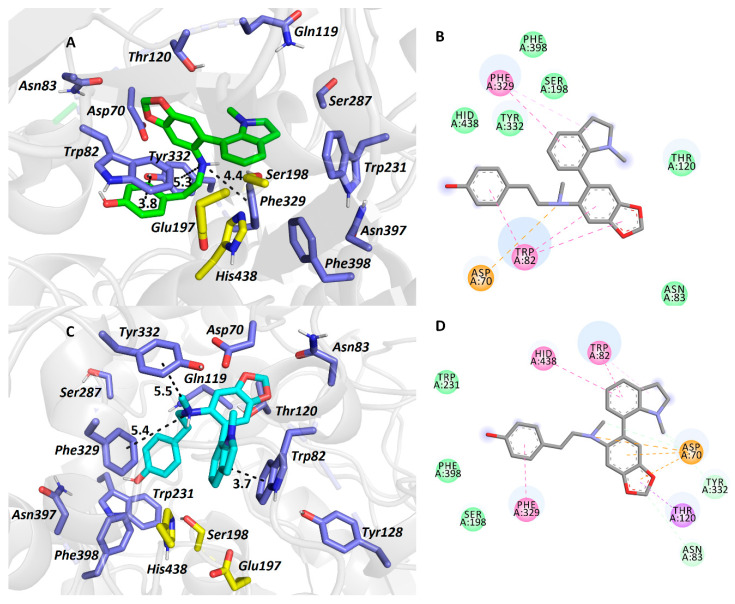
Carltonine A (13) with a central amino moiety in (R) (Figure 4A,B) and (S) (Figure 4C,D) conformations displayed completely different binding modes, even though their estimated energetic balances were equipotent (−10.6 kcal/mol for both). For the (R)-13 pseudo-enantiomer-hBuChE complex (Figure 5A,B), the N-methylindoline moiety occupies the vicinity of the catalytic triad with a T-shaped π-π interaction close to Phe329 (5.0 Å, distance measured from ring-to-ring center). Trp82 faces the dimethoxybenzene ring by displacing π-π interaction, whereas the phenolic appendage is left somewhat vacant. In the (S)-13 pseudo-enantiomer-hBuChE complex (Figure 4C,D), the N-methylindoline moiety is engaged in parallel π-π stacking with Trp82 (3.7 Å). Contrary to the vacant occupancy of the phenolic appendage in the (R)-13 pseudo-enantiomer, in the case of the (S)-13 pseudo-enantiomer, Phe329 (4.9 Å) and Trp231 (5.5 Å) anchor this moiety. The dimethoxybenzene ring is stabilized by π-anion contact to Asp70.
Figure 4.
The hBuChE active site in complex with (R)-13 (in salmon, A,B) and (S)-13 (in purple, C,D) pseudo-enantiomers. All the amino acids exhibiting in the vicinity of the ligands up-to 6.0 Å are rendered. Hydrogen atoms of amino acids are omitted for clarity. Catalytic triad residues are shown in yellow, and amino acid residues in blue (A,C). In 2D diagrams (B,D), crucial amino acid residues are displayed in different colours depending on the nature of the interaction (e.g., purple for π-π, orange for anion-π, dark green for van der Waals contact, and light green for conventional hydrogen bond).
Figure 5.
hBuChE active site in complex with (R)-14 (in green, A,B) and (S)-14 (in light-blue, C,D) pseudo-enantiomers. All the amino acids exhibiting in the vicinity of the ligands up-to 6.0 Å are rendered. Hydrogen atoms of amino acids are omitted for clarity. Catalytic triad residues are shown in yellow and amino acid residues in blue (A,C). In 2D diagrams (B,D), crucial amino acid residues are displayed in different colours depending on the nature of the interaction (e.g., purple for π-π, orange for anion-π, dark green for van der Waals contact, light green for conventional hydrogen bond).
The top-ranked docking poses for (R)-14 and (S)-14 pseudo-enantiomers in hBuChE active site are depicted in Figure 5A–D, respectively. In this case, binding energies differ favoring the (R)-14 pseudo-enantiomer (−11.6 kcal/mol) over the (S)-14 one (–10.9 kcal/mol). Similarly to the (R)-13 pseudo-enantiomer-hBuChE complex, N-methylindoline is stabilized by T-shaped π-π interaction with Phe329 (5.0 Å) and by cation-π formation with the central nitrogen atom of (R)-14 (Figure 5A,B). The protonated amino-group of (R)-14 orchestrates the ligand-enzyme contact by other cation-π interactions with Tyr332 (5.3 Å) and Trp82 (4.5 Å). The latter amino acid residue is in close vicinity to 2H-1,3-benzodioxole displaying T-shaped π-π stacking (4.8 Å), and additionally to the phenolic moiety of the ligand via parallel π-π stacking (3.8 Å). Likewise, the same applies for the (S)-14 pseudo-enantiomer in hBuChE (Figure 5C,D) when observing the central protonated amine of the ligand, i.e., cation-π contact with Tyr332 (5.5 Å) and Phe329 (5.4 Å), but not with Trp82. In this case, Trp82 revealed only an apparently parallel π-π interaction with the N-methylindoline moiety (3.7 Å).
Taken together from all the in silico observations above, it can be deduced that the higher inhibition potency of 14 over 13 might be attributed to the presence of the 2H-1,3-benzodioxole moiety for its “extended” aromatic properties that, especially in the (R)-14 pseudo-enantiomer, broaden the range of hydrophobic interactions between the ligand and enzyme (Table 3).
Table 3.
The best obtained calculated binding energies with Autodock Vina software for the carltonine derivatives under the in silico study within hBuChE enzyme.
| Carltonine Enantiomer | Calculated Binding Energy (kcal/mol) |
|---|---|
| (R)-13 | −10.6 |
| (S)-13 | −10.6 |
| (R)-14 | −11.6 |
| (S)-14 | −10.9 |
3. Experimental
3.1. General Experimental Procedures
All solvents were treated using standard techniques before use. All reagents and catalysts were purchased from Sigma Aldrich, Czech Republic and used without purification. The NMR spectra were obtained in CDCl3 and CD3OD at ambient temperature on a VNMR S500 (Varian, Palo Alto, CA, USA) spectrometer operating at 500 MHz for 1H and 125.7 MHz for 13C. Chemical shifts were recorded as δ values in parts per million (ppm) and were indirectly referenced to tetramethylsilane (TMS) via the solvent signal (CDCl3—7.26 ppm for 1H and 77.0 ppm for 13C; CD3OD—3.30 ppm for 1H and 49.0 ppm for 13C). Coupling constants (J) are given in Hz. For unambiguous assignment of 1H and 13C signals, 2D NMR experiments, namely gCOSY, gHSQC, gHMBC and NOESY, were measured using standard parameter settings and standard pulse programs provided by the producer of the spectrometer. ESI-HRMS were obtained with a Waters Synapt G2-Si hybrid mass analyzer of a quadrupole-time-of-flight (Q-TOF) type, coupled to a Waters Acquity I-Class UHPLC system. The EI-MS were obtained on an Agilent 7890A GC 5975 inert MSD operating in EI mode at 70 eV (Agilent Technologies, Santa Clara, CA, USA). A DB-5 column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies, USA) was used with a temperature program: 100–180 °C at 15 °C/min, 1 min hold at 180 °C, and 180–300 °C at 5 °C/min and 5 min hold at 300 °C; and detection range m/z 40–600. The injector temperature was 280 °C. The flow-rate of carrier gas (helium) was 0.8 mL/min. A split ratio of 1:15 was used. TLC was carried out on Merck precoated silica gel 60 F254 plates. Compounds on the plate were observed under UV light (254 and 366 nm) and visualized by spraying with Dragendorff’s reagent.
3.2. Plant Material
The fresh bulbs of Narcissus pseudonarcissus cv. Carlton were obtained from the herbal dealer Lukon Glads (Sadská, Czech Republic). Botanical identification was performed by Prof. L. Opletal. A voucher specimen is deposited in the Herbarium of the Faculty of Pharmacy in Hradec Králové under number: CUFPH-16130/AL-654.
3.3. Extraction and Isolation of Alkaloids
Fresh bulbs (30 kg) were minced and completely extracted with ethanol (EtOH) (96%, v/v, 3×) by boiling for 30 min under reflux; the combined extract was filtered and evaporated to dryness under reduced pressure. The crude extract (485 g) was acidified to pH 1–2 with 2% hydrochloric acid (HCl; 1 L) and the volume of the suspension was made up to 5 L with water. The suspension was filtered; the filtrate was defatted by diethyl ether (Et2O, 3 × 1.5 L), alkalized to pH 9–10 with a 10% solution of sodium carbonate (Na2CO3) and extracted with ethyl acetate (EtOAc; 3 × 1.5L). The organic layer was evaporated to give 245 g of dark brown fluid residue. The alkaloid summary extract was again dissolved in 2% HCl (1000 mL), washed with Et2O (3 × 300 mL) and alkalized to pH 9–10 with 10% Na2CO3. The water layer was extracted with Et2O (4 × 350 mL) and chloroform (CHCl3; 4 × 350 mL). Both Dragendorff positive parts were evaporated and pooled. A concentrated alkaloid extract (187 g) in the form of brown syrup was obtained.
The alkaloid extract was further fractionated by column chromatography on aluminum oxide (Al2O3; 5800 g), eluting with light petrol gradually enriched with CHCl3 (1:1, 2:3, 1:4; each 5000 mL), followed by CHCl3 (3000 mL) and finally by CHCl3 enriched with EtOH (99:1, 49:1, 97:3, 12:1, 1:1; 0:100 each 3000 mL). Fractions were collected in amounts of 1000 mL and monitored by TLC. Finally, 423 fractions were collected, combined into 25 fractions, and analyzed by GC-MS. Fractions with similar profile were pooled together to give nine final fractions (I–IX).
Fraction I (650 mg) was recrystallized from an ethanol/chloroform mixture (1:1, 100 mL) to give lycosinine B (1, 48 mg). Preparative TLC of the mother liquor (To: cHx: Et2NH, 60:40:5, 1×) led to the isolation of trispheridine (2, 5 mg). Repeated preparative TLC of the mother liquor gave 3,4-anhydrogalanthamine (3, 5 mg).
Fraction II (500 mg) was fractionated by preparative TLC (cHx: Me2CO: NH3; 30: 60: 1; 1×). Three subfractions were obtained (IIa–c). Repeated preparative TLC of subfraction IIb (cHx: Me2CO: NH3, 30: 60: 1; 2×) led to the isolation of oduline (4; 11 mg). Additional preparative TLC of subfraction IIc gave masonine (5; 44 mg).
Fraction III (16.3 g) was crystallized and recrystallized from EtOH and, finally, 6.39 g of galanthamine (6) was obtained.
Fraction IV (1.7 g) was further chromatographed by preparative TLC (To: cHx: Et2NH, 10:10:2, 2×) to obtain subfractions Iva–b. Repeated preparative TLC of IVa (320 mg; cHx: Et2NH, 9:1; 2×) led to the isolation of galanthine (7; 120 mg).
Fraction V (3.05 g) was crystallized and recrystallized three times from EtOH to give lycorenine (9; 981 mg).
Fraction VI (950 mg) was subjected to preparative TLC (To: cHx: Et2NH, 50:50: 5; 2×) to obtain four subfractions VIa-d. Subfraction VIa (120 mg) was treated by preparative TLC (cHx: Me2CO: NH3, 20:80:1; 2x) to give lycoramine (9; 20 mg). Subfraction VIb (150 mg) was subjected to preparative TLC (To: cHx: Et2NH; 40: 60: 5; 2×), crystallized from EtOH, and 45 mg of homolycorine (10) was obtained.
Fraction VII (25 g) was dissolved in hot EtOH and crystallized. Crude haemanthamine (11; 14.5 g) was obtained. This was repetitively (3×) recrystallized from hot EtOH to give 10.5 g of pure haemanthamine.
Repetitive preparative TLC (EtOAc: cHx: Et2NH; 70: 20: 10; 3×) of fraction VIII (3.25 g) led to the isolation of five subfractions (VIIIa-e). Subfraction VIIIa (545 mg) was subjected to preparative TLC (CH3CN: MeOH: NH3; 70: 20: 0.3; 2×) to give vitattine (12; 150 mg). Subfraction VIIIc (550 mg) was chromatographed by preparative TLC (EtOAc: cHx: NH3; 40: 24: 0.6; 2×) to give carltonine A (13; 70 mg) and subfraction VIIIc/1. Subfraction VIIIc/1 (80 mg) was subjected to preparative TLC (EtOAc: cHx: NH3; 40: 24: 0.6; 1×) and carltonine B (14; 6 mg) was obtained. Subfraction VIIId (75 mg) was further treated by preparative TLC (EtOAc: Chx: NH3; 40: 24: 0.6; 2×) to yield carltonine C (15; 7 mg).
Fraction IX (1.65 g) was subjected to preparative TLC (To: Me2CO: MeOH: NH3; 50:60: 10:1; 1×) to give two subfractions IXa-b. Subfraction IXa (320 mg) was recrystallized from hot EtOH and separated by preparative TLC (To: Me2CO: NH3; 40:60:1; 2×) to give 9-O-demethylhomolycorine (16; 35 mg).
Carltonine A (13): pale yellow oil; for 1H and 13C NMR data see Table 1; HRMS m/z 433.2488 [M + H]+ (calculated for C27H33N2O3+, 433.2486).
Carltonine B (14): pale yellow oil; for 1H and 13C NMR data see Table 1; HRMS m/z 417.2172 [M + H]+ (calculated for C26H29N2O3+, 417.2173).
Carltonine C (15): yellowish amorphous solid; for 1H and 13C NMR data see Table 1; HRMS m/z 700.3743 [M + H]+ (calculated for C44H50N3O5+, 700.3745).
3.4. Biological Assays
3.4.1. hAChE and hBuChE Inhibition Assay
The hAChE and hBuChE activities were determined using a modified method of Ellman, as described [36,43,44]. Briefly, hAChE and hBuChE activities were determined using a modified method of Ellman, with acetylthiocholine iodide (ATChI) and butyrylthiocholine iodide (BuTChI) as substrates, respectively. Briefly, 8.3 μL of either blood cell lysate or plasma dilutions (at least six different concentrations), 283 μL of 5 mM 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) and 8.3 μL of the sample dilution in dimethyl sulfoxide (DMSO) (40 mM, 10 mM, 4 mM, 1 mM, 0.4 mM, and 0 mM) were added to the semi-micro cuvette. The reaction was initiated by addition of 33.3 μL 10 mM substrate (ATChI or BuTChI). The final proportion of DTNB and substrate was 1:1. The increase of absorbance (ΔA) at 436 nm for AChE and 412 nm for BuChE was measured for 1 min at 37 °C using a spectrophotometer (SynergyTM HT Multi-Detection Microplate Reader). Each measurement was repeated six times for every concentration of enzyme preparation. The % inhibition was calculated according to the following formula:
| (1) |
where ΔABl is the increase of absorbance of the blank sample and ΔASa is the increase of absorbance of the measured sample. Inhibition potency of the tested compounds was expressed as an IC50 value (concentration of inhibitor, which causes 50% cholinesterase inhibition).
3.4.2. POP Inhibition Assay
POP (EC 3.4.21.26) was dissolved in phosphate-buffered saline (PBS; 0.01 M Na/K phosphate buffer, pH 7.4, containing 137 mM NaCl and 2.7 mM KCl); the specific activity of the enzyme was 0.2 U/mL. The assay was performed in standard polystyrene 96-well microplates with a flat and clear bottom. Stock solutions of tested compounds were prepared in DMSO (10 mM). Dilutions (10−3 to 10−7 M) were prepared from the stock solution with deionized H2O; the control was performed with the same DMSO concentration. POP substrate, (Z)-Gly-Pro-p-nitroanilide, was dissolved in 50% 1,4-dioxane (5 mM). For each reaction, PBS (170 μL), tested compound (5 μL), and POP (5 μL) were incubated for 5 min at 37 °C. Then, substrate (20 μL) was added, and the microplate was incubated for 30 min at 37 °C. The formation of p-nitroanilide, directly proportional to the POP activity, was measured spectrophotometrically at 405 nm using a microplate ELISA reader (multimode microplate reader Synergy 2, BioTek Instruments, Winooski, VT, USA). The inhibition potency of tested compounds was calculated by nonlinear regression analysis and was expressed as an IC50 value (concentration of inhibitor which causes 50% POP inhibition). All calculations were performed using GraphPad Prism software version 7.03 for Windows (GraphPad Software Inc., San Diego, California, USA) [44].
3.4.3. Molecular Modelling Studies
Two structures of hAChE and hBuChE were gained from RCSB Protein Data Bank—PDB ID: 4EY6 (crystal structure of hAChE) and 4BDS (crystal structure of hBuChE) [42,45]. All receptor structures were prepared by DockPrep function of UCSF Chimera (v. 1.4) and converted to pdbqt-files by AutodockTools (v. 1.5.6) [46,47]. Flexible residues selection was based on previous experience with either hAChE, hBuChE or the spherical region around the binding cavity [48,49,50]. Three-dimensional structures of ligands were built by Open Babel (v. 2.3.1), minimized by Avogadro (v 1.1.0) and converted to pdbqt-file format by AutodockTools [51]. The docking calculations were made by Autodock Vina (v. 1.1.2) with the exhaustiveness of 8 [52]. Calculation was repeated 20 times for each ligand and receptor and the best-scored result was selected for manual inspection. The visualization of enzyme-ligand interactions was prepared using The PyMOL Molecular Graphics System (Version 2.0, Schrödinger, LLC, Mannheim, Germany). 2D diagrams were created with Dassault Systèmes BIOVIA, Discovery Studio Visualizer (v 17.2.0.16349, Dassault Systèmes, 2016, San Diego, CA, USA).
4. Conclusions
The phytochemical study of the alkaloidal extract of Narcissus pseudonarcissus cv. Carlton resulted in the isolation of thirteen previously described AAs, and three new AAs of belladine-type, named carltonine A–C (13-15). Their structures were elucidated using a combination of NMR and MS analysis. Compounds isolated in sufficient quantity and not previously tested for their biological activities in relation to AD, were screened for their potential to inhibit hAChE, hBuChE and POP. Significant and selective hBuChE inhibitory activity was demonstrated by the newly described alkaloids carltonine A (13) and carltonine B (14) with IC50 values of 0.91 ± 0.02 µM and 0.031 ± 0.001 µM, respectively. The in vitro results were justified by computational studies predicting plausible binding modes of compounds 13 and 14 in the active site of hBuChE. The new compounds exerted an interesting biological profile deserving further lead-optimization. The next step will be the development of an appropriate synthetic route leading to carltonine derivatives with follow-up preparation of semi-synthetic derivatives.
Acknowledgments
The authors wish to thank Gerald Blunden for critical reading of the manuscript and English corrections.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/10/5/800/s1, Figure S1-1: ESI-HRMS spectrum of carltonine A (13); Figure S1-2: 1H NMR spectrum of carltonine A (13) in CDCl3; Figure S1-3: 13C NMR spectrum of carltonine A (13) in CDCl3; Figure S1-4: gCOSY spectrum of carltonine A (13) in CDCl3; Figure S1-5: gHSQC spectrum of carltonine A (13) in CDCl3; Figure S1-6: gHMBCAD spectrum of carltonine A (13) in CDCl3; Figure S1-7: gH2BC spectrum of carltonine A (13) in CDCl3; Figure S2-1: ESI-HRMS spectrum of carltonine B (14); Figure S2-2: 1H NMR spectrum of carltonine B (14) in CDCl3; Figure S2-3: 13C NMR spectrum of carltonine B (14) in CDCl3; Figure S2-4: gCOSY spectrum of carltonine B (14) in CDCl3; Figure S2-5: gHSQC spectrum of carltonine B (14) in CDCl3; Figure S2-6: gHMBCAD spectrum of carltonine B (14) in CDCl3; Figure S2-7: gH2BC spectrum of carltonine B (14) in CDCl3; Figure S3-1: ESI-HRMS spectrum of carltonine C (15); Figure S3-2: 1H NMR spectrum of carltonine C (15) in CDCl3; Figure S3-3: 13C NMR spectrum of carltonine C (15) in CDCl3; Figure S3-4: gCOSY spectrum of carltonine C (15) in CDCl3; Figure S3-5: gHSQC spectrum of carltonine C (15) in CDCl3; Figure S3-6: gHMBCAD spectrum of carltonine C (15) in CDCl3; Figure S3-7: gH2BC spectrum of carltonine C (15) in CDCl3; Figure S3-8: 1H NMR spectrum of carltonine C (15) in CDCl3 at 50 °C; Figure S3-9: 13C NMR spectrum of carltonine C (15) in CDCl3 at 50 °C; Figure S4: Overlapped pseudo-enantiomers in the hBuChE active site and their topology difference.
Author Contributions
A.A.M., M.Š. contributed to the isolation of Amaryllidaceae alkaloids. J.M., J.K. (Jiří Kuneš), L.N. and M.Š. contributed to the identification of isolated alkaloids (such as MS, NMR). D.H., J.J. and M.H. were involved with the measurement of various biological activities of all isolated compounds. J.K. (Jan Korábečný), T.K. carried out the docking studies. L.C. and J.K. (Jan Korábečný) designed the study, supervised the laboratory work and contributed to critical reading of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by Charles University grants (GA UK no. 178518, SVV UK 260 412, 260 401; Progres/UK Q40 and Q42), by MH CZ—DRO (University Hospital Hradec Kralove, no. 00179906), by the Czech Science Foundation (project no. 20-29633J), by the Long-term Development Plan (Faculty of Military Health Sciences), and by EFSA-CDN (no. CZ.02.1.01/0.0/0.0/16_019/0000841) co-funded by ERDF and by MICU (grant no. SAF2016-76693-R to A.M.). Computational resources were provided by CESNET LM2015042 and the CERIT Scientific Cloud LM2015085, provided under the program “Projects of Large Research, Development, and Innovations Infrastructures”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Burns A., Jacoby R., Levy R. Psychiatric phenomena in Alzheimer’s disease. I: Disorders of thought content. Br. J. Psychiatry J. Ment. Sci. 1990;157 doi: 10.1192/bjp.157.1.72. [DOI] [PubMed] [Google Scholar]
- 2.Nichols E., Szoeke C.E.I., Vollset S.E., Abbasi N., Abd-Allah F., Abdela J., Aichour M.T.E., Akinyemi R.O., Alahdab F., Asgedom S.W., et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cimler R., Maresova P., Kuhnova J., Kuca K. Predictions of Alzheimer’s disease treatment and care costs in European countries. PLoS ONE. 2019;14:e0210958. doi: 10.1371/journal.pone.0210958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A., Singh A., Ekavali A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. PR. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Hampel H., Mesulam M.-M., Cuello A.C., Farlow M.R., Giacobini E., Grossberg G.T., Khachaturian A.S., Vergallo A., Cavedo E., Snyder P.J., et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain J. Neurol. 2018;141:1917–1933. doi: 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zemek F., Drtinova L., Nepovimova E., Sepsova V., Korabecny J., Klimes J., Kuca K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin. Drug Saf. 2014;13:759–774. doi: 10.1517/14740338.2014.914168. [DOI] [PubMed] [Google Scholar]
- 7.Nachon F., Brazzolotto X., Trovaslet M., Masson P. Progress in the development of enzyme-based nerve agent bioscavengers. Chem. Biol. Interact. 2013;206:536–544. doi: 10.1016/j.cbi.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Nordberg A., Ballard C., Bullock R., Darreh-Shori T., Somogyi M. A Review of Butyrylcholinesterase as a Therapeutic Target in the Treatment of Alzheimer’s Disease. Prim. Care Companion CNS Disord. 2013;15 doi: 10.4088/PCC.12r01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inestrosa N.C., Alvarez A., Pérez C.A., Moreno R.D., Vicente M., Linker C., Casanueva O.I., Soto C., Garrido J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron. 1996;16:881–891. doi: 10.1016/S0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 10.Babkova K., Korabecny J., Soukup O., Nepovimova E., Jun D., Kuca K. Prolyl oligopeptidase and its role in the organism: Attention to the most promising and clinically relevant inhibitors. Future Med. Chem. 2017;9:1015–1038. doi: 10.4155/fmc-2017-0030. [DOI] [PubMed] [Google Scholar]
- 11.Szeltner Z., Polgár L. Structure, function and biological relevance of prolyl oligopeptidase. Curr. Protein Pept. Sci. 2008;9:96–107. doi: 10.2174/138920308783565723. [DOI] [PubMed] [Google Scholar]
- 12.Wang S., Dong G., Sheng C. Structural Simplification of Natural Products. Chem. Rev. 2019;119:4180–4220. doi: 10.1021/acs.chemrev.8b00504. [DOI] [PubMed] [Google Scholar]
- 13.Iranshahy M., Quinn R.J., Iranshahi M. Biologically active isoquinoline alkaloids with drug-like properties from the genus Corydalis. RSC Adv. 2014;4:15900–15913. doi: 10.1039/C3RA47944G. [DOI] [Google Scholar]
- 14.Nair J.J., van Staden J. Pharmacological and toxicological insights to the South African Amaryllidaceae. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;62:262–275. doi: 10.1016/j.fct.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Goietsenoven G.V., Mathieu V., Lefranc F., Kornienko A., Evidente A., Kiss R. Narciclasine as well as other Amaryllidaceae Isocarbostyrils are Promising GTP-ase Targeting Agents against Brain Cancers. Med. Res. Rev. 2013;33:439–455. doi: 10.1002/med.21253. [DOI] [PubMed] [Google Scholar]
- 16.Stafford G.I., Pedersen M.E., van Staden J., Jäger A.K. Review on plants with CNS-effects used in traditional South African medicine against mental diseases. J. Ethnopharmacol. 2008;119:513–537. doi: 10.1016/j.jep.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Olin J., Schneider L. Galantamine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2002:CD001747. doi: 10.1002/14651858.CD001747. [DOI] [PubMed] [Google Scholar]
- 18.Bastida J., Viladomat F., Codina C. Narcissus alkaloids. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry. Volume 20. Elsevier; Amsterdam, The Netherlands: 1997. pp. 323–405. Structure and Chemistry (Part F) [Google Scholar]
- 19.Nair J.J., Rárová L., Strnad M., Bastida J., van Staden J. Mechanistic insights to the cytotoxicity of Amaryllidaceae alkaloids. Nat. Prod. Commun. 2015;10:171–182. doi: 10.1177/1934578X1501000138. [DOI] [PubMed] [Google Scholar]
- 20.Bastida J., Lavilla R., Viladomat F. Chemical and biological aspects of Narcissus alkaloids. Alkaloids Chem. Biol. 2006;63:87–179. doi: 10.1016/s1099-4831(06)63003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hulcová D., Maříková J., Korábečný J., Hošťálková A., Jun D., Kuneš J., Chlebek J., Opletal L., De Simone A., Nováková L., et al. Amaryllidaceae alkaloids from Narcissus pseudonarcissus L. cv. Dutch Master as potential drugs in treatment of Alzheimer’s disease. Phytochemistry. 2019;165:112055. doi: 10.1016/j.phytochem.2019.112055. [DOI] [PubMed] [Google Scholar]
- 22.Torras-Claveria L., Berkov S., Codina C., Viladomat F., Bastida J. Daffodils as potential crops of galanthamine. Assessment of more than 100 ornamental varieties for their alkaloid content and acetylcholinesterase inhibitory activity. Ind. Crops Prod. 2013;43:237–244. doi: 10.1016/j.indcrop.2012.07.034. [DOI] [Google Scholar]
- 23.Breiterová K., Ločárek M., Kohelová E., Talácková M., Hulcová D., Opletal L., Cahlíková L. Daffodils as Potential Crops of Biologically-active Compounds: Assessment of 40 Ornamental Taxa for their Alkaloid Profile and Cholinesterases Inhibition Activity. Nat. Prod. Commun. 2018;13 doi: 10.1177/1934578X1801300410. [DOI] [Google Scholar]
- 24.Pellegrino S., Meyer M., Zorbas C., Bouchta S.A., Saraf K., Pelly S.C., Yusupova G., Evidente A., Mathieu V., Kornienko A., et al. The Amaryllidaceae Alkaloid Haemanthamine Binds the Eukaryotic Ribosome to Repress Cancer Cell Growth. Structure. 2018;26:416–425. doi: 10.1016/j.str.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Sener B., Orhan I., Satayavivad J. Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytother. Res. PTR. 2003;17:1220–1223. doi: 10.1002/ptr.1346. [DOI] [PubMed] [Google Scholar]
- 26.Havelek R., Seifrtova M., Kralovec K., Bruckova L., Cahlikova L., Dalecka M., Vavrova J., Rezacova M., Opletal L., Bilkova Z. The effect of Amaryllidaceae alkaloids haemanthamine and haemanthidine on cell cycle progression and apoptosis in p53-negative human leukemic Jurkat cells. Phytomedicine Int. J. Phytother. Phytopharm. 2014;21:479–490. doi: 10.1016/j.phymed.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Kohelová E., Peřinová R., Maafi N., Korábečný J., Hulcová D., Maříková J., Kučera T., Martínez González L., Hrabinova M., Vorčáková K., et al. Derivatives of the β-Crinane Amaryllidaceae Alkaloid Haemanthamine as Multi-Target Directed Ligands for Alzheimer’s Disease. Molecules. 2019;24:1307. doi: 10.3390/molecules24071307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y., Huang S.-X., Zhao Y.-M., Zhao Q.-S., Sun H.-D. Alkaloids from the Bulbs of Lycoris aurea. Helv. Chim. Acta. 2005;88:2550–2553. doi: 10.1002/hlca.200590193. [DOI] [Google Scholar]
- 29.Fan-Chiang T.-T., Wang H.-K., Hsieh J.-C. Synthesis of phenanthridine skeletal Amaryllidaceae alkaloids. Tetrahedron. 2016;72:5640–5645. doi: 10.1016/j.tet.2016.07.065. [DOI] [Google Scholar]
- 30.Bozkurt B., Çoban G., Kaya G., Onur M., Unver-Somer N. Alkaloid profiling, anticholinesterase activity and molecular modeling study of Galanthus elwesii. South Afr. J. Bot. 2017 doi: 10.1016/j.sajb.2017.08.004. [DOI] [Google Scholar]
- 31.Huang S.-D., Zhang Y., He H.-P., Li S.-F., Tang G.-H., Chen D.-Z., Cao M.-M., Di Y.-T., Hao X.-J. A new Amaryllidaceae alkaloid from the bulbs of Lycoris radiata. Chin. J. Nat. Med. 2013;11:406–410. doi: 10.3724/SP.J.1009.2013.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pigni N.B., Ríos-Ruiz S., Martínez-Francés V., Nair J.J., Viladomat F., Codina C., Bastida J. Alkaloids from Narcissus serotinus. J. Nat. Prod. 2012;75:1643–1647. doi: 10.1021/np3003595. [DOI] [PubMed] [Google Scholar]
- 33.Chen J.-Q., Xie J.-H., Bao D.-H., Liu S., Zhou Q.-L. Total Synthesis of (−)-Galanthamine and (−)-Lycoramine via Catalytic Asymmetric Hydrogenation and Intramolecular Reductive Heck Cyclization. Org. Lett. 2012;14:2714–2717. doi: 10.1021/ol300913g. [DOI] [PubMed] [Google Scholar]
- 34.Berkov S., Bastida J., Sidjimova B., Viladomat F., Codina C. Phytochemical differentiation of Galanthus nivalis and Galanthus elwesii (Amaryllidaceae): A case study. Biochem. Syst. Ecol. 2008;8:638–645. doi: 10.1016/j.bse.2008.04.002. [DOI] [Google Scholar]
- 35.Bohno M., Sugie K., Imase H., Yusof Y.B., Oishi T., Chida N. Total synthesis of Amaryllidaceae alkaloids, (+)-vittatine and (+)-haemanthamine, starting from d-glucose. Tetrahedron. 2007;63:6977–6989. doi: 10.1016/j.tet.2007.05.041. [DOI] [Google Scholar]
- 36.Ellman G.L., Courtney K.D., Andres V., Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 37.Vaněčková N., Hošt‘álková A., Šafratová M., Kuneš J., Hulcová D., Hrabinová M., Doskočil I., Štěpánková Š., Opletal L., Nováková L., et al. Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W. Watson and their biological activities. RSC Adv. 2016;6:80114–80120. doi: 10.1039/C6RA20205E. [DOI] [Google Scholar]
- 38.Tarrago T., Kichik N., Seguí J., Giralt E. The Natural Product Berberine is a Human Prolyl Oligopeptidase Inhibitor. ChemMedChem. 2007;2:354–359. doi: 10.1002/cmdc.200600303. [DOI] [PubMed] [Google Scholar]
- 39.Breiterová K., Koutová D., Maříková J., Havelek R., Kuneš J., Majorošová M., Opletal L., Hošťálková A., Jenčo J., Řezáčová M., et al. Amaryllidaceae Alkaloids of Different Structural Types from Narcissus L. cv. Professor Einstein and Their Cytotoxic Activity. Plants Basel Switz. 2020;9:137. doi: 10.3390/plants9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cahlíková L., Hrabinová M., Kulhánková A., Benesová N., Chlebek J., Jun D., Novák Z., Macáková K., Kunes J., Kuca K., et al. Alkaloids from Chlidanthus fragrans and their acetylcholinesterase, butyrylcholinesterase and prolyl oligopeptidase activities. Nat. Prod. Commun. 2013;8:1541–1544. doi: 10.1177/1934578X1300801110. [DOI] [PubMed] [Google Scholar]
- 41.Šafratová M., Hošťálková A., Hulcová D., Breiterová K., Hrabcová V., Machado M., Fontinha D., Prudêncio M., Kuneš J., Chlebek J., et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch. Pharm. Res. 2018;41:208–218. doi: 10.1007/s12272-017-1000-4. [DOI] [PubMed] [Google Scholar]
- 42.Nachon F., Carletti E., Ronco C., Trovaslet M., Nicolet Y., Jean L., Renard P.-Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013;453:393–399. doi: 10.1042/BJ20130013. [DOI] [PubMed] [Google Scholar]
- 43.Pohanka M., Karasova J.Z., Kuca K., Pikula J., Holas O., Korabecny J., Cabal J. Colorimetric dipstick for assay of organophosphate pesticides and nerve agents represented by paraoxon, sarin and VX. Talanta. 2010;81:621–624. doi: 10.1016/j.talanta.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 44.Hostalkova A., Marikova J., Opletal L., Korabecny J., Hulcova D., Kunes J., Novakova L., Perez D.I., Jun D., Kucera T., et al. Isoquinoline Alkaloids from Berberis vulgaris as Potential Lead Compounds for the Treatment of Alzheimer’s Disease. J. Nat. Prod. 2019;82:239–248. doi: 10.1021/acs.jnatprod.8b00592. [DOI] [PubMed] [Google Scholar]
- 45.Cheung J., Rudolph M.J., Burshteyn F., Cassidy M.S., Gary E.N., Love J., Franklin M.C., Height J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012;55:10282–10286. doi: 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- 46.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 47.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panek D., Więckowska A., Wichur T., Bajda M., Godyń J., Jończyk J., Mika K., Janockova J., Soukup O., Knez D., et al. Design, synthesis and biological evaluation of new phthalimide and saccharin derivatives with alicyclic amines targeting cholinesterases, beta-secretase and amyloid beta aggregation. Eur. J. Med. Chem. 2017;125:676–695. doi: 10.1016/j.ejmech.2016.09.078. [DOI] [PubMed] [Google Scholar]
- 49.Hepnarova V., Korabecny J., Matouskova L., Jost P., Muckova L., Hrabinova M., Vykoukalova N., Kerhartova M., Kucera T., Dolezal R., et al. The concept of hybrid molecules of tacrine and benzyl quinolone carboxylic acid (BQCA) as multifunctional agents for Alzheimer’s disease. Eur. J. Med. Chem. 2018;150:292–306. doi: 10.1016/j.ejmech.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 50.Svobodova B., Mezeiova E., Hepnarova V., Hrabinova M., Muckova L., Kobrlova T., Jun D., Soukup O., Jimeno M.L., Marco-Contelles J., et al. Exploring Structure-Activity Relationship in Tacrine-Squaramide Derivatives as Potent Cholinesterase Inhibitors. Biomolecules. 2019;9:379. doi: 10.3390/biom9080379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: An open chemical toolbox. J. Cheminformatics. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.